Chemical Profiling of Monoterpenes and Genome-Wide Discovery of Monoterpene Synthases in Artemisia annua
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Chemicals
2.2. Establishment and Validation of GC-MS Methodology for Monoterpenoids Analysis
2.3. Profiling A. annua Volatile Monoterpenoids Via GC-MS
2.4. Compound Identification and Quantification
2.5. Genome-Wide Identification of Monoterpene Synthase (TPS) Genes in A. annua
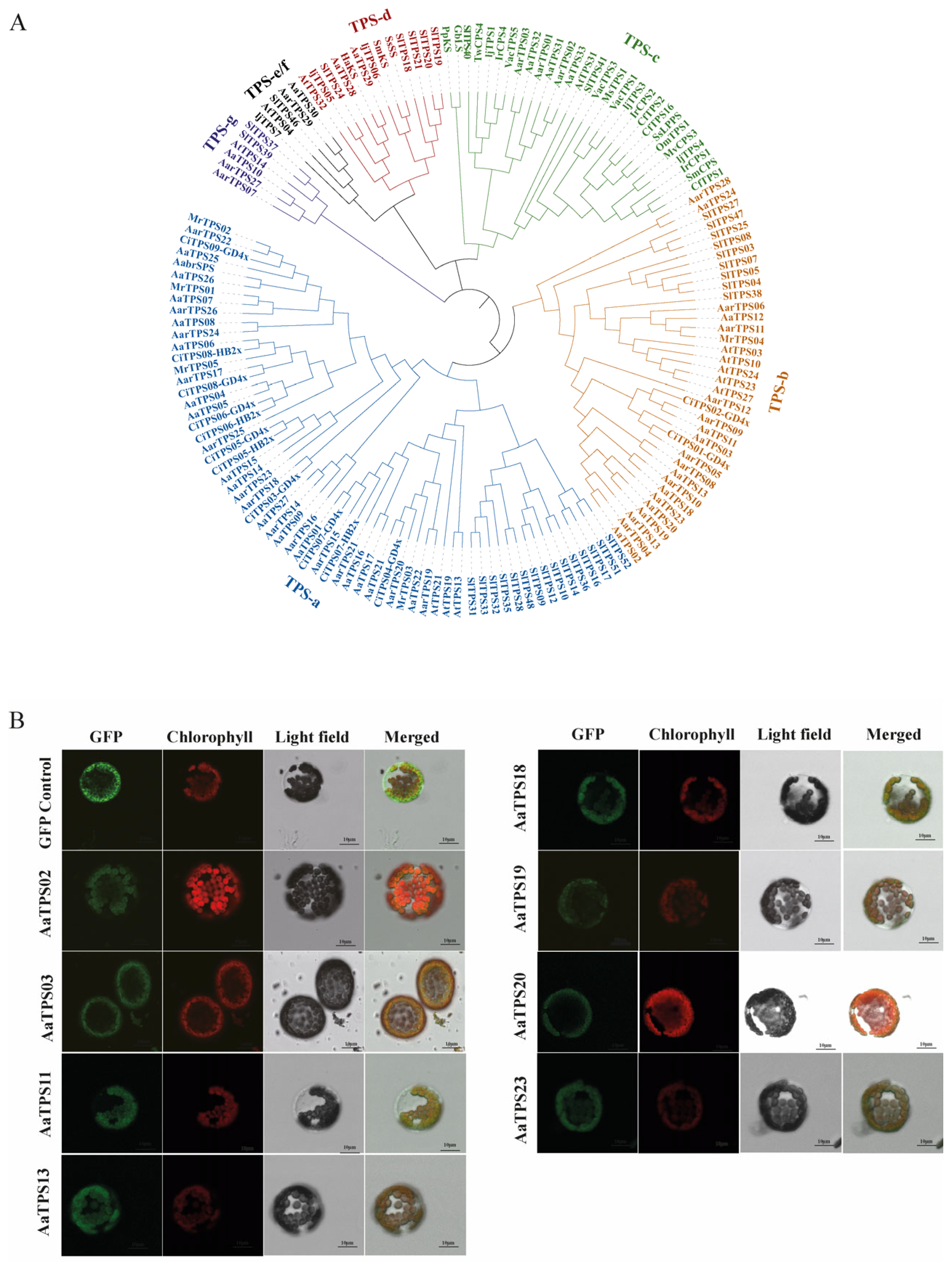
2.6. Subcellular Localization
2.7. Isolation of Full-Length TPS Transcripts and qRT-PCR Validation
2.8. Recombinant Protein Expression and Enzymatic Characterization
3. Results
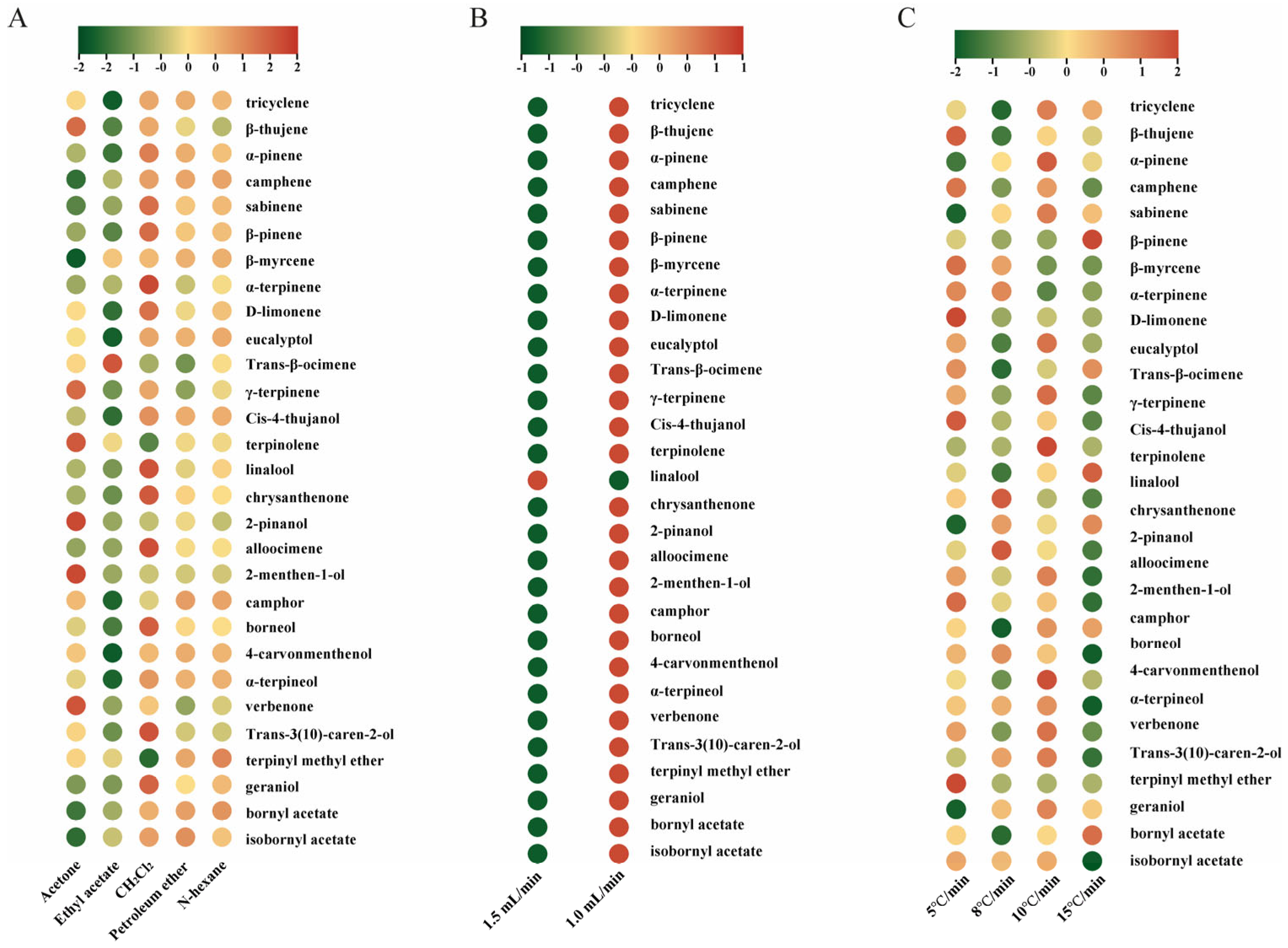
3.1. Optimization of Extraction and GC-MS Conditions for Monoterpenoids Analysis
3.2. Monoterpenoid Profiling in A. annua Under Optimized Extraction and GC-MS Conditions
3.3. Genome-Wide Identification of Monoterpene Synthase Candidates and Subcellular Localization
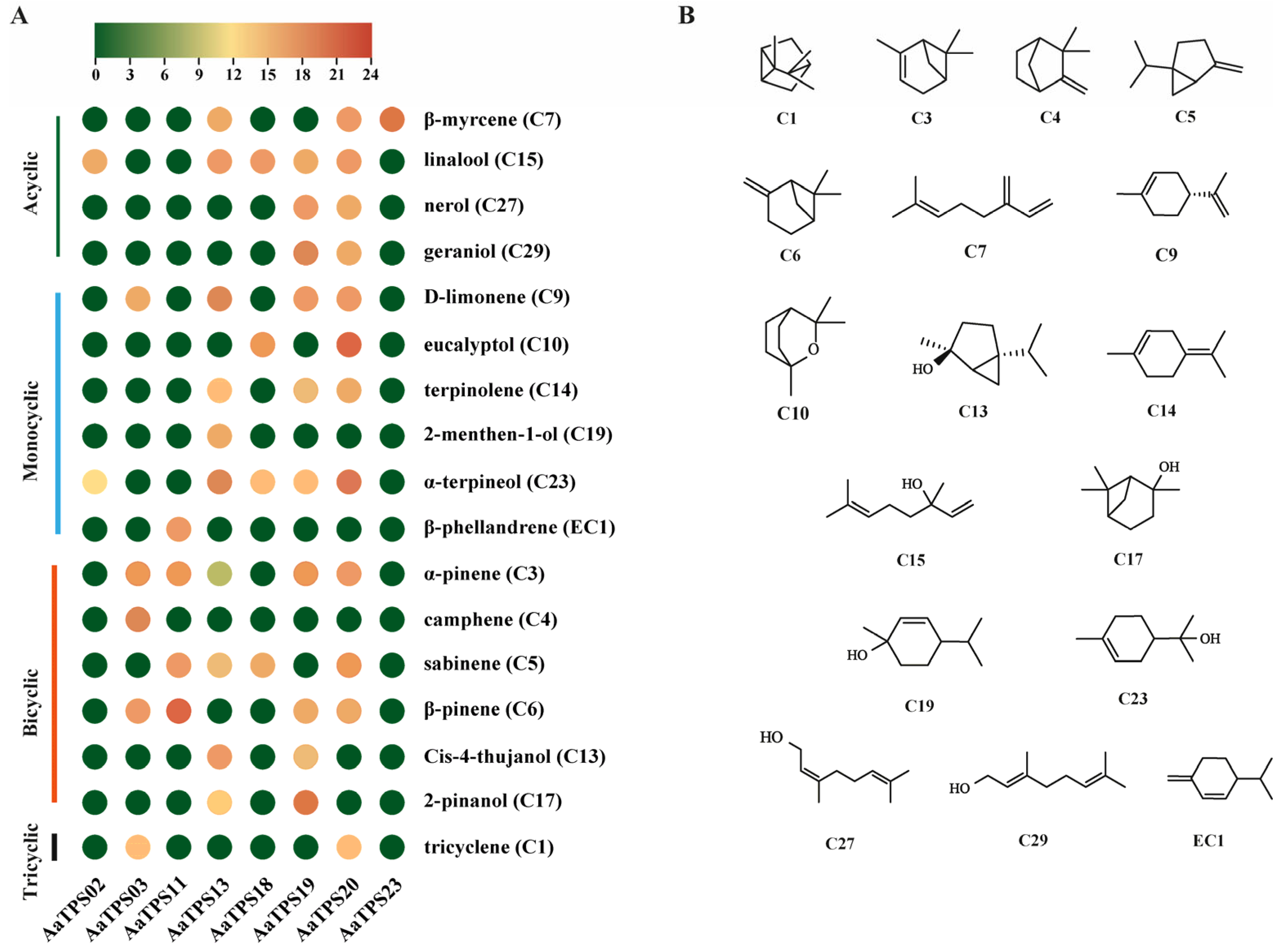
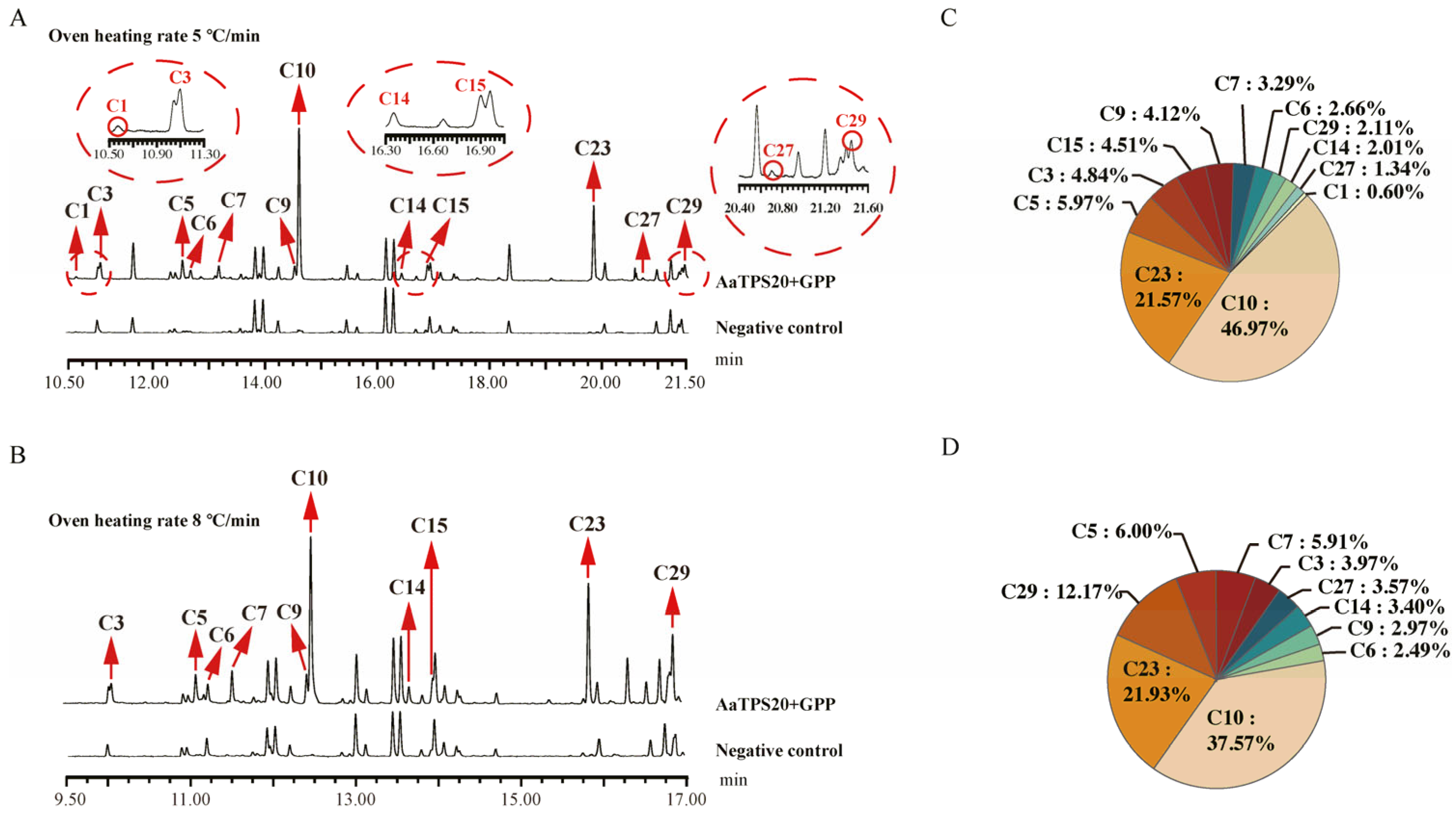
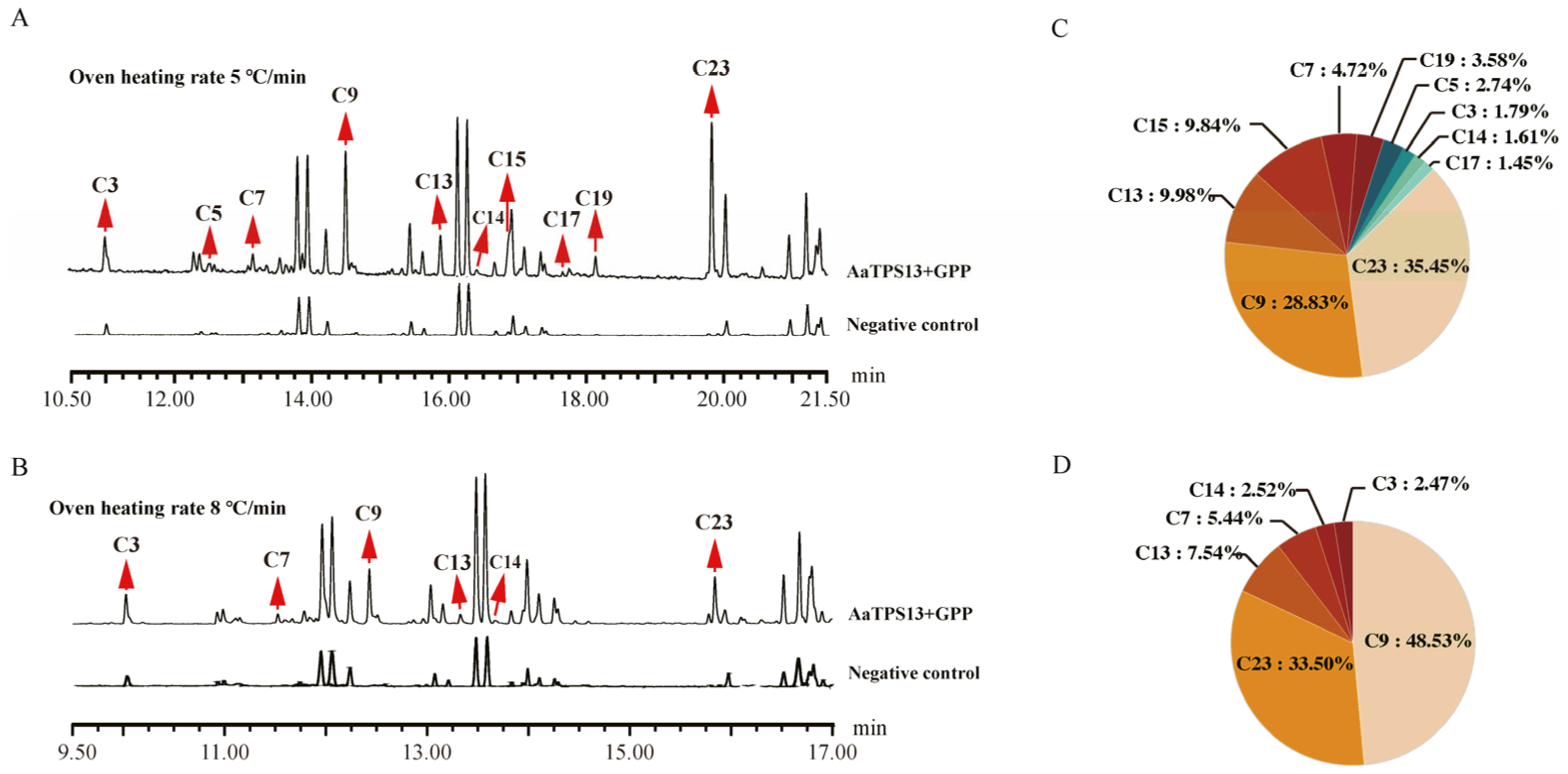
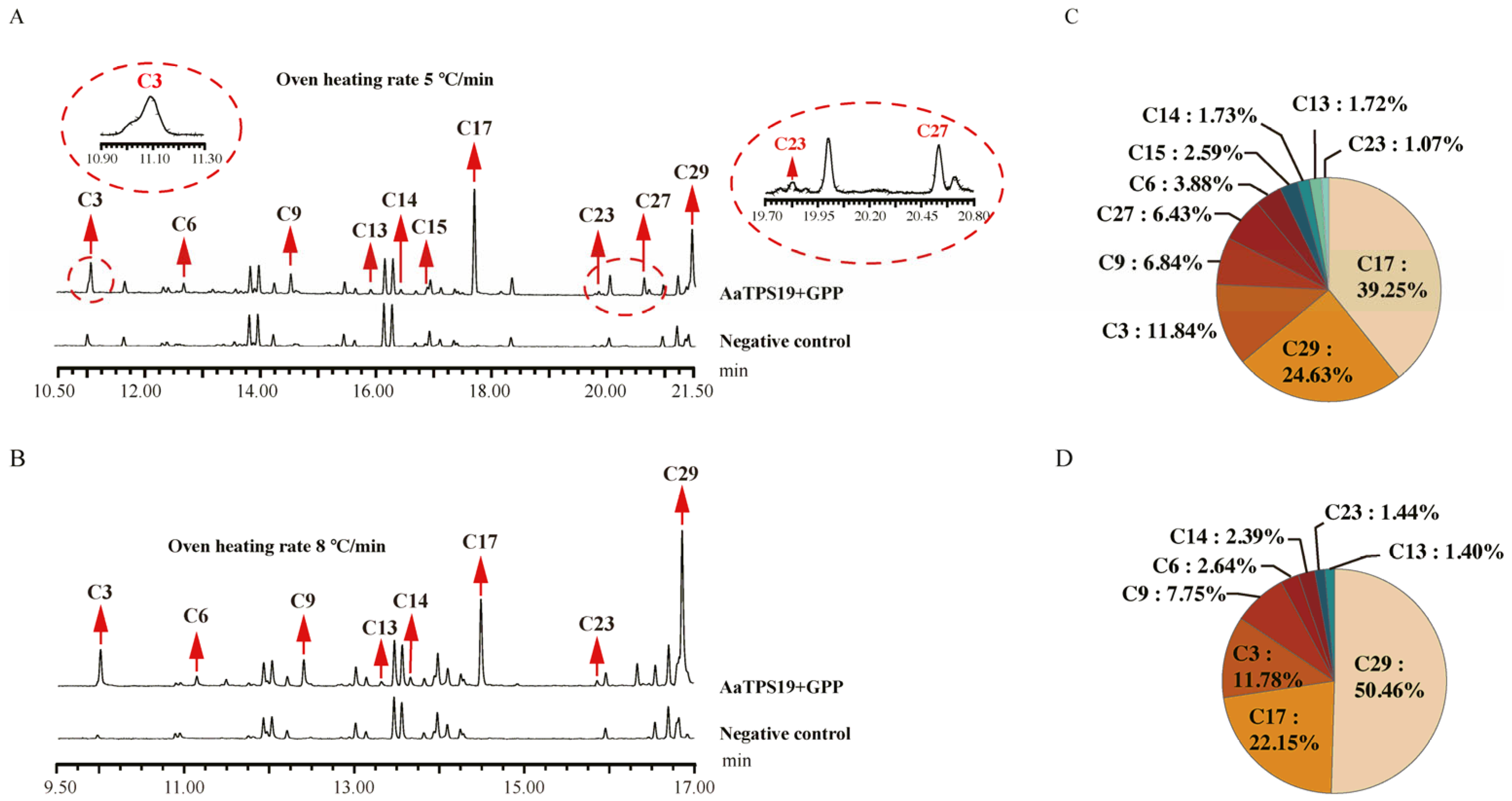
3.4. In Vitro Characterization of Monoterpene Synthase in Artemisia annua
3.5. Expression Pattern Analysis of Monoterpene Synthase in Artemisia annua
4. Discussion
4.1. Optimized GC–MS Enables First Identification of a 2-Pinanol Synthase
4.2. Interpreting Expression–Metabolite Incongruence
4.3. Concordance with Prior AaTPS Reports and Effects of Analytical/Construct Design
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TPS | terpene synthase |
| IPTG | β-D-1-thiogalactopyranoside |
| GPP | geranyl pyrophosphate |
| FPP | farnesyl diphosphate |
| qRT-PCR | quantitative real-time PCR |
| GC–MS | gas chromatography–mass spectrometry |
| RSD | relative standard deviation |
References
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Cao, Q.; Liu, L.; Ma, X.; Zhong, C.; Tang, M.; Liu, M.; Qu, L.B.; Wei, B.; Xu, X. 1, 8-Cineole Ameliorated Staphylococcus aureus-Induced Pneumonia through Modulation of TRP-KYN and Arginine-NO Reprogramming. J. Agric. Food Chem. 2025, 73, 11670–11683. [Google Scholar] [CrossRef]
- Abbas, F.; O’Neill Rothenberg, D.; Zhou, Y.; Ke, Y.; Wang, H.C. Volatile organic compounds as mediators of plant communication and adaptation to climate change. Physiol. Plant 2022, 174, e13840. [Google Scholar] [CrossRef]
- Raguso, R.A. More lessons from linalool: Insights gained from a ubiquitous floral volatile. Curr. Opin. Plant Biol. 2016, 32, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Yahyaa, M.; Ibdah, M.; Marzouk, S.; Ibdah, M. Profiling of the Terpene Metabolome in Carrot Fruits of Wild (Daucus carota L. ssp. carota) Accessions and Characterization of a Geraniol Synthase. J. Agric. Food Chem. 2018, 66, 2378–2386. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, X.; Wang, Z.; Wang, T.; Pan, Y.; Fang, K.; Wang, S.; Xi, J. High Nitrogen Enhances Maize Susceptibility to Holotrichia parallela via beta-Caryophyllene-Mediated Olfactory Recognition and Jasmonate Suppression. J. Agric. Food Chem. 2025, 73, 8204–8213. [Google Scholar] [CrossRef]
- Cascone, P.; Iodice, L.; Maffei, M.E.; Bossi, S.; Arimura, G.; Guerrieri, E. Tobacco overexpressing beta-ocimene induces direct and indirect responses against aphids in receiver tomato plants. J. Plant Physiol. 2015, 173, 28–32. [Google Scholar] [CrossRef]
- Zhang, L.; Su, Q.F.; Wang, L.S.; Lv, M.W.; Hou, Y.X.; Li, S.S. Linalool: A ubiquitous floral volatile mediating the communication between plants and insects. J. Syst. Evol. 2022, 61, 538–549. [Google Scholar] [CrossRef]
- Judd, R.; Bagley, M.C.; Li, M.; Zhu, Y.; Lei, C.; Yuzuak, S.; Ekelof, M.; Pu, G.; Zhao, X.; Muddiman, D.C.; et al. Artemisinin Biosynthesis in Non-glandular Trichome Cells of Artemisia annua. Mol. Plant 2019, 12, 704–714. [Google Scholar] [CrossRef]
- Abdallah, I.I.; Mahmoud, H.A.; El-Sebakhy, N.A.; Mahgoub, Y.A. Comparative phytochemical profiling and authentication of four Artemisia species using integrated GC-MS, HPTLC and NIR spectroscopy approach. BMC Chem. 2025, 19, 100. [Google Scholar] [CrossRef]
- Ma, D.M.; Wang, Z.; Wang, L.; Alejos-Gonzales, F.; Sun, M.A.; Xie, D.Y. A Genome-Wide Scenario of Terpene Pathways in Self-pollinated Artemisia annua. Mol. Plant 2015, 8, 1580–1598. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, L.; Yang, Y.; Xiang, H.; Yang, P. Plant secondary metabolites-mediated plant defense against bacteria and fungi pathogens. Plant Physiol. Biochem. 2024, 217, 109224. [Google Scholar] [CrossRef]
- Xu, M.; Jiang, Y.; Chen, S.; Chen, F.; Chen, F. Herbivory-Induced Emission of Volatile Terpenes in Chrysanthemum morifolium Functions as an Indirect Defense against Spodoptera litura Larvae by Attracting Natural Enemies. J. Agric. Food Chem. 2021, 69, 9743–9753. [Google Scholar] [CrossRef]
- Zhou, Z.; Xian, J.; Wei, W.; Xu, C.; Yang, J.; Zhan, R.; Ma, D. Volatile metabolic profiling and functional characterization of four terpene synthases reveal terpenoid diversity in different tissues of Chrysanthemum indicum L. Phytochemistry 2021, 185, 112687. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Ling, X.Y.; Zhou, X.F.; Chen, Y.X.; Wang, T.T.; Lin, X.J.; Zhao, Y.Y.; Ye, Y.S.; Huang, L.X.; Sun, Y.W.; et al. Comparing genomes of Fructus Amomi-producing species reveals genetic basis of volatile terpenoid divergence. Plant Physiol. 2023, 193, 1244–1262. [Google Scholar] [CrossRef] [PubMed]
- Luck, K.; Chen, X.; Norris, A.M.; Chen, F.; Gershenzon, J.; Kollner, T.G. The reconstruction and biochemical characterization of ancestral genes furnish insights into the evolution of terpene synthase function in the Poaceae. Plant Mol. Biol. 2020, 104, 203–215. [Google Scholar] [CrossRef]
- Yan, X.M.; Zhou, S.S.; Liu, H.; Zhao, S.W.; Tian, X.C.; Shi, T.L.; Bao, Y.T.; Li, Z.C.; Jia, K.H.; Nie, S.; et al. Unraveling the evolutionary dynamics of the TPS gene family in land plants. Front. Plant Sci. 2023, 14, 1273648. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef]
- Hansen, N.L.; Heskes, A.M.; Hamberger, B.; Olsen, C.E.; Hallstrom, B.M.; Andersen-Ranberg, J.; Hamberger, B. The terpene synthase gene family in Tripterygium wilfordii harbors a labdane-type diterpene synthase among the monoterpene synthase TPS-b subfamily. Plant J. 2017, 89, 429–441. [Google Scholar] [CrossRef]
- Vidic, D.; Copra-Janicijevic, A.; Milos, M.; Maksimovic, M. Effects of Different Methods of Isolation on Volatile Composition of Artemisia annua L. Int. J. Anal. Chem. 2018, 2018, 9604183. [Google Scholar] [CrossRef]
- D’Atri, V.; Fekete, S.; Clarke, A.; Veuthey, J.L.; Guillarme, D. Recent Advances in Chromatography for Pharmaceutical Analysis. Anal. Chem. 2019, 91, 210–239. [Google Scholar] [CrossRef]
- Peng, Y.; Feng, X.; Feng, Y.; Li, L.; Chen, Y.; Yu, J. Optimization of two-stage thermal desorption combined with pentafluorophenyl hydrazine derivatization-gas chromatography/mass spectrometry analytical method of atmospheric carbonyl compounds. Microchemical Journal 2024, 197. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Pandey, P.; Meitei, R.; Cardona, D.; Gujar, A.C.; Shulaev, V. GC-MS/MS Profiling of Plant Metabolites. In Plant Metabolic Engineering: Methods and Protocols; Springer: New York, NY, USA, 2022; pp. 101–115. [Google Scholar] [CrossRef]
- Xiang, L.; He, P.; Shu, G.; Yuan, M.; Wen, M.; Lan, X.; Liao, Z.; Tang, Y. AabHLH112, a bHLH transcription factor, positively regulates sesquiterpenes biosynthesis in Artemisia annua. Front. Plant Sci. 2022, 13, 973591. [Google Scholar] [CrossRef] [PubMed]
- Gou, Y.; Zhang, F.; Tang, Y.; Jiang, C.; Bai, G.; Xie, H.; Chen, M.; Liao, Z. Engineering Nootkatone Biosynthesis in Artemisia annua. ACS Synth. Biol. 2021, 10, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Cassia de Souza Schneider, R.; Zanon Baldissarelli, V.; Trombetta, F.; Martinelli, M.; Bastos Caramão, E. Optimization of gas chromatographic–mass spectrometric analysis for fatty acids in hydrogenated castor oil obtained by catalytic transfer hydrogenation. Analytica Chimica Acta 2004, 505, 223–226. [Google Scholar] [CrossRef]
- Zeki, O.C.; Eylem, C.C.; Recber, T.; Kir, S.; Nemutlu, E. Integration of GC-MS and LC-MS for untargeted metabolomics profiling. J. Pharm. Biomed. Anal. 2020, 190, 113509. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics by Gas Chromatography-Mass Spectrometry: Combined Targeted and Untargeted Profiling. Curr. Protoc. Mol. Biol. 2016, 114, 30–34. [Google Scholar] [CrossRef]
- Ye, P.; Liang, S.; Wang, X.; Duan, L.; Jiang-Yan, F.; Yang, J.; Zhan, R.; Ma, D. Transcriptome analysis and targeted metabolic profiling for pathway elucidation and identification of a geraniol synthase involved in iridoid biosynthesis from Gardenia jasminoides. Ind. Crops Prod. 2019, 132, 48–58. [Google Scholar] [CrossRef]
- Zhou, F.; Pichersky, E. The complete functional characterisation of the terpene synthase family in tomato. New Phytol. 2020, 226, 1341–1360. [Google Scholar] [CrossRef]
- Zhi, Y.; Dai, C.; Fang, X.; Xiao, X.; Lu, H.; Chen, F.; Chen, R.; Ma, W.; Deng, Z.; Lu, L.; et al. Gene-Directed In Vitro Mining Uncovers the Insect-Repellent Constituent from Mugwort (Artemisia argyi). J. Am. Chem. Soc. 2024, 146, 30883–30892. [Google Scholar] [CrossRef]
- Ruan, J.X.; Li, J.X.; Fang, X.; Wang, L.J.; Hu, W.L.; Chen, X.Y.; Yang, C.Q. Isolation and Characterization of Three New Monoterpene Synthases from Artemisia annua. Front. Plant Sci. 2016, 7, 638. [Google Scholar] [CrossRef]
- Lu, S.; Xu, R.; Jia, J.W.; Pang, J.; Matsuda, S.P.; Chen, X.Y. Cloning and functional characterization of a beta-pinene synthase from Artemisia annua that shows a circadian pattern of expression. Plant Physiol. 2002, 130, 477–486. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, W.; Lin, X.; Le, Z.; Wang, M.; Qin, X.; Zeng, L.; Qian, Y.; Shu, G.; Chen, M.; Lan, X.; et al. Chemical Profiling of Monoterpenes and Genome-Wide Discovery of Monoterpene Synthases in Artemisia annua. Horticulturae 2025, 11, 1083. https://doi.org/10.3390/horticulturae11091083
Wei W, Lin X, Le Z, Wang M, Qin X, Zeng L, Qian Y, Shu G, Chen M, Lan X, et al. Chemical Profiling of Monoterpenes and Genome-Wide Discovery of Monoterpene Synthases in Artemisia annua. Horticulturae. 2025; 11(9):1083. https://doi.org/10.3390/horticulturae11091083
Chicago/Turabian StyleWei, Wuke, Xinyue Lin, Zijian Le, Mengxue Wang, Xingyan Qin, Lingjiang Zeng, Yan Qian, Guoping Shu, Min Chen, Xiaozhong Lan, and et al. 2025. "Chemical Profiling of Monoterpenes and Genome-Wide Discovery of Monoterpene Synthases in Artemisia annua" Horticulturae 11, no. 9: 1083. https://doi.org/10.3390/horticulturae11091083
APA StyleWei, W., Lin, X., Le, Z., Wang, M., Qin, X., Zeng, L., Qian, Y., Shu, G., Chen, M., Lan, X., Wang, B., Liao, Z., Hou, Y., Mao, J., & Zhang, F. (2025). Chemical Profiling of Monoterpenes and Genome-Wide Discovery of Monoterpene Synthases in Artemisia annua. Horticulturae, 11(9), 1083. https://doi.org/10.3390/horticulturae11091083







