Abstract
Extreme climate events—such as heatwaves, floods, and droughts—are increasingly affecting ecosystems, with the global average temperature projected to rise by up to 3 °C (IPCC, 2023) due to anthropogenic greenhouse gas emissions. These changes pose critical challenges to food security, as evidenced by 733 million people facing hunger in 2024. In response, crop modeling considering different climate change scenarios has become a valuable tool to guide the development of climate-resilient agricultural strategies. Despite its nutritional importance and capacity to thrive across diverse environments, Ipomoea batatas (sweet potato) remains understudied in terms of potential spatial distribution forecasting, particularly in regions of high agrobiodiversity such as northwestern South America. Therefore, in this study we modeled the projected distribution of wild and landrace sweet potato genepools in the northern Andes under four future timeframes using seven machine learning algorithms. Our results predicted a 50% reduction in the climatically suitable range for the wild genepool by 2081, coupled with an average altitudinal shift from 1537 to 2216 m above sea level (a.s.l.). For landraces, a 36% reduction was projected by 2080, with a shift from 62 to 1995 m a.s.l. By the end of the century, suitable zones for both wild and cultivated genepools are expected to converge in high-altitude regions such as the Colombian Massif, with additional remnants of wild populations near the mountain range of Farallones de Cali. This modeling approach provides essential insights into the spatial dynamics of I. batatas under climate change, highlighting the need for ex situ conservation planning in vulnerable regions as well as assisted migration to more suitable areas. Future research should integrate edaphic and biotic interaction data to better approach the realized niche of the species and understand potential responses under a niche conservatism assumption, as well as genomic data to account for the species’ intrinsic adaptative potential, overall informing conservation, germplasm mobilization, and pre-breeding strategies that may ultimately secure the role of sweet potato in resilient food systems.
1. Introduction
A pressing global emergency is the alarming growth in environmental alterations that directly affect ecosystems, which are mainly related to extreme climate events, such as heat waves, devastating floods, intense rainfall, and prolonged droughts. The current global average temperature is notably 1.1–1.3 °C higher than the preindustrial baseline (1850–1900) of 14 ± 0.5 °C [1,2]. The Sixth Assessment Report (AR6) of the Intergovernmental Panel on Climate Change (IPCC) of 2023 ratified the interdependence of biotic and abiotic factors on climate change, indicating that human activities are the main source of greenhouse gas (GHG) emissions, representing the primary driver of ongoing global warming [2]. GHG emissions are generally related to the use of energy, land use change, lifestyles, and consumption and production patterns in all regions of the world [1,2]. The effects of climate alterations are further exacerbated in regions where El Niño Southern Oscillation (ENSO) occurs [1].
The impacts of extreme climate events are multidimensional, affecting food security, public health, economic stability, and biodiversity. In particular, global food security has been a high-alert concern in recent years due to the remanent effects of the COVID-19 pandemic on top of the already persistent crises caused by climate variability, with 733 million people in the world facing hunger in 2024 [3]. To face these environmental and social-economic challenges, mitigation instruments seek to reduce food insecurity and limited agricultural production. In this context, modeling tools that simulate potential crop distribution under changing climatic scenarios are essential for anticipating risks and supporting climate-resilient agriculture [4]. Forecasting crop responses to climate variability will enable prioritizing strategies capable of articulating standing agrobiodiversity with community initiatives customized for their own agroecosystems, leading to climate-resilient agricultural recommendations for more reliable and sustainable food access and distribution.
Modeling crop production under current conditions has already enabled the design and implementation of new agronomic strategies [4]. However, understanding how climate change will affect the geographic distribution of species and cropland suitability remains a key challenge, especially in highly environmentally heterogeneous and species-rich regions. Species distribution models (SDMs), and particularly ecological niche models (ENMs), are commonly used to project potential shifts in habitat suitability under future climate scenarios [5]. These forecasting models estimate a species’ potential ecological niche based on known occurrences and environmental predictors of the sampling locality. The models assume niche conservatism—that is, that a species’ ecological preferences remain stable over time [6], implicating that range shifts would only happen through selection, extinction at the trailing edge, and migration to the leading edge.
In this context, ENMs typically approximate the fundamental niche—the range of abiotic environmental conditions in which a species can persist in the absence of biotic constraints such as competition, predation, or symbiosis. However, because ENMs do not usually incorporate biotic interactions due to lack of comprehensive data, they do not capture the full extent of the realized niche, which is the portion of the fundamental niche that a species is at the end able to occupy in nature, shaped by both abiotic and biotic factors. As a result, ENM outputs represent potential areas of environmental suitability, but not necessarily actual occupancy [7]. Furthermore, under the niche conservatism assumption, ENMs do not account for evolutionary adaptation, but rather project range shifts through migration based on environmental constraints derived from current conditions [6]. Therefore, interpreting model outputs as evidence of adaptive potential, plasticity or resilience must be avoided unless supported by additional ecological, experimental or genomic data.
A relevant case study for agri-food systems is the sweet potato (Ipomoea batatas L.), a crop with high phenotypic plasticity and a broad distribution across diverse altitudinal and latitudinal gradients. It is considered an important, versatile, and underutilized crop for food security due to its nutritional value and adaptability [8,9]. China is currently the largest global producer, contributing approximately 57% of total production [10]. Sweet potato provides a valuable source of energy and essential nutrients [11] and is cultivated in a wide range of environments that vary in CO2 concentration [12], water availability [13], temperature, humidity, and solar radiation—factors that influence its phenology and productivity [14,15]. To model its potential distribution under climate change, it is essential to consider not only the current cultivation areas but also its center of domestication in South America, as well as the distribution of its closest wild relatives, such as Ipomoea trifida, found in the Caribbean, Central, and South America [12]. Its genetic diversity is conserved in international genebanks such as International Potato Center (CIP), which holds over 5500 accessions (GRIN-Global 2020 https://npgsweb.ars-grin.gov/gringlobal/search, accessed on 13 January 2025) [16]. Still, in countries like Colombia, many local landraces and wild populations persist at low density in semi-wild conditions and are at risk of extinction [17]. Understanding their current and potential distribution is thus critical for designing and prioritizing in situ and ex situ conservation strategies.
In this species, leaf traits and other morphological differences are related with growth efficiency and, to some extent, with root yield [18]. The ability of sweet potato to thrive across different altitudinal and environmental conditions is of great agronomic interest, as it supports its cultivation in a wide range of agro-ecological zones [14,15,19]. Some provenances exhibit tolerance to varying altitude conditions relative to their origin, which has been linked to differences in physiological responses—particularly those related to photosynthesis [18]. Rather than conclusively implying evolutionary adaptation, these responses may reflect reaction norms and ultimately phenotypic plasticity, as this mechanism can enable populations to perform under a wide range of environments. Disentangling these processes would require physiological and genomic evidence ideally gathered from multi-locality common garden experiments [20,21]. Nonetheless, understanding distribution shifts that only assume niche conservatism is an achievable task already informative enough to guide conservation strategies aimed at preserving the broad diversity found in South America.
Mapping efforts conducted between 1998 and 2000 revealed variation in sweet potato cultivation, with major concentrations in the subtropics and at mid-elevations of the tropics [22,23]. Among regions with high production, the Caribbean stood out after China, especially the Greater Antilles like Cuba and Haiti. In Asia high production was also observed in Indonesia, New Guinea, and Vietnam, while in Africa, cultivation was prominent in the African Great Lakes region, particularly around Lake Victoria (i.e., Burundi, Rwanda, Uganda, and the Democratic Republic of Congo), as well as in Ghana, Nigeria, and Madagascar. In terms of broader dispersal patterns, the crop was also reported in India, North America, and parts of northeastern Brazil, Argentina, Peru, and Bolivia. Besides mapping distributions of cultivated sweet potato, Khoury et al. (2015) [24] modeled the potential distribution of its landraces and wild relatives using Maxent [25], a widely accepted species distribution modeling algorithm based on the principle of maximum entropy [26]. The model was calibrated with 5614 georeferenced occurrence records compiled from various databases, covering both cultivated and wild taxa, while environmental predictors were gathered from the WorldClim database. The study produced spatial projections of habitat suitability and identified regions with high taxonomic richness in Mesoamerica and the southeastern United States, emphasizing their relevance for ex situ conservation, as only 79% of the recorded diversity had been collected [24].
Numerous other studies have highlighted the effectiveness of the maximum entropy (MaxEnt) algorithm in predicting the potential distribution of plant species under climate change scenarios [27,28,29]. For sweet potato, regional-scale projections using MaxEnt have proven valuable for identifying environmentally suitable zones and quantifying potential habitat loss under future climates [24]. Nonetheless, recent comparative studies have shown that alternative supervised learning algorithms—such as Random Forest and Gradient Boosting Machines (GBMs)—can achieve equal or even superior predictive performance [30,31]. As emphasized by Qiao et al. (2015) [32], there are no “silver bullets” in species distribution modeling since model performance is often context-dependent. Therefore, adopting a multi-model framework represents a sound practice for sweet potato that would enable more robust predictions, better generalization across environmental gradients, and increased confidence in model-based inferences under climate change.
Updated estimates of habitat contraction under a multi-model framework will have direct implications for species richness and extinction risk, reinforcing conservation assessments at both regional and local scales. Specifically, while the commercial cultivation of sweet potato is being promoted, parallel in situ conservation of local varieties and landraces has also been recognized as a milestone to prevent genetic erosion of this neglected and underutilized crop [17]. This is particularly relevant for Colombia, where some native populations persist at low frequencies in wild habitats and are occasionally classified as weeds [17]. Yet, the effects of climate change on sweet potato distribution remain poorly understood in this hyper-agrobiodiverse region of northwest South America, a gap that a multi-model approach could bridge. Therefore, this study aimed to forecast potential shifts in the geographic distribution of Colombian sweet potato germplasm under various timeframes of climate change, using a wide mosaic of ecological niche modeling techniques, ultimately supporting the prioritization of conservation targets.
2. Materials and Methods
2.1. Study Area and Data Compilation
Geolocation data was compiled from two open platforms and the germplasm bank of the Colombian nation managed by AGROSAVIA. The first platform was Global Biodiversity Information Facility or GBIF, which is an international organization and in turn a data network, with the aim of providing free information on any type of existing biological data (https://www.gbif.org/, accessed on 27 July 2024). The second was BIEN or Botanical Information and Ecology Network (https://bien.nceas.ucsb.edu/bien/ accessed on 27 July 2024), a collaborative network that has been formed by the union of disaggregated networks of botanical researchers with the aim to understand the impact of climate change on plant diversity [33]. GBIF and BIEN initially provided 44 non-redundant wild observations (Table S1). On the other hand, the passport data of the Colombian germplasm bank is the result of expeditions conducted in prioritized areas of Colombia between the years 2013 and 2016 [17] in 131 villages in 8 provinces of the Caribbean region (i.e., Antioquia, Atlántico, Bolívar, César, Córdoba, La Guajira, Magdalena, and Sucre) and 12 provinces of the Andean region (i.e., Antioquia, Boyacá, Caldas, Cauca, Huila, Nariño, North of Santander, Santander, Quindío, Risaralda, Tolima, and Valle del Cauca). A total of 658 observations were obtained from the AGROSAVIA database, out of which 345 corresponded to wild accessions and 313 to landraces. To reduce spatial redundancy and align the occurrence data with the resolution of the environmental variables, exact duplicate records with identical geographic coordinates were removed. Additionally, we retained only one occurrence per 1 km2 grid cell, matching the resolution of the WorldClim environmental layers. After these data capture and depuration steps, the final dataset of 702 occurrences included 389 wild and 313 landrace records (Table S1).
2.2. Compilation and Prioritization of Bioclimatic Variables
Contemporary bioclimatic data was retrieved from the WorldClim repository [34], using a 30-year historical dataset. The selected spatial resolution was 30 s, roughly corresponding to 1 km2 pixels. Nineteen bioclimatic variables were obtained (listed here: https://www.worldclim.org/data/bioclim.html, accessed on 27 July 2024), all derived solely from historical (period 1970–2000) precipitation and temperature data. Additionally, maximum and minimum temperatures (°C) were also used, from the same platform as baseline bioclimatic conditions. Subsequently, projected future values of bioclimatic variables for the periods 2021–2040, 2041–2060, 2061–2080, and 2081–2100 were retrieved, also at a resolution of 30 s. These periods were selected to study short, medium, and long-term effects. Projected data were obtained from the MPI-ESM1-2-HR model under the Shared Socioeconomic Pathway SSP5–8.5, which represents a high-emissions scenario consistent with continued fossil fuel development [35]. This scenario uses historical data to simulate climate change and is recommended for the northern Andes [36] due to its accurate predictions of regional seasonality [37].
The complexity of models used for climate sensitivity assessments is influenced by the prioritization of environmental factors [38]. Therefore, it is important to minimize collinearity [39] and sample bias [40] and to improve the selection of variables [41]. Collinearity refers to a strong relationship between two or more predictor variables, which can cause instability in parameter estimates [42]. The Variance Inflation Factor (VIF), which is the square of the multiple correlation coefficient from regressing a predictor variable against all other predictor variables, serves as a measure of collinearity [43]. Hence, variable selection was performed by retaining those with a VIF < 10 [42,44], following the guidelines of the usdm package [45] in R v.3.3.1 (R Core Team). Pairwise Pearson correlation coefficients (r) among all variables were also calculated using the same software. This process allowed for the selection of an optimal set of climatic variables to minimize collinearity. Additionally, the absolute correlation coefficients (r) among these variables were examined. A subset of 11 variables from the original 19 was identified as non-collinear and kept in subsequent modeling analyses for each genepool (i.e., wild and landraces).
2.3. Potential Niche Distribution Modeling
Potential niche distribution modeling was conducted using the maximum entropy algorithm, which applies maximum entropy and Bayesian inference techniques to estimate probability distributions of occurrences. This approach was implemented via the R-function MaxNet, based on the R-package ENMeval, optimizing transformations of predictor variables (L = linear, Q = quadratic, H = hinge, and their combinations) and regularization multipliers (rm = 1:3), selecting the best-performing model configuration based on evaluation metrics. Each genepool (wild and landraces) was separately analyzed using MaxEnt with an average of 5000 pseudo-absence points, which were randomly sampled across the study area using the randomPoints function from the dismo package, excluding known presence locations to prevent overlap. Block partitioning (partitions = “block”) was used to ensure spatially independent model evaluation. Models were calibrated using four-fold cross-validation across 500 iterations and evaluated with the Area Under the Curve (AUC) and the corrected Akaike Information Criterion (AICc). Additionally, six supervised machine learning methods were explored for modeling potential niche distribution: Random Forest, Gradient Boosting Modeling, Logistic Model, Naïve Bayes, Linear Discriminant Analysis, and K-Nearest Neighbors. For each gemepool, pseudo-absence points were randomly generated in equal number to the presence records, ensuring a balanced training dataset. These models were implemented using the caret R package (R Core Team) and optimized through 500 iterations with repeated cross-validation and 20% testing. The best prediction model for wild and landraces was selected based on AUC scores.
The described technique was also applied to data from 2021 to 2040. To further forecast distribution changes for wild and landraces, trained models were projected from current locations to the years 2021–2040, 2041–2060, 2061–2080, and 2081–2100. Projections were made using the predict function of the R-package raster, with maps constructed and summarized using a customized R-script with the ggplot R-package. The maps were then aggregated to produce geographical data on the likelihood of present and future presence of wild and landraces. Resultant distribution maps were compared per genepool to assess their climate sensitivity across all four timeframes. A 95% presence probability threshold was applied to binarize presence–absence predictions. This conservative threshold was selected to delineate areas of high environmental suitability, thereby minimizing false positives and identifying core habitat zones, as recommended in previous studies using MaxEnt for conservation-oriented projections [46,47]. For cells identified as presence, elevation values were extracted using the elevation layer from WorldClim 2.1, which is derived from SRTM (Shuttle Radar Topography Mission) elevation data, to calculate the altitudinal distribution of predicted occurrences.
3. Results
3.1. Current Distribution Ranges of Wild Sweet Potato and Landraces
Sweet potato occurrences were found under two habitat conditions; 389 records were defined as wild and 313 as landraces. Landraces were localized mainly in the Caribbean region, while wild occurrences were found mostly in Andean region. Habitat definition was possibly related to population adaptation but also to human intervention through its use and conservation (Figure 1). Moreover, the presence of occurrences in these regions could also be the result of historical migration events or recent range shifts, in addition to local adaptation to specific environmental conditions.
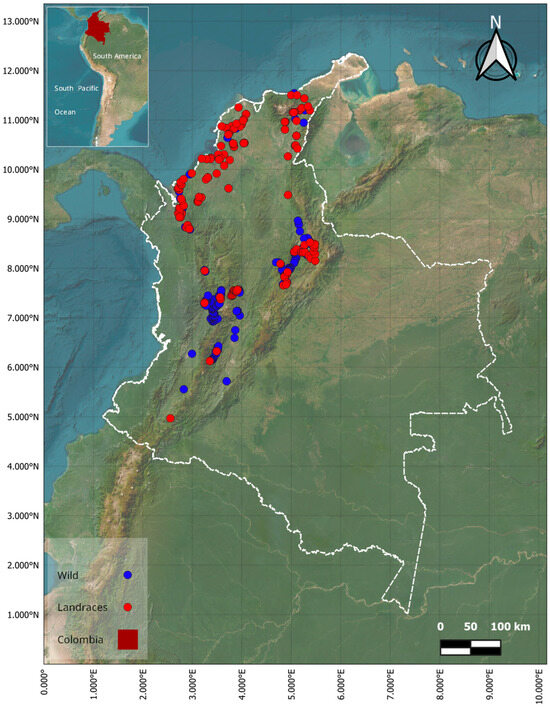
Figure 1.
Geographical representation of the sampling area. Distribution map of sweet potato (Ipomeas batatas L.) in Colombia. Blue and red dots denote wild and landrace occurrences, respectively.
The sweet potato occupied contrasting environments across its wild and landrace genepools, extending from sea level to altitudes that exceed 2500 m a.s.l., with a wide distribution in the Caribbean and Andean regions. Specifically, the bioclimatic variables analyzed in this study showed significant differences between wild and landrace genepools. Mean temperature in the wild genepool distribution was 22.50 ± 3.05 °C, while the landrace distribution exhibited a higher mean of 26.0 ± 4.24 °C (t-welch = 11.17, p-value = 3.53 × 10−25). The average maximum temperature in the areas where the wild occurrences were distributed was 27.32 ± 3.02 °C, also lower compared to 31.08 ± 4.21 °C for the records of landraces (t-welch = 11.81, p-value = 1.57 × 10−27). Similarly, the minimum temperature followed the same trend, 17.68 ± 3.17 °C for the distribution of the wild genepool, reaching up to 21.09 ± 4.38 °C in the distribution of the landraces (t-welch = 10.25, p-value = 6.59 × 10−22). Finally, mean precipitation associated with wild occurrences was 34.92 ± 9.08 mm, and this was higher for the landrace occurrences with a value of 41.63 ± 13.73 mm (t-welch = 6.57, p-value = 1.84 × 10−10). These statistically significant climatic differences highlighted relevant ecological contrasts between wild and cultivated sweet potato genepools. Wild populations, generally located in cooler environments, are likely adapted to narrower ecological niches and less climatic variability. Their persistence may thus depend on the conservation of such specific microhabitats. In contrast, landraces exhibited broader climatic envelopes, including tolerance to higher temperatures and higher precipitation regimes, likely reflecting long-term human selection and cultivation in more heterogeneous environments. These climatic patterns suggest that, under future warming and altered rainfall, the wild genepool may face greater constraints on altitudinal migration or ecological adaptation, while landraces might retain a comparatively higher capacity for persistence or human-assisted translocation.
3.2. Prioritization of Bioclimatic Variables
The variables with a VIF lower than 10 were retained to minimize multicollinearity caused by highly correlated variables. The optimization process left 11 informative bioclimatic variables for both wild occurrences (Figure 2A) and landraces (Figure 2B): Bio1, Bio3, Bio4, Bio5, Bio8, Bio9, Bio10, Bio12, and Bio16, in addition to the limiting bioclimatic variables [i.e., average precipitation (mm) and mean, maximum, and minimum temperatures (°C)].
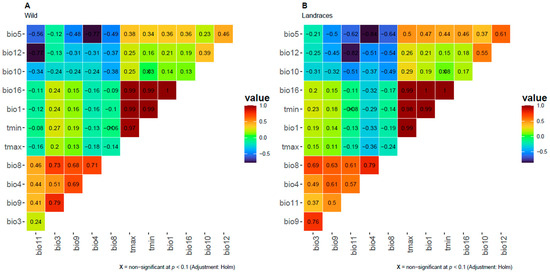
Figure 2.
Correlogram of bioclimatic variables for the current distribution ranges of sweet potato after the VIF test for multicollinearity. Cells are colored according to the absolute Pearson’s correlation score, as marked in the gradient to the right of each subpanel. Non-significant correlations are strikethrough after Holm test. Variables are sorted according to the hclust function from R in order to maximize groupings and visualize more straightforward concerted tendencies in the among-variable correlations. Variable names are after WorldClim (https://www.worldclim.org/data/bioclim.html, accessed on 13 January 2025) as follows: Bio1: annual mean temperature; Bio4: temperature seasonality (standard deviation ×100); Bio5: max temperature of warmest month; Bio8: mean temperature of wettest quarter; Bio9: mean temperature of driest quarter; Bio10: mean temperature of warmest quarter; Bio11: mean temperature of coldest quarter; Bio12: annual precipitation; and Bio16: precipitation of wettest quarter. Also retrieved from the same WorldClim platform, tmax: maximum temperature (°C) and tmin: minimum temperature (°C) are depicted as boundary bioclimatic variables.
3.3. Model Accuracy
The traditional MaxEnt software provided a satisfactory assessment of the accuracy of the 12 models, including the prediction area and omission rate according to AUC scores (Figure 3A,B, Table S2), even when compared to machine learning models (Figure 3C,D). In each figure, the blue bar shows the model’s fit in the training dataset and the green indicates the model’s fit to the testing dataset. For the wild genepool, all MaxEnt models demonstrated quite high predictive power (up to 0.957 for the fc.H_rm.1 model). For the landrace genepool, the trend slightly shifted but still showed good predictive power (up to 0.948 for the fc.LQH_rm.1 model). Although some machine learning approaches like RF, GBM, or KNN scored higher AUCs in the training set than the MaxEnt model, they exhibited greater overfitting when compared to the testing dataset, while MaxEnt showed more stability.
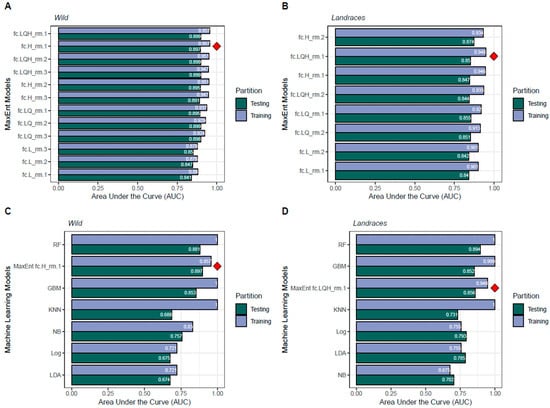
Figure 3.
Summary runaway plots (i.e., horizontal barplots) of omission rate charts as synthetized by the AUC score for modeling approaches with near-to-present bioclimatic variables. Graphs for (A) wild and (B) landrace genepools using various parametric combinations of the MaxEnt algorithm, and graphs for (C) wild and (D) landrace genepools using machine learning alternatives. Parametric combinations of the MaxEnt algorithm rely on the optimization of transformations (L: linear, Q: quadratic, H: hinge, P: product, and T: threshold) of the original predictor variables (‘feature classes’) using the R package ENMeval. Red rhombus mark the preferred alternatives according to the AUC in the testing dataset (green horizontal bars). The six supervised machine learning approaches included the following: RF: Random Forest; GBM: Gradient Boosting Modeling; Log: Logistic Model; BY: Naïve Bayes; LDA: Linear Discriminant Analysis; and KNN: K-Nearest Neighbors algorithm.
To better understand model behavior, we performed hyperparameter tuning for each machine learning model using repeated cross-validation. For the wild dataset, the best-performing Random Forest model used an mtry of 3, a min.node.size of 3, and 700 trees; the optimal KNN model used k = 2; and the best GBM model combined a learning rate (shrinkage) of 0.1, an interaction depth of 2, and 500 trees.
For the landrace dataset, the best Random Forest configuration used an mtry of 7, a min.node.size of 3, and 700 trees; the best KNN model also used k = 2; and the optimal GBM model used a learning rate of 0.1, an interaction depth of 2, and 1000 trees. Naive Bayes, logistic regression, and LDA were implemented using default or non-tunable parameters in both cases. Although some of these machine learning models achieved higher training AUCs, they consistently showed higher variance between training and test performance, highlighting their susceptibility to overfitting. In contrast, MaxEnt models displayed more stable behavior across datasets. Consequently, these maximum entropy modeling approaches were consistently implemented in the downstream pipeline for both wild and landrace datasets.
3.4. Forecasted Spatial Distribution of Wild Sweet Potato Under Climate Change
The analysis across the temporal timeframes of 2021–2040, 2041–2060, 2061–2080, and 2081–2100 identified various responses of the wild genepool to climate changes under predicted short-, medium-, and long-term conditions. Consistently, the estimated spatial distribution of the wild genepool of sweet potato in the first timeframe (2021–2040) showed the potential to extend beyond initially explored areas, with climatic factors favoring survival at a 95% probability, covering a geographic area of 19,523 km2. Within this area, optimal areas were notable in the Andean region, particularly along the central and western mountain ranges, with smaller distributions in high-altitude remnants of the eastern mountain range, both in the north and the south of the country. A small remnant was observed in the Colombian Massif, specifically in the eastern mountain range (Figure 4A). In contrast, the Caribbean region showed little to no environmental suitability for wild genepools, given their current minimal distribution in this region.
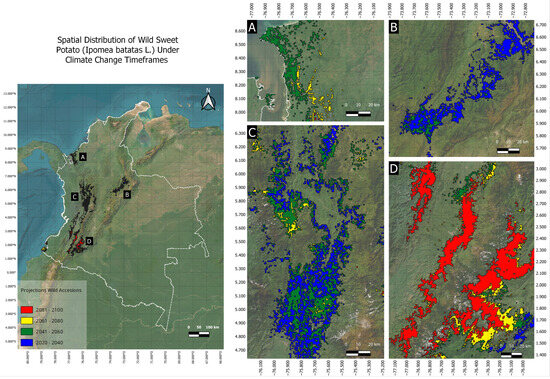
Figure 4.
Summarized range shifts in suitable conditions for the establishment of wild sweet potato. Subpanels (A–D) zoom in on different regions according to the map on the left. Blue, green, yellow, and red shadows mark the potential presence probability (>95%) of the wild genepool for the years 2021–2040 (19,523 km2), 2041–2060 (23,519 km2), 2061–2080 (15,069 km2), and 2081–2100 (10,655 km2).
In the second timeframe, projected for the period between 2041 and 2060, wild-type sweet potatoes would occupy an area of 23,519 km2 (Figure 4B). Although the overall distribution remained within the same regions as in the first timeframe, an increase in area was observed, with a tendency to extend the range to higher altitudes than those recorded in the previous period. A notable expansion of the suitable area was projected near the Gulf of Urabá in the Caribbean region, and a new area appeared along the Pacific coast of Nariño, indicating geographical expansion, so far absent in the previous timeframe.
In the third timeframe (between 2061 and 2080), the area decreased to 15,069 km2 (Figure 4C). A continued southward shift of wild genepools was observed, specifically towards the Andean region within the Colombian Massif, with small remnants in the Central and Western Cordilleras and parts of the Pacific. Finally, in the fourth timeframe, projected between 2081 and 2100, the area was further reduced to 10,655 km2 (Figure 4D), with the potential distribution concentrated exclusively in the Colombian Massif. The contraction of the distribution area and confinement to a single geographical point at altitudes approaching 3000 m above sea level indicated increased vulnerability, as the intrinsic environmental factors of this area may continue to fluctuate due to climate change.
3.5. Forecasted Spatial Distribution of Sweet Potato Landraces Under Climate Change
The potential distribution of sweet potato landraces in Colombia during the most proximal timeframe (2021–2040) projected a high presence probability and broad distribution (>95%, covering 33,710 km2) primarily in the Caribbean region (Figure 5A), aligning with the current distribution, as indicated by the high density of collection points in this area. In the Andean region, optimal areas were identified at altitudes around 2500 m a.s.l., with remnants located in the northern Eastern Cordillera, southern Western Cordillera, and near the Colombian Massif. Given the temperature increase, the persistence of suitable conditions within current lowland areas suggested broad plastic responses by the landrace genepool. Also, an upward shift in climatic suitability was projected in the Andean region, indicating that landraces could potentially expand into higher altitudes where environmental conditions are predicted to resemble those currently favorable.
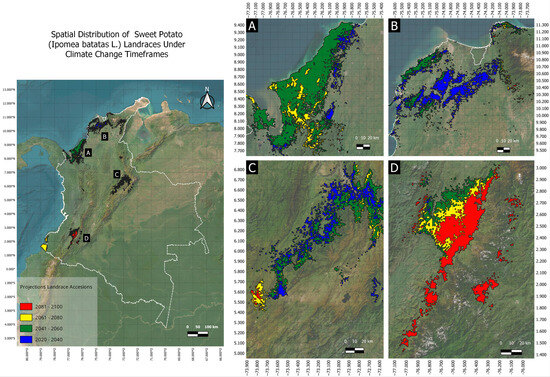
Figure 5.
Summarized range shifts in suitable conditions for the establishment of landraces of sweet potato. Subpanels (A–D) zoom in on different regions according to the map on the left. Blue, green, yellow, and red shadows mark the potential presence probability (>95%) for the years 2021–2040 (33,710 km2), 2041–2060 (40,142 km2), 2061–2080 (37,223 km2), and 2081–2100 (25,584 km2).
In the second timeframe, covering the period between 2041 and 2060, an increase in the distribution was observed, extending up to 40,142 km2 (Figure 5B). A hotspot of presence probability was identified near the Gulf of Urabá in the Caribbean region, while the distribution areas in the Andean region remained stable. In the third timeframe, which projected distributions under climatic conditions between 2061 and 2080, the potential area was reduced to 37,223 km2 (Figure 5C). Both the probability and the amplitude of areas with high potential identified in the second timeframe (i.e., Gulf of Urabá, Eastern, and Western Cordilleras) decreased in the third timeframe. However, a new suitable zone appeared in the southern Pacific region, where environmental conditions were projected to match the already plastic potential niche of the landrace genepool. In the fourth timeframe, spanning from 2081 to 2100, the distribution area contracted further to 25,584 km2 (Figure 5D), with a single potential distribution zone in the south of the country, between the Western Cordillera and the Colombian Massif, and small remnants located above 3000 m a.s.l. In this timeframe, adverse environmental conditions led to high climatic vulnerability. Thus, it is crucial to explore new areas for collecting cultivated germplasm and assessing its production potential in other natural regions of the country.
3.6. Altitudinal Shifts During Climate Change
The altitudinal distribution showed that the wild genepool tended to migrate slightly above 1500 m a.s.l., occupying an area of 10,655 km2 (Figure 6A) during the 2021–2040 period. Projected climate changes for 2041–2060 suggested a slight increase in this area while maintaining altitude (i.e., migration in the leading edge without extinction in the trailing edge), confirming an upward shift to higher altitudes in the central mountain range, covering approximately 23,519 km2. During the 2061–2080 timeframe the wild genepool moved southward, particularly within the Andean region of the Colombian Massif, leaving small remnants in the Central Cordillera. This shift raised the average altitude to 1920 m a.s.l., with persistent populations in the trailing edge. During 2081–2100, wild sweet potato may experience a forced altitudinal increase with extinctions in the trailing edge, reaching an average of 2211 m a.s.l. While altitudinal migration may help survival, the reduction in the trailing edge is a risk of extinction in low-altitude areas.
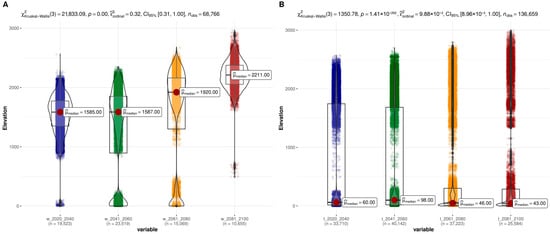
Figure 6.
Summarized range shifts in suitable conditions for the establishment of sweet potato across the altitudinal gradient. Presence probability (>95%) for (A) wild and (B) landrace genepools given near-to-present bioclimatic variables 2021–2040 (blue), and predicted bioclimatic scores for the years 2041–2060 (green), 2061–2080 (orange), 2081–2100 (red).
On the other hand, the optimal areas for sweet potato landraces were identified at both low and high altitudes, resulting in two population types and an average altitude of 60 m a.s.l., covering an estimated area of 33,710 km2 (Figure 6B). Interestingly, climate changes projected for the 2041–2060 period indicated favorable conditions for the crop’s expansion, with an increase to 40,142 km2, with overall marginal extinctions in the trailing edge together with a slight altitudinal migration in both population types. In contrast, a decrease in mean altitude was observed in the 2061–2080 period, reducing the geographical area occupied by the species to 37,223 km2 at an average of 46 m a.s.l. Last, environmental pressures in the 2081–2100 period caused a further reduction in suitable areas, a marked migration to higher altitudes, and slight extinction in low-altitude regions, where the area occupied shrank to 25,584 km2 with an average altitude of 43 m a.s.l.
4. Discussion
The capacity of native sweet potato varieties to withstand elevated temperatures and other adverse climatic conditions is a key feature for their selection and cultivation under a changing climate. In particular, landraces demonstrate notable phenotypic plasticity, positioning them as a promising genepool for addressing the challenges posed by climate variability, particularly in high-temperature environments. According to Olivas-Aguirre et al. [48], this response capacity is particularly significant because sweet potato is native to the Americas and has evolved under a wide range of environmental and geographical conditions. Moreover, there is considerable diversity within the genus Ipomoea, which comprises approximately 600 species, including both wild and domesticated genepools. Within the Batatas series, 13 species are related to the cultivated sweet potato, although its wild ancestor has yet to be identified [49]. Improved sweet potato varieties have also shown physiological traits associated with efficient water use, which is particularly valuable in regions such as the semi-arid eastern areas of Kenya [50].
In scenarios of climate change and variability, sweet potato landraces exhibit physiological plasticity related to drought tolerance. This translates into a resilient response to atmospheric and hydric stress (high temperatures and low soil water availability leading to increased evapotranspiration and a net negative water flux from the system), supporting projections of its continued presence at varying altitudes along Colombia’s Caribbean and Pacific coasts. This has been previously reported by Motsa et al. [51], who noted that this species, as a C3 plant, shows photosynthetic plasticity that enables acclimation to arid environments. This plasticity, along with nutritional value, underpins the classification of sweet potato as a drought-resilient crop, with important implications for food security in highly climate-vulnerable areas of the world. Additionally, Yagaso et al. [52] emphasized the importance of integrating climate-resilient varieties into farmers’ adaptation strategies, with institutional support.
Meanwhile, under various timeframes of global warming, the wild sweet potato genepool currently distributed in the Andean region of Colombia is projected to shift toward higher altitudes. While these spatial shifts may reflect the climatic suitability of highland environments, they cannot be directly interpreted as adaptive evolutionary responses. Instead, they likely result from changes in environmental compatibility across altitudinal gradients, favoring the migration potential of the species under a niche conservatism assumption. The projected migration towards ecological niches with favorable climatic conditions may be facilitated by dispersal, historical biogeography, ecological tolerances, or even introgression. For instance, in relation to the latter driver, not surprisingly chloroplast genomes have revealed high similarity between sweet potato cultivars and their wild relatives, including Ipomoea trifida and I. tabascana, which are distributed across the high mountains of Central and South America [53].
In the context of climate change and global warming, the priority of preserving the plant genetic resources of species vital to food security demands a multifaceted approach. This is why the current research effort in sweet potato adds to an integration of phenotypic, genotypic, and ecogeographic data that aims to identify areas of high morphotype and ecotype diversity [54]. Still, the sustainable management of sweet potato and its phenotypic variability requires research efforts that not only examine the migration potential of the species but also focus on intrinsic adaptation and mitigation strategies. According to Konko et al. [55], it is essential to have information that reveals trends in climatic parameters at a local scale, in addition to data on a wider spatial distribution, to fully understand the dynamics of plant populations. Zapata-Caldas et al. [56] further suggests that in order to assess the degree of agricultural vulnerability in the tropical Andes, it is necessary not only to understand the potential impacts of climate change in terms of distribution shifts due to extinctions at the trailing edge and migration into uncolonized areas at the leading edge, but also to recognize multiple adaptation trajectories, including genetic and farmers’ adaptation, to effectively address the multidimensional impacts of climate change on agri-food systems.
Moreover, we advocate for multi-model comparisons in ecological niche modeling efforts. In our study, MaxEnt did not consistently achieve the highest predictive performance across all metrics when compared to algorithms such as Random Forest or Gradient Boosting Machines. However, it exhibited the most stable behavior, with minimal differences between training and testing performance, suggesting lower susceptibility to overfitting. This stability increases confidence in its projections, particularly in contexts involving moderate sample sizes and high environmental heterogeneity, as observed in northwest South America. Anyhow, we cannot discard that the outcome of this study may be specific to the structure and quality of our dataset. Therefore, we strongly encourage other research groups to continue implementing analytical model batteries and comparative assessments, as the most appropriate algorithm can vary depending on the species, data structure, and environmental context. Such rigorous and integrative modeling practices will ultimately enhance the predictive power, reliability, and practical utility of ENMs in the face of climate change.
Overall, our findings provide valuable projections of the potential geographic distribution of sweet potato landraces and wild relatives in Colombia across four timeframes of climate change. However, as highlighted by the BAM framework (Biotic–Abiotic–Mobility) of Peterson et al. [6], the ecological niche estimated by SDMs—particularly those implemented here through MaxEnt and supervised machine learning algorithms—primarily approximates the fundamental niche, i.e., the range of abiotic conditions suitable for the species’ persistence. These models rely solely on abiotic predictors such as temperature and precipitation, thus omitting key biotic interactions (e.g., competition, herbivory, facilitation, and symbiosis) and dispersal limitations that constrain the realized niche in nature. They also exclude edaphic variables, which—while they could improve present-day model accuracy [57,58]—are currently limited by their static nature and would constrain the reliability of long-term projections. In the future, such variables could enhance model realism if integrated into more dynamic datasets. As a result, the temporal and spatial dynamics revealed in this study must be interpreted as projections of climatic suitability rather than actual occupancy patterns. This limitation emphasizes the need for future modeling efforts that incorporate biotic variables—when available—and that explore how the inclusion of species interactions could refine projections and improve sensitivity to environmental constraints. Doing so would help constrain models toward more realistic estimates of species range that better reflect the spatial complexity of ecological systems.
In parallel, the integration of genomic data, particularly from wild and landrace genepools, is needed to develop models of adaptive potential [59], linking in this way patterns of environmental suitability to the underlying standing genetic diversity [60,61]. Such integrative approaches could reveal evolutionary responses to climate pressures [62] and inform the design of germplasm conservation and mobilization strategies [63] that preserve both ecological function and genetic integrity. Yet, due to the impracticality of mobilizing and utilizing wild and landrace genepools in regions where farmers’ and markets’ adoption may be poor [64], and to effectively harness the potential of sweet potato genetic resources under climate change, it is crucial to introgress into more accepted cultivars phenotypic traits related to drought tolerance, heat resistance, and altitude adaptation, which directly influence plant resilience in increasingly harsh environments. These traits can be better targeted through genome–environment association (GEA) analyses [59], which offer a powerful framework for identifying genomic regions linked to adaptive responses, going in this way one step further than the dichotomic extinction vs. migration responses assumed by SDMs and ENMs under a niche conservatism paradigm. Approaches like GEA have been successfully applied in crops like common bean for heat tolerance [65,66] and in forest trees like Fagus sylvatica for altitudinal shifts under climate pressure [67]. These studies reveal the genetic architecture underlying climate adaptation, thereby paving the way for future pre-breeding strategies that can effectively utilize wild relatives and landraces as reservoirs of adaptive alleles [68]. Such strategies may further harness marker-assisted selection (MAS) and genomic selection (GS) in recurrent backcrossing schemes or advanced biotechnological tools like CRISPR/Cas-mediated gene editing, which in turn may boost the development of climate-resilient sweet potato varieties adapted to future agroecological realities.
5. Conclusions
This study provides a comprehensive and unprecedented assessment of the potential impact of climate change on the geographic distribution of sweet potato germplasm in Colombia, distinguishing between wild and landrace genepools. The results reveal a projected contraction in environmentally suitable areas for wild genepools, together with an altitudinal shift toward areas above 2200 m a.s.l. by the end of the 21st century. In contrast, landraces showed greater projected stability in lowland regions such as the Caribbean and Pacific coasts, although they may also face risks of extinction under extreme climate scenarios. This divergent patterns likely reflects differences in environmental tolerance and human management history rather than adaptive capacity.
Last but not least, our findings highlight the urgent need to design differentiated strategies for the conservation and use of sweet potato germplasm. For wild genepools, priority should be given to refugial zones such as the Colombian Massif, while for landraces, efforts should focus on identifying new climatically suitable cultivation areas and collecting germplasm from vulnerable regions. Additionally, this study recommends incorporating these spatial projections into pre-breeding programs capable of exploring the potential of wild relatives and landraces through phenotypic and genomic characterizations, and their ultimate utilization through backcrossing schemes, in order to enrich recurrent breeding schemes that target future climatic conditions.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae11091080/s1. Table S1. Georeferencing for the occurrences of 389 wild and 313 landraces; Table S2. Summary of performance statistics for the potential distribution models implemented in MaxEnt using 12 parameter combinations for wild populations and 8 for landraces, as shown in Figure 3A,B; Figure S1. Marginal response curves of the bioclimatic variables utilized in the training datasets of the species distribution models for (A) wild and (B) landraces occurrences.
Author Contributions
F.L.-H., M.G.R.-A., A.R., and A.J.C. conceived and designed the study proposal. F.L.-H. and A.R. compiled and curated all data. F.L.-H. performed the analyses with conceptual input from A.R. and A.J.C. All authors contributed to the interpretation and discussion of the results. F.L.-H. and M.G.R.-A. drafted the initial version of the manuscript, with substantial input from A.R. and A.J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Ministerio de Agricultura y Desarrollo Rural (MADR) of Colombia (TV24). The funding agency had no role in the study design, data collection and analysis, interpretation of results, nor in the decision to publish or prepare the manuscript. The APC was funded by the journal Horticulturae from MDPI.
Data Availability Statement
Raw georeferencing data is available under the “Supplementary Materials”. The analytical pipeline designed and utilized in this study is available upon request to the authors.
Acknowledgments
The authors thank the curators and staff of the Colombian Plant Germplasm Bank, administered by the Corporación Colombiana de Investigación Agropecuaria (AGROSAVIA), specifically for their support in providing and managing the plant accessions. F.L.-H. and A.J.C. also acknowledge the valuable input and suggestions received during the Genomics and Computational Biology in Plant Sciences workshop (Rionegro, Colombia, 2024), which contributed to the refinement of the analytical scripts used in this study. The authors gratefully acknowledge the support of AGROSAVIA and the Ministerio de Agricultura y Desarrollo Rural (MADR) of Colombia (TV24), which funded the research that led to this publication. The authors also thank the journal Horticulturae (MDPI) for covering the article processing charge (APC) as part of the Special Issue on “Insights to Optimize Sweet Potato Production and Transformation”.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shakoor, A.; Albasher, G.; Farooq, T.H. Climate change on the brink: Time for urgent action. Ecol. Inform. 2023, 78, 102286. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2024; FAO: Rome, Italy, 2024; ISBN 978-92-5-138882-2. [Google Scholar]
- Abbas, G.; Ahmed, M.; Fatima, Z.; Hussain, S.; Kheir, A.M.S.; Ercişli, S.; Ahmad, S. Modeling the potential impact of climate change on maize-maize cropping system in semi-arid environment and designing of adaptation options. Agric. For. Meteorol. 2023, 341, 109674. [Google Scholar] [CrossRef]
- Pardi, F.; Ruziman, H.H.; Suratman, M.N. The vulnerability of forest resources to climate change. In Land and Environmental Management Through Forestry; Wiley: Hoboken, NJ, USA, 2023; pp. 103–131. ISBN 9781119910527. [Google Scholar]
- Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B.; Peterson, A.T.; Soberón, J.; Pearson, R.G. Ecological Niches and Geographic Distributions (MPB-49); Princeton University Press: Princeton, NJ, USA, 2011; ISBN 9781400840670. [Google Scholar]
- Soberon, J.; Peterson, A.T. Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodivers. Inform. 2005, 2. [Google Scholar] [CrossRef]
- Woolfe, J. Sweet Potato an Untapped Food Resource; Cambridge University Press: Cambridge, UK, 1992; ISBN 0521402956. [Google Scholar]
- Bovell-Benjamin, A.C. Sweet potato: A review of its past, present, and future role in human nutrition. Adv. Food Nutr. Res. 2007, 52, 1–59. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Yao, Z.; Yang, Y.; Wang, Z.; Zou, H.; Zhang, X.; Chen, J.; Fang, B.; Huang, L. Genetic fingerprint construction and genetic diversity analysis of sweet potato (Ipomoea batatas) germplasm resources. BMC Plant Biol. 2023, 23, 355. [Google Scholar] [CrossRef] [PubMed]
- Galvao, A.C.; Nicoletto, C.; Zanin, G.; Vargas, P.F.; Sambo, P. nutraceutical content and daily value contribution of sweet potato accessions for the european market. Horticulturae 2021, 7, 23. [Google Scholar] [CrossRef]
- Velumani, R.; Raju, S.; Gangadharan, B.; Pallavi Nair, K.; George, J. Photosynthetic response of sweet potato (Ipomoea batatas) to photon flux density and elevated carbon dioxide. Indian J. Agric. Sci. 2017, 87, 1231–1237. [Google Scholar] [CrossRef]
- Van Heerden, P.D.R.; Laurie, R. Effects of prolonged restriction in water supply on photosynthesis, shoot development and storage root yield in sweet potato. Physiol. Plant. 2008, 134, 99–109. [Google Scholar] [CrossRef]
- Alemu, T.; Amsalu, R. Ultraviolet-B, end of day light and exclusion effect on photosynthetic efficiency of sweet potato (Ipomoea batatas L.) based on altitude. J. Hortic. Postharvest Res. 2020, 3, 1–10. [Google Scholar] [CrossRef]
- Adebola, P.O.; Shegro, A.; Laurie, S.M.; Zulu, L.N.; Pillay, M. Genotype x environment interaction and yield stability estimate of some sweet potato [Ipomoea batatas (L.) Lam] breeding lines in South Africa. J. Plant Breed. Crop Sci. 2013, 5, 182–186. [Google Scholar] [CrossRef]
- Byrne, P.F.; Volk, G.M.; Gardner, C.; Gore, M.A.; Simon, P.W.; Smith, S. Sustaining the future of plant breeding: The critical role of the USDA-ARS national plant germplasm system. Crop Sci. 2018, 58, 451–468. [Google Scholar] [CrossRef]
- Rosero, A.; Rodríguez, E.; Aguilera-Arango, G.; Rosero, M.; Granda, L.; Pastrana, I.; Martínez, R.; Perez, J.; Espitia, L.; Gomez, E.; et al. Assessment of the current state of in situ conservation and use of sweet potato (Ipomoea batatas L.) in Colombia. Cult. Agric. Food Environ. 2022, 44, 76–89. [Google Scholar] [CrossRef]
- Pérez-Pazos, J.V.; Rosero, A.; Martínez, R.; Pérez, J.; Morelo, J.; Araujo, H.; Burbano-Erazo, E. Influence of morpho-physiological traits on root yield in sweet potato (Ipomoea batatas Lam.) genotypes and its adaptation in a sub-humid environment. Sci. Hortic. 2021, 275, 109703. [Google Scholar] [CrossRef]
- Sulistiani, R.; Rosmayati; Siregar, L.A.M.; Harahap, F. Differences in morphology and sugar content of purple sweet potato (Ipomoea batatas L.) with potassium treatment at several altitudes. IOP Conf. Ser. Earth Environ. Sci. 2018, 122, 012050. [Google Scholar] [CrossRef]
- Booth, T.H. Perspective: Home and Away: The bioclimatology of acacia species in Australia and overseas. For. Ecol. Manag. 2024, 565, 122042. [Google Scholar] [CrossRef]
- Booth, T.H. Forestry trials and species adaptability to climate change. Glob. Change Biol. 2024, 30, 122042. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Huaccho, L.; Zhang, D.P. Global Distribution of Sweetpotato. Available online: https://cgspace.cgiar.org/items/2f01885b-7533-4596-9cc1-73e03fe5cf75 (accessed on 17 April 2025).
- Huaccho Huatuco, L.D.; Hijmans, R.J. A Geo-Referenced Database of Global Sweetpotato Distribution: Production Systems and Natural Resource Management Department. Available online: https://cipotato.org/wp-content/uploads/2014/10/cip_psnrmd_wp_no4.pdf (accessed on 27 July 2025).
- Khoury, C.K.; Heider, B.; Castañeda-Álvarez, N.P.; Achicanoy, H.A.; Sosa, C.C.; Miller, R.E.; Scotland, R.W.; Wood, J.R.I.; Rossel, G.; Eserman, L.A.; et al. Distributions, ex situ conservation priorities, and genetic resource potential of crop wild relatives of sweetpotato [Ipomoea batatas (L.) Lam., I. Series Batatas]. Front. Plant Sci. 2015, 6, 251. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Bedoya-Canas, L.E.; López-Hernández, F.; Cortés, A.J. Climate change responses of high-elevation polylepis forests. Forests 2024, 15, 811. [Google Scholar] [CrossRef]
- Carnaval, A.C.; Moritz, C. Historical climate modelling predicts patterns of current biodiversity in the Brazilian Atlantic forest. J. Biogeogr. 2008, 35, 1187–1201. [Google Scholar] [CrossRef]
- Williams, J.N.; Seo, C.; Thorne, J.; Nelson, J.K.; Erwin, S.; O’Brien, J.M.; Schwartz, M.W. Using species distribution models to predict new occurrences for rare plants. Divers. Distrib. 2009, 15, 565–576. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiao, N.; Shen, M.; Li, J. Comparison between optimized maxent and random forest modeling in predicting potential distribution: A case study with Quasipaa Boulengeri in China. Sci. Total Environ. 2022, 842, 156867. [Google Scholar] [CrossRef] [PubMed]
- Tong, R.; Yesson, C.; Yu, J.; Luo, Y.; Zhang, L. Key Factors for species distribution modeling in benthic marine environments. Front. Mar. Sci. 2023, 10, 1222382. [Google Scholar] [CrossRef]
- Qiao, H.; Soberón, J.; Peterson, A.T. No silver bullets in correlative ecological niche modelling: Insights from testing among many potential algorithms for niche estimation. Methods Ecol. Evol. 2015, 6, 1126–1136. [Google Scholar] [CrossRef]
- Enquist, B.J.; Condit, R.; Peet, R.K.; Schildhauer, M.; Thiers, B.M. Cyberinfrastructure for an integrated botanical information network to investigate the ecological impacts of global climate change on plant biodiversity. PeerJ Preprints 2016. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Müller, W.A.; Jungclaus, J.H.; Mauritsen, T.; Baehr, J.; Bittner, M.; Budich, R.; Bunzel, F.; Esch, M.; Ghosh, R.; Haak, H.; et al. A higher-resolution version of the Max Planck Institute Earth system model (MPI-ESM1.2-HR). J. Adv. Model. Earth Syst. 2018, 10, 1383–1413. [Google Scholar] [CrossRef]
- Arias, P.A.; Ortega, G.; Villegas, L.D.; Martínez, J.A. Colombian climatology in CMIP5/CMIP6 models: Persistent biases and improvements. Rev. Fac. Ing. Univ. 2021, 100, 75–96. [Google Scholar] [CrossRef]
- Tovar, C.; Arnillas, C.A.; Cuesta, F.; Buytaert, W. Diverging responses of tropical Andean biomes under future climate conditions. PLoS ONE 2013, 8, e63634. [Google Scholar] [CrossRef]
- Zeng, Y.; Low, B.W.; Yeo, D.C.J. Novel methods to select environmental variables in maxent: A case study using invasive crayfish. Ecol. Model. 2016, 341, 5–13. [Google Scholar] [CrossRef]
- Feng, X.; Park, D.S.; Liang, Y.; Pandey, R.; Papeş, M. Collinearity in ecological niche modeling: Confusions and challenges. Ecol. Evol. 2019, 9, 10365–10376. [Google Scholar] [CrossRef]
- Syfert, M.M.; Smith, M.J.; Coomes, D.A. The effects of sampling bias and model complexity on the predictive performance of maxent species distribution models. PLoS ONE 2013, 8, e55158. [Google Scholar] [CrossRef]
- Cobos, M.E.; Peterson, A.T.; Osorio-Olvera, L.; Jiménez-García, D. An exhaustive analysis of heuristic methods for variable selection in ecological niche modeling and species distribution modeling. Ecol. Inform. 2019, 53, 100983. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hadi, A.S. Regression Analysis by Example; Wiley: Hoboken, NJ, USA, 2006; ISBN 9780.470905845. [Google Scholar]
- Guisan, A.; Thuiller, W.; Zimmermann, N.E. Habitat Suitability and Distribution Models; Cambridge University Press: Cambridge, UK, 2017; ISBN 9781139028271. [Google Scholar]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where is positional uncertainty a problem for species distribution modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A Practical guide to maxent for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.; Quintero-Vargas, J.; Escobar-Puentes, A.; Wall-Medrano, A. Bioactive Compounds and Biological Activities of Sweet Potato (Ipomoea batatas (L.) Lam.); Springer: Berlin/Heidelberg, Germany, 2024; pp. 877–900. ISBN 978-3-031-44746-4. [Google Scholar]
- Nimmakayala, P.; Vajja, G.; Reddy, U.K. Wild Crop Relatives: Genomic and Breeding Resources; Springer: Berlin/Heidelberg, Germany, 2011; pp. 123–132. ISBN 978-3-642-21102-7. [Google Scholar]
- Mbayaki, C.W.; Kinama, J.M. More crop per drop: The magic of sweet potato (Ipomoea batatas L.). Trop. Subtrop. Agroecosystems 2021, 25. [Google Scholar] [CrossRef]
- Motsa, N.M.; Modi, A.T.; Mabhaudhi, T. Sweet potato (Ipomoea batatas L.) as a drought tolerant and food security crop. S. Afr. J. Sci. 2015, 111, 8. [Google Scholar] [CrossRef]
- Yagaso, Z.S.; Bayu, T.Y.; Bedane, M.D. Assessing the current status of food security under climate variability and the role of household-level adaption strategies near Ghibe III Hydroelectric Dam, Ethiopia. Discov. Food 2024, 4, 77. [Google Scholar] [CrossRef]
- Xiao, S.; Xu, P.; Deng, Y.; Dai, X.; Zhao, L.; Heider, B.; Zhang, A.; Zhou, Z.; Cao, Q. Comparative analysis of chloroplast genomes of cultivars and wild species of sweetpotato (Ipomoea batatas [L.] Lam). BMC Genomics 2021, 22, 262. [Google Scholar] [CrossRef] [PubMed]
- Vásquez Hernández, L.; Paredes, D.; Otero González, J.C.; Tapia, C.; Pabón Garcés, G.J. Diversidad de morfotipos de camote Ipomoea batatas (Convolvulaceae), y determinación de áreas óptimas para su conservación en Ecuador. Rev. Cuba. De Cienc. Biológicas 2019, 7, 1–11. [Google Scholar]
- Konko, Y.; Umaru, E.T.; Adjoussi, P.; Okhimamhe, A. Climate change and coastal population dynamics in Togo (West Africa). J. Coast. Conserv. 2023, 27, 47. [Google Scholar] [CrossRef]
- Zapata-Caldas, E.; Jarvis, A.; Ramirez, J.; Lau, C. Análisis de los impactos de cambio climático sobre cultivos andinos. Available online: https://ambienteycomercio.org/impacto-del-cambio-climatico-sobre-cultivos-andinos/ (accessed on 20 April 2025).
- Raymundo, R.; Asseng, S.; Cammarano, D.; Quiroz, R. Potato, sweet potato, and yam models for climate change: A review. Field Crop Res. 2014, 166, 173–185. [Google Scholar] [CrossRef]
- Khalil, T.; Asad, S.A.; Khubaib, N.; Baig, A.; Atif, S.; Umar, M.; Kropp, J.P.; Pradhan, P.; Baig, S. climate change and potential distribution of potato (Solanum Tuberosum) crop cultivation in Pakistan using maxent. AIMS Agric. Food 2021, 6, 663–676. [Google Scholar] [CrossRef]
- Cortés, A.J.; López-Hernández, F.; Blair, M.W. Genome–environment associations, an innovative tool for studying heritable evolutionary adaptation in orphan crops and wild relatives. Front. Genet. 2022, 13, 910386. [Google Scholar] [CrossRef]
- Cortés, A.J. Unlocking genebanks for climate adaptation. Nat. Clim. Change 2025, 15, 590–592. [Google Scholar] [CrossRef]
- Campbell, Q.; Castañeda-Álvarez, N.; Domingo, R.; Bishop-von Wettberg, E.; Runck, B.; Nandkangré, H.; Halpin-McCormick, A.; Fumia, N.; Neyhart, J.; Kilian, B.; et al. Prioritizing parents from global genebanks to breed climate-resilient crops. Nat. Clim. Chang. 2025, 15, 673–681. [Google Scholar] [CrossRef]
- Cortés, A.J.; López-Hernández, F.; Osorio-Rodriguez, D. Predicting thermal adaptation by looking into populations’ genomic past. Front. Genet. 2020, 11, 564515. [Google Scholar] [CrossRef] [PubMed]
- McCouch, S.; Navabi, Z.K.; Abberton, M.; Anglin, N.L.; Barbieri, R.L.; Baum, M.; Bett, K.; Booker, H.; Brown, G.L.; Bryan, G.J.; et al. Mobilizing crop biodiversity. Mol. Plant 2020, 13, 1341–1344. [Google Scholar] [CrossRef] [PubMed]
- Kholová, J.; Urban, M.O.; Bavorová, M.; Ceccarelli, S.; Cosmas, L.; Desczka, S.; Grando, S.; Lensink, R.; Nchanji, E.; Pavlík, J.; et al. Promoting new crop cultivars in low-income countries requires a transdisciplinary approach. Nat. Plants 2024, 10, 1610–1613. [Google Scholar] [CrossRef]
- López-Hernández, F.; Cortés, A.J. Last-generation genome–environment associations reveal the genetic basis of heat tolerance in Common Bean (Phaseolus vulgaris L.). Front. Genet. 2019, 10, 954. [Google Scholar] [CrossRef]
- Buitrago-Bitar, M.A.; Cortés, A.J.; López-Hernández, F.; Londoño-Caicedo, J.M.; Muñoz-Florez, J.E.; Muñoz, L.C.; Blair, M.W. Allelic diversity at abiotic stress responsive genes in relationship to ecological drought indices for cultivated tepary bean, Phaseolus acutifolius A. Gray, and its wild relatives. Genes 2021, 12, 556. [Google Scholar] [CrossRef] [PubMed]
- Frank, A.; Oddou-Muratorio, S.; Lalagüe, H.; Pluess, A.R.; Heiri, C.; Vendramin, G.G. Genome-environment association study suggests local adaptation to climate at the regional scale in Fagus Sylvatica. New Phytol. 2016, 210, 589–601. [Google Scholar] [CrossRef]
- Cortés, A.J.; López-Hernández, F. Harnessing crop wild diversity for climate change adaptation. Genes 2021, 12, 783. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).