Differential Systemic Translocation of Oxathiapiprolin, Benthiavalicarb, and Their Mixture to Tomato Leaves and Fruits as Evidenced by Their Differential Protection from Late Blight Caused by Phytophthora infestans
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants
2.2. Fruits
2.3. Fungicides
2.4. Fungicide Application
2.5. Pathogen and Inoculation
2.6. Assessment of Systemic Translocation
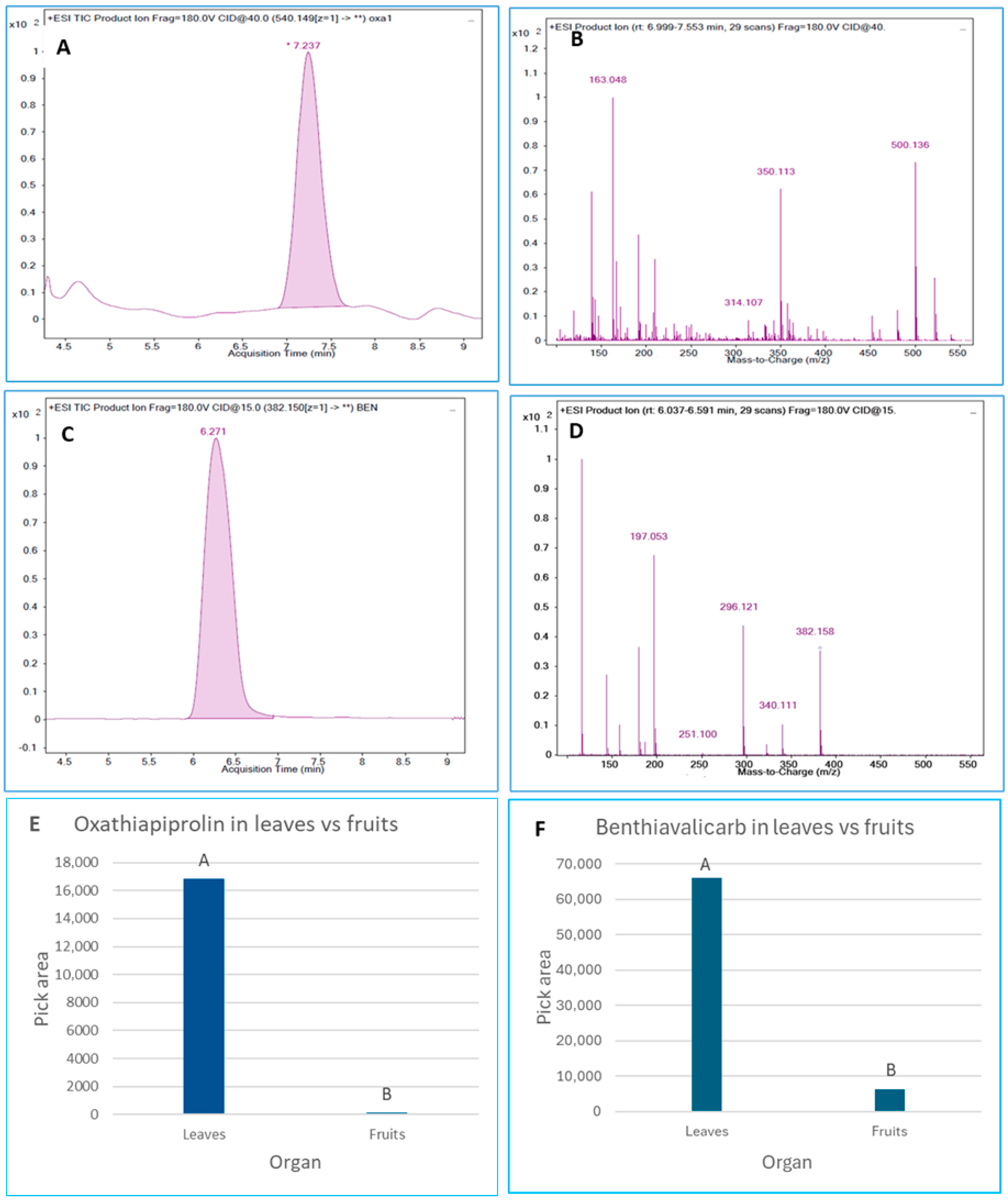
2.7. LC–MS/MS Analysis
2.8. Data Analysis
3. Results
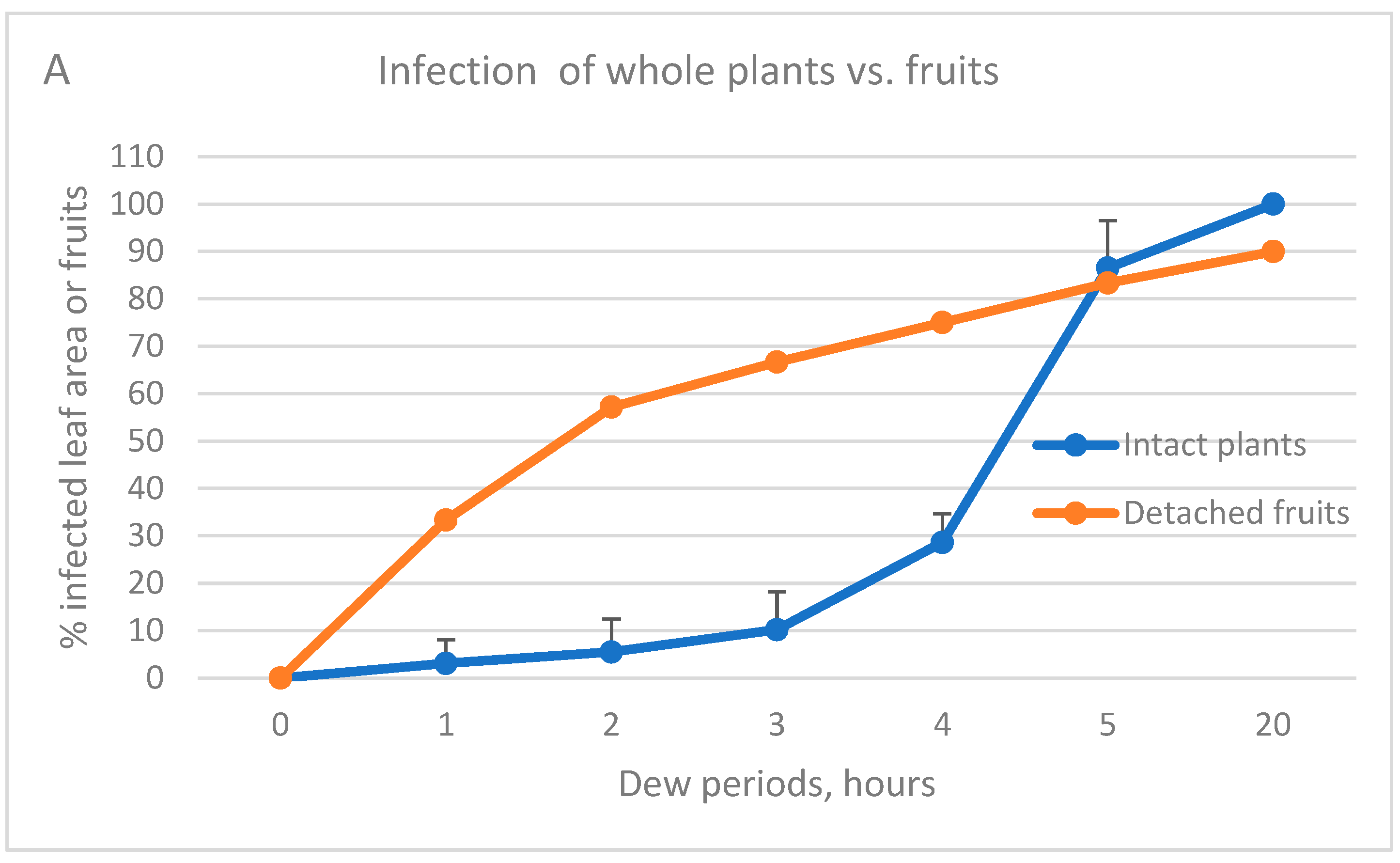
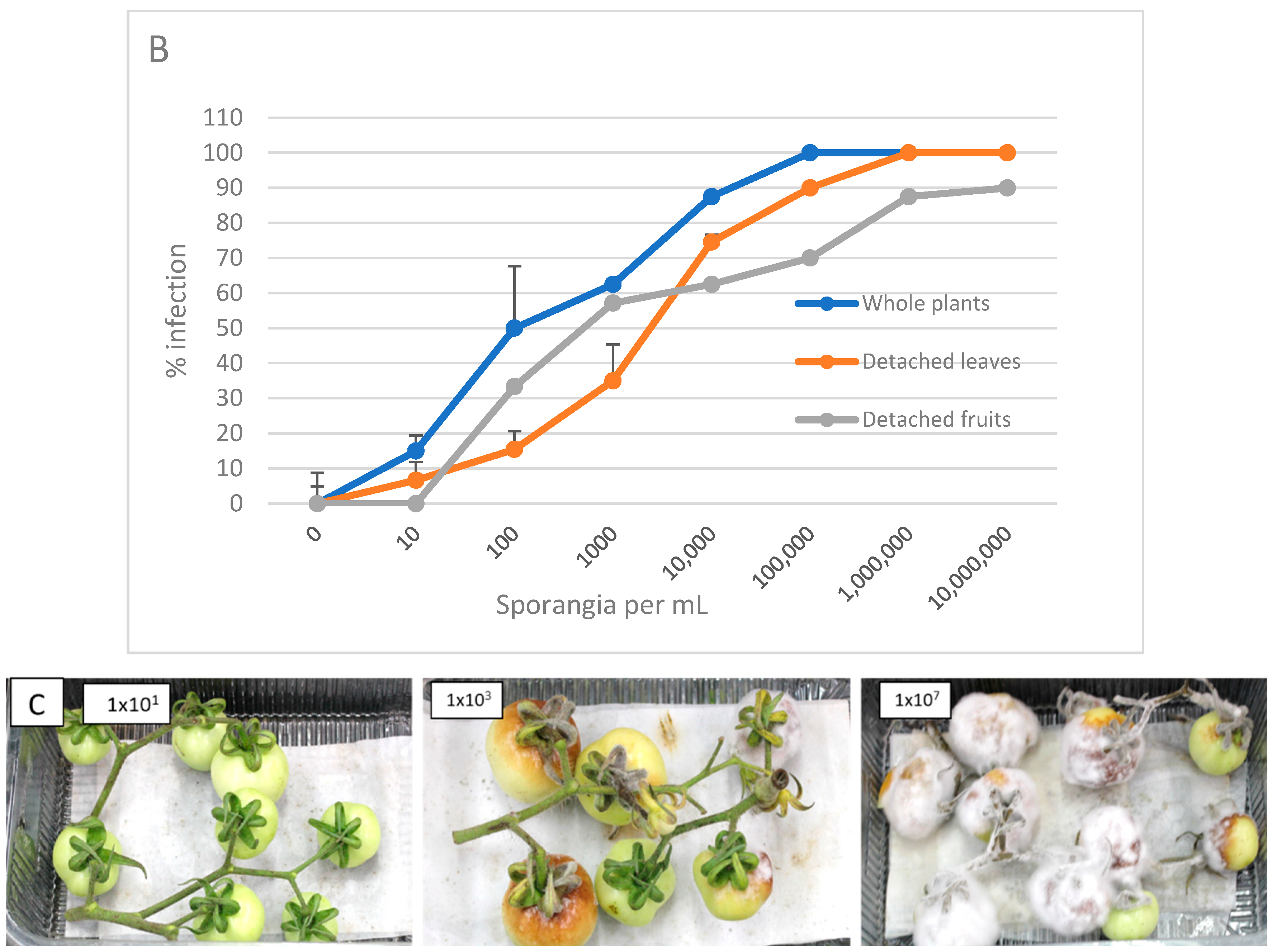
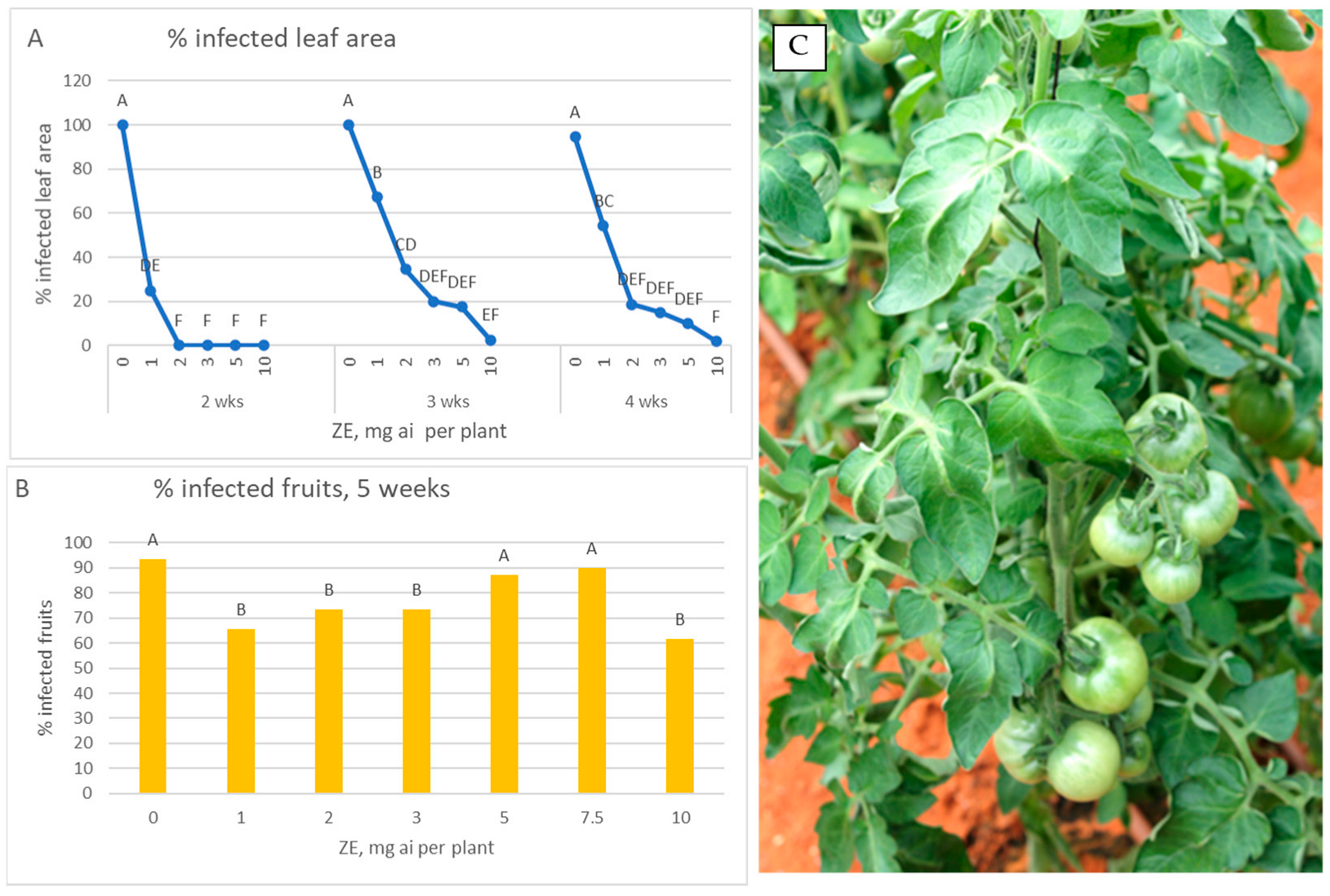
3.1. Proneness of Green Fruits to Infection
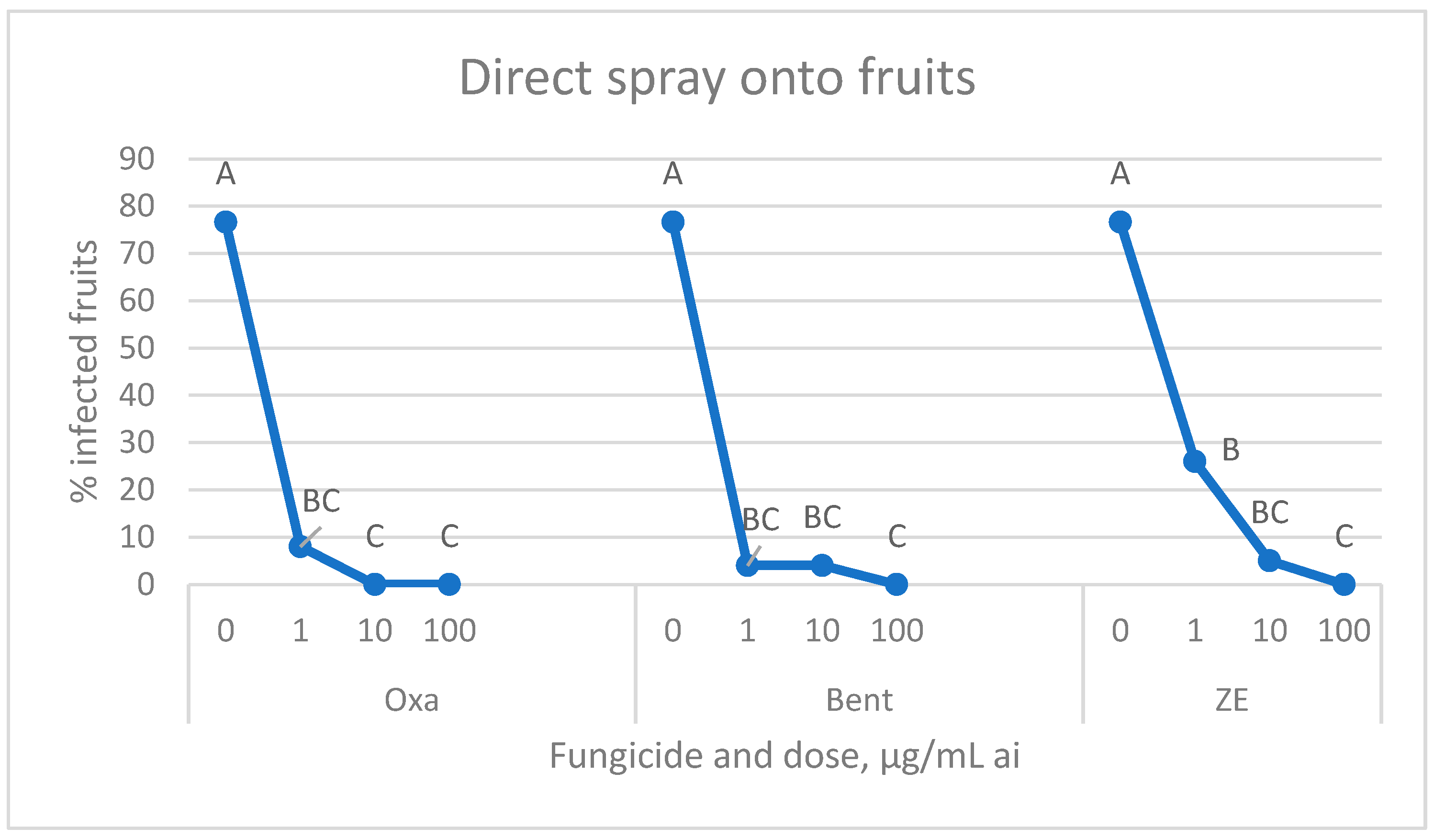
3.2. Fungicide Efficacy—Spray Application to Green Fruits
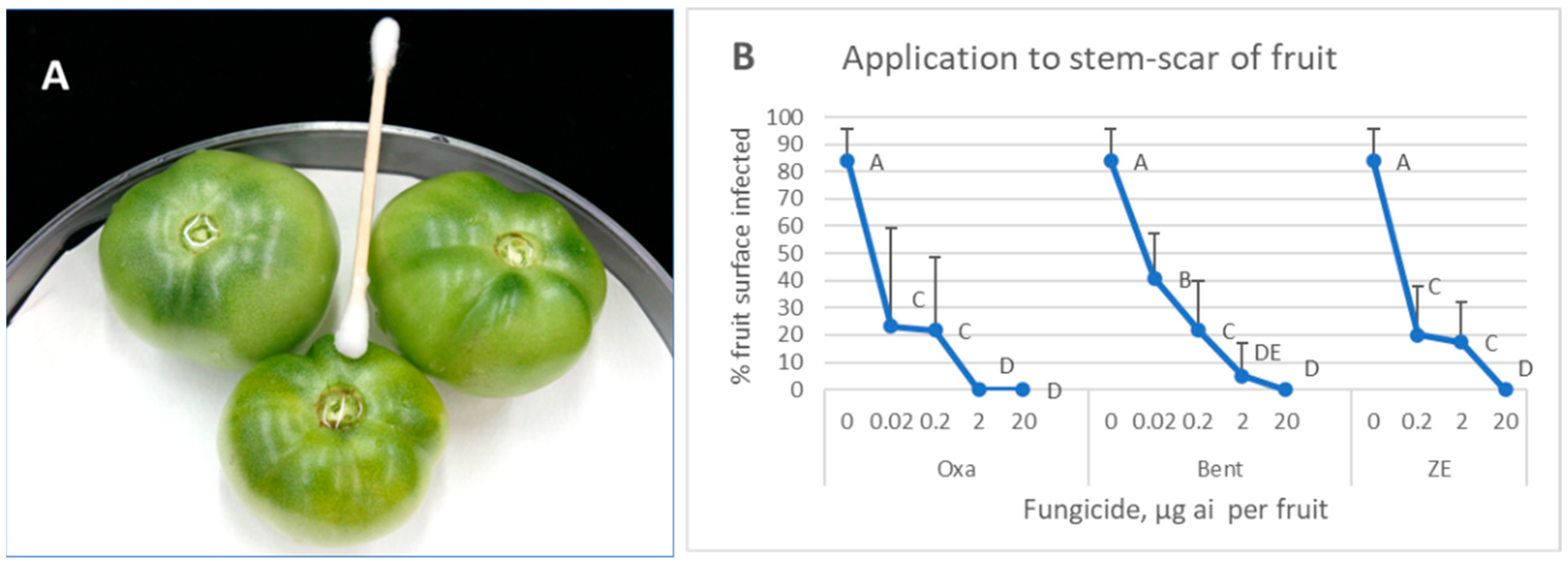
3.3. Application of Fungicides to the Fruit Stem Scar
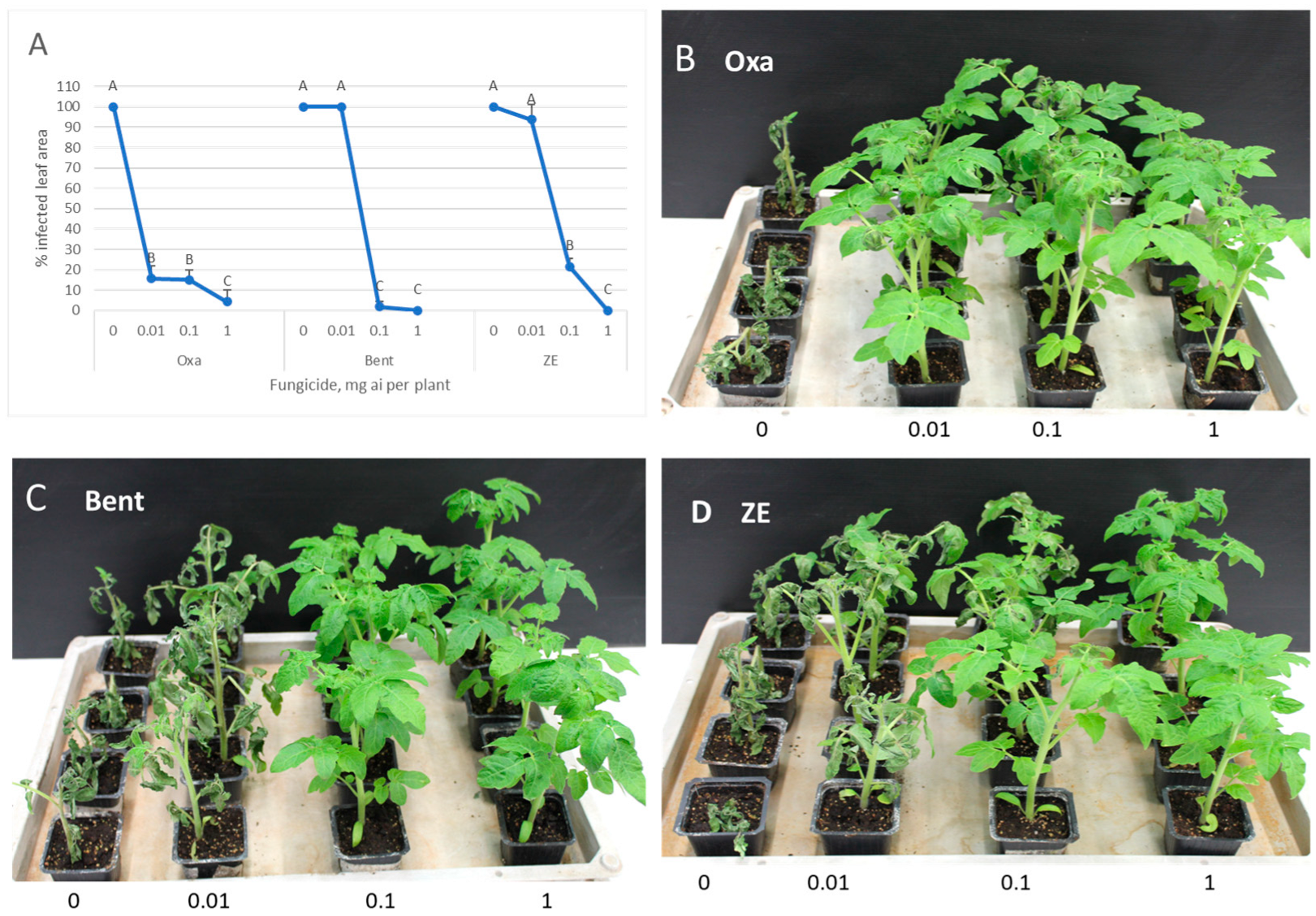
3.4. Systemic Translocation of Fungicides from Foliage to Fruits
3.5. Systemic Translocation of Fungicides from Roots to Leaves and Fruits
4. Discussion
- (a)
- Soil application of systemic fungicides effectively controls foliage blight but not fruit blight.
- (b)
- Fruits, while less protected, may consequently retain lower fungicide residues, enhancing consumer safety.
- (c)
- Efficient foliar protection may indirectly prevent fruit infection unless external inoculum sources introduce the pathogen.
- (d)
- Reduced fungicide accumulation in fruits may lower the selection pressure on P. infestans, potentially delaying the development of fungicide resistance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warneke, B.; Pscheidt, J.W.; Nackley, L. Pesticide Redistribution and Its Implications on Pesticide Efficacy; Oregon State University Extension Service: St. Helens, OR, USA, 2023; Available online: https://extension.oregonstate.edu/catalog/pub/pnw-772-pesticide-redistribution-its-implications-pesticide-efficacy (accessed on 18 June 2025).
- Cohen, Y.; Reuveni, M.; Eyal, H. The systemic antifungal activity of Ridomil against Phytophthora infestans on tomato plants. Phytopathology 1979, 69, 433–436. [Google Scholar] [CrossRef]
- Cohen, Y. The novel oomycide oxathiapiprolin inhibits all stages in the asexual life cycle of Pseudoperonospora cubensis—Causal agent of cucurbit downy mildew. PLoS ONE 2015, 10, e014001. [Google Scholar] [CrossRef]
- Cohen, Y.; Rubin, A.E.; Galperin, M. Oxathiapiprolin-based fungicides provide enhanced control of tomato late blight induced by mefenoxam-insensitive Phytophthora infestans. PLoS ONE 2018, 13, e0204523. [Google Scholar] [CrossRef]
- Wang, Z.X.; Lv, X.; Wang, R.B.; He, Z.B.; Feng, W.Z.; Liu, W.J.; Yang, C.X.; Wang, Z.Y.; Ke, Q.H.; Tao, K.Z.; et al. Use of oxathiapiprolin for controlling soybean root rot caused by Phytophthora sojae: Efficacy and mechanism of action. Pest Manag. Sci. 2023, 79, 381–390. [Google Scholar] [CrossRef]
- Lacey, R.F.; Fairhurst, M.J.; Daley, K.J.; Ngata-Aerengamate, T.A.; Patterson, H.R.; Patrick, W.M.; Gerth, M.L. Assessing the effectiveness of oxathiapiprolin toward Phytophthora agathidicida, the causal agent of kauri dieback disease. FEMS Microbes 2021, 2, xtab016. [Google Scholar] [CrossRef]
- Cohen, Y.; Weitman, M. Mobility of oxathiapiprolin in and between tomato plants. Pest Manag. Sci. 2023, 79, 1102–1112. [Google Scholar] [CrossRef]
- Salas, S.E.; Shepherd, C.P.; Ngugi, H.K.; Genet, J.L. Disease control attributes of oxathiapiprolin fungicides for management of cucurbit downy mildew. Plant Dis. 2019, 103, 2812–2820. [Google Scholar] [CrossRef]
- Cohen, Y.; Rubin, A.E. A new strategy for durable control of late blight in potato by a single soil application of an oxathiapiprolin mixture in early season. PLoS ONE 2020, 15, e0238148. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.; Rubin, A.E.; Galperin, M. Root treatment with oxathiapiprolin, benthiavalicarb or their mixture provides prolonged systemic protection against oomycete foliar pathogens. PLoS ONE 2020, 15, e0227556. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.; Rubin, A.E.; Galperin, M. Novel synergistic fungicidal mixtures of oxathiapiprolin protect sunflower seeds from downy mildew caused by Plasmopara halstedii. PLoS ONE 2019, 14, e0222827. [Google Scholar] [CrossRef] [PubMed]
- Gordan, H.; Mahdavi, V.; Behbahan, A.K. Pesticides residue analysis and associated human health risk assessment in tomato and onion. Environ. Monit. Assess. 2025, 197, 718. [Google Scholar] [CrossRef]
- Wu, C.; Zheng, Y.; Sun, T.; He, M.; Zhang, L.; Mao, L.; Zheng, Y. Simultaneous determination of benthiavalicarb-isopropyl and its four metabolites in fruits and vegetables using ultrahigh-performance liquid chromatography/tandem mass spectrometry. Int. J. Environ. Anal. Chem. 2022, 104, 2523–2533. [Google Scholar] [CrossRef]
- Yu, P.; Jia, C.; Zhao, E.; Chen, L.; He, H.; Jing, J.; He, M. Determination of oxathiapiprolin concentration and dissipation in grapes and soil by ultrahigh-performance liquid chromatography-tandem mass spectrometry. J. Sci. Food Agric. 2017, 97, 3294–3299. [Google Scholar] [CrossRef]
- Ji, P.; Grey, T.L.; Csinos, A.S.; Ji, P. Translocation of oxathiapiprolin in bell pepper plants and systemic protection of plants against Phytophthora blight. Plant Dis. 2016, 100, 1931–1936. [Google Scholar] [CrossRef]
- Sakai, J.; Miura, I.; Shibata, M.; Yonekura, N.; Hiyoshi, H.; Takagaki, M. Development of a new fungicide, benthiavalicarb-isopropyl. J. Pestic. Sci. 2010, 35, 488–489. [Google Scholar] [CrossRef]
- Rubin, E.; Baider, A.; Cohen, Y. Phytophthora infestans produces oospores in fruits and seeds of tomato. Phytopathology 2001, 91, 1074–1080. [Google Scholar] [CrossRef]
- Rubin, E.; Cohen, Y. Oospores associated with tomato seeds may lead to seedborne transmission of Phytophthora infestans. Phytoparasitica 2004, 32, 237–245. [Google Scholar] [CrossRef]
- Evangelisti, E.; Govers, F. Roadmap to success: How oomycete plant pathogens invade tissues and deliver effectors. Annu. Rev. Microbiol. 2024, 78, 24.1–24.20. [Google Scholar] [CrossRef]
- Fich, E.A.; Fisher, J.; Zamir, D.; Rose, J.K.C. Transpiration from tomato fruit occurs primarily via trichome-associated transcuticular polar pores. Plant Physiol. 2020, 184, 1840–1852. [Google Scholar] [CrossRef]
- Cohen, Y.; Rubin, A.E.; Galperin, M. Effective control of two genotypes of Phytophthora infestans in the field by three oxathiapiprolin fungicidal mixtures. PLoS ONE 2021, 16, e0258280. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.A.; Nguyen, K.A.; Hao, W.; Belisle, R.J.; Forster, H.; Adaskaveg, J.E. Mobility of oxathiapiprolin and mefenoxam in citrus seedlings after root application and implications for managing Phytophthora root rot. Plant Dis. 2020, 104, 3159–3165. [Google Scholar] [CrossRef]
- Pasteris, R.J.; Hanagan, M.A.; Bisaha, J.J.; Finkelstein, B.L.; Hoffman, L.E.; Gregory, V.; Andreassi, J.L.; Sweigard, J.A.; Klyashchitsky, B.A.; Henry, Y.T.; et al. Discovery of oxathiapiprolin, a new oomycete fungicide that targets an oxysterol binding protein. Bioorg. Med. Chem. 2016, 24, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Abuley, I.K.; Lynott, J.S.; Hansen, J.G.; Cooke, D.E.L.; Lees, A.K. The EU43 genotype of Phytophthora infestans displays resistance to mandipropamid. Plant Pathol. 2023, 72, 1305–1313. [Google Scholar] [CrossRef]
- Derpmann, J.; Leonard, S.; Böhm, J.; Mehl, A. Characterization of CAA-, OSBPI- and double-resistant field isolates of Phytophthora infestans and their impact on late blight control in potatoes. Res. Sq. 2025. [Google Scholar] [CrossRef]



| Experiment 2 | Experiment 3 | Experiment 4 | ||||
|---|---|---|---|---|---|---|
| 2 Days | 3 Days | 4 Days | ||||
| Leaves | Fruits | Leaves | Fruits | Leaves | Fruits | |
| Control | 89.5 ± 14.2 a | 89.9 ± 7.8 a | 56.5 ± 26.4 a | 69.2 (n = 52) | 97.5 ± 3.5 a | 95.5 ± 6.4 a |
| Oxa | nt | 88.1 ± 3.2 a | 6.5 ± 16.5 b | 52.4 (n = 103) | nt | nt |
| ZE | 4.9± 4.4 b | 62.7 ± 15.9 b | 0.5 ± 0 bc | 44.9 (n = 89) | 12.8 ± 12.5 b | 62.5 ± 15.9 ab |
| Bent | 1.7 ± 2.9 b | 79.2 ± 10.9 aba | nt | nt | nt | nt |
| LEAVES | |||||
| % infected leaf area | |||||
| 30 d | 41 d | 50 d | 68 d | 78 d | |
| Control | 95.0 ± 4.2 a | 97.8 ± 8.3 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a |
| Oxa | 0 b | 1.1 ± 4.7 b | 4.3 ± 8.6 b | 4.3 ± 6.2 b | 5.6 ± 9.2 c |
| ZE | 0 b | 1.1 ± 4.7 b | 8.0 ± 16.0 b | 4.3 ± 3.5 b | 28.1 ± 15.3 b |
| FRUITS | |||||
| % infected fruits | |||||
| 30 d | 41 d | 50 d | 68 d | 78 d | |
| Control | nt | nt | nt | 98.3 ± 3.7 a | 95.6 ± 3.9 a |
| Oxa | nt | nt | nt | 67.6 ± 9.1 b | 88.0 ± 14.5 a |
| ZE | nt | nt | nt | 65.9 ± 17.6 b | 88.2 ± 3.9 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen, Y.; Cohen, R. Differential Systemic Translocation of Oxathiapiprolin, Benthiavalicarb, and Their Mixture to Tomato Leaves and Fruits as Evidenced by Their Differential Protection from Late Blight Caused by Phytophthora infestans. Horticulturae 2025, 11, 1050. https://doi.org/10.3390/horticulturae11091050
Cohen Y, Cohen R. Differential Systemic Translocation of Oxathiapiprolin, Benthiavalicarb, and Their Mixture to Tomato Leaves and Fruits as Evidenced by Their Differential Protection from Late Blight Caused by Phytophthora infestans. Horticulturae. 2025; 11(9):1050. https://doi.org/10.3390/horticulturae11091050
Chicago/Turabian StyleCohen, Yigal, and Reut Cohen. 2025. "Differential Systemic Translocation of Oxathiapiprolin, Benthiavalicarb, and Their Mixture to Tomato Leaves and Fruits as Evidenced by Their Differential Protection from Late Blight Caused by Phytophthora infestans" Horticulturae 11, no. 9: 1050. https://doi.org/10.3390/horticulturae11091050
APA StyleCohen, Y., & Cohen, R. (2025). Differential Systemic Translocation of Oxathiapiprolin, Benthiavalicarb, and Their Mixture to Tomato Leaves and Fruits as Evidenced by Their Differential Protection from Late Blight Caused by Phytophthora infestans. Horticulturae, 11(9), 1050. https://doi.org/10.3390/horticulturae11091050








