Management of Postharvest Diseases via Eco-Friendly Technologies: A Review of Recent Research
Abstract
1. Introduction
1.1. Effects on Agricultural Economy
1.2. Effects on Public Health and Food Safety
1.3. Challenges of Conventional Methods
1.3.1. Overuse of Chemical Fungicides
1.3.2. Resistance Development and Residual Problems
1.4. Rise and Challenges of Environmentally Friendly Approaches
2. Biological Control Approaches
2.1. Antagonistic Microorganisms
2.1.1. Yeast Species
2.1.2. Bacterial Biocontrol Agents
2.1.3. Mechanisms of Action
2.2. Plant Extracts and Natural Compounds
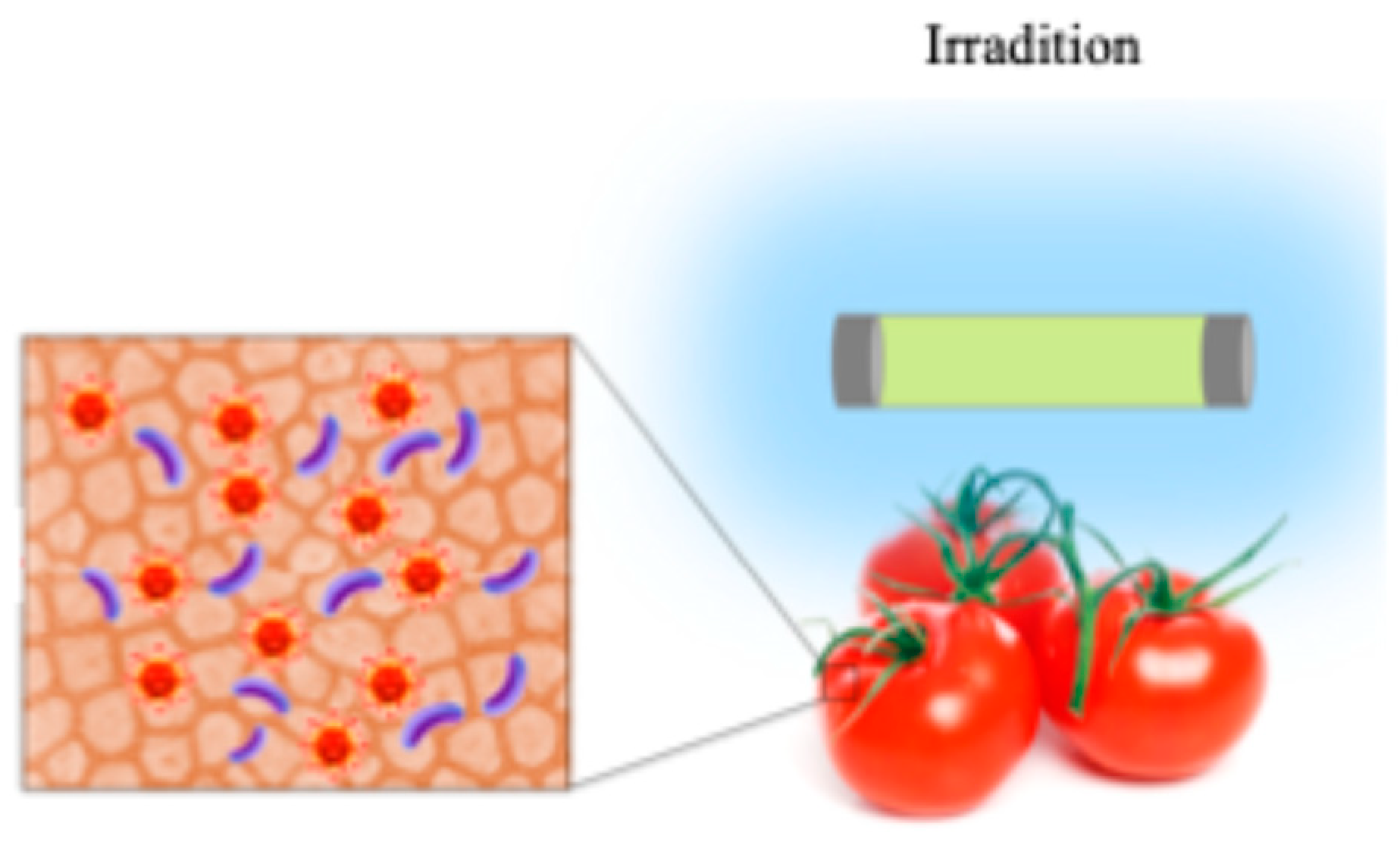
3. Physical and Physicochemical Methods
4. Chemical Alternatives: Natural-Origin Compounds
5. Hurdle Technologies
6. Advantages and Limitations
7. Future Perspectives
8. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Coates, L.; Johnson, G.I. Postharvest diseases of fruit and vegetables. In Postharvest Handling: A Systems Approach, 2nd ed.; Johnson, G.I., Le Van, G.R., Hofman, A.D., Bachmann, N.J., Eds.; Academic Press: Cambridge, MA, USA, 2007; pp. 533–580. [Google Scholar]
- Schneider, K.; Barreiro-Hurle, J.; Rodriguez-Cerezo, E. Pesticide reduction amidst food and feed security concerns in Europe. Nat. Food 2023, 6, 746–750. [Google Scholar] [CrossRef]
- Yin, Y.; Miao, J.; Shao, W.; Liu, X.; Zhao, Y.; Ma, Z. Fungicide resistance: Progress in understanding mechanisms, monitoring, and management. Phytopathology 2023, 113, 707–718. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; Macarisin, D.; Wilson, C. Twenty years of postharvest biocontrol research: Is it time for a new paradigm? Postharvest Biol. Technol. 2009, 52, 137–145. [Google Scholar] [CrossRef]
- Janisiewicz, W.J.; Conway, W.S.; Leverentz, B. Biological control of postharvest decays of apple can prevent growth of Escherichia coli O157:H7 in apple wounds. J. Food Prot. 1999, 62, 1372–1375. [Google Scholar] [CrossRef]
- Wang, S.; Tan, Z.; Wang, C.; Liu, W.; Hang, F.; He, X.; Ye, D.; Li, L.; Sun, J. Iron competition as an important mechanism of pulcherrimin-producing Metschnikowia sp. strains for controlling postharvest fungal decays on citrus fruit. Foods 2023, 12, 4249. [Google Scholar] [CrossRef]
- He, Y.; Degraeve, P.; Oulahal, N. Bioprotective yeasts: Potential to limit postharvest spoilage and to extend shelf life or improve microbial safety of processed foods. Heliyon 2024, 10, e24929. [Google Scholar] [CrossRef]
- Sharma, R.R.; Singh, D.; Singh, R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biol. Control 2009, 50, 205–221. [Google Scholar] [CrossRef]
- Hariharan, G.; Rifnas, L.M.; Prasannath, K. Role of Trichoderma spp. in biocontrol of plant diseases. In Microbial Biocontrol: Food Security and Post-Harvest Management; Kumar, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 39–78. [Google Scholar]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Kebriti, I.; Solgi, M.; Velashjerdi, M. Improving quality of strawberry by novel essential oil nanoemulsions of Echinophora platyloba combined with Aloe vera gel and gum arabic. Sci. Rep. 2025, 15, 1731. [Google Scholar] [CrossRef]
- Dutta, P.K.; Tripathi, S.; Mehrotra, G.K.; Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Romanazzi, G.; Sanzani, S.M.; Bi, Y.; Tian, S.; Martínez, P.G.; Alkan, N. Induced resistance to control postharvest decay of fruit and vegetables. Postharvest Biol. Technol. 2016, 122, 82–94. [Google Scholar] [CrossRef]
- Javanmardi, Z.; Koushesh Saba, M.; Nourbakhsh, H.; Amini, J. Efficiency of nanoemulsion of essential oils to control Botrytis cinerea on strawberry surface and prolong fruit shelf life. Int. J. Food Microbiol. 2023, 384, 109979. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Filho, J.G.; Da Cruz Silva, G.; Oldoni, F.C.A.; Miranda, M.; Florencio, C.; De Oliveira, R.M.D.; De Paula Gomes, M.; Ferreira, M.D. Edible coating based on carnauba wax nanoemulsion and Cymbopogon martinii essential oil on papaya postharvest preservation. Coatings 2022, 12, 1700. [Google Scholar] [CrossRef]
- Karimi Dehbakri, S.; Ehtesham Nia, A.; Mumivand, H.; Rastegar, S. Effect of nanocellulose-based edible coatings enriched with α-pinene and myrtle essential oil on the postharvest quality of strawberry. BMC Plant Biol. 2025, 25, 812. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture 2019: Moving Forward on Food Loss and Waste Reduction; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Gamagae, S.U.; Sivakumar, D.; Wijesundera, R.L.C. Evaluation of postharvest application of sodium bicarbonate incorporated wax formulation and Candida oleophila for the control of anthracnose of papaya. Crop Prot. 2004, 23, 575–579. [Google Scholar] [CrossRef]
- Romanazzi, G.; Smilanick, J.L.; Feliziani, E.; Droby, S. Integrated management of postharvest gray mold on fruit crops. Postharvest Biol. Technol. 2016, 113, 69–76. [Google Scholar] [CrossRef]
- Raynaldo, R.; Luan, F.; Zhang, J.; Wang, H.; Xu, X. Biological control and other alternatives to chemical fungicides in postharvest management of fruit diseases: A review. Food Innov. Adv. 2024, 3, 135–143. [Google Scholar] [CrossRef]
- Ahmad, T.; Xing, F.; Nie, C.; Cao, C.; Xiao, Y.; Yu, X.; Moosa, A.; Liu, Y. Biocontrol potential of lipopeptides produced by the novel Bacillus subtilis strain Y17B against postharvest Alternaria fruit rot of cherry. Front. Microbiol. 2023, 14, 1150217. [Google Scholar] [CrossRef]
- Beg, M.A.; Lewis, K.J.; Oliver, J.E. Fungicide resistance profiles of Alternaria spp. associated with fruit rot of blueberry in Georgia, USA. Front. Plant Sci. 2025, 16, 1524586. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, M.; Liao, Q.; Wang, Y.; Sui, Y.; Gong, C. Current status and future trends of eco-friendly management of postharvest fungal decays in tomato fruit. NPJ Sci. Food 2025, 9, 104. [Google Scholar] [CrossRef]
- Bardas, G.A.; Veloukas, T.; Koutita, O.; Karaoglanidis, G.S. Multiple resistance of Botrytis cinerea from kiwifruit to SDHIs, QoIs and fungicides of other chemical groups. Pest Manag. Sci. 2010, 66, 967–973. [Google Scholar] [CrossRef]
- Loncaric, A.; Sarkanj, B.; Gotal, A.M.; Kovac, M.; Nevistic, A.; Fruk, G.; Babojelic, M.S.; Babic, J.; Milicevic, B.; Kovac, T. Penicillium expansum impact and patulin accumulation on conventional and traditional apple cultivars. Toxins 2021, 13, 703. [Google Scholar] [CrossRef] [PubMed]
- Troncoso-Rojas, R.; Tiznado-Hernández, M.E. Alternaria alternata (Black Rot, Black Spot). In Postharvest Decay: Control Strategies; Bautista-Baños, S., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 147–187. [Google Scholar]
- Li, J.; Yang, T.; Yuan, F.; Lv, X.; Zhou, Y. Inhibitory effect and potential antagonistic mechanism of isolated epiphytic yeasts against Botrytis cinerea and Alternaria alternata in postharvest blueberry fruits. Foods 2024, 13, 1334. [Google Scholar] [CrossRef] [PubMed]
- Janisiewicz, W.J.; Korsten, L. Biological control of postharvest diseases of fruits. Annu. Rev. Phytopathol. 2002, 40, 411–441. [Google Scholar] [CrossRef] [PubMed]
- Feliziani, E.; Santini, M.; Landi, L.; Romanazzi, G. Pre- and postharvest treatment with alternatives to synthetic fungicides to control postharvest decay of sweet cherry. Postharvest Biol. Technol. 2013, 78, 133–138. [Google Scholar] [CrossRef]
- Tripathi, P.; Dubey, N.K. Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biol. Technol. 2004, 32, 235–245. [Google Scholar] [CrossRef]
- De Chiara, M.L.V.; Castagnini, J.M.; Capozzi, V. Cutting-edge physical techniques in postharvest for fruits and vegetables: Unveiling their power, inclusion in ‘hurdle’ approach, and latest applications. Postharvest Biol. Technol. 2024, 151, 104619. [Google Scholar] [CrossRef]
- Zhou, Y.; Deng, L.; Zeng, K. Enhancement of biocontrol efficacy of Pichia membranaefaciens by hot water treatment in postharvest diseases of citrus fruit. Crop Prot. 2014, 63, 89–96. [Google Scholar] [CrossRef]
- Paull, R.E.; Chen, N.J. Heat treatment and fruit ripening. Postharvest Biol. Technol. 2000, 21, 21–37. [Google Scholar] [CrossRef]
- Conway, W.S.; Leverentz, B.; Janisiewicz, W.J.; Saftner, R.A.; Camp, M.J. Improving biocontrol using antagonist mixtures with heat and/or sodium bicarbonate to control postharvest decay of apple fruit. Postharvest Biol. Technol. 2005, 36, 235–244. [Google Scholar] [CrossRef]
- Allende, A.; Tomas-Barberan, F.A.; Gil, M.I. Minimal processing for healthy traditional foods. Trends Food Sci. Technol. 2006, 17, 513–519. [Google Scholar] [CrossRef]
- Jiang, A.; Zuo, J.; Zheng, Q.; Guo, L.; Gao, L.; Zhao, S.; Wang, Q.; Hu, W. Red LED irradiation maintains the postharvest quality of broccoli by elevating antioxidant enzyme activity and reducing the expression of senescence-related genes. Sci. Hortic. 2019, 251, 73–79. [Google Scholar] [CrossRef]
- Misra, N.N.; Tiwari, B.K.; Raghavarao, K.S.M.S.; Cullen, P.J. Nonthermal plasma inactivation of food-borne pathogens. Food Eng. Rev. 2011, 3, 159–170. [Google Scholar] [CrossRef]
- Sandhya. Modified atmosphere packaging of fresh produce: Current status and future needs. LWT-Food Sci. Technol. 2010, 43, 381–392. [Google Scholar] [CrossRef]
- Chavan, P.; Lata, K.; Kaur, T.; Jambrak, A.R.; Sharma, S.; Roy, S.; Sinhmar, A.; Thory, R.; Singh, G.P.; Aayush, K.; et al. Recent advances in the preservation of postharvest fruits using edible films and coatings: A comprehensive review. Food Chem. 2023, 418, 135916. [Google Scholar] [CrossRef]
- Sholberg, P.L. Fumigation of fruit with short-chain organic acids to reduce the potential of postharvest decay. Plant Dis. 1998, 82, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Romanazzi, G.; Gabler, F.M.; Margosan, D.; Mackey, B.E.; Smilanick, J.L. Effect of chitosan dissolved in different acids on its ability to control postharvest gray mold of table grape. Postharvest Pathol. Mycotoxins 2009, 99, 1028–1036. [Google Scholar] [CrossRef]
- Wanakamol, W.; Kongwong, P.; Chuamuangphan, C.; Bundhurat, D.; Boonyakiat, D.; Poonlarp, P. Hurdle approach for control of enzymatic browning and extension of shelf life of fresh-cut leafy vegetables using vacuum precooling and modified atmosphere packaging: Commercial application. Horticulturae 2022, 8, 745. [Google Scholar] [CrossRef]
- Cheng, L.; Zhou, L.; Li, D.; Gao, Z.; Teng, J.; Nie, X.; Guo, F.; Wang, C.; Wang, X.; Li, S.; et al. Combining the biocontrol agent Meyerozyma guilliermondii with UV-C treatment to manage postharvest gray mold on kiwifruit. Biol. Control 2023, 180, 105198. [Google Scholar] [CrossRef]
- Leng, J.; Dai, Y.; Qiu, D.; Zou, Y.; Wu, X. Utilization of the antagonistic yeast, Wickerhamomyces anomalus, combined with UV-C to manage postharvest rot of potato tubers caused by Alternaria tenuissima. Int. J. Food Microbiol. 2022, 377, 109782. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, K.; Wang, G. Combination of the biocontrol yeast Cryptococcus laurentii with UV-C treatment for control of postharvest diseases of tomato fruit. BioControl 2013, 58, 269–281. [Google Scholar] [CrossRef]
- Masilamani, S.; Natarajan, V.; Radhakrishnan, M. Recent advances in nonthermal hurdle approach in the food sector. Qual. Assur. Saf. Crops Foods 2025, 17, 19–36. [Google Scholar] [CrossRef]
- Wu, F.; Wang, H.; Lin, Y.; Feng, S.; Li, X. Biocontrol mechanisms of antagonistic yeasts on postharvest fruits and vegetables and the approaches to enhance the biocontrol potential of antagonistic yeasts. Int. J. Food Microbiol. 2025, 430, 111038. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.B.; Ferreira, M.S.; de Oliveira, T.A.M.F.; Viana, A.C.; de Araújo, A.A.; Soares, B.L.; Pereira, A.G. Antifungal edible coatings for fruits based on zein and chitosan nanowhiskers. J. Food Sci. 2024, 89, 793–804. [Google Scholar] [CrossRef]
- Sasikumar, R.; Kumar, S.T.; Mangang, I.B.; Kaviarasu, G. A comprehensive review on cold plasma applications in the food industry. Sustain. Food Technol. 2025, 3, D5FB00148J. [Google Scholar] [CrossRef]
- Xiang, Q.; Zhang, R.; Fan, L.; Ma, Y.; Wu, D.; Li, K.; Bai, Y. Microbial inactivation and quality of grapes treated by plasma-activated water combined with mild heat. LWT-Food Sci. Technol. 2020, 126, 109336. [Google Scholar] [CrossRef]
- Bremenkamp, I.; Ramos, A.V.; Lu, P.; Patange, A.; Bourke, P.; Sousa-Gallagher, M.J. Combined effect of plasma treatment and equilibrium modified atmosphere packaging on safety and quality of cherry tomatoes. Future Foods 2021, 3, 100011. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Z. Combined use of ultrasound-assisted washing with in-package atmospheric cold plasma processing as a novel non-thermal hurdle technology for ready-to-eat blueberry disinfection. Ultrason. Sonochem. 2022, 84, 105960. [Google Scholar] [CrossRef]
- Bigi, F.; Maurizzi, E.; Quartieri, A.; De Leo, R.; Gullo, M.; Pulvirenti, A. Non-thermal techniques and the “hurdle” approach: How is food technology evolving? Trends Food Sci. Technol. 2023, 132, 11–39. [Google Scholar] [CrossRef]
| Fruits and Vegetables | Disease | Pathogen(s) |
|---|---|---|
| Pome fruits | Blue mold | Penicillium spp. |
| Gray mold | Botrytis cinerea, Botryotinia fuckeliana | |
| Bitter rot | Colletotrichum gloeosporioides, Glomerella cingulata | |
| Alternaria rot | Alternaria spp. | |
| Mucor rot | Mucor piriformis | |
| Stone fruits | Brown rot | Monilia spp., Monilinia fructicola |
| Rhizopus rot | Rhizopus spp. | |
| Gray mold | Botrytis cinerea | |
| Blue mold | Penicillium spp. | |
| Alternaria rot | Alternaria alternata | |
| Grapes | Gray mold | Botrytis cinerea, Botryotinia fuckeliana |
| Blue mold | Penicillium spp. | |
| Rhizopus rot | Rhizopus spp. | |
| Strawberries and similar fruits | Gray mold | Botrytis cinerea |
| Rhizopus rot | Rhizopus spp. | |
| Cladosporium rot | Cladosporium spp. | |
| Blue mold | Penicillium spp. | |
| Subtropical citrus fruits | Blue mold | Penicillium italicum |
| Green mold | Penicillium digitatum | |
| Black center rot | Alternaria citri | |
| Stem end rot | Phomopsis citri, Diaporthe citri | |
| Brown rot | Phytophthora citrophthora, P. parasitica | |
| Avocado | Anthracnose | Colletotrichum gloeosporioides, C. acutatum, Glomerella cingulata |
| Stem end rot | Dothiorella spp., Botryosphaeria spp., Lasiodiplodia theobromae, Stilbella cinnabarina, Thyronectria pseudotrichia, Phomopsis perseae | |
| Bacterial soft rot | Erwinia carotovora | |
| Tropical fruits | Banana anthracnose | Colletotrichum musae |
| Crown rot | Fusarium spp., Verticillium spp., Acremonium sp., Colletotrichum musae | |
| Ceratocystis fruit rot | Thielaviopsis paradoxa, Ceratocystis paradoxa | |
| Mango | Anthracnose | Colletotrichum gloeosporioides, C. acutatum, Glomerella cingulata |
| Stem end rot | Dothiorella spp., Botryosphaeria spp., Lasiodiplodia theobromae, Phomopsis mangiferae, Pestalotiopsis mangiferae | |
| Other pathogens | Rhizopus stolonifer, Aspergillus niger, Alternaria alternata, Botrytis cinerea, Penicillium expansum, Mucor circinelloides | |
| Papaya | Anthracnose | Colletotrichum spp. |
| Black rot | Phoma caricae-papayae, Mycosphaerella caricae | |
| Phomopsis rot | Phomopsis caricae-papayae | |
| Rhizopus rot | Rhizopus stolonifer | |
| Phytophthora fruit rot | Phytophthora palmivora | |
| Pineapple | Water blister rot | Thielaviopsis paradoxa, Ceratocystis paradoxa |
| Fruit core rot | Penicillium funiculosum, Fusarium moniliforme var. subglutinans, Gibberella fujikuroi var. subglutinans | |
| Yeast rot | Saccharomyces spp. | |
| Bacterial brown rot | Erwinia spp. | |
| Cucurbits | Bacterial soft rot | Erwinia spp., Bacillus polymyxa, Pseudomonas syringae, Xanthomonas campestris |
| Gray mold | Botrytis cinerea, Botryotinia fuckeliana | |
| Fusarium rot | Fusarium spp. | |
| Alternaria rot | Alternaria spp. | |
| Charcoal rot | Macrophomina phaseolina | |
| Cottony rot | Pythium spp. | |
| Rhizopus rot | Rhizopus spp. | |
| Tomato, eggplant, pepper | Bacterial soft rot | Erwinia spp., Bacillus polymyxa, Pseudomonas spp., Xanthomonas campestris |
| Gray mold | Botrytis cinerea, Botryotinia fuckeliana | |
| Fusarium rot | Fusarium spp. | |
| Alternaria rot | Alternaria alternata | |
| Cladosporium rot | Cladosporium spp. | |
| Rhizopus rot | Rhizopus spp. | |
| Watery soft rot | Sclerotinia spp. | |
| Cottony rot | Pythium spp. | |
| Sclerotium rot | Sclerotium rolfsii, Athelia rolfsii | |
| Legumes | Gray mold | Botrytis cinerea, B. fabae, Botryotinia fuckeliana |
| White mold/Watery soft rot | Sclerotinia spp. | |
| Cottony rot | Pythium spp. | |
| Sclerotium rot | Sclerotium rolfsii, Athelia rolfsii | |
| Crucifers | Bacterial soft rot | Erwinia spp., Bacillus spp., Pseudomonas spp., Xanthomonas campestris |
| Gray mold | Botrytis cinerea, Botryotinia fuckeliana | |
| Alternaria rot | Alternaria spp. | |
| Watery soft rot | Sclerotinia spp. | |
| Phytophthora rot | Phytophthora spp. | |
| Leafy vegetables | Bacterial soft rot | Erwinia spp., Pseudomonas spp., Xanthomonas spp. |
| Gray mold | Botrytis cinerea | |
| Water rot | Sclerotinia spp. | |
| Onion | Bacterial soft rot | Erwinia spp., Lactobacillus spp., Pseudomonas spp. |
| Black mold rot | Aspergillus niger | |
| Fusarium basal rot | Fusarium oxysporum f. sp. cepae | |
| Smudge disease | Colletotrichum circinans | |
| Carrot | Bacterial soft rot | Erwinia spp., Pseudomonas spp. |
| Rhizopus rot | Rhizopus spp. | |
| Gray mold | Botrytis cinerea, Botryotinia fuckeliana | |
| Watery rot | Sclerotinia spp. | |
| Chalara and Thielaviopsis rots | Chalara thielavioides, Thielaviopsis basicola | |
| Potato | Bacterial soft rot | Erwinia spp. |
| Dry rot | Fusarium spp. | |
| Gangrene | Phoma exigua var. exigua, var. foveata | |
| Black scurf | Rhizoctonia solani | |
| Silver scurf | Helminthosporium solani | |
| Skin spot | Polyscytalum pustulans |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalkan, F. Management of Postharvest Diseases via Eco-Friendly Technologies: A Review of Recent Research. Horticulturae 2025, 11, 1056. https://doi.org/10.3390/horticulturae11091056
Kalkan F. Management of Postharvest Diseases via Eco-Friendly Technologies: A Review of Recent Research. Horticulturae. 2025; 11(9):1056. https://doi.org/10.3390/horticulturae11091056
Chicago/Turabian StyleKalkan, Fatih. 2025. "Management of Postharvest Diseases via Eco-Friendly Technologies: A Review of Recent Research" Horticulturae 11, no. 9: 1056. https://doi.org/10.3390/horticulturae11091056
APA StyleKalkan, F. (2025). Management of Postharvest Diseases via Eco-Friendly Technologies: A Review of Recent Research. Horticulturae, 11(9), 1056. https://doi.org/10.3390/horticulturae11091056





