Abstract
The yellow passion fruit is a key crop in irrigated areas of Northeast Brazil, but production is challenged by limited water availability and high salinity in groundwater used for irrigation. This study evaluated the effects of grafting Passiflora edulis f. flavicarpa Degener (E) onto P. foetida L. (F) rootstock to reduce the impact of saline stress. Conducted in a greenhouse using a 3 × 2 factorial design with four replications, the experiment tested three grafting combinations (F + F, E + E and E + F) under two salinity levels (0.5 and 6.0 dS m−1). Key parameters measured included SPAD index, soluble protein content, hydrogen peroxide (H2O2) levels, catalase enzyme activity, plant height, and leaf number. Salinity significantly reduced plant height, especially at 6.0 dS m−1. The E + E combination had the highest protein content in roots (23.8%). However, grafting P. edulis onto P. foetida (E + F) enhanced catalase activity and reduced H2O2 accumulation, indicating improved tolerance to salt stress. The findings suggest that using P. foetida as a rootstock may help mitigate oxidative damage and promote better physiological performance of yellow passion fruit under saline conditions, offering a strategy to sustain cultivation in stress-prone environments.
1. Introduction
Brazil is the largest producer of yellow passion fruit in the world, responsible for 65% of total production [1], and Northeast Brazil is the country’s largest producer, accounting for approximately 70% of national production [2]. Despite this, this region has a wide range of climates and is very susceptible to irregular rainfall, and generally the use of supplementary irrigation water, especially underground, has a high content of soluble salts which reduces the productivity of the crop and the interest of the rural producer [3].
High electrical conductivity increases the soil’s osmotic potential, hindering water absorption and causing toxic effects on passion fruit crops, which are considered sensitive, with a salinity threshold of 1.3 dS m−1 [4]. Salt stress can negatively impact the efficiency of photosynthesis because a reduction in stomatal conductance limits water loss but also results in a decrease in CO2 in the substomatal chamber and consequently an increase in photorespiration and subsequent interruption of photosynthetic activity [5], changes in root growth patterns and shoot development [6], and an imbalance due to excess sodium ions (Na+) with other essential cations, such as potassium (K+), affecting cellular functions and enzymatic activities [7].
Plants exposed to salinity synthesize reactive oxygen species (ROS) in their tissues, notably, superoxide (O2−), hydroxyl radical (OH−) and hydrogen peroxide (H2O2) [8]. As a strategy to protect plant cells, H2O2 is used as a signaling molecule to promote the action of antioxidant enzymes [9]. The exacerbated presence of ROS in cells results in changes in metabolism and even interactions with organic molecules, causing cell death [10], and the balance of ROS levels is carefully maintained by a complex system of antioxidant enzymes, especially catalases and ascorbate peroxidase, which play a vital role in breaking down H2O2 into H2O and O2− [11]. These enzymes act at the cellular level in plant organelles such as chloroplasts, mitochondria and peroxisomes, acting as essential defenses against the damage caused by ROS [12].
A number of strategies can be adopted, such as soil and water management techniques [13], the use of microorganisms and biostimulants [14,15], genetic improvement [16] and grafting techniques using salinity-resistant rootstocks [17,18]. There are approximately 530 species belonging to the Passifloraceae family, of which 400 belong to the genus Passiflora, among which the species Passiflora foetida L., an inedible wild passion fruit, is an excellent option as it has a tolerance of up to 4.0 dS m−1 [5,6].
The aim of this study was to evaluate the ability of grafting yellow passion fruit onto P. foetida L. rootstock and its effects on initial growth to reduce the effects of salt stress by increasing antioxidant activities, specifically the enzymatic activity of catalase, and reduce the accumulation of H2O2.
2. Materials and Methods
2.1. Location and Experimental Period
The work was carried out from January to May 2023 in a greenhouse at the Institute of Plant Physiology of the National University of La Plata, 34°54′46.06″ S, 57°55′50.82″ O, Argentina.
2.2. Plant Material
Two types of plant material were used for the experiment: the commercial sour passion fruit cultivar cv. SCS437 Catarina (Passiflora edulis f. flavicarpa Degener), which was used as a scion. The seeds were harvested from fully ripe and healthy fruits from a commercial orchard. The second species was the passion fruit tree (Passiflora foetida L., rootstock), the seeds of which were collected from native plants with mature and healthy fruit in the municipality of Upanema/RN/Brazil.
2.3. Growth Conditions
The region’s climate is classified as temperate, specifically humid subtropical temperate, with the months of December, January and February being the hottest, reaching 35 °C. The greenhouse where the experiment was conducted was built on the second floor of the building with ample sunlight. It has a masonry base, an iron structure and walls and a roof made of semitransparent polycarbonate tiles, allowing for the greenhouse effect. The house has a humidifier and heat exhaust for more efficient control of temperature (25 ± 2 and 20 ± 3 °C, day and night, respectively) and humidity (60 and 85%). The daily light/dark periods were approximately 14 and 10 h, respectively.
2.4. Experimental Design
The experiment was conducted in a completely randomized design (CRD) with four replications in a 3 × 2 double factorial design, with three combinations of grafting species (CGS): P. edulis grafted onto itself (E + E), P. foetida L. grafted onto itself (F + F) and P. edulis grafted onto P. foetida L. (F + E) and two levels of irrigation water salinity (S1: 0.5 dS m−1, local supply water; and S2: 6.0 dS m−1, prepared by dissolving 1929 g m−3 NaCl, 588 g m−3 CaCl2, and 492 g m−3 MgCl2, in a 7:2:1 ratio, which is predominant in groundwater sources of the Brazilian Northeast [19]). The experiment included 24 useful plots, each with an experimental unit of one plant.
2.5. Seedling Production and Transplanting
To obtain the seedlings, three seeds per cup were sown in 150 mL disposable cups with Plantmax® commercial substrate (Plantmax, Cascavel, PR, Brazil). The P. foetida species was sown 10 days before P. edulis because of its delayed germination and thicker stem. On the fifth day after germination (DAG), the excess plants were thinned, leaving just one plant per cup. Once the seedlings were established in the substrate, at 25 DAG, grafting was carried out via a full cleft according to each treatment, and five days later, they were transplanted into 3-L pots containing a mixture of virgin-area vertisol and vermiculite in a 2:1 ratio, respectively.
2.6. Salinity Treatments and Water Management
The treatments were applied daily for 20 days starting at 50 DAG, when all the plants were well established and vigorous in the pots. The water requirement was determined via lysimetry by collecting and weighing the water in an impermeable container. This determination was performed in pots containing the same soil and plants as the treatments, in which the water balance of inflow and outflow from the soil profile was recorded.
2.7. Variables Analyzed
After the treatment period, the SPAD index was determined for the third fully expanded leaf, starting from the apex. Measurements were taken between 7 and 9 a.m. via a SPAD-502 portable chlorophyll meter (Minolta Camera Co., Ltd., Osaka, Japan). Four SPAD index measurements were taken per leaf in the central region of the leaf limbus of each plant in the useful plot, totaling 16 measurements per plot, in each treatment, and the average was used to represent the treatments.
Immediately after the readings were taken, the plants were harvested, the substrate was removed from the root system under running water, and the material was transported to the laboratory for further analysis. Fresh weight and shoot length were measured. The total soluble protein content was then determined via the use of bovine serum albumin (Sigma-Aldrich®, St. Louis, MO, USA) as a standard [20].
To determine the content of H2O2 in the leaves and roots, three leaf limb discs (approximately 0.02 g) and three cm of roots (approximately 0.01 g) were placed in 2 mL Eppendorf microtubes containing 2 mL of Amplex Red™ solution with 10.000 U/L horseradish peroxidase, 10 µmol/L Amplex Red (N-acetyl-3,7-dihydroxyphenoxazine) and 50 mmol/L Tris-HCl buffer, pH 7 (Thermo Fisher Scientific, Waltham, MA, USA). The tubes were then placed in a shaker at 100 rpm for incubation for 100 min. After this period, the fluorescence of the solution was measured via a CLARIOstarPlus multimode microplate reader (Ortenberg, Germany) in fluorescence mode at a wavelength of 560 nm [21]. The H2O2 content was estimated by comparing the H2O2 standard curve with the values observed in the fluorescence changes in the samples (Amplex Red™) [22].
For catalase (CAT) enzyme activity, approximately 500 mg of leaves and 500 mg of roots were macerated in liquid nitrogen and placed in separate 2 mL Eppendorf microtubes, and 1 mL of 100 mM bicine buffer solution, pH 7.5, containing 10% (v/v) glycerol and protease inhibitors (1 nM phenylmethylsulfonyl fluoride; 1 µM E-64 and 1 µM EDTA, from Sigma-Aldrich®) was added. The homogenized mixture was centrifuged at 13,000 rpm for 10 min in a refrigerated centrifuge at 4 °C, and the resulting supernatant was subsequently used to analyze the enzymatic activity via a UV-visible spectrophotometer (Shimadzu, Kyoto, Japan, model: AV-160A).
CAT activity was measured according to the methodology recommended by [23], which consists of monitoring the decomposition of H2O2 via a spectrophotometer at a wavelength of 240 nm. For this purpose, 50 mM potassium phosphate-buffer solution (pH 7.0), 15 mM H2O2, up to 100 μL of homogenate (1 mg/mL protein) and 0.1% (v/v) Triton X-100 were used. The activity was monitored for 40 s by reducing the absorbance.
The activity was calculated according to Equation (1):
where
(α tg) = ΔA/Δt; where
α tg = change in tangent;
ΔA = the change in absorbance;
Δt = the change in time (minutes).
2.8. Statistical Analyses
The data were tested for ANOVA assumptions of normality (Shapiro–Wilk test) and homogeneity of variances (Levene’s test). Variables that did not meet these assumptions (H2O2 in the leaves and roots, CAT activity in roots, and number of leaves) were transformed using a quadratic equation. ANOVA was then performed, and means were compared using Tukey’s test (p ≤ 0.05) to reduce type I error. Analyses were conducted in RStudio version 3.6.0, and the tables and figures present the original (non-transformed) means.
3. Results
3.1. Total Soluble Proteins
Significant differences in total soluble protein content (TSPC) were observed among the grafting combination (p < 0.05), as shown in Table 1, especially for the total soluble protein content in roots (TSPCR). No significant effects of salinity or interactions among species and salinity were observed on total soluble protein content in leaves and roots (p > 0.05). The coefficient of variation was 8.25% for TSPCL and 16.69% for TSPCR, indicating acceptable experimental precision (Table 1).

Table 1.
F test, Tukey’s test, and standard deviation for total soluble protein content in yellow passion fruit under different grafting combinations and salinity levels.
According to the Tukey test, the combination P. edulis grafted on itself presented the highest mean TSPCR value (0.26 ± 0.03 mg g−1), followed by P. foetida L. grafted on itself (0.21 ± 0.05 mg g−1) and P. edulis grafted on P. foetida L. (0.17 ± 0.02 mg g−1), which presented the lowest value. The last two combinations did not differ significantly from each other but were significantly lower than the self-grafted P. edulis (p < 0.05). The TSPCR of the combination P. edulis grafted on itself was 36.8% higher than the other combinations that presented an average of 0.19 mg g−1 (Table 1).
3.2. Hydrogen Peroxide (H2O2) and Catalase (CAT)
For hydrogen peroxide in leaves and roots, isolated effects of species combinations and salinity levels were significant, as well as their interaction (p < 0.05) (Table 2). The highest H2O2 content was detected in the leaves of the P. edulis grafted onto itself (E + E) under irrigation water with EC of 6.0 dS m−1, representing an increase of 314.2% compared with the EC of 0.5 dS m−1 (S1) for the same grafting combination (E + E). This value was also 240.5% higher than the average all grafting combinations under the same salinity condition (Figure 1A).

Table 2.
F test for hydrogen peroxide in leaves (H2O2 leaves) and roots (H2O2 roots) in yellow passion fruit under different grafting combinations and salinity levels.
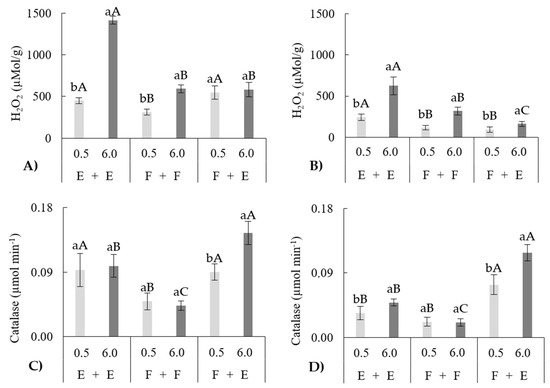
Figure 1.
Hydrogen peroxide (H2O2) content in leaves (A) and roots (B) and catalase (µmol min−1) content in leaves (C) and roots (D) of passion fruit plants under different grafting conditions and different salinity levels (dS m−1). Averages followed by the same lowercase letter for the same species combination and uppercase letter for the same salinity level do not differ according to Tukey’s test (p < 0.05).
At the basal salinity level (S1), the F + F combination exhibited H2O2 content in leaves 37.5% lower than the averages observed for the E + E and F + E combinations, which did not differ from each other. At 6.0 dS m−1 (S2), the F + F and F + E combinations presented similar to each other H2O2 content, 58.1% lower than that observed for E + E combination (Figure 1A).
Evaluating the different species combinations under S1, the H2O2 synthesis for the combination F + F was 37.5% lower than the average for the other combinations (equal to each other). Under S2, the H2O2 synthesis for the combination E + E was 240.5% higher than the average for the other combinations (equal to each other) p < 0.05 (Figure 1A).
With respect to the content of H2O2 in the roots, at the basal salinity level, the F + F and F + E combinations resulted in reductions in H2O2 compared with E + E of 53.3 and 61.9%, respectively. For S2, the F + E combinations presented the lowest average value, which was 49% lower than that of F + F and 74.1% lower than that of E + E. For E + E, F + F and F + E, there were increases of 256.1, 278.9 and 174.2%, respectively, for S2 compared with S1 (Figure 1B). The levels of H2O2 in the roots were significantly lower than those in the leaves, with a 2.5-fold reduction (Figure 1A,B).
Evaluating the activity of CAT in the leaves, it was observed for S1 that the F + F combination had a catalase content in the leaves that was 47.3% lower than that of the E + E combination and 45.5% lower than that of F + E. Similar behavior occurred for S2, where the F + F combination had a catalase content in the leaves that was 56.6% lower than that of the E + E combination and 70.3% lower than that of F + E (Figure 1C).
The evaluation of the activity of catalase in the roots revealed that at 0.5 dS m−1, the F + F and E + E combinations did not differ from each other but had lower averages than the F + E combinations, with values in the order of 70.3 and 50%, respectively. For an S2, the F + E combinations presented the highest average, which was 240.8% greater than that of the E + E combinations and 561.9% greater than that of the F + F combinations. In addition, only the F + F combinations presented no difference between the different ECs tested. On the other hand, when exposed to S2, E + E and F + E resulted in increases of 44.1 and 59.4%, respectively, in catalase activity compared with that resulting from S1 (Figure 1D). The catalase activity in the roots was significantly lower than that in the leaves, with a reduction of 38.7% (Figure 1C,D).
3.3. SPAD Index
The SPAD index and plant height (PH) were influenced separately by the tested factors: different species combinations and salinity level. The number of leaves (NL) and catalase (CAT) in the leaf and root underwent a double interaction of the factors (Table 3). Regarding the SPAD index, the E + E combination presented a dense green coloration (Figure 2), higher than the SPAD indices of the F + F and F + E combinations, by 20.8 and 12.9%, respectively (Table 3).

Table 3.
F test for growth parameters, catalase activity and Tukey’s test and standard deviation for in passion fruit under different grafting combinations and salinity levels.

Figure 2.
Passiflora edulis grafted onto itself (A and B), P. foetida L. grafted onto itself (C and D) and P. edulis grafted onto P. foetida L. (E and F). Irrigation water level salinity: 0.5 dS m−1 (A, C and E) and 6.0 dS m−1 (B, D and F).
3.4. Plant Height
For this variable, there was no interaction between the sources of variation tested. However, there was an isolated effect of the different combinations of species, where E + E and F + F were statistically equal to each other. However, both were significantly superior to F + E, with increases of 13.7 and 18.5 cm in plants height, respectively (Figure 3A). Concerning the performance of the plants in the face of salinity, there was a significant reduction of 9.6 cm when they were exposed to irrigation water of 6.0 dS m−1 compared with those irrigated with 0.5 dSm−1 (Figure 3B).
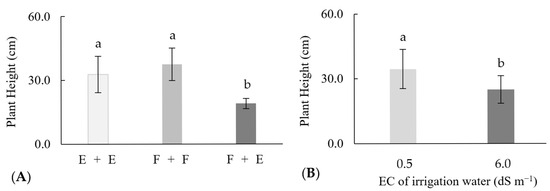
Figure 3.
Height (cm) of passion fruit plants under different grafting conditions (A) and different levels of salinity (B) of the irrigation water (EC). Averages followed by the same lowercase letters do not differ according to Tukey’s test (p < 0.05).
3.5. Number of Leaves
The number of leaves was significantly affected by both salinity and the different combinations of species, and a significant interaction effect was detected between the factors (p < 0.05). Compared with the salinity levels, only the F + F species combination differed (Table 4). In this case, plants subjected to irrigation water with S2 presented a significant reduction in the number of leaves, on the order of 30%. Evaluating the different combinations of species within each EC level, it can be seen that at both 0.5 and 6.0 dS m−1, the F + F combination was superior for this variable; however, for the lowest EC, the amplitude of the data was more prominent, with a difference between the three combinations with a reduction of 65 and 78%, respectively, for E + E and F + E, whereas for the highest EC, there was no difference between E + E and F + E.

Table 4.
Number of leaves of passion fruit plants under different grafting combinations and different levels of irrigation water salinity.
4. Discussion
Saline stress causes significant damage to agriculture in arid and semiarid regions worldwide. The Brazilian semiarid region is affected by saline stress, primarily due to the use of brackish water in agriculture. Yellow passion fruit is an important crop in the irrigated areas of Northeast Brazil, but limited water availability and high salinity of groundwater used for irrigation reduce crop production. Research on salinity management strategies in agriculture is important, especially those testing genotypes with the potential to tolerate saline stress. We studied this, using Passiflora rootstock combinations to increase salinity tolerance. We found that using P. foetida L. as a rootstock for P. edulis can improve plant performance under saline stress conditions.
The results revealed significant impacts of irrigation water salinity and different scion/scion combinations on the initial growth and antioxidant responses of yellow passion fruit. The total soluble protein content (TSPC) in passion fruit has not received considerable attention, but it plays a crucial role in the physiological response to abiotic and biotic stresses and allows us to assess the health and adaptability of plants in different growing environments [24,25]. In addition, variation in TSPC has been associated with different stages of plant development and various levels of abiotic stress, providing valuable insights into the mechanisms of molecular regulation during growth, development and response to adverse conditions [26]. The total soluble protein content in roots (TSPCR) data are similar to those presented by the SPAD index, indicating that these plants have higher levels of chlorophyll, a higher concentration of nitrogen and greater protein synthesis distributed throughout the plant tissues, especially in the roots. Nitrogen acts as a structural component in the formation of proteins and consequently affects the vegetative development of plants by stimulating the emission and growth of buds [27], whereas potassium plays a fundamental role in the synthesis of proteins, carbohydrates, sugars and organic acids [28]. The production of H2O2 in plants is a natural process of photosynthesis, but various sources of biotic and abiotic disturbances can intensify its production, causing oxidative damage to various plant tissues. One of the main sources of disturbance is exposure to higher electrical conductivity (EC) of irrigation water, as observed in this study, where plants exposed to an EC of 6.0 dS m−1 (S2) presented greater production of H2O2, especially for P. edulis grafted onto itself.
The result obtained in this study is a direct consequence of the impact of salinity on the stomatal aperture, resulting in an imbalance between photochemical energy consumption and CO2 fixation in the Calvin cycle [29]. This condition reduces the availability of NADP+, the main electron acceptor, while promoting photorespiration, making the intracellular environment electrically unstable and resulting in an increase in the production of various reactive oxygen species (ROS) [29]. This was confirmed when we compared the levels of H2O2 expressed in the leaves and roots. The highest concentration was clearly detected in the leaves, where the metabolic processes of photosynthesis and respiration are most active. Salinity causes an imbalance in the electron transport chain (ETC) of photosystem I, increasing the availability of electrons in the system, which results in the reduction of O2− to superoxide (O2−) and subsequent dismutation to H2O2, i.e., the highest concentration of H2O2 is close to the production site and photosynthetically active areas and is increased by exposure to adverse salinity conditions [30,31].
The levels of H2O2 associated with the P. foetida L. species as a scion under both low-salinity and high-soluble salt conditions are in line with previous studies that recommend using the species for this purpose up to 4.0 dS m−1 [17]. This lower expression of H2O2 could be associated with structures such as glands, papillae and trichomes that allow tolerance to excess salts through the salt exclusion mechanism, which would allow the photosynthetic apparatus to function better, increasing the tolerance of the plant to salinity [24,25]. Compared with less adapted plants, plants that are more adaptable to stress conditions tend to present lower levels of H2O2 [17]. This is due, among other factors, to the increased activity of antioxidant enzymes, mainly peroxidase and catalase [32]. The low expression of H2O2 in grafted plants of P. foetida L. may be associated with two adaptive responses of this plant, the first related to the low concentration of intrinsic H2O2, which acts as a signaling molecule for the action of enzymes capable of dismutating these molecules, thus keeping their levels below critical levels [33], as well as those related to the activation of a powerful antioxidant system that modulates numerous physiological processes, including photosynthesis [28,34].
The activity of the catalase enzyme in the aerial part and roots observed in this study for F + E exposed to S2 implies a potential adaptive response of P. foetida L. to the higher EC of the irrigation water [35]. These high levels of the CAT enzyme contribute to the detoxification of H2O2, which is present at relatively low levels, suggesting that the plants respond to the potential oxidative stress induced by changes in the concentration of ions with greater expression of antioxidant enzymes, especially CAT, which dismutates reactive oxygen species [36]. From the behavior observed in our study, it can be concluded that the P. foetida L. rootstock increases resistance to abiotic stress, reduces the accumulation of ROS, notably hydrogen peroxide, and improves the capacity of its ROS elimination system by increasing the synthesis of antioxidant enzymes, including catalase and nonenzymatic compounds. However, in plants sensitive to salt stress, such as P. edulis, the increase in CAT activity was not sufficient for reducing hydrogen peroxide levels compared with the more resistant P. foetida L. [37].
Catalase activity is a variable that responds to ROS levels, notably H2O2, and shows a positive correlation under salt stress [38], as observed only for the F + E combination. This behavior may be associated with a greater capacity of the rootstock to maintain the balance of reactive oxygen species (ROS) and antioxidant enzymes, regulating them at levels where they act as signaling molecules, leading to adaptive responses [6,39]. However, H2O2 is not the only variable responsible for the oscillation in CAT levels; the action of this biochemical antioxidant agent is also related to other agents, such as superoxide dismutase and ascorbate peroxidase, which together contribute to redox homeostasis, as well as nonenzymatic antioxidants (ascorbic acid, carotenoids, flavonoids and phenolic compounds), which together act to prevent oxidative damage and maintain low concentrations of ROS [39,40]. The present study addressed the catalase activity and H2O2. Therefore, it is premature to conclude that the low level of H2O2 in F + E is a direct consequence of the action of catalase.
The data this study showed that different combinations of species significantly influence the SPAD index in passion fruit plants under conditions of salt stress through the loss of chlorophyll and the consequent decline in photosynthetic activity [41,42]. Compared with the F + F and F + E combinations, the E + E combination resulted in a more intense green color. This result can be attributed to various factors, including the intrinsic characteristics of the grafted species and their interactions [27,29]. Studies have indicated that the appropriate selection of rootstocks can improve the efficiency of nutrient and water absorption, influencing the quality and quantity of chlorophyll in the leaves; i.e., photosynthesis, leaf transpiration and instantaneous water use efficiency are indirectly altered by the rootstock [43,44]. When we evaluate this variable in isolation, we suggest that the E + E combination may have shown greater genetic compatibility, but the F + F combination does not show a correlated value, making it possible to conclude that this behavior is due to an intrinsic characteristic of the species and not a more effective response to grafting. Furthermore, the variation in the SPAD index between the combinations of species did not result in different responses to salt stress. Previous studies have suggested that different plant species have different mechanisms for adapting to salt stress, including the regulation of photosynthetic activity and the production of photosynthetic pigments [45]. These different combinations may lead to the development of more efficient adaptive strategies to maintain chlorophyll integrity under saline conditions, resulting in a more balanced SPAD index even under stress conditions.
Our results are different from those obtained by previous studies, which highlight the differences in chlorophyll A, B and total levels between the P. edulis and P. foetida L. species, where the latter presented significantly higher values than did the former, leading us to propose that the E + E combination, which results in a denser green color, indicates better maintenance of photosynthetic activity [9]. In summary, the observation of the isolated effect on the SPAD index between different combinations of species highlights the importance of careful rootstock selection, providing valuable insights for optimizing passion fruit production in challenging environments. However, associations with other variables are essential for more conclusive analyses, as are further studies to better highlight the interactions between plants, the variables evaluated and stress conditions.
The results obtained in this study indicate that although no significant interaction was identified between the sources of variation tested, the different combinations of species played a particular role in the growth characteristics of passion fruit plants as well as under conditions of salt stress. The literature shows reduced growth of passion fruit (P. edulis) plants under salt stress [46,47]. However, our results reveal that grafting P. edulis onto P. foetida improves plant growth under salt stress. Genetic compatibility studies are extremely important for optimizing plant growth and development through successful grafting strategies [48]. The statistical similarity between the E + E and F + F combinations suggests that these two genetic configurations have similar characteristics that favor plant performance. The significant reduction in plant height when plants were exposed to irrigation water with relatively high salinity (S2) is in line with studies reporting negative effects of salt stress on plant growth [30,33]. These findings confirm the sensitivity of passion fruit plants to relatively high salinity conditions, resulting in an adverse effect on the vertical development of the plants. If we relate these results to the observations of the SPAD index, we can see consistency in the response patterns of the plants. Compared with the F + F and F + E combinations, the E + E combination, which resulted in greater height, also resulted in a denser green color, as indicated by the greater SPAD index. This consistency suggests that taller plants may also have greater photosynthetic efficiency [49].
In addition, the results related to the total soluble protein content (TSPCL and TSPCR) reinforce the idea that the E + E combination has superior characteristics. The 23.8% increase in total soluble protein content in the roots of this combination compared with the other combinations highlights its particular ability to respond positively to grafting onto itself, possibly through specific adaptive mechanisms. Taken together, these results suggest that the different plant combinations used for grafting play critical roles, affecting not only growth characteristics such as height but also essential physiological processes such as photosynthetic efficiency and total soluble protein production. This study revealed that the number of leaves on passion fruit plants is sensitive to salinity and to different combinations of species. The results revealed a reduction in the number of leaves in plants subjected to irrigation water with a relatively high EC, which was even more pronounced in the F + F combination.
The analysis of the different species combinations further reinforced the importance of studies evaluating the interactions between species in the face of salinity stress. The F + F combination showed consistent superiority, regardless of the salinity level, and the F + E combination was satisfactory, with an increase in the number of leaves at the highest salinity. These results are in line with the observations on plant height and the SPAD index discussed earlier. The E + E combination, which was positive in terms of height and SPAD index, was less efficient in terms of leaf production; on the other hand, it did not reduce at the highest salinity, similar to the F + F combination. This pattern suggests a complexity in the physiological responses to both different grafting combinations and salt stress, showing that specific characteristics can be favored or harmed depending on the context evaluated [50]. Previous studies have highlighted the variability in the responses of different plant species to salt stress, which are influenced by adaptation mechanisms specific to each species [36,38]. Compared with the other combinations, the F + F combination had a more robust response to salt stress. This is due to adaptive mechanisms such as trichomes and leaf glands that act to exclude sodium [33]. In summary, this research highlights the importance of carefully selecting combinations of passion fruit species to optimize performance under conditions of salt stress. Understanding the specific interactions that favor the maintenance of leaf number and other morphophysiological characteristics is crucial for advancing sustainable passion fruit cultivation in challenging environments.
5. Conclusions
Salt stress decreases plant height and leaf number in passion fruit, regardless of the scion/rootstock combination used. Salt stress causes hydrogen peroxide (H2O2) accumulation in P. edulis plants grafted onto themselves. However, H2O2 levels are controlled in P. edulis grafted onto P. foetida L. due to increased catalase activity. Grafting yellow passion fruit onto P. foetida L. rootstock is viable because it reduces the oxidative stress caused by salt stress in passion fruit plants.
Author Contributions
Conceptualization, A.A.d.S. and J.F.d.M.; methodology, C.S.; formal analysis, R.R.d.S. and W.A.O.d.S.; investigation, A.A.d.S. and C.S.; data curation, C.S.; writing—original draft preparation, A.A.d.S. and M.d.M.D.; writing—review and editing, C.G.B. and F.V.d.S.S.; supervision, C.G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed in part by the Coordination for the Improvement of Higher Education Personnel—Brazil (CAPES), Finance Code 001.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank the Postgraduate Program in Phytotechnics of the Federal Rural University of the Semi-Arid (UFERSA), and the Coordination for the Improvement of Higher Education Personnel (CAPES) and the Institute of Plant Physiology, Faculty of Agricultural and Forestry Sciences, National University of La Plata for providing structural and technical resources.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAO. Food Outlook—Biannual Report on Global Food Markets; Food Outlook; FAO: Rome, Italy, 2023. [Google Scholar]
- IBGE. Brazilian Institute of Geography and Statistics—IBGE. Available online: https://www.ibge.gov.br/explica/producao-agropecuaria/maracuja/br (accessed on 10 January 2024).
- Gheyi, H.R.; da Silva Dias, N.; De Lacerda, C.F. Manejo da Salinidade na Agricultura: Estudos Básicos e Aplicados, 2nd ed.; INCTSal: Fortaleza, CE, Brazil, 2016; 504p. [Google Scholar]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 1985; Volume 29, ISBN 9251022631. [Google Scholar]
- Pecherina, A.; Dimitrieva, A.; Mudrilov, M.; Ladeynova, M.; Zanegina, D.; Brilkina, A.; Vodeneev, V. Salt-Induced Early Changes in Photosynthesis Activity Caused by Root-to-Shoot Signaling in Potato. Int. J. Mol. Sci. 2024, 25, 1229. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M.; Ahmad, P.; Chandna, R.; Prasad, M.N.V.; Ozturk, M. Enhancing Plant Productivity under Salt Stress: Relevance of Poly-Omics. In Salt Stress in Plants: Signalling, Omics and Adaptations; Ahmad, P., Azooz, M.M., Prasad, M.N.V., Eds.; Springer New York: New York, NY, USA, 2013; pp. 113–156. ISBN 9781461461081. [Google Scholar]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of Reactive Oxygen Species and Antioxidant Defense in Plants under Salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef] [PubMed]
- Contiero, L.F.; Cavichioli, J.C.; Aparecido Manzani Lisboa, L.; Vitorino, R.A.; Ramos, S.B.; de Figueiredo, P.A.M. Water Stress in Passion Fruit Cropping: An Approach to Its Development. Rev. Eng. Agric. Reveng. 2021, 29, 245–253. [Google Scholar] [CrossRef]
- Zandi, P.; Schnug, E. Reactive Oxygen Species, Antioxidant Responses and Implications from a Microbial Modulation Perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef]
- Barbosa, M.R.; de Souza, L.M.; Nascimento, K.R.P. Ros E O Estresse Oxidativo Por Seca Em Plantas. Multidiscip. Sci. Rep. 2023, 3, 1–17. [Google Scholar] [CrossRef]
- Davar, R.; Darvishzadeh, R.; Majd, A. Changes in Antioxidant Systems in Sunflower Partial Resistant and Susceptible Lines as Affected by Sclerotinia Sclerotiorum. Biologia 2013, 68, 821–829. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular Mycorrhizal Fungi Act as Biostimulants in Horticultural Crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic Action of a Microbial-Based Biostimulant and a Plant Derived-Protein Hydrolysate Enhances Lettuce Tolerance to Alkalinity and Salinity. Front. Plant Sci. 2017, 8, 131. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Y.; Wang, F.; Li, T.; Chen, Z.; Kong, D.; Bi, J.; Zhang, F.; Luo, X.; Wang, J.; et al. Enhanced Rice Salinity Tolerance via CRISPR/Cas9-Targeted Mutagenesis of the OsRR22 Gene. Mol. Breed. 2019, 39, 47. [Google Scholar] [CrossRef]
- de Souza, G.L.F.; Nascimento, A.P.J.; de Andrade Silva, J.; Bezerra, F.T.C.; da Silva, R.Í.L.; Cavalcante, L.F.; Mendonça, R.M.N. Growth of Wild Passion Fruit (Passiflora foetida L.) Rootstock under Irrigation Water Salinity. Rev. Bras. Eng. Agric. E Ambient. 2023, 27, 114–120. [Google Scholar] [CrossRef]
- Penella, C.; Landi, M.; Guidi, L.; Nebauer, S.G.; Pellegrini, E.; Bautista, A.S.; Remorini, D.; Nali, C.; López-Galarza, S.; Calatayud, A. Salt-Tolerant Rootstock Increases Yield of Pepper under Salinity through Maintenance of Photosynthetic Performance and Sinks Strength. J. Plant Physiol. 2016, 193, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, J.F.; Lisboa, R.A.; Oliveira, M.; Silva Júnior, M.J.; Alves, L.P. Caracterização das águas subterrâneas usadas para irrigação na área produtora de melão da Chapada do Apodi. Rev. Bras. De Eng. Agric. E Ambient. 2003, 7, 469–472. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Steelheart, C.; Alegre, M.L.; Baldet, P.; Rothan, C.; Bres, C.; Just, D.; Okabe, Y.; Ezura, H.; Ganganelli, I.; Gergoff Grozeff, G.E.; et al. The Effect of Low Ascorbic Acid Content on Tomato Fruit Ripening. Planta 2020, 252, 36. [Google Scholar] [CrossRef]
- Zhou, M.; Diwu, Z.; Panchuk-Voloshina, N.; Haugland, R.P. A Stable Nonfluorescent Derivative of Resorufin for the Fluorometric Determination of Trace Hydrogen Peroxide: Applications in Detecting the Activity of Phagocyte NADPH Oxidase and Other Oxidases. Anal. Biochem. 1997, 253, 162–168. [Google Scholar] [CrossRef]
- Bartoli, C.G.; Simontacchi, M.; Tambussi, E.; Beltrano, J.; Montaldi, E.; Puntarulo, S. Drought and Watering-Dependent Oxidative Stress: Effect on Antioxidant Content in Triticum aestivum L. Leaves. J. Exp. Bot. 1999, 50, 375–383. [Google Scholar] [CrossRef]
- El-Badri, A.M.; Batool, M.; Mohamed, I.A.A.; Wang, Z.; Khatab, A.; Sherif, A.; Ahmad, H.; Khan, M.N.; Hassan, H.M.; Elrewainy, I.M.; et al. Antioxidative and Metabolic Contribution to Salinity Stress Responses in Two Rapeseed Cultivars during the Early Seedling Stage. Antioxidants 2021, 10, 1227. [Google Scholar] [CrossRef]
- Ashraf, M.; O’Leary, J.W. Changes in Soluble Proteins in Spring Wheat Stressed with Sodium Chloride. Biol. Plant. 1999, 42, 113–117. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, R.; Jain, V.; Jain, S. Differential Behavior of the Antioxidant System in Response to Salinity Induced Oxidative Stress in Salt-Tolerant and Salt-Sensitive Cultivars of Brassica juncea L. Biocatal. Agric. Biotechnol. 2018, 13, 12–19. [Google Scholar] [CrossRef]
- Bianchi, L.; Henrique Germino, G.; de Almeida Silva, M. Plant Adaptation to Water Deficit. Acta Iguazu 2017, 5, 15–32. [Google Scholar]
- Oliveira Novais Araújo, B.; Andrade Monteiro, M.; Celente Martins, A.; Lacerda Fonseca, L.; Somavilla Uliana, A.; Diel de Oliveira, V.; Pedó, T.; Zanatta Aumonde, T. Biochemical Performance of Bean Seedlings Under Water Restriction in Early Development. Rev. La Fac. Agron. 2021, 120, 070. [Google Scholar] [CrossRef]
- Suzuki, N.; Miller, G.; Morales, J.; Shulaev, V.; Torres, M.A.; Mittler, R. Respiratory Burst Oxidases: The Engines of ROS Signaling. Curr. Opin. Plant Biol. 2011, 14, 691–699. [Google Scholar] [CrossRef]
- Dietz, K.J. Thiol-Based Peroxidases and Ascorbate Peroxidases: Why Plants Rely on Multiple Peroxidase Systems in the Photosynthesizing Chloroplast? Mol. Cells 2016, 39, 20–25. [Google Scholar] [CrossRef]
- Raja, V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic Stress: Interplay between ROS, Hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [Google Scholar] [CrossRef]
- de Araújo, N.O.; de Sousa Santos, M.N.; de Araujo, F.F.; Véras, M.L.M.; de Jesus Tello, J.P.; da Silva Arruda, R.; Fugate, K.K.; Finger, F.L. Balance between Oxidative Stress and the Antioxidant System Is Associated with the Level of Cold Tolerance in Sweet Potato Roots. Postharvest Biol. Technol. 2021, 172, 111359. [Google Scholar] [CrossRef]
- Hurtado-Salazar, A.; Pereira da Silva, D.F.; Ceballos-Aguirre, N.; Ocampo, J.; Bruckner, C.H. Tolerancia a La Salinidad de Passiflora Tarminiana Coppens & Barney. Rev. Colomb. Cienc. Hortícolas 2018, 12, 11–19. [Google Scholar] [CrossRef]
- Kotula, L.; Clode, P.L.; Jimenez, J.D.L.C.; Colmer, T.D. Salinity Tolerance in Chickpea Is Associated with the Ability to “exclude” Na from Leaf Mesophyll Cells. J. Exp. Bot. 2019, 70, 4991–5002. [Google Scholar] [CrossRef]
- Azevedo Neto, A.D.d.; Pereira, P.P.A.; Costa, D.P.; dos Santos, A.C.C. Fluorescência Da Chlorofila Como Uma Possível Ferramenta Para Seleção de Tolerância à Salinidade Em Girassol. Rev. Ciência Agronômica 2011, 42, 893–897. [Google Scholar] [CrossRef]
- Ben Rejeb, I.; Pastor, V.; Mauch-Mani, B. Plant Responses to Simultaneous Biotic and Abiotic Stress: Molecular Mechanisms. Plants 2014, 3, 458–475. [Google Scholar] [CrossRef]
- Liu, B.; Li, M.; Cheng, L.; Liang, D.; Zou, Y.; Ma, F. Influence of Rootstock on Antioxidant System in Leaves and Roots of Young Apple Trees in Response to Drought Stress. Plant Growth Regul. 2012, 67, 247–256. [Google Scholar] [CrossRef]
- Aydın, A. The Growth, Leaf Antioxidant Enzymes and Amino Acid Content of Tomato as Affected by Grafting on Wild Tomato Rootstocks 1 (S. Pimpinellifolium and S. Habrochaites) Under Salt Stress. Sci. Hortic. 2024, 325, 112679. [Google Scholar] [CrossRef]
- Yao, Z.; Rao, Z.; Hou, S.W.; Tian, C.; Liu, C.Y.; Yang, X.; Zhu, G. The Appropriate Expression and Coordination of Glycolate Oxidase and Catalase Are Vital to the Successful Construction of the Photorespiratory Metabolic Pathway. Front. Plant Sci. 2022, 13, 999757. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, R.; Uzilday, B.; Sekmen, A.H.; Turkan, I. Reactive Oxygen Species Regulation and Antioxidant Defense in Halophytes. Funct. Plant Biol. 2013, 40, 832–847. [Google Scholar] [CrossRef]
- Li, R.H.; Guo, P.; Michael, B.; Stefania, G.; Salvatore, C. Evaluation of Chlorophyll Content and Fluorescence Parameters as Indicators of Drought Tolerance in Barley. Agric. Sci. China 2006, 5, 751–757. [Google Scholar] [CrossRef]
- Netto, A.T.; Campostrini, E.; De Oliveira, J.G.; Bressan-Smith, R.E. Photosynthetic Pigments, Nitrogen, Chlorophyll a Fluorescence and SPAD-502 Readings in Coffee Leaves. Sci. Hortic. 2005, 104, 199–209. [Google Scholar] [CrossRef]
- Jifon, J.L.; Syvertsen, J.P.; Whaley, E. Growth Environment and Leaf Anatomy Affect Nondestructive Estimates of Chlorophyll and Nitrogen in Citrus sp. Leaves. J. Am. Soc. Hortic. Sci. 2005, 130, 152–158. [Google Scholar] [CrossRef]
- Leão, P.C.d.S.; Chaves, A.R.d.M. Sistemas de Condução e suas Influências na Produtividade e Desempenho Agronômico de Videiras ‘Syrah’ e ‘Chenin Blanc’ no Vale do São Francisco; Embrapa Semiárido: Petrolina, Brazil, 2020; 32p. [Google Scholar]
- Abdelrady, W.A.; Ma, Z.; Elshawy, E.E.; Wang, L.; Askri, S.M.H.; Ibrahim, Z.; Dennis, E.; Kanwal, F.; Zeng, F.; Shamsi, I.H. Physiological and Biochemical Mechanisms of Salt Tolerance in Barley under Salinity Stress. Plant Stress 2024, 11, 100403. [Google Scholar] [CrossRef]
- Oliveira, F.A.; Lopes, M.A.C.; Sá, F.V.S.; Nobre, R.G.; Moreira, R.C.L.; Silva, L.A.; Paiva, E.P. Interaction of irrigation water salinity and substrate on the production of yellow passion fruit seedlings. Comum. Sci. 2015, 6, 471–478. [Google Scholar] [CrossRef]
- Souza, T.M.A.; Mendonça, V.; Sá, F.V.S.; Silva, M.J.; Dourado, C.S.T. Calcium silicate as salt stress attenuator in seedlings of yellow passion fruit cv. BRS GA1. Rev. Caatinga 2020, 33, 509–517. [Google Scholar] [CrossRef]
- Lee, J.M.; Kubota, C.; Tsao, S.J.; Bie, Z.; Echevarria, P.H.; Morra, L.; Oda, M. Current Status of Vegetable Grafting: Diffusion, Grafting Techniques, Automation. Sci. Hortic. 2010, 127, 93–105. [Google Scholar] [CrossRef]
- Lee, J.; Rai, P.K.; Jeon, Y.J.; Kim, K.H.; Kwon, E.E. The Role of Algae and Cyanobacteria in the Production and Release of Odorants in Water. Environ. Pollut. 2017, 227, 252–262. [Google Scholar] [CrossRef]
- Da Silva, R.M.; de Aguiar Cardoso, E.; Faleiro, F.G.; Linhares, P.C.F.; Barreto, É.d.S.; De Sousa, R.P.; De Assis, J.P.; Lobato, L.V.d.C. Grafting of Passion Fruit Cultivars on Passiflora foetida L. and Influence of Grafting Age. Obs. La Econ. Latinoam. 2023, 21, 6152–6167. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).