Study on the Compatibility of Distant Hybridization Between Rhododendron Subgenus Tsutsusi and R. moulmainense, a Fragrant Rhododendron from China
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Hybridization Pollination
2.3. Fluorescence Observation of Pollen Tubes
2.4. Observation of Paraffin Sections of the Ovary
2.5. Statistical Indicators of Hybridization Compatibility
2.6. Statistical Analysis
3. Results
3.1. Effect of Pollination Methods on Ovary Swelling Rate and Fruit Bearing Rate
3.2. Indices of Hybrid Compatibility and Evaluation of Fertility
3.3. Analysis of Pollen Tube Growth
3.4. Analysis of the Ovary’s Development
4. Discussion
4.1. Analysis of Indicators for Hybrid Compatibility
4.2. Overcoming Pre-Fertilization Barriers in Rhododendron Fertilization
4.3. Overcoming Post-Fertilization Barriers in Rhododendron Fertilization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, M.; Fang, R.; He, M.; Hu, L.; Yang, H.; Chamberlain, D. Flora of China; Science Press: Beijing, China, 2005; Volume 14. [Google Scholar]
- Zhang, Y.; Wang, J.; Wang, X.; Wang, L.; Wang, Y.; Wei, J.; Niu, Z.; Jian, L.; Jin, B.; Chen, C.; et al. Population Structures and Dynamics of Rhododendron Communities with Different Stages of Succession in Northwest Guizhou, China. Plants 2024, 13, 946. [Google Scholar] [CrossRef]
- Wang, D.; Ma, Y.; Zhao, X.; Wang, L. Genetic Diversity of Rhododendron Dauricum Based on Morphological Traits and SSR Markers. Front. Plant Sci. 2025, 16, 1533824. [Google Scholar] [CrossRef]
- Qi, J.; Wang, L.; Li, W.; Song, J.; Li, S.; Guan, W. Phenotypic Character Analysis and Comprehensive Evaluation of 9 Encore® azalea Varieties. Mol. Plant Breed. 2024. [Google Scholar]
- Bai, Y.; Wang, D.; Xie, L. Studies on the Research Method of Pollen Vitality of Rhododendron moulmainense Hook.f. Bai Yuqing1 et al. Anhui Agric. Bull. 2019, 25, 13–15. [Google Scholar] [CrossRef]
- Tao, D.; Kalendar, R.; Paterson, A.H. Editorial: Interspecific Hybridization in Plant Biology, Volume II. Frontiers in Plant Science 2024, 15, 1412622. [Google Scholar] [CrossRef]
- Manzoor, S.A.; Griffiths, G.; Obiakara, M.C.; Esparza-Estrada, C.E.; Lukac, M. Evidence of Ecological Niche Shift in Rhododendron Ponticum (L.) in Britain: Hybridization as a Possible Cause of Rapid Niche Expansion. Ecol. Evol. 2020, 10, 2040–2050. [Google Scholar] [CrossRef] [PubMed]
- Leslie, A.C. The International Rhododendron Register and Checklist; The Royal Horticultural Society: London, UK, 2008. [Google Scholar]
- Zhang, C. Major Rhododendron Varieties and Their Cultivation History. China Flowers Hortic. 2022, 52–57. [Google Scholar]
- Ye, Y.; Sun, L.; Zhang, Y.; Li, Y.; Xu, J.; Ma, C.; Niu, Y.; Zhang, M.; Dai, S.; Huang, H. Studies on Distant Crossing Compatibility and Hybrid Ploidy in Different Ploidy Chrysanthemums. Acta Hortic. Sin. 2024, 51, 2329–2342. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, G.; Li, K.; Zhou, G.; Zhou, S. Genomic Variation of New Cultivars Selected from Distant Hybridization in Lilium. Plant Breed. 2012, 131, 227–230. [Google Scholar] [CrossRef]
- Li, Z.; Chen, M.; Zhou, L.; Sun, M.; Xiang, W.; Ma, J.; Zheng, S.; Zeng, J.; Li, Y. Identification and Fusarium wilt resistance evaluation of distant hybrid offspring of Lilium brownii var. viridulum. Guihaia 2024, 44, 2172–2186. [Google Scholar]
- Ma, C.; Zhao, X.; Ruan, F.; Li, Z.; Li, C. Studies on Immature Hybrid Embryo Culture of Distant Hybridization in Rosa. Mol. Plant Breed. 2021, 19, 6468–6475. [Google Scholar] [CrossRef]
- Ou, Z.; Yang, Y.; Feng, C.; Jiang, L.; Zhang, Y.; Zhuang, Y.; Luo, L.; Yu, C. Fluorescent microscope observation on growth of pollen tube on distant hybridization in Rosa persica. J. Northeast. Agric. Univ. 2022, 53, 18–26. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, L.; Teixeira da Silva, J.A.; Yu, X. The Plastome Reveals New Insights into the Evolutionary and Domestication History of Peonies in East Asia. BMC Plant Biol. 2023, 23, 243. [Google Scholar] [CrossRef]
- Wang, E.; Wang, X.; Ji, H.; Han, K.; Lu, L.; Wang, Z. Analysis on the Measures to Overcome the Obstacles of Distant Hybridization in Tree Peony. Spec. Wild Econ. Anim. Plant Res. 2022, 44, 115–120. [Google Scholar] [CrossRef]
- Kho, Y.O.; Baër, J. A Microscopical Research on the Incompatibility in the cross Rhododendron Impeditum × R. Williamsianum. Euphytica 1970, 19, 303–309. [Google Scholar] [CrossRef]
- Ureshino, K.; Miyajima, I.; Ozaki, Y.; Kobayashi, N.; Michishita, A.; Akabane, M. Appearance of Albino Seedlings and ptDNA Inheritance in Interspecific Hybrids of Azalea. Euphytica 1999, 110, 61–66. [Google Scholar] [CrossRef]
- Moyle, L.C.; Jewell, C.P.; Kostyun, J.L. Fertile Approaches to Dissecting Mechanisms of Premating and Postmating Prezygotic Reproductive Isolation. Curr. Opin. Plant Biol. 2014, 18, 16–23. [Google Scholar] [CrossRef]
- Xie, W.; Li, S.; Oba, E.G.; Peng, L.; Wang, J.; Zhang, L.; Song, J.; Huang, H. Microscopic Observation and Transcriptome Analysis Provide Insights into Mechanisms of Hybrid Incompatibility in Rhododendron. Sci. Hortic. 2024, 336, 113417. [Google Scholar] [CrossRef]
- Gibbs, P.E. Late-Acting Self-Incompatibility—The Pariah Breeding System in Flowering Plants. New Phytol. 2014, 203, 717–734. [Google Scholar] [CrossRef]
- Huang, J.; Yang, L.; Yang, L.; Wu, X.; Cui, X.; Zhang, L.; Hui, J.; Zhao, Y.; Yang, H.; Liu, S.; et al. Stigma Receptors Control Intraspecies and Interspecies Barriers in Brassicaceae. Nature 2023, 614, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liu, F.; Qi, X.; Xu, W.; Yang, L. Salt Solution Treatment Plays an Important Role in Overcoming Pre-Fertilization Barriers during Asiatic and Oriental Lily Crossbreeding. Sci. Hortic. 2021, 288, 110343. [Google Scholar] [CrossRef]
- Geng, X.; Zhao, H.; Zhang, Y.; Wang, L.; Zhang, L. Effects of Different Pollination Methods on Fruit Setting of Different Hybrid Combinations in Wild Rhododendron. J. Yunnan Agric. Univ. (Nat. Sci.) 2017, 32, 83–88. [Google Scholar] [CrossRef]
- Yin, Y.; Song, W.; Song, J.; Du, Y.; Du, J. Studies on the Cross Affinity of Rhododendron hybridum and Rhododendron spinuliferum (Rhododendron). J. Southwest For. Univ. 2023, 43, 173–178. [Google Scholar]
- Maryenti, T.; Kato, N.; Ichikawa, M.; Okamoto, T. Establishment of an In Vitro Fertilization System in Wheat (Triticum Aestivum L.). Plant Cell Physiol. 2019, 60, 835–843. [Google Scholar] [CrossRef]
- Maryenti, T.; Koshimizu, S.; Onda, N.; Ishii, T.; Yano, K.; Okamoto, T. Wheat Cybrid Plants, OryzaWheat, Regenerated from Wheat–Rice Hybrid Zygotes via in Vitro Fertilization System Possess Wheat–Rice Hybrid Mitochondria. Plant Cell Physiol. 2024, 65, 1344–1357. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, X.; Zhu, J.; Jin, Y.; Xia, C.; Zheng, B.; Silvestri, C.; Cui, F. Modern Technologies Provide New Opportunities for Somatic Hybridization in the Breeding of Woody Plants. Plants 2024, 13, 2539. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.H.; Scher, C.L. Exploring the Potential of Gametic Reconstruction of Parental Genotypes by F1 Hybrids as a Bridge for Rapid Introgression. Genome 2017, 60, 713–719. [Google Scholar] [CrossRef]
- Eeckhaut, T.; De Keyser, E.; Van Huylenbroeck, J.; De Riek, J.; Van Bockstaele, E. Application of Embryo Rescue after Interspecific Crosses in the Genus Rhododendron. Plant Cell Tissue Organ Cult. 2007, 89, 29–35. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Hu, L.; Zhang, J.; Xiao, F.; Zhang, S.; Shao, F.; Huang, L. Embryological Observations on Seed Abortion in Hibiscus syriacus L. and Physiological Studies on Nutrients, Enzyme Activity and Endogenous Hormones. BMC Plant Biol. 2023, 23, 665. [Google Scholar] [CrossRef]
- Geng, X.; Zhang, C.; Luo, F.; Wang, L. Study on Hybrid Seed Set of Wild Rhododendron in China. Jiangsu Agric. Sci. 2013, 41, 159–161. [Google Scholar] [CrossRef]
- Sun, C.-Q.; Chen, F.-D.; Teng, N.-J.; Liu, Z.-L.; Fang, W.-M.; Hou, X.-L. Factors Affecting Seed Set in the Crosses between Dendranthema Grandiflorum (Ramat.) Kitamura and Its Wild Species. Euphytica 2010, 171, 181–192. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, M.; Li, S. Research progress on mechanism of plant embryo abortion. J. Northeast. Agric. Univ. 2021, 52, 89–96. [Google Scholar] [CrossRef]
- Wang, C.T.; Wang, X.Z.; Wang, Z.W.; Yu, Q.M.; Tang, Y.Y.; Wu, Q.; Yu, S.T. Realizing Hybrids between the Cultivated Peanut (Arachis hypogaea L.) and Its Distantly Related Wild Species Using in Situ Embryo Rescue Technique. Genet. Resour. Crop Evol. 2020, 67, 1–8. [Google Scholar] [CrossRef]
- Geng, X.; Zhao, H.; Wu, Y.; Zhang, Y. Cross-compatibility of wild Rhododendron and the effective evaluation indicators. Guihaia 2017, 37, 979–988. [Google Scholar]
- Lattier, J.D.; Contreras, R.N. Intraspecific, Interspecific, and Interseries Cross-Compatibility in Lilac. J. Am. Soc. Hortic. Sci. 2017, 142, 279–288. [Google Scholar] [CrossRef]
- Gao, X.; Hu, Y.; Li, F.; Cao, F.; Guo, Q. Sex Identification and Male–Female Differences in Ginkgo Biloba Hybrid F1 Generation Seedlings. Forests 2024, 15, 1636. [Google Scholar] [CrossRef]
- Hao, Z.; Li, Y.; Yang, Y.; Song, J.; Meng, J.; Guan, W. Studies on Distant Hybridization Compatibility between the Azalea (Rhododendron × hybridum hort.) and the Rhododendron decorum Franch. Native to China. Horticulturae 2024, 10, 1089. [Google Scholar] [CrossRef]
- Bai, Y.; Lin, B.; Xu, T.; Zhang, K. Research on hybridization of Rhododendron moulmainense. Hunan For. Sci. Technol. 2025, 52, 66–72. [Google Scholar]
- Zhuang, P. Progress on the fertility of Rhododendron. Biodivers. Sci. 2019, 27, 327–338. [Google Scholar] [CrossRef]
- Christie, K.; Fraser, L.S.; Lowry, D.B. The Strength of Reproductive Isolating Barriers in Seed Plants: Insights from Studies Quantifying Premating and Postmating Reproductive Barriers over the Past 15 Years. Evolution 2022, 76, 2228–2243. [Google Scholar] [CrossRef] [PubMed]
- Dhooghe, E.; Reheul, D.; Van Labeke, M.-C. Overcoming Pre-Fertilization Barriers in Intertribal Crosses between Anemone coronaria L. and Ranunculus asiaticus L. Horticulturae 2021, 7, 529. [Google Scholar] [CrossRef]
- Kaur, K.; Gupta, M.; Vikal, Y.; Singh, K.; Neelam, K. Callose Depositions Underlie the Incompatible Reaction in Intergeneric Crosses of Rice. Plant Genet. Resour. 2021, 19, 447–452. [Google Scholar] [CrossRef]
- Xie, W.; Song, J.; Tang, L.; Peng, L.; Li, S.; Wang, J. Cross Compatibility of the Cross between Rhododendron “XXL” and R. cyanocarpum. Mol. Plant Breed. 2023, 21, 582–588. [Google Scholar] [CrossRef]
- Zhou, P.; Li, J.; Jiang, H.; Yang, Z.; Sun, C.; Wang, H.; Su, Q.; Jin, Q.; Wang, Y.; Xu, Y. Hormone and Transcriptomic Analysis Revealed That ABA and BR Are Key Factors in the Formation of Inter-Subgeneric Hybridization Barrier in Water Lily. Physiol. Plant. 2024, 176, e14177. [Google Scholar] [CrossRef]
- Hao, Q.; Xu, L.; Wang, H.; Liu, Q.; Wang, K. Evaluation of Pollen Viability, Stigma Receptivity, and the Cross Barrier between Tropical and Hardy Water Lily Cultivars. Flora 2022, 290, 152046. [Google Scholar] [CrossRef]
- Shen, X. Cytological Study on Abortion of Intersectional Distant Hybrid of Paeonia lactiflora cv. ‘Hangbaishao’ × Paeonia ostii cv. ‘Fengdan’. Master’s thesis, Henan University of Science and Technology, Luoyang, China, 2025. [Google Scholar]
- Geng, X.; Huan, Z.; Su, J.; Liu, X. Researches Advances in Germplasm Innovation of Rhododendron. Mol. Plant Breed. 2021, 19, 604–613. [Google Scholar] [CrossRef]
- Deng, Y.; Ye, X. The Post-fertilization Reproductive Barriers and Overcoming Methods of Horticultural Crops Distant Hybridization. Acta Agric. Boreali-Sin. 2013, 28, 120–124. [Google Scholar]
- Eeckhaut, T.; Samyn, G.; Van Bockstaele, E. Interspecific Breeding in the Rhododendron Genus Involving R. Simsii Hybrids. Acta Hortic. 2003, 612, 165–172. [Google Scholar] [CrossRef]
- Gao, J.; Xiao, Z.; Dong, B.; Zhao, H. Study on Cross-compatibility Among the Three Subgenera of Rhododendron subgen. Tsutsusi, subgen. Azaleastrum and subgen. Pentanthera. J. Agric. Biotechnol. 2024, 32, 1049–1060. [Google Scholar]
- Xie, W.-J.; Leus, L.; Wang, J.-H.; Van Laere, K. Fertility Barriers in Interspecific Crosses within Viburnum. Euphytica 2017, 213, 34. [Google Scholar] [CrossRef]
- Feng, J.; Chen, S.; Chen, H.; Dai, L.; Qi, X.; Ahmad, M.Z.; Gao, K.; Qiu, S.; Jin, Y.; Deng, Y. Metabolomics Reveals a Key Role of Salicylic Acid in Embryo Abortion Underlying Interspecific Hybridization between Hydrangea macrophylla and H. arborescens. Plant Cell Rep. 2024, 43, 248. [Google Scholar] [CrossRef]
- Geng, X.; Wu, Y.; Zhao, H. Preliminary Study on Hybrid Embryo Rescue of Rhododendron. J. Yunnan Agric. Univ. (Nat. Sci.) 2014, 29, 533–539. [Google Scholar]
- El-Tantawy, A.A.; Xie, W.; Li, S.; Wang, J.; Peng, L.; Song, J.; Zhang, L.; Cai, Y.; Yang, X. Changes of Endogenous Hormones during Ovary Development in Rhododendron delavayi × Rhododendron sinofalconeri and Rhododendron delavayi × Rhododendron cyanocarpum. Eur. J. Hortic. Sci. 2020, 85, 248–257. [Google Scholar] [CrossRef]
- Zheng, W.; Yan, L.J.; Burgess, K.S.; Luo, Y.H.; Zou, J.Y.; Qin, H.T.; Wang, J.H.; Gao, L.M. Natural Hybridization among Three Rhododendron Species (Ericaceae) Revealed by Morphological and Genomic Evidence. BMC Plant Biol. 2021, 21, 529. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Zhang, C.; Feng, S. Identification and Genetic Diversity Analysis of Hybrid Offspring of Azalea Based on EST-SSR Markers. Sci. Rep. 2022, 12, 15239. [Google Scholar] [CrossRef] [PubMed]


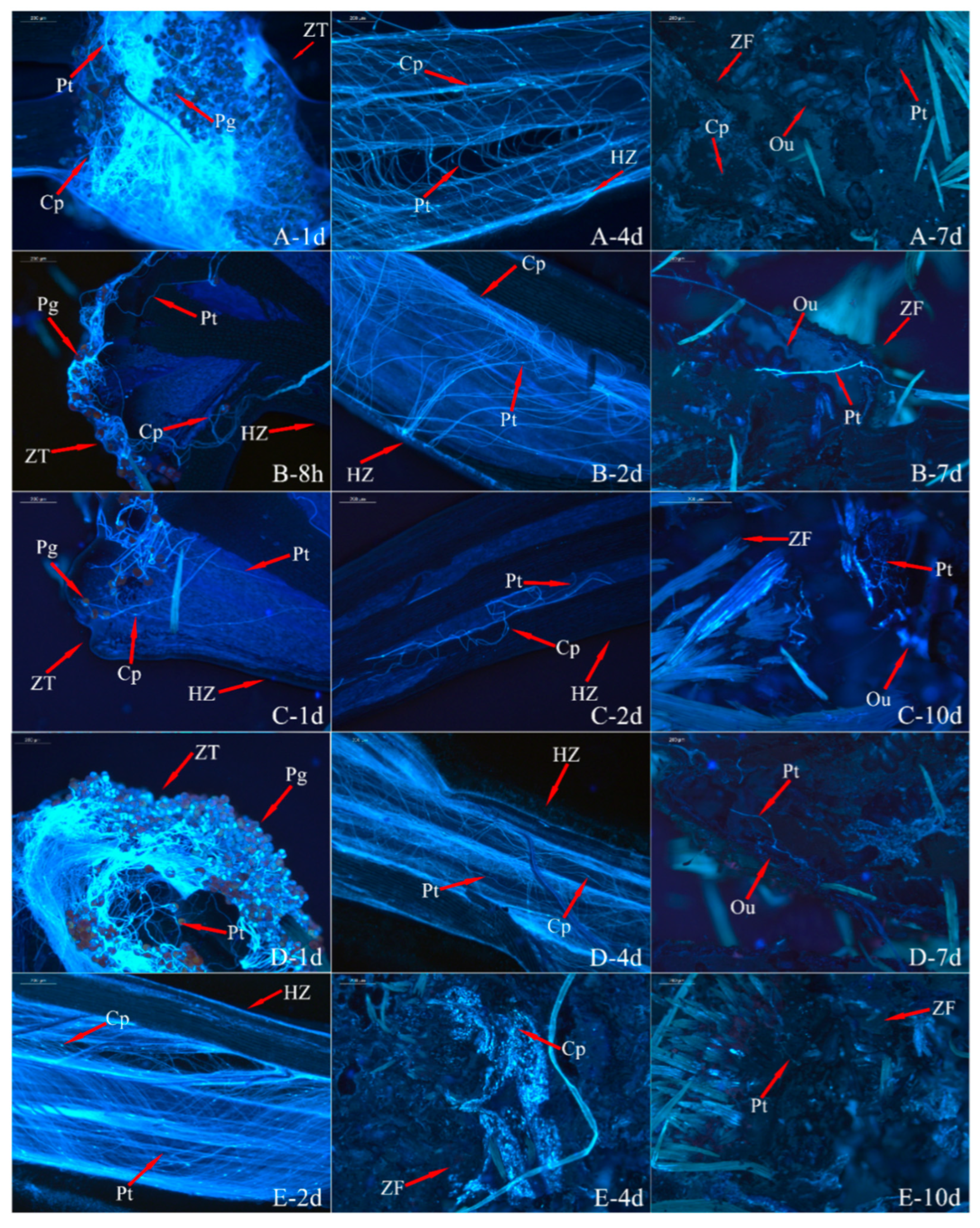
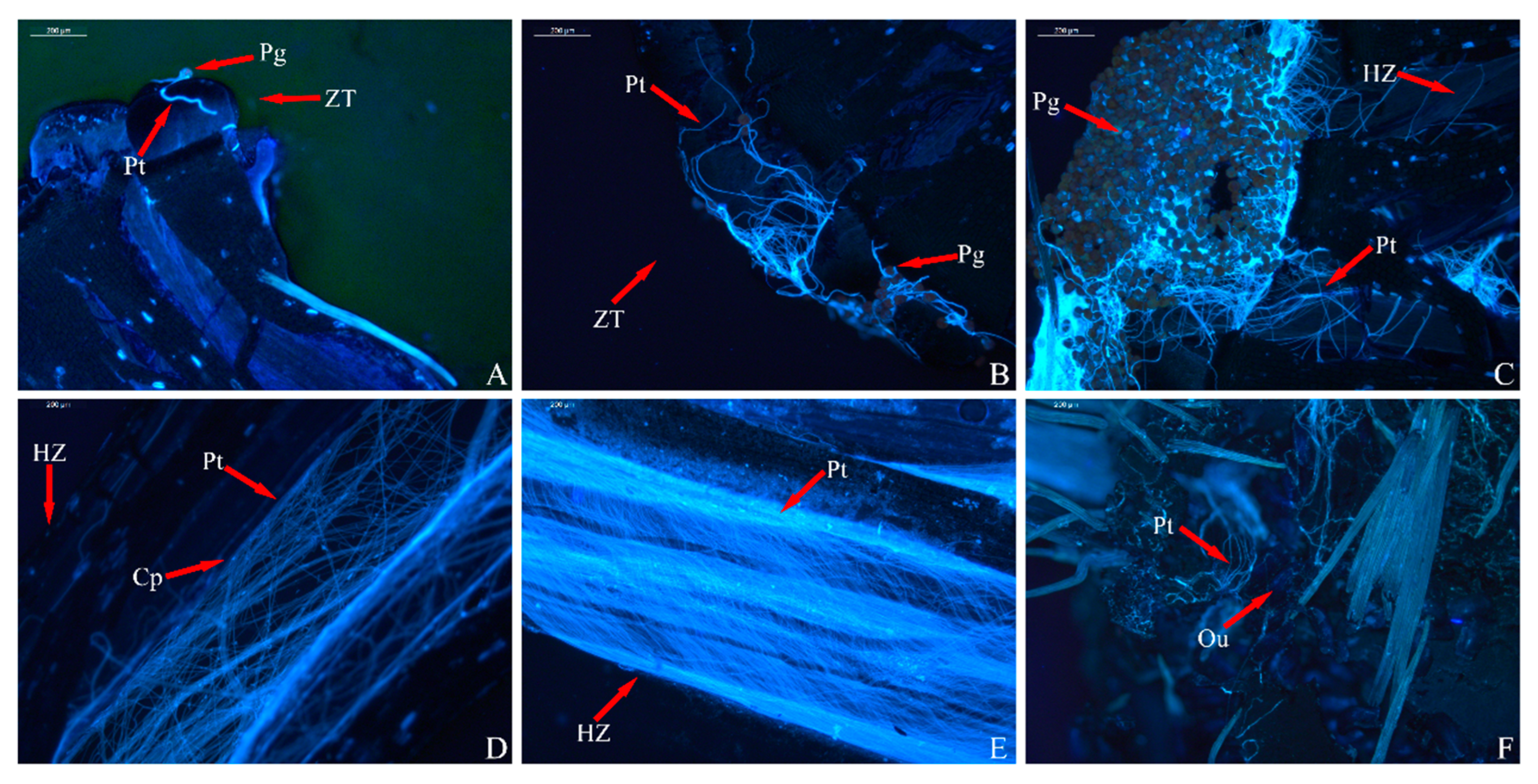
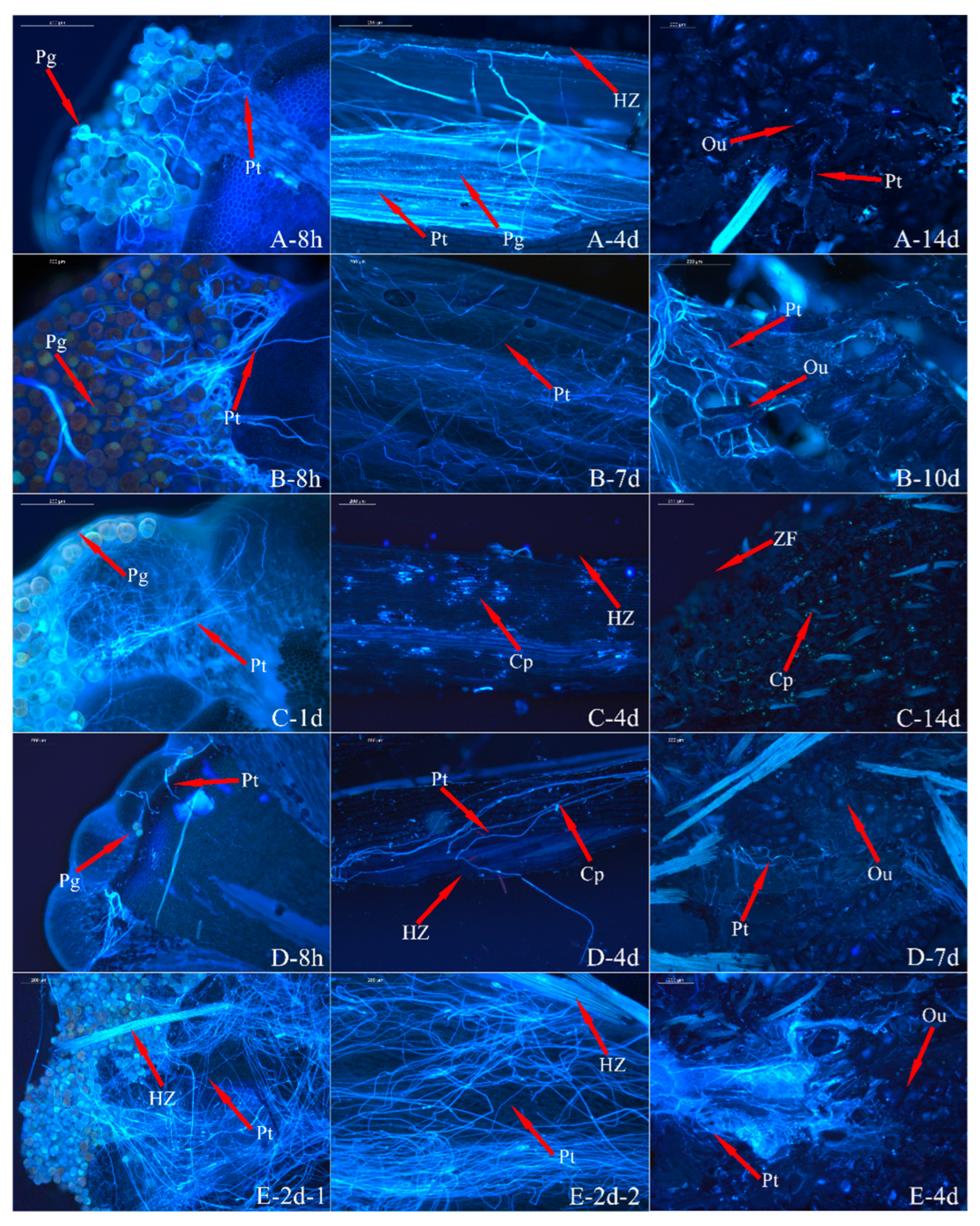
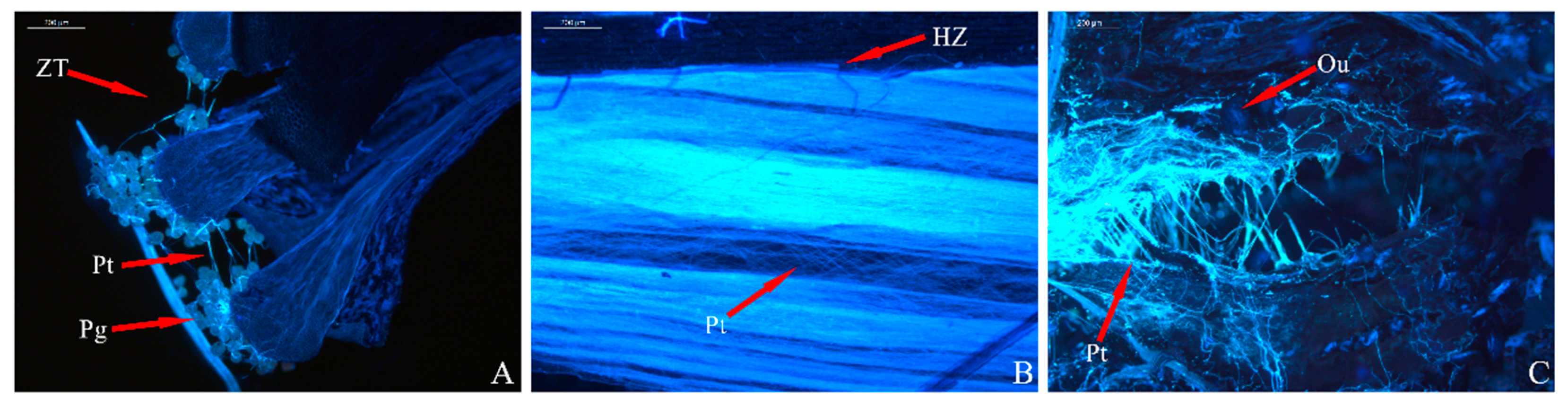
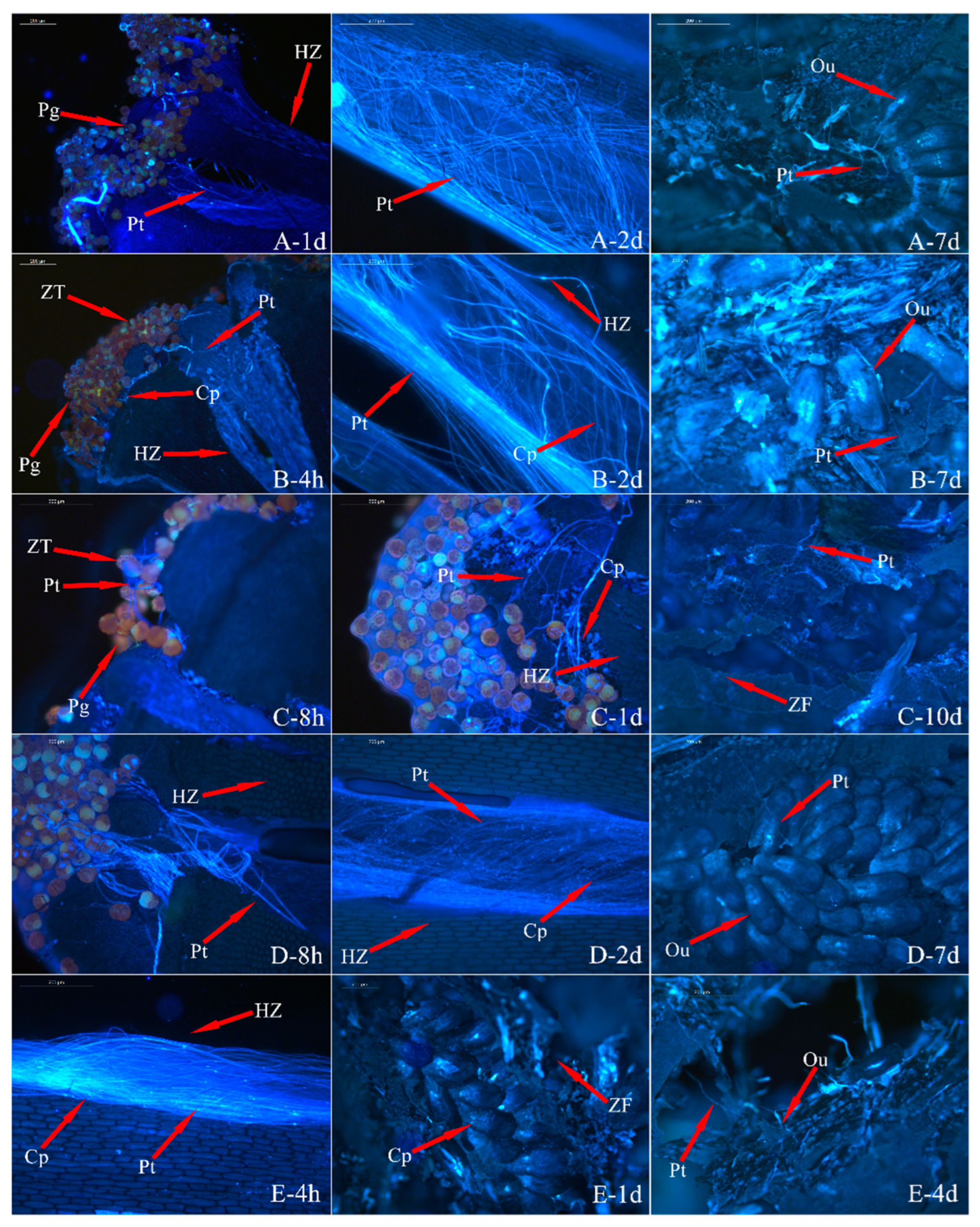
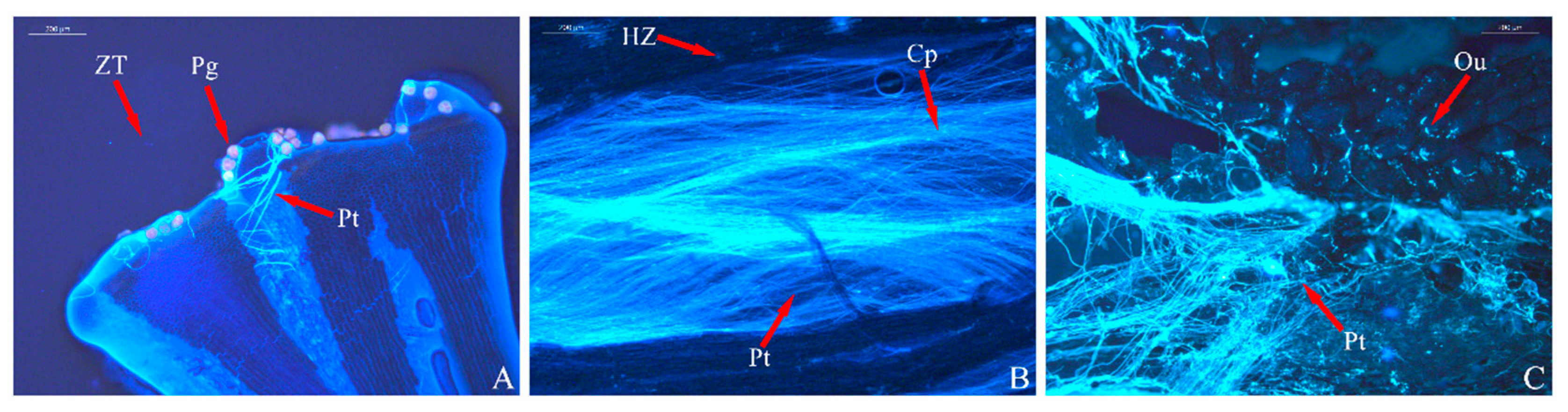
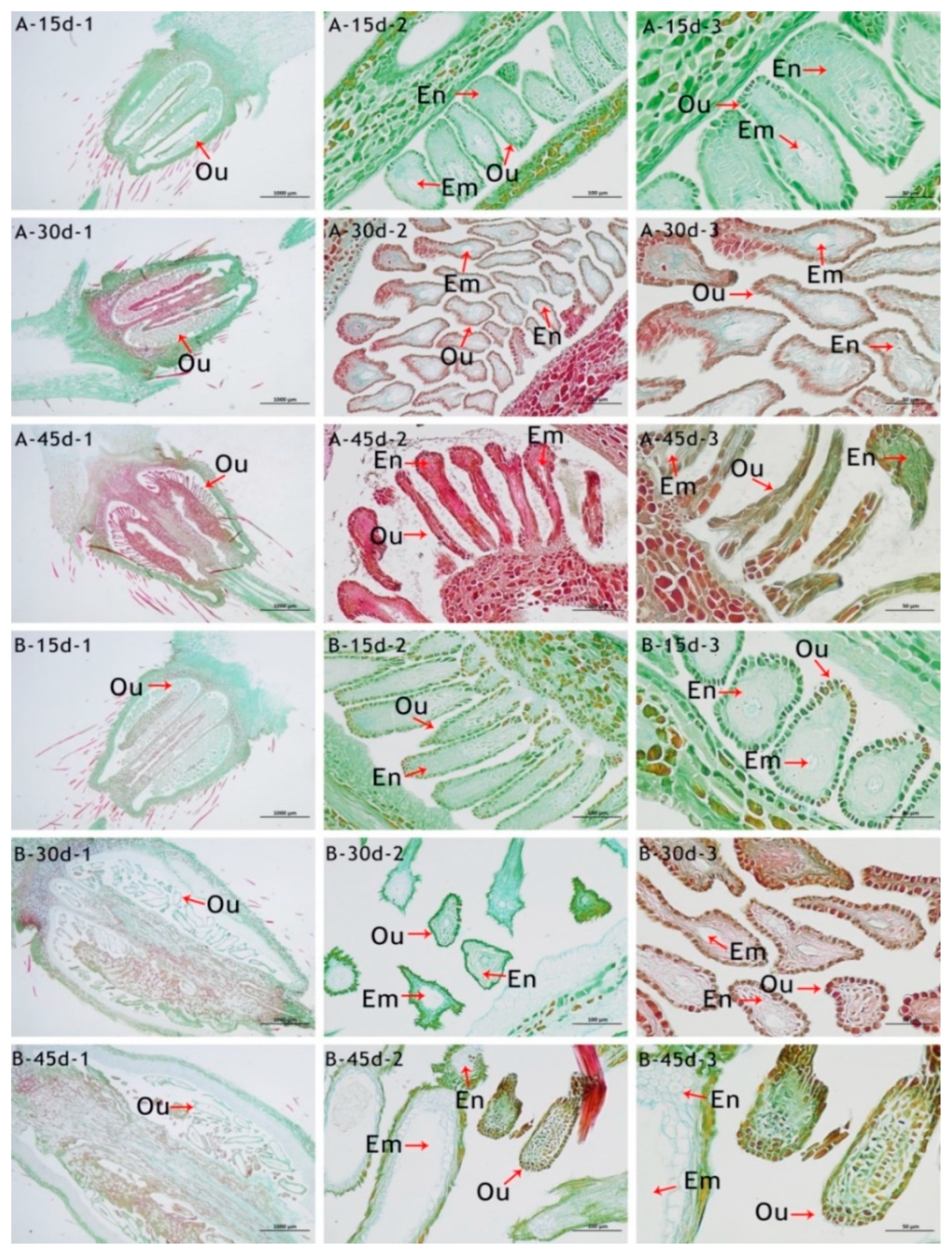
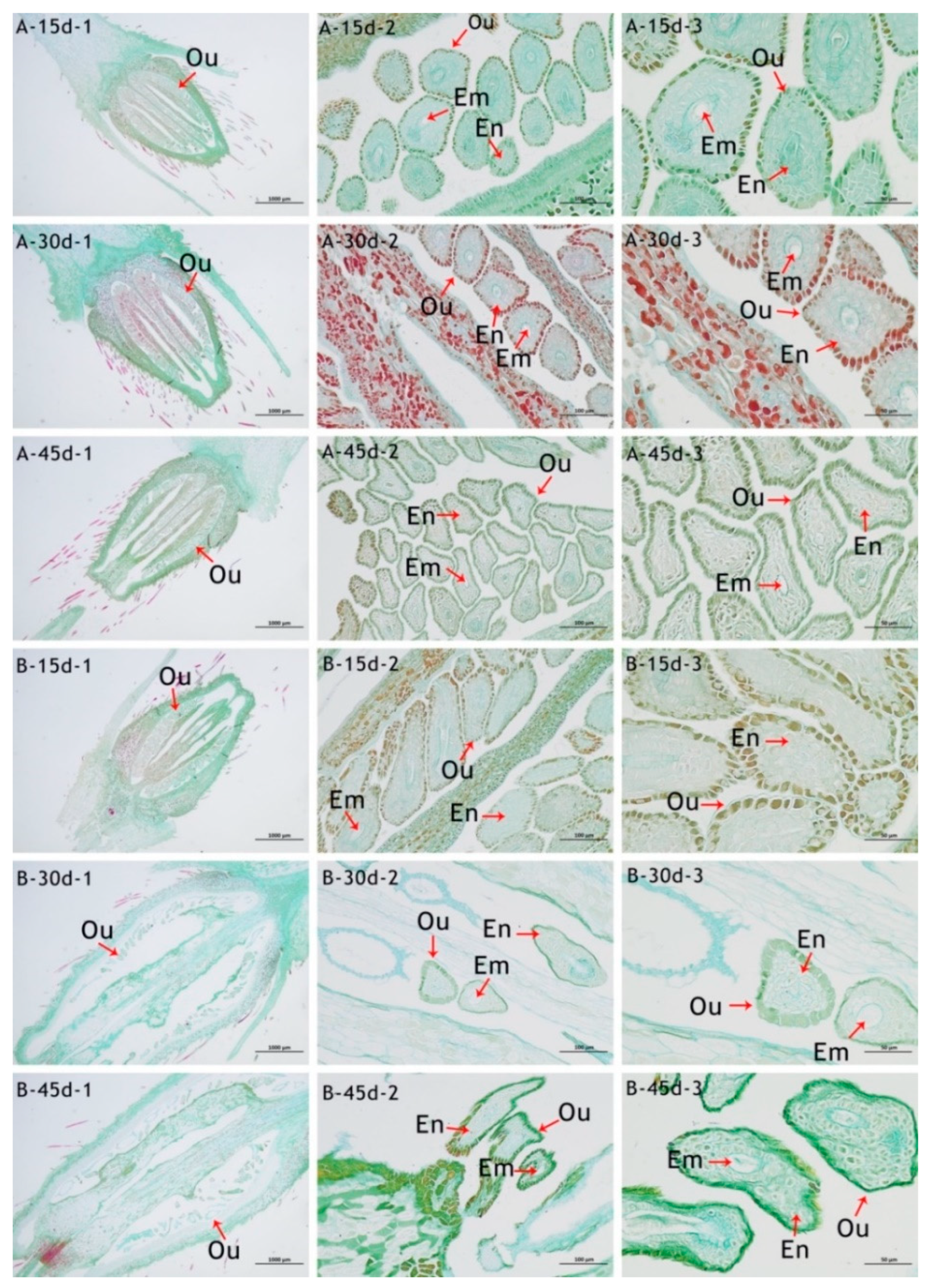
| Index | Sterile | Weakly Fertile | Fertile | |||
|---|---|---|---|---|---|---|
| Threshold | Score | Threshold | Score | Threshold | Score | |
| Green seeding rate | 0 < Gs < 10 | 1 | 10 ≤ Gs < 50 | 3 | Gs ≥ 50 | 5 |
| Green seeding coefficient | 0 < Gc < 0.6 | 0.5 | 0.6 ≤ Gc < 0.9 | 1.5 | Gc ≥ 0.9 | 2 |
| Percentage of fertile fruit | 0 < St < 20 | 0.5 | 20 ≤ St < 40 | 1 | St ≥ 40 | 1.5 |
| Unit number of fertile seeds | 0 < Sf < 20 | 0.5 | 20 ≤ Sf < 200 | 1 | Sf ≥ 200 | 1.5 |
| Hybrid Combination | Conventional Pollination | Pollen Heating Pollination | Early Pollination | Delayed Pollination | Repeat Pollination | |
|---|---|---|---|---|---|---|
| ‘Carnation’ × R. moulmainense | Ovary expansion rate | 10.2 | 3.7 | 3.92 | 9.8 | 11.63 |
| Fruit setting rate | 4.08 | 3.7 | 1.96 | 5.82 | 9.3 | |
| ‘Carnation’ × ‘Fuchsia Parasol’ (ck) | Ovary expansion rate | 81.25 | ||||
| Fruit setting rate | 72.92 | |||||
| ‘Red Tiara’ × R. moulmainense | Ovary expansion rate | 20 | 35.14 | 0 | 26.19 | 44.44 |
| Fruit setting rate | 16.67 | 32.43 | 0 | 21.43 | 35.56 | |
| ‘Red Tiara’ × ‘Red Tiara’ (ck) | Ovary expansion rate | 97.73 | ||||
| Fruit setting rate | 97.73 | |||||
| ‘Fuchsia Parasol’ × R. moulmainense | Ovary expansion rate | 0 | 4.26 | 0 | 35.29 | 23.53 |
| Fruit setting rate | 0 | 4.26 | 0 | 33.33 | 21.57 | |
| ‘Fuchsia Parasol’ × R. rivulare (ck) | Ovary expansion rate | 85.71 | ||||
| Fruit setting rate | 83.67 | |||||
| ‘Pink Ribbons’ × R. moulmainense | Ovary expansion rate | 13.46 | 8 | 0 | 7.69 | 24.07 |
| Fruit setting rate | 7.69 | 6 | 0 | 3.85 | 16.67 | |
| ‘Pink Ribbons’ × ‘Pink Ribbons’ (ck) | Ovary expansion rate | 95.83 | ||||
| Fruit setting rate | 95.83 |
| Hybrid and Selfing Combination | Number of Capsule Seeds (Grain) | 1000-Seed Weight |
|---|---|---|
| ‘Carnation’ × R. moulmainense | 33.33 ± 7.51 d | 0.0260 ± 0.0026 c |
| ‘Carnation’ × ‘Fuchsia Parasol’ (ck) | 360.67 ± 10.97 a | 0.0487 ± 0.0012 a |
| ‘Red Tiara’ × R. moulmainense | 105.33 ± 10.21 c | 0.0387 ± 0.0032 b |
| ‘Red Tiara’ × ‘Red Tiara’ (ck) | 296.00 ± 25.51 b | 0.0540 ± 0.0036 a |
| ‘Fuchsia Parasol’ × R. moulmainense | 98.33 ± 18.04 c | 0.0390 ± 0.0070 b |
| ‘Fuchsia Parasol’ × R. rivulare (ck) | 365.33 ± 66.71 a | 0.0477 ± 0.0021 a |
| ‘Pink Ribbons’ × R. moulmainense | 83.33 ± 14.29 cd | 0.0303 ± 0.0067 c |
| ‘Pink Ribbons’ × ‘Pink Ribbons’ (ck) | 377.33 ± 53.95 a | 0.0487 ± 0.0012 a |
| Hybrid and Selfing Combination | Germination Rate | Green Seeding Rate | Green Seeding Coefficient | Unit Number of Fertile Seeds (Grain) | Comprehensive Score |
|---|---|---|---|---|---|
| ‘Carnation’ × R. moulmainense | 0.00 ± 0.00 d | 0.00 ± 0.00 e | 0 | 0 | 0 |
| ‘Carnation’ × ‘Fuchsia Parasol’ | 48.48 ± 2.99 b | 48.48 ± 2.99 b | 1 | 360.67 | 8 |
| ‘Red Tiara’ × R. moulmainense | 23.96 ± 4.77 c | 21.02 ± 3.03 d | 0.88 | 92.69 | 6 |
| ‘Red Tiara’ × ‘Red Tiara’ | 63.54 ± 6.51 a | 63.54 ± 6.51 a | 1 | 296 | 10 |
| ‘Fuchsia Parasol’ × R. moulmainense | 22.86 ± 2.86 c | 20.00 ± 4.95 d | 0.87 | 85.55 | 5.5 |
| ‘Fuchsia Parasol’ × R. rivulare | 28.15 ± 3.58 c | 27.07 ± 1.74 c | 0.96 | 350.72 | 8 |
| ‘Pink Ribbons’ × R. moulmainense | 3.09 ± 0.05 d | 2.05 ± 1.78 e | 0.66 | 55 | 4 |
| ‘Pink Ribbons’ × ‘Pink Ribbons’ | 53.20 ± 0.23 b | 53.20 ± 0.23 b | 1 | 377.33 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Qi, J.; Wang, L.; Song, J.; Zhao, Y.; Li, Y.; Guan, W. Study on the Compatibility of Distant Hybridization Between Rhododendron Subgenus Tsutsusi and R. moulmainense, a Fragrant Rhododendron from China. Horticulturae 2025, 11, 1116. https://doi.org/10.3390/horticulturae11091116
Li H, Qi J, Wang L, Song J, Zhao Y, Li Y, Guan W. Study on the Compatibility of Distant Hybridization Between Rhododendron Subgenus Tsutsusi and R. moulmainense, a Fragrant Rhododendron from China. Horticulturae. 2025; 11(9):1116. https://doi.org/10.3390/horticulturae11091116
Chicago/Turabian StyleLi, Hongling, Jing Qi, Lele Wang, Jie Song, Yan Zhao, Yefang Li, and Wenling Guan. 2025. "Study on the Compatibility of Distant Hybridization Between Rhododendron Subgenus Tsutsusi and R. moulmainense, a Fragrant Rhododendron from China" Horticulturae 11, no. 9: 1116. https://doi.org/10.3390/horticulturae11091116
APA StyleLi, H., Qi, J., Wang, L., Song, J., Zhao, Y., Li, Y., & Guan, W. (2025). Study on the Compatibility of Distant Hybridization Between Rhododendron Subgenus Tsutsusi and R. moulmainense, a Fragrant Rhododendron from China. Horticulturae, 11(9), 1116. https://doi.org/10.3390/horticulturae11091116





