Variations in C:N:P Stoichiometry and Non-Structural Carbohydrates in Different Parts of Pomelo (Citrus maxima) Flowers at Three Phenophases
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Flower Collection and Measurements
2.3. Determinations of C, N, and P Contents
2.4. Determinations of NSC Contents
2.5. Statistical Analysis
3. Results
3.1. Variations of Morphological Indicators and Biomass
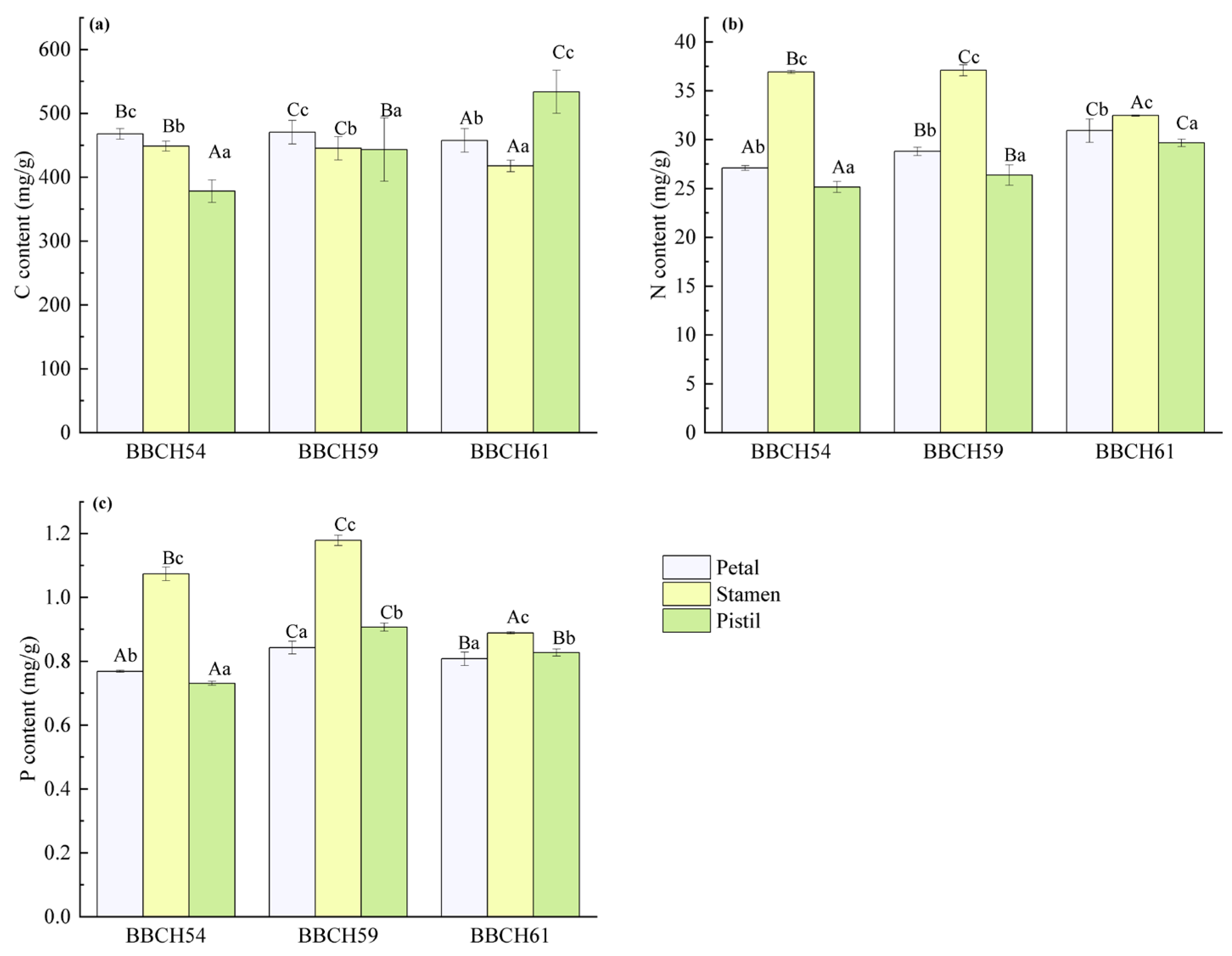
3.2. Variations in C, N, and P Contents
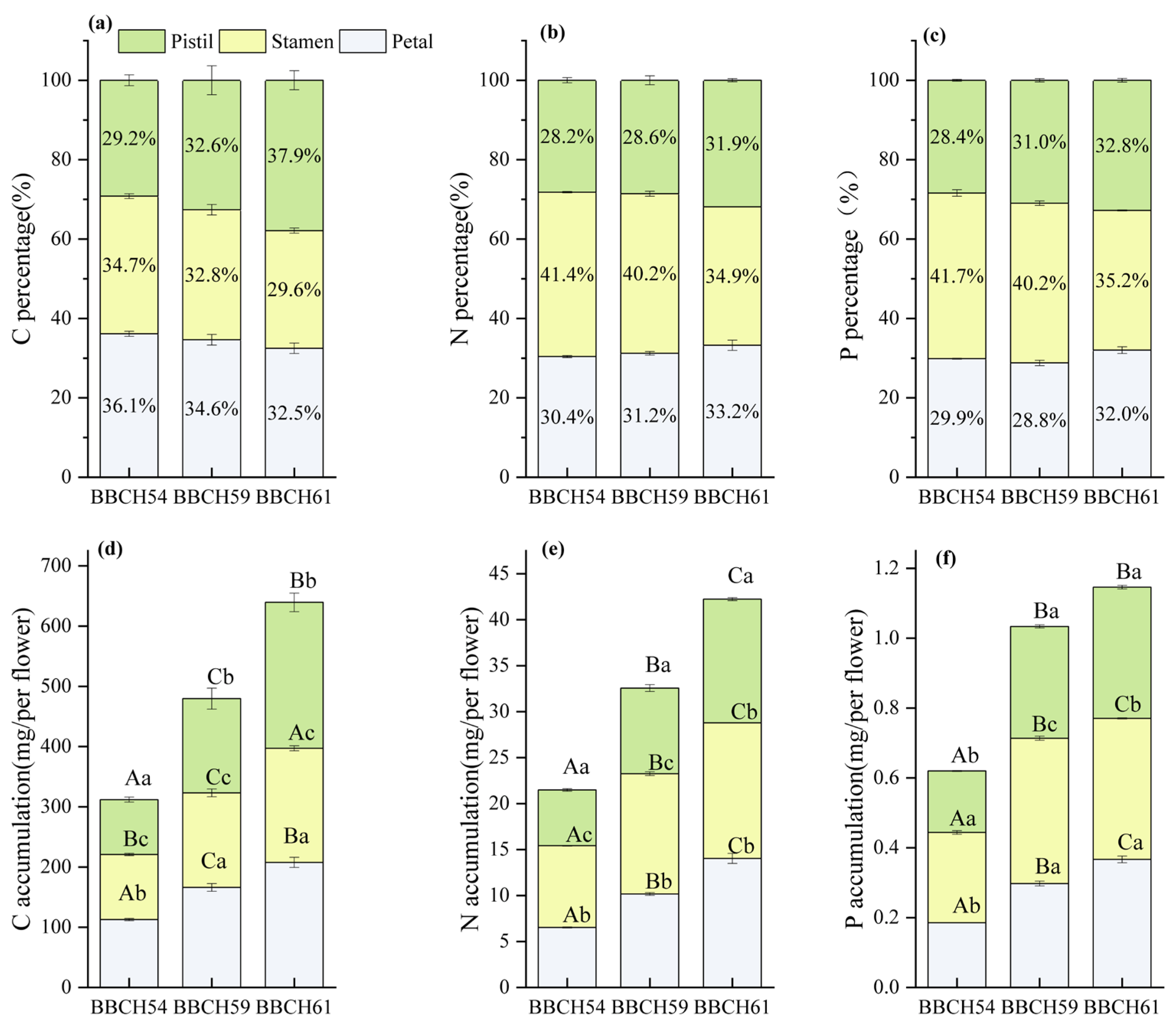
3.3. Variations in C, N, and P Accumulation and Allocation Proportion
3.4. Variations in C, N, and P Stoichiometry
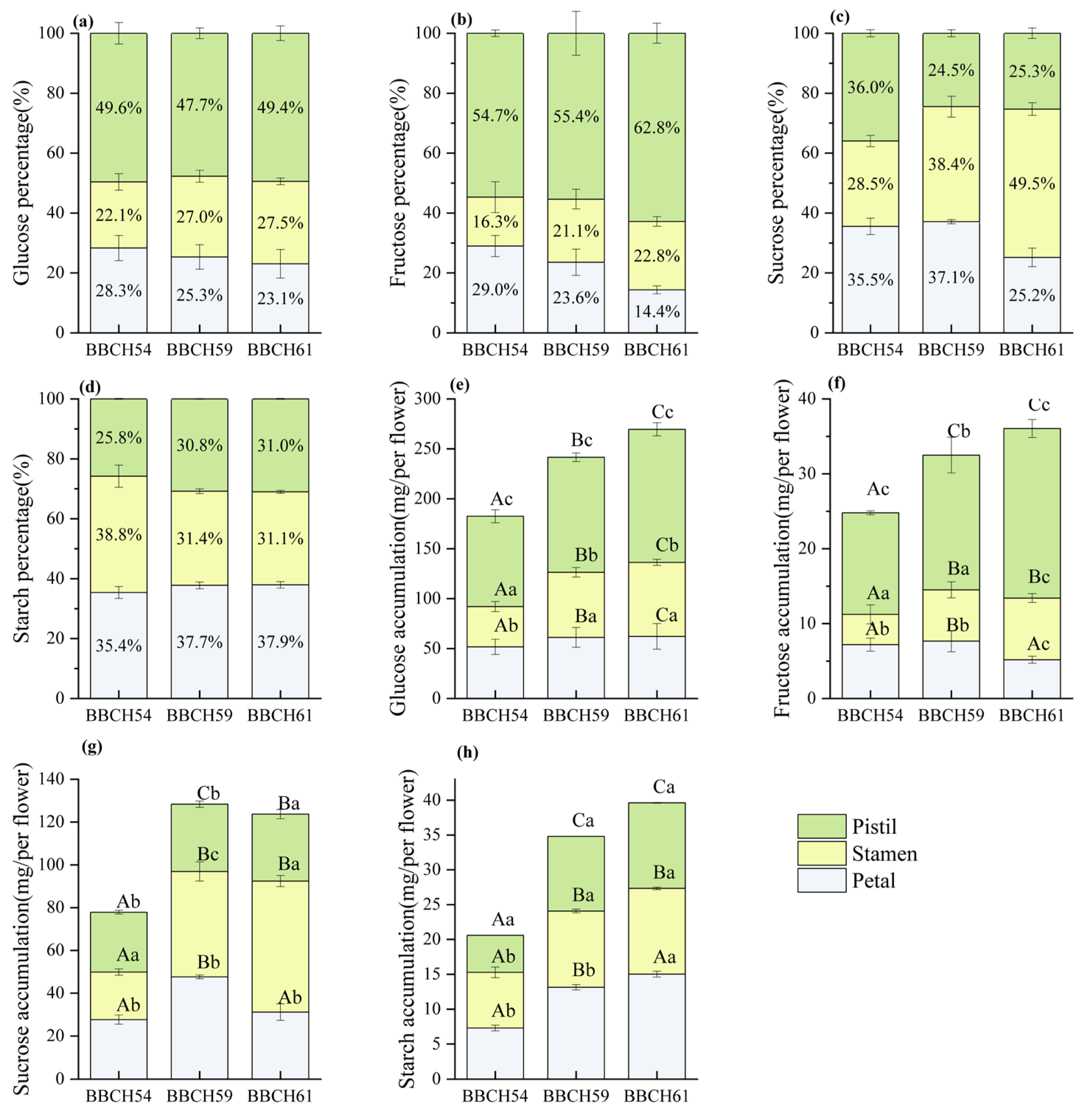
3.5. Non-Structural Sugars (NSC) Contents
3.6. NSC Accumulation and Allocation Proportion
3.7. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baslam, M.; Mitsui, T.; Sueyoshi, K.; Ohyama, T. Recent Advances in Carbon and Nitrogen Metabolism in C3 Plants. Int. J. Mol. Sci. 2019, 22, 318. [Google Scholar] [CrossRef]
- Krouk, G.; Kiba, T. Nitrogen and Phosphorus Interactions in Plants: From Agronomic to Physiological and Molecular Insights. Curr. Opin. Plant Biol. 2020, 57, 104–109. [Google Scholar] [CrossRef]
- Ågren, G.I. Stoichiometry and Nutrition of Plant Growth in Natural Communities. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 153–170. [Google Scholar] [CrossRef]
- Sardans, J.; Rivas-Ubach, A.; Peñuelas, J. The C:N:P Stoichiometry of Organisms and Ecosystems in a Changing World: A Review and Perspectives. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 33–47. [Google Scholar] [CrossRef]
- Ren, H.Y.; Qian, W.Z.; Yi, L.; Ye, Y.L.; Gu, T.; Gao, S.; Cao, G.X. Nutrient Composition and Antioxidant Activity of Cercis chinensis Flower in Response to Different Development Stages. Horticulturae 2023, 9, 961. [Google Scholar] [CrossRef]
- Jia, S.; Wang, Y.; Hu, J.; Ding, Z.; Liang, Q.; Zhang, Y.; Wang, H. Mineral and Metabolic Profiles in Tea Leaves and Flowers During Flower Development. Plant Physiol. Biochem. 2016, 106, 316–326. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Zhang, W.; Njie, A.; Pan, X. The Changes in C/N, Carbohydrate, and Amino Acid Content in Leaves During Female Flower Bud Differentiation of Juglans sigillata. Acta Physiol. Plant. 2022, 44, 19. [Google Scholar] [CrossRef]
- Yu, T.; Yang, Y.; Wang, H.; Qian, W.; Hu, Y.; Gao, S.; Liao, H. The Variations of C/N/P Stoichiometry, Endogenous Hormones, and Non-Structural Carbohydrate Contents in Michelia maudiae ‘Rubicunda’ Flower at Five Development Stages. Horticulturae 2023, 9, 1198. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, Y.; Cheng, Y.; Yu, T.; Zhu, M.; Qian, W.; Gao, S.; Zhuang, G. Non-Structural Carbohydrate Content and C:N:P Stoichiometry in Houpoea officinalis Flowers in Response to Development Stages. Horticulturae 2024, 10, 784. [Google Scholar] [CrossRef]
- Verlinden, S. Changes in Mineral Nutrient Concentrations in Petunia Corollas during Development and Senescence. HortScience 2003, 38, 71–74. [Google Scholar] [CrossRef]
- Wang, T.-W.; Tan, J.; Li, L.-Y.; Yang, Y.; Zhang, X.-M.; Wang, J.-R. Combined Analysis of Inorganic Elements and Flavonoid Metabolites Reveals the Relationship between Flower Quality and Maturity of Sophora japonica L. Front. Plant Sci. 2023, 14, 1255637. [Google Scholar] [CrossRef]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar Sensing and Signaling in Plants: Conserved and Novel Mechanisms. Ann. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef]
- Mishra, B.S.; Sharma, M.; Laxmi, A. Role of Sugar and Auxin Crosstalk in Plant Growth and Development. Physiol. Plant. 2022, 174, e13546. [Google Scholar] [CrossRef]
- Therby-Vale, R.; Lacombe, B.; Rhee, S.Y.; Nussaume, L.; Rouached, H. Mineral Nutrient Signaling Controls Photosynthesis: Focus on Iron Deficiency-Induced Chlorosis. Trends Plant Sci. 2022, 27, 502–509. [Google Scholar] [CrossRef]
- Wang, Y.; Funnell, K.A.; Eason, J.R.; Morgan, E.R.; Woolley, D.J. Osmotic Regulation via Carbohydrate Metabolism Drives Petal Expansion and Floret Opening in Gentian ‘Showtime Spotlight. ’ Sci. Hortic. 2016, 211, 19–25. [Google Scholar] [CrossRef]
- Cho, L.H.; Pasriga, R.; Yoon, J.; Jeon, J.S.; An, G. Roles of Sugars in Controlling Flowering Time. J. Plant Biol. 2018, 61, 121–130. [Google Scholar] [CrossRef]
- Norikoshi, R.; Imanishi, H.; Ichimura, K. Changes in Cell Number, Osmotic Potential and Concentrations of Carbohydrates and Inorganic Ions in Tweedia Caerulea during Flower Opening. J. Jpn. Soc. Hortic. Sci. 2013, 82, 51–56. [Google Scholar] [CrossRef]
- Xu, J.; Li, Q.; Yang, L.; Li, X.; Wang, Z.; Zhang, Y. Changes in Carbohydrate Metabolism and Endogenous Hormone Regulation During Bulblet Initiation and Development in Lycoris radiata. BMC Plant Biol. 2020, 20, 180. [Google Scholar] [CrossRef]
- Önder, S.; Tonguç, M.; Önder, D.; Erbaş, S.; Mutlucan, M. Flower Color and Carbohydrate Metabolism Changes During the Floral Development of Rosa Damascena. S. Afr. J. Bot. 2023, 156, 234–243. [Google Scholar] [CrossRef]
- Roderick, B.; John, E.; Julian, H.; Allan, W. Flower opening in Asiatic lily is a rapid process controlled by dark-light cycling. Ann. Bot. 2000, 86, 1169–1174. [Google Scholar] [CrossRef][Green Version]
- Hedhly, A.; Vogler, H.; Schmid, M.W.; Pazmino, D.; Gagliardini, V.; Santelia, D.; Grossniklaus, U. Starch Turnover and Metabolism During Flower and Early Embryo Development. Plant Physiol. 2016, 172, 2388–2402. [Google Scholar] [CrossRef]
- Fernandes, L.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E.; Casal, S. Phytochemical Characterization of Borago officinalis L. and Centaurea cyanus L. during Flower Development. Food Res. Int. 2019, 123, 771–778. [Google Scholar] [CrossRef]
- Jhanji, S.; Kaur, G.; Kaur, R.; Dhatt, U.K. Physiological and Biochemical Changes during Flower Development and Senescence in Chrysanthemum and Gladiolus. Acta Physiol. Plant. 2023, 45, 14. [Google Scholar] [CrossRef]
- Ulger, S.; Sonmez, S.; Karkacier, M.; Ertoy, N.; Akdesir, O.; Aksu, M. Determination of endogenous hormones, sugars and mineral nutrition levels during the induction, initiation and differentiation stage and their effects on flower formation in olive. Plant Growth Regul. 2004, 42, 89–95. [Google Scholar] [CrossRef]
- Borghi, M. Roles of Sugar Metabolism and Transport in Flower Development. Curr. Opin. Plant Biol. 2025, 85, 102722. [Google Scholar] [CrossRef]
- Wei, S.; Liu, H.; Li, J.; Ren, T.; Xie, J. Metabolite Variations of Sugars, Organic Acids, Fatty Acids and Amino Acids in Flower Buds of Zingiber mioga Roscoe at Different Developmental Stages. J. Food Compos. Anal. 2023, 116, 105050. [Google Scholar] [CrossRef]
- Önder, S.; Tonguç, M.; Erbaş, S.; Önder, D.; Mutlucan, M. Investigation of Phenological, Primary and Secondary Metabolites Changes during Flower Developmental of Rosa damascena. Plant Physiol. Biochem. 2022, 192, 20–34. [Google Scholar] [CrossRef]
- Xiang, N.; Zhao, Y.; Zhang, B.; Gu, Q.; Chen, W.; Guo, X. Volatiles Accumulation during Young Pomelo (Citrus maxima (Burm.) Merr.) Fruits Development. Int. J. Mol. Sci. 2022, 23, 5665. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Wang, P.; Pang, X.; Zhang, L.; Qian, L.; Jia, X.; Chen, W.; Ruan, S.; Sun, L. The New Exploration of Pure Total Flavonoids Extracted from Citrus maxima (Burm.) Merr. as a New Therapeutic Agent to Bring Health Benefits for People. Front. Nutr. 2022, 9, 958329. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Dong, T.; Xiong, B.; Qiu, X.; Sun, G.; Liao, L.; Fan, N.; Wang, X.; Deng, H.; He, S.; et al. Variation in The Content and Composition of Limonoids in Fruits of Four Pomelo Varieties During Fruit Development: The Natural Debittering Process in Pomelo Fruits. J. Food Compos. Anal. 2021, 100, 103928. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Hu, Y.; Gu, Q.; Chen, W.; Li, J.; Guo, X.; Liu, Y. Evaluation of Carotenoids Accumulation and Biosynthesis in Two Genotypes of Pomelo (Citrus maxima) during Early Fruit Development. Molecules 2021, 26, 5054. [Google Scholar] [CrossRef]
- Sapkota, B.; Devkota, H.P.; Poudel, P. Citrus maxima (Brum.) Merr. (Rutaceae): Bioactive Chemical Constituents and Pharmacological Activities. Evid. Based Complement. Altern. Med. 2022, 2022, 8741669. [Google Scholar] [CrossRef]
- Agustí, M.; Reig, C.; Martínez-Fuentes, A.; Mesejo, C. Advances in Citrus Flowering: A Review. Front. Plant Sci. 2022, 13, 868831. [Google Scholar] [CrossRef]
- Zhang, X.; Zhuang, X.; Xiong, T.; Huang, S.; Qian, X.; Guo, T.; Chen, M.; Xie, W.; Huang, Y. Extraction From Different Parts of Citrus maxima Flowers Using Ultrasound as an Aid and Study of Their Composition and Function. Ultrason. Sonochem. 2023, 100, 106632. [Google Scholar] [CrossRef]
- Fadón, E.; Herrero, M.; Rodrigo, J. Flower development in sweet cherry framed in the BBCH scale. Sci. Hortic. 2015, 192, 141–147. [Google Scholar] [CrossRef]
- Qian, W.; Hu, Y.; Lin, X.; Yu, D.; Jia, S.; Ye, Y.; Mao, Y.; Yi, L.; Gao, S. Phenological Growth Stages of Abelmoschus manihot: Codification and Description According to the BBCH Scale. Agronomy 2023, 13, 1328. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Ni, M.; Yu, F. A Study on Petal Morphological and Physiological Characteristics of Styrax Japonicus during the Flowering Period. Agronomy 2021, 11, 1498. [Google Scholar] [CrossRef]
- Önder, D. Variation in antioxidant capacity, antioxidant activity and mineral composition during flower development of oil-bearing rose (Rosa damascena Mill.). Sci. Rep. 2023, 13, 17255. [Google Scholar] [CrossRef]
- Simko, I.; Zhao, R. Phenotypic Characterization, Plant Growth and Development, Genome Methylation, and Mineral Elements Composition of Neotetraploid Lettuce (Lactuca sativa L.). Front. Plant Sci. 2023, 14, 1296660. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.; Rabinovich, M.; Smith, H.M. The Role of Auxin and Sugar Signaling in Dominance Inhibition of Inflorescence Growth by Fruit Load. Plant Physiol. 2021, 187, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Zhu, X.; Zhang, T.; Chen, J.; Chen, J.; Qin, Y. Transcriptome Analysis Reveals Sugar and Hormone Signaling Pathways Mediating Flower Induction in Pitaya (Hylocereus polyrhizus). Int. J. Mol. Sci. 2025, 26, 1250. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Norikoshi, R.; Suzuki, K.; Imanishi, H.; Ichimura, K. Determination of Subcellular Concentrations of Soluble Carbohydrates in Rose Petals during Opening by Nonaqueous Fractionation Method Combined with Infiltration–Centrifugation Method. Planta 2009, 230, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Yap, Y.M.; Loh, C.S.; Ong, B.L. Regulation of Flower Development in Dendrobium crumenatum by Changes in Carbohydrate Contents, Water Status and Cell Wall Metabolism. Sci. Hortic. 2008, 119, 59–66. [Google Scholar] [CrossRef]
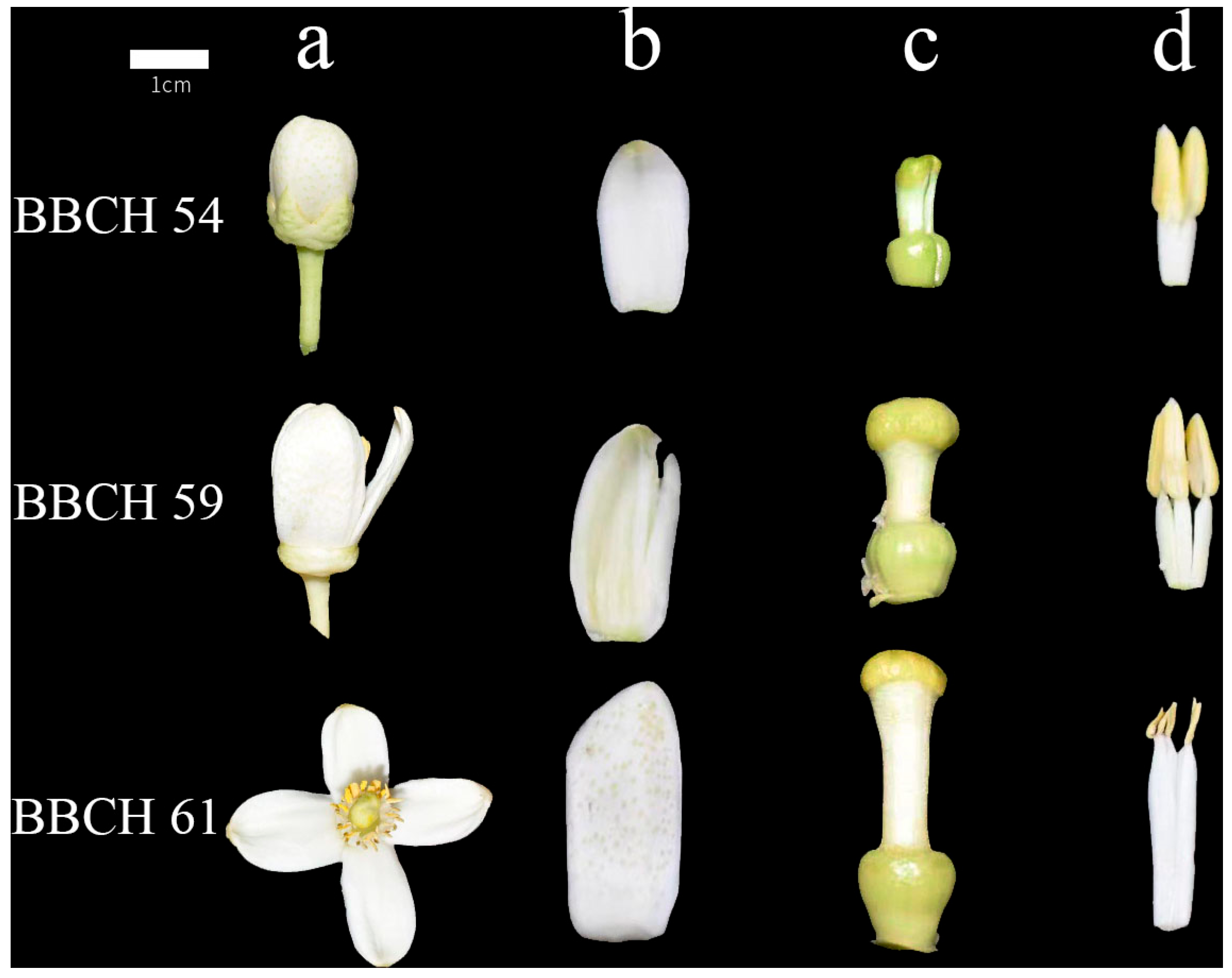



| Organs | BBCH54 | BBCH59 | BBCH61 | |
|---|---|---|---|---|
| Pistil | Fresh mass (g) | 0.34 ± 0.02 b | 0.62 ± 0.04 a | 0.71 ± 0.10 a |
| Dry mass (g) | 0.11 ± 0.01 c | 0.16 ± 0.003 b | 0.19 ± 0.02 a | |
| RWC (%) | 74.37 ± 1.38 a | 73.26 ± 0.41 a | 72.04 ± 1.82 a | |
| Stamen | Fresh mass (g) | 0.25 ± 0.02 b | 0.43 ± 0.01 a | 0.43 ± 0.04 a |
| Dry mass (g) | 0.04 ± 0.03 b | 0.08 ± 0.03 a | 0.08 ± 0.01 a | |
| RWC (%) | 83.49 ± 1.15 a | 81.65 ± 1.27 a | 81.31 ± 0.97 a | |
| Petal | Fresh mass (g) | 0.50 ± 0.09 c | 0.83 ± 0.10 b | 1.27 ± 0.11 a |
| Dry mass (g) | 0.08 ± 0.02 b | 0.11 ± 0.01 b | 0.19 ± 0.02 a | |
| RWC (%) | 83.14 ± 0.54 b | 86.63 ± 1.05 a | 85.62 ± 0.99 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, J.; Hu, S.; Kong, Y.; Pan, H.; Zhu, M.; Yu, T.; Hu, H.; Zhuang, G.; Gao, S. Variations in C:N:P Stoichiometry and Non-Structural Carbohydrates in Different Parts of Pomelo (Citrus maxima) Flowers at Three Phenophases. Horticulturae 2025, 11, 1053. https://doi.org/10.3390/horticulturae11091053
Liao J, Hu S, Kong Y, Pan H, Zhu M, Yu T, Hu H, Zhuang G, Gao S. Variations in C:N:P Stoichiometry and Non-Structural Carbohydrates in Different Parts of Pomelo (Citrus maxima) Flowers at Three Phenophases. Horticulturae. 2025; 11(9):1053. https://doi.org/10.3390/horticulturae11091053
Chicago/Turabian StyleLiao, Jiali, Shiyao Hu, Yiming Kong, Haohao Pan, Maoyuan Zhu, Ting Yu, Hongling Hu, Guoqing Zhuang, and Shun Gao. 2025. "Variations in C:N:P Stoichiometry and Non-Structural Carbohydrates in Different Parts of Pomelo (Citrus maxima) Flowers at Three Phenophases" Horticulturae 11, no. 9: 1053. https://doi.org/10.3390/horticulturae11091053
APA StyleLiao, J., Hu, S., Kong, Y., Pan, H., Zhu, M., Yu, T., Hu, H., Zhuang, G., & Gao, S. (2025). Variations in C:N:P Stoichiometry and Non-Structural Carbohydrates in Different Parts of Pomelo (Citrus maxima) Flowers at Three Phenophases. Horticulturae, 11(9), 1053. https://doi.org/10.3390/horticulturae11091053






