Transcriptome Analysis Reveals Pollination and Fertilization Mechanisms of Paeonia ostii ‘Fengdanbai’
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Pollination
2.2. RNA Extraction, cDNA Library Construction, and Transcriptome Sequencing
2.3. DEG Annotation and Analysis
2.4. Quantitative RT-PCR (qPCR) Analysis of DEGs
2.5. Gene Clone and Phylogenetic Tree Analysis
2.6. Promoter Cis-Acting Elements and Gene Co-Expression Analysis
2.7. Statistical Analysis
3. Results
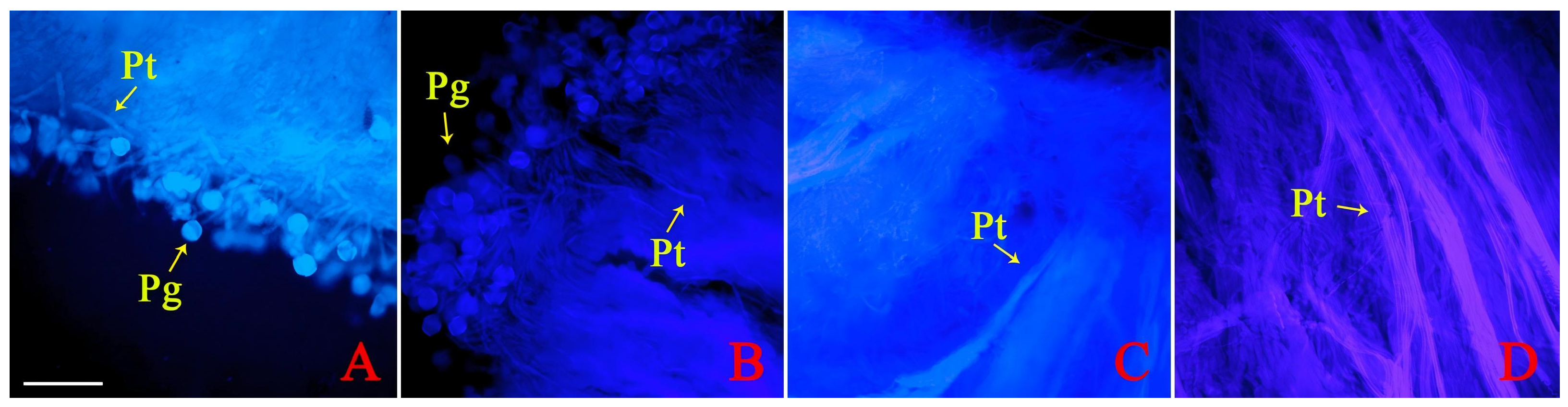
3.1. Critical Periods for the Pollen Tube Growth
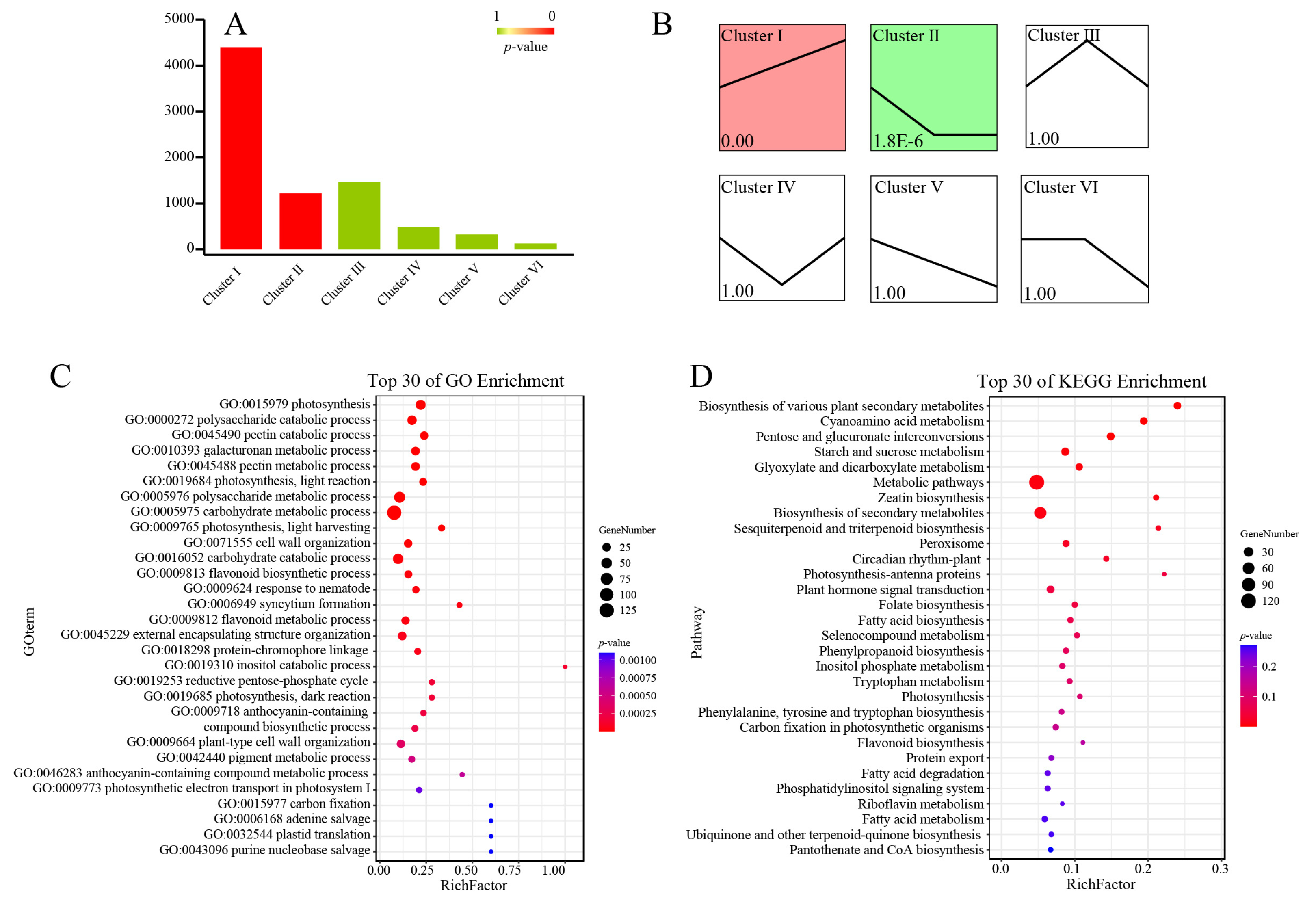
3.2. Pistil Transcriptome Analysis
3.3. DEGs Relating to the Pollination Process of Pistils
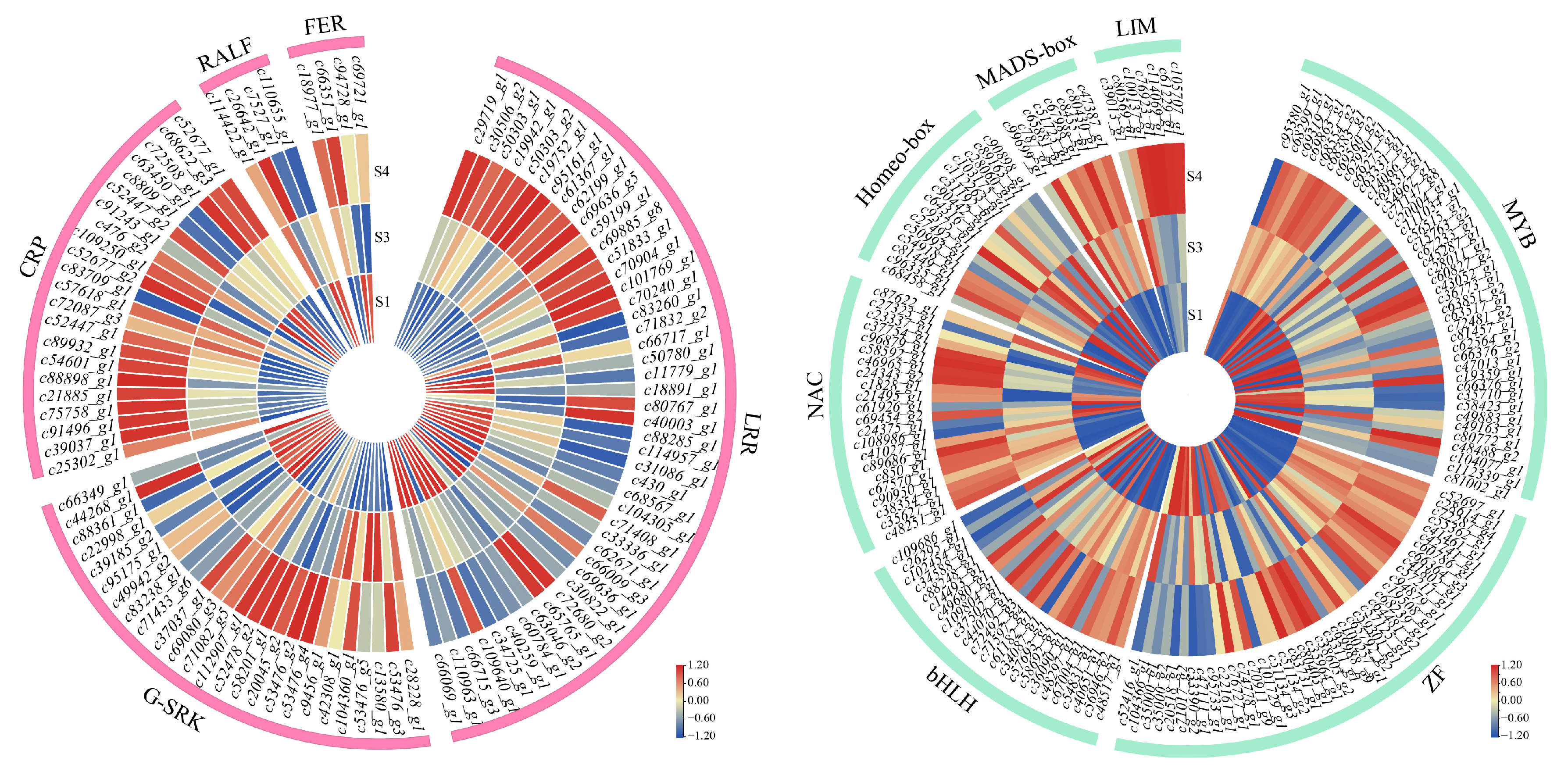
3.4. Expression Patterns of Pollination and Fertilization Related DEGs in P. ostii ‘Fengdanbai’ Pistil
3.5. Validation of DEGs Expression Profiles
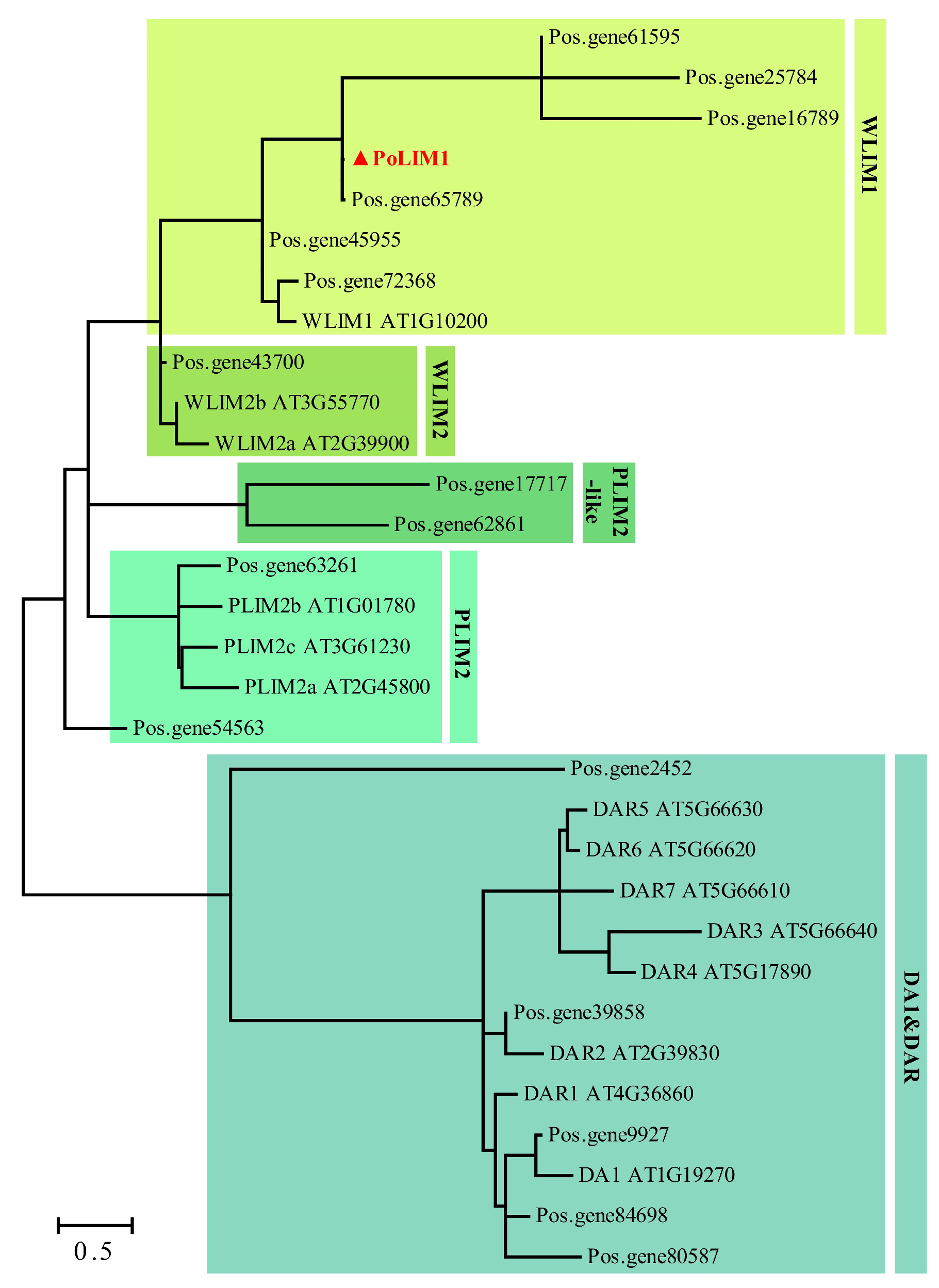
3.6. PobUNE10 and PoLIM1 Are Candidate Regulators of Pollination and Fertilization
4. Discussion
4.1. Hormone Signaling, Carbohydrate Metabolism, and Calcium-Dependent Kinases Are Essential for Pollination and Fertilization
4.2. PoUNE10 and PoLIM1 Are Involved in the Process of Pollination and Fertilization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peng, L.P.; Men, S.Q.; Liu, Z.A.; Tong, N.N.; Imran, M.; Shu, Q.Y. Fatty acid composition, phytochemistry, antioxidant activity on seed coat and kernel of Paeonia ostii from main geographic production areas. Foods 2019, 9, 30. [Google Scholar] [CrossRef]
- Deng, R.; Gao, J.; Yi, J.; Liu, P. Could peony seeds oil become a high-quality edible vegetable oil? The nutritional and phytochemistry profiles, extraction, health benefits, safety and value-added-products. Food Res. Int. 2022, 156, 111200. [Google Scholar] [CrossRef]
- Zhang, K.; Cao, W.; Baskin, J.M.; Baskin, C.C.; Sun, J.; Yao, L.; Tao, J. Seed development in Paeonia ostii (Paeoniaceae), with particular reference to embryogeny. BMC Plant Biol. 2021, 21, 603. [Google Scholar] [CrossRef]
- Liu, N.; Cheng, F.; Zhong, Y.; Guo, X. Comparative transcriptome and coexpression network analysis of carpel quantitative variation in Paeonia rockii. BMC Genom. 2019, 20, 683. [Google Scholar] [CrossRef]
- Wang, J.; Kambhampati, S.; Allen, D.K.; Chen, L.-Q. Comparative metabolic analysis reveals a metabolic switch in mature, hydrated, and germinated pollen in Arabidopsis thaliana. Front. Plant Sci. 2022, 13, 836665. [Google Scholar] [CrossRef]
- Poidevin, L.; Forment, J.; Unal, D.; Ferrando, A. Transcriptome and translatome changes in germinated pollen under heat stress uncover roles of transporter genes involved in pollen tube growth. Plant Cell Environ. 2021, 44, 2167–2184. [Google Scholar] [CrossRef]
- Qin, Y.; Leydon, A.R.; Manziello, A.; Pandey, R.; Mount, D.; Denic, S.; Vasic, B.; Johnson, M.A.; Palanivelu, R. Penetration of the stigma and style elicits a novel transcriptome in pollen tubes, pointing to genes critical for growth in a pistil. PLoS Genet. 2009, 5, e1000621. [Google Scholar] [CrossRef]
- Tang, W.; Kelley, D.; Ezcurra, I.; Cotter, R.; McCormick, S. LeSTIG1, an extracellular binding partner for the pollen receptor kinases LePRK1 and LePRK2, promotes pollen tube growth in vitro. Plant J. 2004, 39, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Kaothien, P.; Ok, S.H.; Shuai, B.; Wengier, D.; Cotter, R.; Kelley, D.; Kiriakopolos, S.; Muschietti, J.; McCormick, S. Kinase partner protein interacts with the LePRK1 and LePRK2 receptor kinases and plays a role in polarized pollen tube growth. Plant J. 2005, 42, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Mecchia, M.A.; Santos-Fernandez, G.; Duss, N.N.; Somoza, S.C.; Boisson-Dernier, A.; Gagliardini, V.; Martínez-Bernardini, A.; Fabrice, T.N.; Ringli, C.; Muschietti, J.P.; et al. RALF4/19 peptides interact with LRX proteins to control pollen tube growth in Arabidopsis. Science 2017, 358, 1600–1603. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Liu, X.; Yan, S.; Liu, B.; Zhong, Y.; Song, W.; Chen, J.; Wang, Z.; Che, G.; Liu, L.; et al. Pollen tube emergence is mediated by ovary-expressed ALCATRAZ in cucumber. Nat. Commun. 2023, 14, 258. [Google Scholar] [CrossRef]
- Rajani, S.; Sundaresan, V. The Arabidopsis myc/bHLH gene ALCATRAZ enables cell separation in fruit dehiscence. Curr. Biol. 2001, 11, 1914–1922. [Google Scholar] [CrossRef]
- Pagnussat, G.C.; Yu, H.J.; Ngo, Q.A.; Rajani, S.; Mayalagu, S.; Johnson, C.S.; Capron, A.; Xie, L.-F.; Ye, D.; Sundaresan, V. Genetic and molecular identification of genes required for female gametophyte development and function in Arabidopsis. Development 2005, 132, 603–614. [Google Scholar] [CrossRef]
- Guyon, V.N.; Astwood, J.D.; Garner, E.C.; Dunker, A.K.; Taylor, L.P. Isolation and characterization of cDNAs expressed in the early stages of flavonol-induced pollen germination in petunia. Plant Physiol. 2000, 123, 699–710. [Google Scholar] [CrossRef]
- Yang, X.; Bu, Y.; Niu, F.; Cun, Y.; Zhang, L.; Song, X. Comprehensive analysis of LIM gene family in wheat reveals the involvement of TaLIM2 in pollen development. Plant Sci. 2022, 314, 111101. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.J.; Kim, E.J.; Yoon, J.; Silva, J.; Moon, S.; Min, C.W.; Cho, L.-H.; Kim, S.T.; Park, S.K.; Kim, Y.-J.; et al. A myosin XI adaptor, TAPE, is essential for pollen tube elongation in rice. Plant Physiol. 2022, 190, 562–575. [Google Scholar] [CrossRef]
- Dean Rider, S., Jr.; Henderson, J.T.; Jerome, R.E.; Edenberg, H.J.; Romero-Severson, J.; Ogas, J. Coordinate repression of regulators of embryonic identity by PICKLE during germination in Arabidopsis. Plant J. 2003, 35, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Li, N.; Chen, L.; Xu, Y.; Li, Y.; Zhang, Y.; Li, C.; Li, Y. The ubiquitin receptor DA1 regulates seed and organ size by modulating the stability of the ubiquitin-specific protease UBP15/SOD2 in Arabidopsis. Plant Cell. 2014, 26, 665–677. [Google Scholar] [CrossRef]
- Chen, X.; Liu, C.; Guo, P.; Hao, X.; Pan, Y.; Zhang, K.; Liu, W.; Zhao, L.; Luo, W.; He, J.; et al. Differential SW16.1 allelic effects and genetic backgrounds contributed to increased seed weight after soybean domestication. J. Integr. Plant Biol. 2023, 65, 1734–1752. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, S.; Li, W.; Liu, A.; Jiang, Y.; Gan, G.; Li, W.; Liang, X.; Yu, N.; Chen, R.; et al. Comparative transcriptome analysis provides insights into the molecular mechanism underlying double fertilization between self-crossed Solanum melongena and that hybridized with Solanum aethiopicum. PLoS ONE 2020, 15, e0235962. [Google Scholar] [CrossRef]
- Xie, X.; Cheng, T.; Yan, Y.; Zhu, C.; Zhang, M.; Sun, Z.; Wang, T. Integrated metabolomic and transcriptomic analysis of the anthocyanin regulatory networks in Lagerstroemia indica petals. BMC Plant Biol. 2025, 25, 316. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, X.; Yang, K.; Zhu, C.; Liu, Y.; Gao, Z. Multifaceted analyses reveal carbohydrate metabolism mainly affecting the quality of postharvest bamboo shoots. Front. Plant Sci. 2022, 13, 1021161. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Zhang, X.; Liu, Z.; Peng, L.; Hao, Q.; Liu, Z.; Men, S.; Tong, N.; Shu, Q. ABSCISIC ACID-INSENSITIVE 5-ω3 FATTY ACID DESATURASE3 module regulates unsaturated fatty acids biosynthesis in Paeonia ostii. Plant Sci. 2022, 317, 111189. [Google Scholar] [CrossRef]
- Chen, M. Study on the Development characteristics of Paeonia ostii ‘Feng Dan’ seeds and its yield components. Master’s Thesis, Yangzhou University, Yangzhou, China, 2020. [Google Scholar]
- Dan, H.; Xie, M.; Lu, B.; Wang, Z.; Liu, Y.; He, S. Analysis of pollination affinity performance and its physiological mechanism in Paeonia suffruticosa and Paeonia lactiflora. J. Northwest A F Univ. 2017, 45, 129–136. [Google Scholar]
- Çetinbaş-Genç, A.; Vardar, F. Effect of methyl jasmonate on in-vitro pollen germination and tube elongation of Pinus nigra. Protoplasma 2020, 257, 1655–1665. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Lu, M.; Yang, J.; Tan, X. The core jasmonic acid-signalling module CoCOI1/CoJAZ1/CoMYC2 are involved in jas mediated growth of the pollen tube in Camellia oleifera. Curr. Issues Mol. Biol. 2022, 44, 5405–5415. [Google Scholar] [CrossRef]
- Hu, W.; Liu, Y.; Loka, D.A.; Zahoor, R.; Wang, S.; Zhou, Z. Drought limits pollen tube growth rate by altering carbohydrate metabolism in cotton (Gossypium hirsutum) pistils. Plant Sci. 2019, 286, 108–117. [Google Scholar] [CrossRef]
- You, L.; Yu, L.; Liang, R.; Sun, R.; Hu, F.; Lu, X.; Zhao, J. Identification and analysis of genes involved in double fertilization in rice. Int. J. Mol. Sci. 2021, 22, 12850. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, Z.; Yao, F.; Gao, L.; Ma, S.; Sui, X.; Zhang, Z. Down-regulating CsHT1, a cucumber pollen-specific hexose transporter, inhibits pollen germination, tube growth, and seed development. Plant Physiol. 2015, 168, 635–647. [Google Scholar] [CrossRef]
- Li, C.; Meng, D.; Piñeros, M.A.; Mao, Y.; Dandekar, A.M.; Cheng, L. A sugar transporter takes up both hexose and sucrose for sorbitol-modulated in vitro pollen tube growth in apple. Plant Cell. 2020, 32, 449–469. [Google Scholar] [CrossRef]
- Brazel, A.J.; Ó’Maoiléidigh, D.S. Photosynthetic activity of reproductive organs. J. Exp. Bot. 2019, 70, 1737–1754. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Fatima, M.; Li, J.; Zhou, P.; Zaynab, M.; Ming, R. Post-pollination sepal longevity of female flower co-regulated by energy-associated multiple pathways in dioecious spinach. Front. Plant Sci. 2022, 13, 1010149. [Google Scholar] [CrossRef]
- Wang, H.; Schauer, N.; Usadel, B.; Frasse, P.; Zouine, M.; Hernould, M.; Latché, A.; Pech, J.-C.; Fernie, A.R.; Bouzayen, M. Regulatory features underlying pollination-dependent and -independent tomato fruit set revealed by transcript and primary metabolite profiling. Plant Cell. 2009, 21, 1428–1452. [Google Scholar] [CrossRef]
- Seth, R.; Bhandawat, A.; Parmar, R.; Singh, P.; Kumar, S.; Sharma, R.K. Global transcriptional insights of pollen-pistil interactions commencing self-incompatibility and fertilization in tea [Camellia sinensis (L.) O. Kuntze]. Int. J. Mol. Sci. 2019, 20, 539. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Li, L.; Ye, H.; Yu, X.; Algreen, A.; Yin, Y. Three related receptor-like kinases are required for optimal cell elongation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2009, 106, 7648–7653. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Liu, M.J.; Kita, D.; Jordan, S.S.; Yeh, F.J.; Yvon, R.; Carpenter, H.; Federico, A.N.; Garcia-Valencia, L.E.; Eyles, S.J.; et al. FERONIA controls pectin- and nitric oxide-mediated male-female interaction. Nature 2020, 579, 561–566. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, C.; Xi, Y.; Shao, Q.; Li, L.; Luan, S. A receptor-channel trio conducts Ca2+ signalling for pollen tube reception. Nature 2022, 607, 534–539. [Google Scholar] [CrossRef]
- Song, Y.; Wang, J.; Zhu, J.; Shang, W.; Jia, W.; Sun, Y.; He, S.; Yang, X.; Wang, Z. Functional analysis of the PoSERK-interacting protein PorbcL in the embryogenic callus formation of tree peony (Paeonia ostii T. Hong et J. X. Zhang). Plants 2024, 13, 2697. [Google Scholar] [CrossRef]
- Groszmann, M.; Paicu, T.; Alvarez, J.P.; Swain, S.M.; Smyth, D.R. SPATULA and ALCATRAZ, are partially redundant, functionally diverging bHLH genes required for Arabidopsis gynoecium and fruit development. Plant J. 2011, 68, 816–829. [Google Scholar] [CrossRef]
- Cheng, Z.; Song, X.; Liu, X.; Yan, S.; Song, W.; Wang, Z.; Han, L.; Zhao, J.; Yan, L.; Zhou, Z.; et al. SPATULA and ALCATRAZ confer female sterility and fruit cavity via mediating pistil development in cucumber. Plant Physiol. 2022, 189, 1553–1569. [Google Scholar] [CrossRef]
- Jeong, J.; Choi, G. Phytochrome-interacting factors have both shared and distinct biological roles. Mol. Cells 2013, 35, 371–380. [Google Scholar] [CrossRef]
- Yang, C.; Huang, S.; Zeng, Y.; Liu, C.; Ma, Q.; Pruneda-Paz, J.; Kay, S.A.; Li, L. Two bHLH transcription factors, bHLH48 and bHLH60, associate with phytochrome interacting factor 7 to regulate hypocotyl elongation in Arabidopsis. Cell Rep. 2021, 35, 109054. [Google Scholar] [CrossRef]
- Ortiz-Ramírez, C.I.; Giraldo, M.A.; Ferrándiz, C.; Pabón-Mora, N. Expression and function of the bHLH genes ALCATRAZ and SPATULA in selected Solanaceae species. Plant J. 2019, 99, 686–702. [Google Scholar] [CrossRef]
- Park, J.I.; Ahmed, N.U.; Jung, H.J.; Arasan, S.K.; Chung, M.Y.; Cho, Y.-G.; Watanabe, M.; Nou, I.-S. Identification and characterization of LIM gene family in Brassica rapa. BMC Genom. 2014, 15, 641. [Google Scholar] [CrossRef]
- Srivastava, V.; Verma, P.K. The plant LIM proteins: Unlocking the hidden attractions. Planta 2017, 246, 365–375. [Google Scholar] [CrossRef]
- Khatun, K.; Robin, A.H.K.; Park, J.I.; Ahmed, N.U.; Kim, C.K.; Lim, K.B.; Kim, M.-B.; Lee, D.-J.; Nou, I.S.; Chung, M.-Y. Genome-wide identification, characterization and expression profiling of LIM family genes in Solanum lycopersicum L. Plant Physiol. Biochem. 2016, 108, 177–190. [Google Scholar]
- Hewedy, O.A.; Elsheery, N.I.; Karkour, A.M.; Elhamouly, N.; Arafa, R.A.; Mahmoud, G.A.-E.; Dawood, M.; Hussein, W.E.; Mansour, A.; Hatem, D.; et al. Jasmonic acid regulates plant development and orchestrates stress response during tough times. Environ. Exp. Bot. 2023, 208, 105260. [Google Scholar] [CrossRef]
- Li, H.; Yang, Y.; Zhang, H.; Li, C.; Du, P.; Bi, M.; Chen, T.; Qian, D.; Niu, Y.; Ren, H.; et al. The Arabidopsis GPI-anchored protein COBL11 is necessary for regulating pollen tube integrity. Cell Rep. 2023, 42, 113353. [Google Scholar] [CrossRef] [PubMed]
- Papuga, J.; Hoffmann, C.; Dieterle, M.; Moes, D.; Moreau, F.; Tholl, S.; Steinmetz, A.; Thomas, C. Arabidopsis LIM proteins: A family of actin bundlers with distinct expression patterns and modes of regulation. Plant Cell. 2010, 22, 3034–3052. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Xu, C.; Gu, C.; Wang, S.; Li, W.; Jiang, X.; Zhang, W.; Hao, Q. Transcriptome Analysis Reveals Pollination and Fertilization Mechanisms of Paeonia ostii ‘Fengdanbai’. Horticulturae 2025, 11, 1082. https://doi.org/10.3390/horticulturae11091082
Li Z, Xu C, Gu C, Wang S, Li W, Jiang X, Zhang W, Hao Q. Transcriptome Analysis Reveals Pollination and Fertilization Mechanisms of Paeonia ostii ‘Fengdanbai’. Horticulturae. 2025; 11(9):1082. https://doi.org/10.3390/horticulturae11091082
Chicago/Turabian StyleLi, Zhen, Chi Xu, Cancan Gu, Shengxin Wang, Wei Li, Xiaolei Jiang, Wanqiu Zhang, and Qing Hao. 2025. "Transcriptome Analysis Reveals Pollination and Fertilization Mechanisms of Paeonia ostii ‘Fengdanbai’" Horticulturae 11, no. 9: 1082. https://doi.org/10.3390/horticulturae11091082
APA StyleLi, Z., Xu, C., Gu, C., Wang, S., Li, W., Jiang, X., Zhang, W., & Hao, Q. (2025). Transcriptome Analysis Reveals Pollination and Fertilization Mechanisms of Paeonia ostii ‘Fengdanbai’. Horticulturae, 11(9), 1082. https://doi.org/10.3390/horticulturae11091082





