Yield and Bioactive Compounds of Asparagus (Asparagus officinalis L.) Grown in Open Field and Rain Shelter Systems on Reclaimed Land in Saemangeum
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site, Asparagus Cultivar, and Cultivation System
2.2. Abiotic Factors During the Cultivation Period
2.3. Determination of Yield Parameters
2.4. Determination of Polyphenols and Flavonoids
2.5. Determination of Antioxidant Enzyme Activities
2.6. Determination of the Antioxidant Capacity
2.7. Statistics and Analysis
3. Results and Discussion
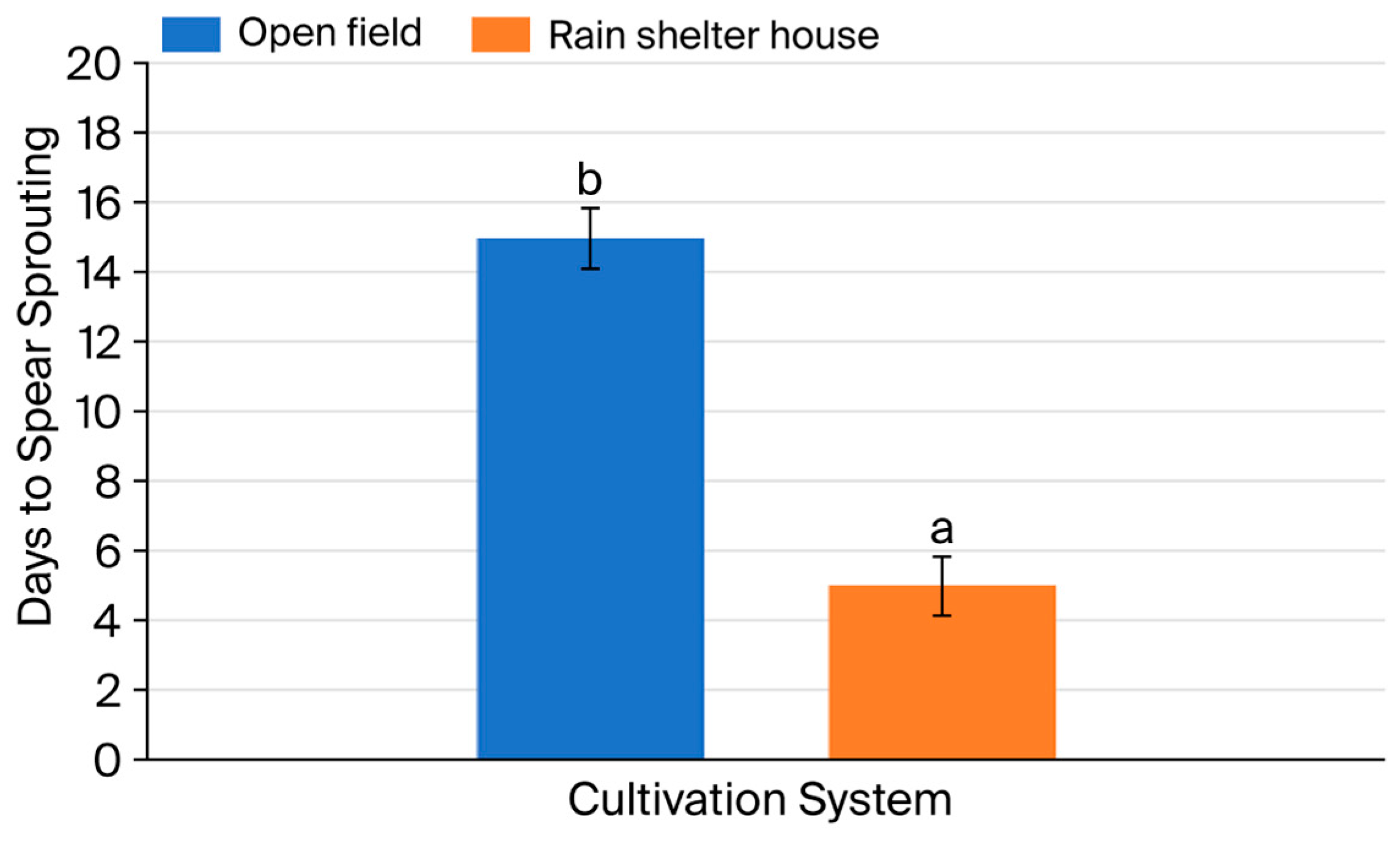
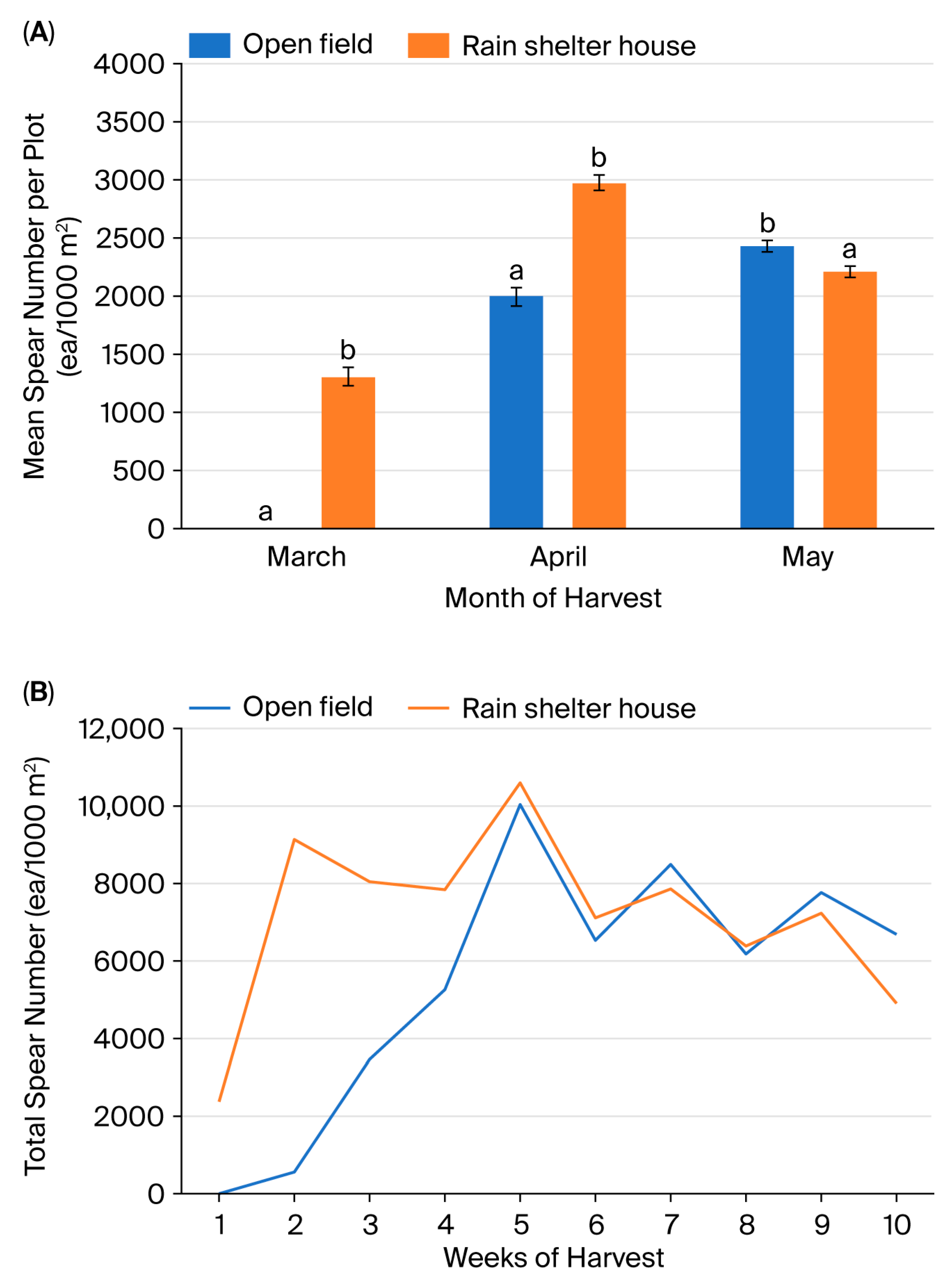
3.1. Growth Characteristics of Asparagus
3.2. Polyphenols and Flavonoids
3.3. Antioxidant Enzymes
3.4. Antioxidant Capacity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jang, J.R.; Lee, S.H.; Cho, Y.K.; Choi, J.Y. A calculation of agricultural water demand according to the farmland developing plan on the Saemangeum tidal land reclamation project. Korean Natl. Comm. Irrig. Drain. J. 2014, 21, 1–16. (In Korean) [Google Scholar]
- Ryu, J.H.; Oh, Y.Y.; Lee, S.H.; Lee, K.D.; Kim, Y.J. Annual changes of soil salinity of the Saemangeum reclaimed tide land during last 10 years. Korean J. Environ. Agric. 2020, 39, 327–333. (In Korean) [Google Scholar] [CrossRef]
- Kwon, B.S.; Baek, S.Y.; Shin, J.S.; Lim, J.T.; Shin, D.Y.; Kim, H.J.; Hyun, K.H. Effects of salinity content on soil chemical composition and productivity of rice in reclaimed saline paddy field. Korean J. Plant Res. 2003, 16, 181–186. (In Korean) [Google Scholar]
- Lee, S.H.; Bae, H.; Lee, S.H.; Oh, Y.Y.; Kim, Y.D.; Chun, H.C.; Choi, Y.D.; Jung, K.Y.; Kang, H.W. Effect of irrigation on soil salinity and corn (Zea mays) growth at coarse-textured tidal saline soil. J. Korean Soc. Int. Agric. 2016, 28, 526–532. (In Korean) [Google Scholar] [CrossRef]
- Kwon, O.D.; Kuk, Y.I.; Kim, S.W.; Park, H.G.; Shin, H.R.; Choi, H.G. Yield of rice as affected by density of common reeds (Phragmites communis Trin.) and controlling efficacy of common reeds by foliar applied herbicides in reclaimed paddy fields. Korean J. Weed Sci. 2006, 26, 56–63. (In Korean) [Google Scholar]
- Park, H.J.; Choi, W.J. Agricultural water salinity of reclaimed tidelands in southwestern coastal areas of South Korea. Korean J. Soil Sci. Fert. 2023, 56, 101–108. (In Korean) [Google Scholar] [CrossRef]
- Lee, S.H.; Hong, B.D.; Ahn, Y.; Ro, H.M. Relation between growth condition of six upland-crops and soil salinity in reclaimed land. J. Korean Soc. Soil Sci. Fert. 2003, 36, 66–71. (In Korean) [Google Scholar]
- Bae, H.S.; Hwang, J.B.; Kim, H.S.; Gu, B.I.; Choi, I.B.; Park, T.S.; Park, H.K.; Lee, S.H.; Oh, Y.Y.; Lee, S.H.; et al. Effect of drip irrigation level on soil salinity and growth of broccoli (Brassica oleracea L. var. italica) in Saemangeum reclaimed tidal land. Prot. Hortic. Plant Fact. 2015, 24, 275–280. (In Korean) [Google Scholar] [CrossRef]
- Lee, I.B.; Jung, D.H.; Yi, P.H.; Lee, J.G.; Bhandari, S.R. Analysis of changes in growth, nutrient absorption characteristics, and glucosinolate contents of chinese cabbage (Brassica rapa pekinensis) grown in reclaimed land according to irrigation with different salt concentrations. Hortic. Sci. Technol. 2021, 39, 572–582. (In Korean) [Google Scholar] [CrossRef]
- Jung, E.T.; Park, N.S.; Park, J.H. Composite modeling for evaluation of groundwater and soil salinization on the multiple reclaimed land due to sea-level rise. Transp. Porous Media 2021, 136, 271–293. [Google Scholar] [CrossRef]
- Shannon, M.C.; Grieve, C.M. Tolerance of vegetable crops to salinity. Sci. Horti 1998, 78, 5–38. [Google Scholar] [CrossRef]
- Kwon, S.B.; Kwon, H.J.; Jeon, S.J.; Seo, H.T.; Kim, H.Y.; Lim, J.G.; Park, J.S. Analysis of biological activities and functional components in different parts of asparagus. Korean J. Food Sci. Technol. 2020, 52, 67–74. (In Korean) [Google Scholar] [CrossRef]
- Chin, C.K.; Garrison, S.A. Functional elements from asparagus for human health. Acta Hortic. 2008, 776, 219–226. [Google Scholar] [CrossRef]
- Lee, J.W.; Heo, B.G.; Bae, J.H.; Ku, Y.G. Comparison of plant growth, dormancy breaking, yield, and biological activities of extracts in four asparagus cultivars. Korean J. Hortic. Sci. Technol. 2015, 33, 796–804. (In Korean) [Google Scholar] [CrossRef]
- Baiano, A.; Bevilacqua, L.; Terracone, C.; Contò, F.; del Nobile, M.A. Single and interactive effects of process variables on microwave-assisted and conventional extractions of antioxidants from vegetable solid wastes. J. Food Eng. 2014, 120, 135–145. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Z.; Wan, Y.; Zhang, Y.; Abdu, H.I.; Yang, M.; Pei, J.; Yue, T.; Zhang, X.; Hacimuftuoglu, A.; et al. Literature analysis on asparagus roots and review of its functional characterizations. Front. Nutr. 2023, 9, 1024190. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactive on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Ali, M.A.M. Effect of some bio-stimulants on growth, yield and bulb quality of garlic grown in newly reclaimed soil, new valley-Egypt. J. Plant Prod. 2017, 8, 1285–1294. [Google Scholar] [CrossRef][Green Version]
- Motoki, S.; Kazuki, O.; Shumpei, I.; Takumi, T.; Akira, K. Effects of cultivar and cropping type on the growth and yield of female and male asparagus plants. HortScience 2022, 57, 1460–1465. [Google Scholar] [CrossRef]
- Ha, S.Y.; Lee, T.H.; Shawon, R.A.; Heo, B.G.; Kim, H.C.; Bae, J.H.; Ku, Y.G. Growth characteristics and yield of asparagus ‘Atlas’ grown in an open field and rain-shelter house. Hortic. Sci. Technol. 2020, 38, 169–176. (In Korean) [Google Scholar] [CrossRef]
- Shawon, R.A.; Ha, S.Y.; Lee, T.H.; Cao, T.L.; Kim, H.C.; Bae, J.H.; Ku, Y.G. Influence of shade treatment on plant growth characteristics and spear production in five asparagus (Asparagus officinalis L.) cultivars. Hortic. Sci. Technol. 2021, 39, 37–48. [Google Scholar] [CrossRef]
- Cao, T.L.; Cho, N.; Lee, T.H.; Ahn, S.J.; Lee, D.J.; Ku, Y.G. Bioactive substances, antioxidant enzymes, and anti-cancer activity of asparagus ‘Atlas’ grown in an open field and rain-shelter house system. Hortic. Environ. Biotechnol. 2022, 63, 809–821. [Google Scholar] [CrossRef]
- Ku, Y.G.; Kang, D.H.; Lee, C.K.; Lee, S.Y.; Ryu, C.S.; Kim, D.; Polovka, M.; Namieśnik, J.; Gorinstein, S. Influence of different cultivation systems on bioactivity of asparagus. Food Chem. 2018, 244, 349–358. [Google Scholar] [CrossRef]
- Ku, Y.G.; Bae, J.H.; Namieśnik, J.; Barasch, D.; Nemirovski, A.; Katrich, E.; Gorinstein, S. Detection of bioactive compounds in organi- cally and conventionally grown asparagus spears. Food Anal. Methods 2018, 11, 309–318. [Google Scholar] [CrossRef]
- Ku, Y.G.; Woolley, D.J.; Hughes, A.R.; Nichols, M.A. Temperature effects on dormancy, bud break and spear growth in asparagus (Asparagus officinalis L.). J. Hortic. Sci. Biotechnol. 2007, 82, 446–450. [Google Scholar] [CrossRef]
- Lee, J.H.; Bae, J.H.; Ku, Y.G. Effect of two male cultivars of asparagus with low temperature treatment on bud breaking and spear growth. Korean J. Hortic. Sci. Technol. 2013, 31, 141–145. (In Korean) [Google Scholar] [CrossRef][Green Version]
- Nie, L.C.; Chen, Y.H.; Liu, M. Effects of low temperature and chilling duration on bud break and changes of endogenous hormones of asparagus. Eur. J. Hortic. Sci. 2016, 81, 22–26. [Google Scholar] [CrossRef]
- Ku, Y.G.; Woolley, D.J.; Nichols, M.A. The effect of chilling duration and temperature on asparagus spear growth. Acta Horti. 2008, 776, 445–452. [Google Scholar] [CrossRef]
- Seong, K.C.; Kim, C.H.; Lee, J.S.; Kim, D.S.; Eum, Y.C. Optimum heating date for off-season asparagus (Asparagus officinalis L.) production in December. J. Bio-Environ. Control 2006, 15, 196–200. (In Korean) [Google Scholar]
- Seong, K.C.; Kim, C.H.; Lee, J.S.; Eum, Y.C.; Moon, D.K. Determination of optimum heating date for off-season production of Asparagus (Asparagus officinalis L.). J. Bio-Environ. Control 2012, 21, 276–280. (In Korean) [Google Scholar]
- Feller, C.; Graefe, J.; Fink, M. Bud and spear developement of asparagus under constant temperature. Agric. Sci. 2012, 3, 455–461. [Google Scholar] [CrossRef][Green Version]
- Seong, K.C.; Lee, C.S.; Lee, S.K.; Yoo, B.C. Comparison of growth characteristics by varieties and effects of rain shelter and mulching on the production of asparagus (Asparagus officinalis L.). J. Bio-Environ. Control 2001, 10, 187–196. (In Korean) [Google Scholar][Green Version]
- Kim, H.C.; Heo, B.G.; Bae, J.H.; Lee, S.Y.; Kang, D.H.; Ryu, C.S.; Kim, D.E.; Choi, I.J.; Ku, Y.G. Comparison of plant growth characteristics and biological activities of four asparagus cultivars by cultural method. Korean J. Plant Res. 2016, 29, 495–503. (In Korean) [Google Scholar] [CrossRef]
- Kohmura, H.; Watanabe, Y.; Muto, N. Polyphenol content, antioxidant activity and surface colour of asparagus spears cultivated under different conditions of sunlight. Acta Hortic. 2008, 776, 255–260. [Google Scholar] [CrossRef]
- Dixson, R.A.; Pavia, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H. Dietary polyphenol, gut microbiota, and health benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef]
- Papoulias, E.; Siomos, A.S.; Koukounaras, A.; Gerasopoulos, D.; Kazakis, E. Effects of genetic, pre-and post-harvest factors on phenolic content and antioxidant capacity of white asparagus spears. Int. J. Mol. Sci. 2009, 10, 5370–5380. [Google Scholar] [CrossRef]
- Takács-Hájos, M.; Zsombik, L. Total polyphenol, flavonoid and other bioactive materials in different asparagus cultivars. Not. Bot. Horti Agrobo. 2015, 43, 59–63. [Google Scholar] [CrossRef][Green Version]
- Cha, J.Y.; Cho, Y.S. Biofunctional activities of citrus flavonoids. J. Korean Soc. Agric. Chem. Biotechnol. 2001, 44, 122–128. (In Korean) [Google Scholar][Green Version]
- Kawaguchi, K.; Mizuno, T.; Aida, K.; Uchino, K. Hesperidin as an inhibitor of lipases from porcine pancreas and Pseudomonas. Biosci. Biotechnol. Biochem. 1997, 61, 102–104. [Google Scholar] [CrossRef]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. J. Sci. Food Agric. 2015, 95, 869–877. [Google Scholar] [CrossRef]
- Laura, A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Phenolic compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Ed.; Woodhead Publishing, Elsevier: Amsterdam, The Netherlands, 2018; pp. 253–271. [Google Scholar]
- Maeda, T.; Honda, K.; Sonoda, T.; Motoki, S.; Inoue, K.; Suzuki, T.; Oosawa, K.; Suzuki, M. Light condition influences rutin and polyphenol contents in asparagus spears in the mother-fern culture system during the summer–autumn harvest. J. Jpn. Soc. Hortic. Sci. 2010, 79, 161–167. [Google Scholar] [CrossRef]
- Stoffyn, O.M.; Tsao, R.; Liu, R.; Wolyn, D.J. The effects of environment and storage on rutin concentration in two asparagus cultivars grown in southern Ontario. Can. J. Plant Sci. 2012, 92, 901–912. [Google Scholar] [CrossRef]
- Elmer, W.H.; Johnson, D.A.; Mink, G.I. Epidemiology and management of the diseases causal to asparagus decline. Plant Dis. 1996, 80, 117–125. [Google Scholar] [CrossRef]
- López-Moreno, F.J.; Navarro-León, E.; Soriano, T.; Ruiz, J.M. Physiological characterization of asparagus decline syndrome. Plant Soil 2025, 1–12. [Google Scholar] [CrossRef]
- Liu, J.; Matsubara, Y. Induced systemic resistance to fusarium root rot and changes in antioxidative ability by arbuscular mycorrhizal fungus and non-pathogenic fusarium oxysporum in asparagus plants. J. Jpn. Soc. Agric. Technol. Manag. 2016, 23, 21–29. [Google Scholar] [CrossRef]
- Sahoo, M.R.; DasGupta, M.; Kole, P.C.; Bhat, J.S.; Mukherjee, A. Antioxidative enzymes and isozymes analysis of taro genotypes and their implications in Phytophthora blight disease resistance. Mycopathologia 2007, 163, 241–248. [Google Scholar] [CrossRef]
- Boo, H.O.; Hwang, S.J.; Bae, C.S.; Park, S.H.; Song, W.S. Antioxidant activity according to each kind of natural plant pigments. Korean J. Plant Res. 2011, 24, 105–112. (In Korean) [Google Scholar] [CrossRef]
- Roy, Z.; Bansal, R.; Siddiqui, L.; Chaudhary, N. Understanding the role of free radicals and antioxidant enzymes in human diseases. Curr. Pharm. Biotechnol. 2023, 24, 1265–1276. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Kang, N.J.; Kwon, J.K.; Rhee, H.C.; Jeong, H.B.; Kim, H.T. Antioxidant enzymes as defense mechanism against oxidative stress induced by chilling in Cucurbita ficifolia leaves. J. Korean Horti. Sci. 2003, 44, 605–610. (In Korean) [Google Scholar]
- Lee, J.E.; Yun, S.I. Effects of compost and gypsum on soil water movement and retention of a reclaimed tidal land. Korean J. Soil Sci. Fert. 2014, 47, 340–344. (In Korean) [Google Scholar] [CrossRef][Green Version]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- García-Caparrós, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative stress and antioxidant metabolism under adverse environmental conditions: A review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
- Eichholz, I.; Rohn, S.; Gamm, A.; Beesk, N.; Herppich, W.B.; Kroh, L.W.; Ulrichs, C.; Huyskens-Keil, S. UV-B-mediated flavonoid synthesis in white asparagus (Asparagus officinalis L.). Food Res. Int. 2012, 48, 196–201. [Google Scholar] [CrossRef]
- Hemeda, H.M.; Klein, B.P. Inactivation and regeneration of peroxidase activity in vegetable extracts treated with antioxidants. J. Food Sci. 1991, 56, 68–71. [Google Scholar] [CrossRef]
- Zheng, H.; Lu, H. Effect of microwave pretreatment on the kinetics of ascorbic acid degradation and peroxidase inactivation in different parts of green asparagus (Asparagus officinalis L.) during water blanching. Food Chem. 2011, 128, 1087–1093. [Google Scholar] [CrossRef]
- Jansen, M.A.K.; van den Noort, R.E.; Tan, M.Y.A.; Prinsen, E.; Lagrimini, L.M.; Thorneley, R.N.F. Phenol-oxidizing peroxidases contribute to the protection of plants from ultraviolet radiation stress. Plant Physiol. 2001, 126, 1012–1023. [Google Scholar] [CrossRef]
- KMA (Korea Meteorological Administration). Weather Data Service Open MET Data Portal. Available online: https://data.kma.go.kr/stcs/grnd/grndTaList.do?pgmNo=70 (accessed on 12 December 2023).
- Wołosiak, R.; Drużyńska, B.; Derewiaka, D.; Piecyk, M.; Majewska, E.; Ciecierska, M.; Worobiej, E.; Pakosz, P. Verification of the conditions for determination of antioxidant activity by ABTS and DPPH assays—A practical approach. Molecules 2022, 27, 50. [Google Scholar] [CrossRef]
- Cao, T.L.; Shawon, R.A.; Cho, N.; Lee, T.H.; Ko, B.; Kim, H.C.; Bae, J.H.; Ku, Y.G. Effects of shade treatment on plant growth characteristics, phenolic contents, and antioxidant activities of all-male cultivars of asparagus (Asparagus officinalis L.). Hortic. Sci. Technol. 2022, 40, 273–285. [Google Scholar] [CrossRef]
- Rodríguez, R.; Jaramillo, S.; Rodríguez, G.; Espejo, J.A.; Guillén, R.; Fernández-Bolaños, J.; Heredia, A.; Jiménez, A. Antioxidant activity of ethanolic extracts from several asparagus cultivars. J. Agric. Food Chem. 2005, 53, 5212–5217. [Google Scholar] [CrossRef]
- Coklar, H.; Akbulut, M. Changes in phenolic acids, flavonoids, anthocyanins, and antioxidant activities of Mahonia aquifolium berries during fruit development and elucidation of the phenolic biosynthetic pathway. Hortic. Environ. Biotechnol. 2021, 62, 785–794. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Junior, M.R.M. Phenolic compounds: Structure, classification, and antioxidant power. In Bioactive Compounds; Woodhead Publishing: Sawston, UK, 2019; pp. 33–50. [Google Scholar] [CrossRef]
- Joung, Y.M.; Park, S.J.; Lee, K.Y.; Lee, J.Y.; Suh, J.K.; Hwang, S.Y.; Park, K.E.; Kang, M.H. Antioxidative and antimicrobial activities of Lilium species extracts prepared from different aerial parts. Korean J. Food Sci. Technol. 2007, 37, 452–457. (In Korean) [Google Scholar]
- Kim, H.K.; Kim, Y.E.; Do, J.R.; Lee, Y.C.; Lee, B.Y. Antioxidative activity and physiological activity of some Korean medicinal plants. Korean J. Food Sci. Technol. 1995, 27, 80–86. (In Korean) [Google Scholar]
- Lee, S.O.; Kim, M.J.; Kim, D.G.; Choi, H.J. Antioxidative activities of temperature-stepwise water extracts from Inonotus obliquus. J. Korean Soc. Food Sci. Nutr. 2005, 34, 139–147. (In Korean) [Google Scholar] [CrossRef]
- Matkowski, A.; Piotrowska, M. Antioxidant and free radical scavenging activities of some medicinal plants from the Lamiaceae. Fitoterapia 2006, 77, 346–353. [Google Scholar] [CrossRef]
- Rhim, T.J.; Choi, M.Y. The antioxidative effects of Ampelopsis brevipedunculata extracts. Korean J. Plant Res. 2010, 23, 445–450. (In Korean) [Google Scholar]




| Cultivation System | pH (1:5) | EC (dS/m) | OM (%) | Av. P2O5 (mg/kg) | Ex. Cation (cmol/kg) | |||
|---|---|---|---|---|---|---|---|---|
| K | Ca | Mg | Na | |||||
| Before experiment | ||||||||
| 6.3 ± 0.3 z | 0.7 ± 0.1 | 9.8 ± 1.6 | 118.0 ± 40.2 | 0.81 ± 0.08 | 2.63 ± 0.45 | 2.60 ± 0.79 | 0.15 ± 0.09 | |
| During experiment | ||||||||
| Open field | 7.1 ± 0.2 | 0.2 ± 0.1 | 3.8 ± 3.0 | 313.8 ± 101.9 | 1.27 ± 0.14 | 5.58 ± 0.47 | 2.82 ± 0.49 | 0.61 ± 0.11 |
| Rain shelter house | 6.3 ± 0.4 | 0.9 ± 0.3 | 4.6 ± 2.9 | 349.0 ± 132.6 | 1.05± 0.20 | 2.60 ± 0.83 | 2.62 ± 1.07 | 0.57 ± 0.23 |
| Cultivation System | Polyphenol (mg GAE g−1) | Flavonoids (mg GAE g−1) |
|---|---|---|
| Open field | 42.58a z | 28.27a |
| Rain shelter house | 36.54b | 24.69b |
| Cultivation System | CAT (μmol H2O2 Decomposed min−1 mg Protein−1) | APX (μmol Ascorbate Oxidized min−1 mg Protein−1) | POD (μmol Tetraguiacol Formed min−1 mg Protein−1) | SOD (% Inhibition mg Protein−1) |
|---|---|---|---|---|
| Open field | 19.19a z | 789.53a | 4.95a | 82.19a |
| Rain shelter house | 17.55b | 582.83b | 3.49b | 75.71b |
| Cultivation System | DPPH Radical Scavenging (%) | |||||
|---|---|---|---|---|---|---|
| Asparagus Extracts (mg/L) | ||||||
| 250 | 500 | 1000 | 2500 | 5000 | 10,000 | |
| Open field | 15.44a z | 25.03a | 42.21a | 62.68a | 80.77a | 96.55a |
| Rain shelter house | 13.59b | 22.93a | 33.99b | 57.10b | 73.50b | 94.56b |
| Cultivation System | ABTS Radical Scavenging (%) | |||||
|---|---|---|---|---|---|---|
| Asparagus Extracts (mg/L) | ||||||
| 500 | 1000 | 2500 | 5000 | 10,000 | 20,000 | |
| Open field | 25.79a z | 35.83a | 49.48a | 74.79a | 90.34a | 95.21a |
| Rain shelter house | 23.00b | 30.14b | 42.31b | 69.06b | 86.48b | 92.33b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.Y.; Jang, H.J.; Lee, H.N.; Choi, S.W.; Jung, H.H.; Ahn, M.S.; Lee, I.B.; Ku, Y.G. Yield and Bioactive Compounds of Asparagus (Asparagus officinalis L.) Grown in Open Field and Rain Shelter Systems on Reclaimed Land in Saemangeum. Horticulturae 2025, 11, 1067. https://doi.org/10.3390/horticulturae11091067
Hong JY, Jang HJ, Lee HN, Choi SW, Jung HH, Ahn MS, Lee IB, Ku YG. Yield and Bioactive Compounds of Asparagus (Asparagus officinalis L.) Grown in Open Field and Rain Shelter Systems on Reclaimed Land in Saemangeum. Horticulturae. 2025; 11(9):1067. https://doi.org/10.3390/horticulturae11091067
Chicago/Turabian StyleHong, Ju Young, Hyo Jung Jang, Han Na Lee, Seung Wook Choi, Hyun Hwan Jung, Myung Suk Ahn, In Bog Lee, and Yang Gyu Ku. 2025. "Yield and Bioactive Compounds of Asparagus (Asparagus officinalis L.) Grown in Open Field and Rain Shelter Systems on Reclaimed Land in Saemangeum" Horticulturae 11, no. 9: 1067. https://doi.org/10.3390/horticulturae11091067
APA StyleHong, J. Y., Jang, H. J., Lee, H. N., Choi, S. W., Jung, H. H., Ahn, M. S., Lee, I. B., & Ku, Y. G. (2025). Yield and Bioactive Compounds of Asparagus (Asparagus officinalis L.) Grown in Open Field and Rain Shelter Systems on Reclaimed Land in Saemangeum. Horticulturae, 11(9), 1067. https://doi.org/10.3390/horticulturae11091067




