Postharvest Quality Maintenance of Traditional Serbian Peppers: The Impact of Heat Treatment and Storage Temperature
Abstract
1. Introduction
2. Materials and Methods
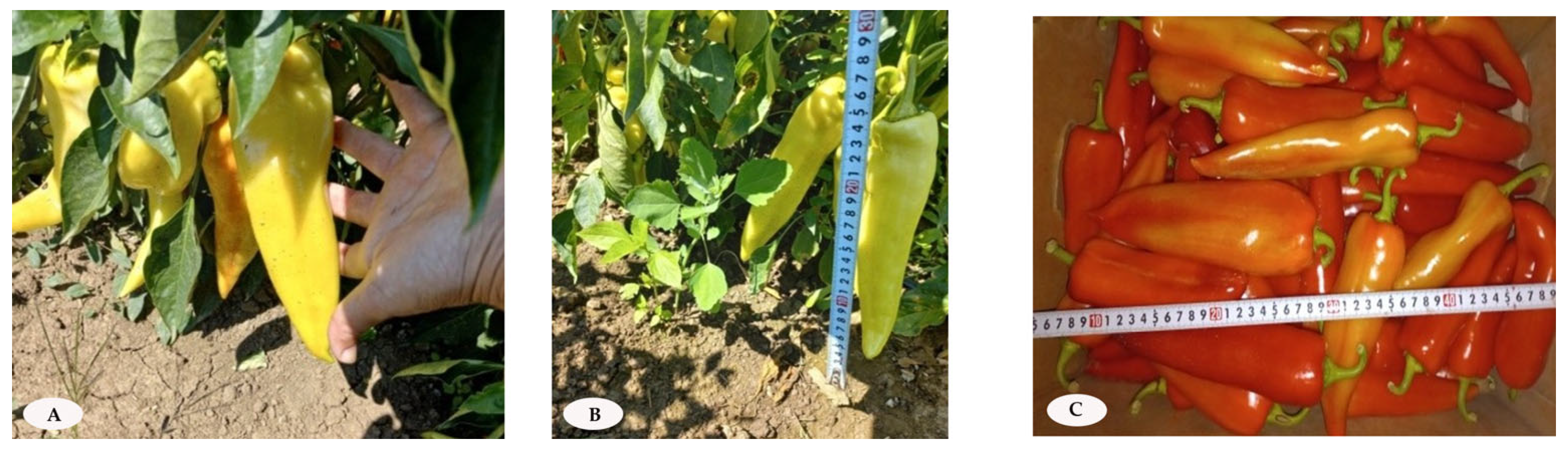
2.1. Plant Material and Cultivation
2.2. Experimental Design
2.3. Weight Loss
2.4. Visual Quality Assessment
2.5. Color
- L* = lightness;
- a* = red/green value;
- b* = yellow/blue value;
- Number 1—the reference/initial color measurement;
- Number 2—the second color measurement.
2.6. Analysis of Fruit Anatomy
2.7. Chemical Analysis
2.8. Statistical Analysis
3. Results
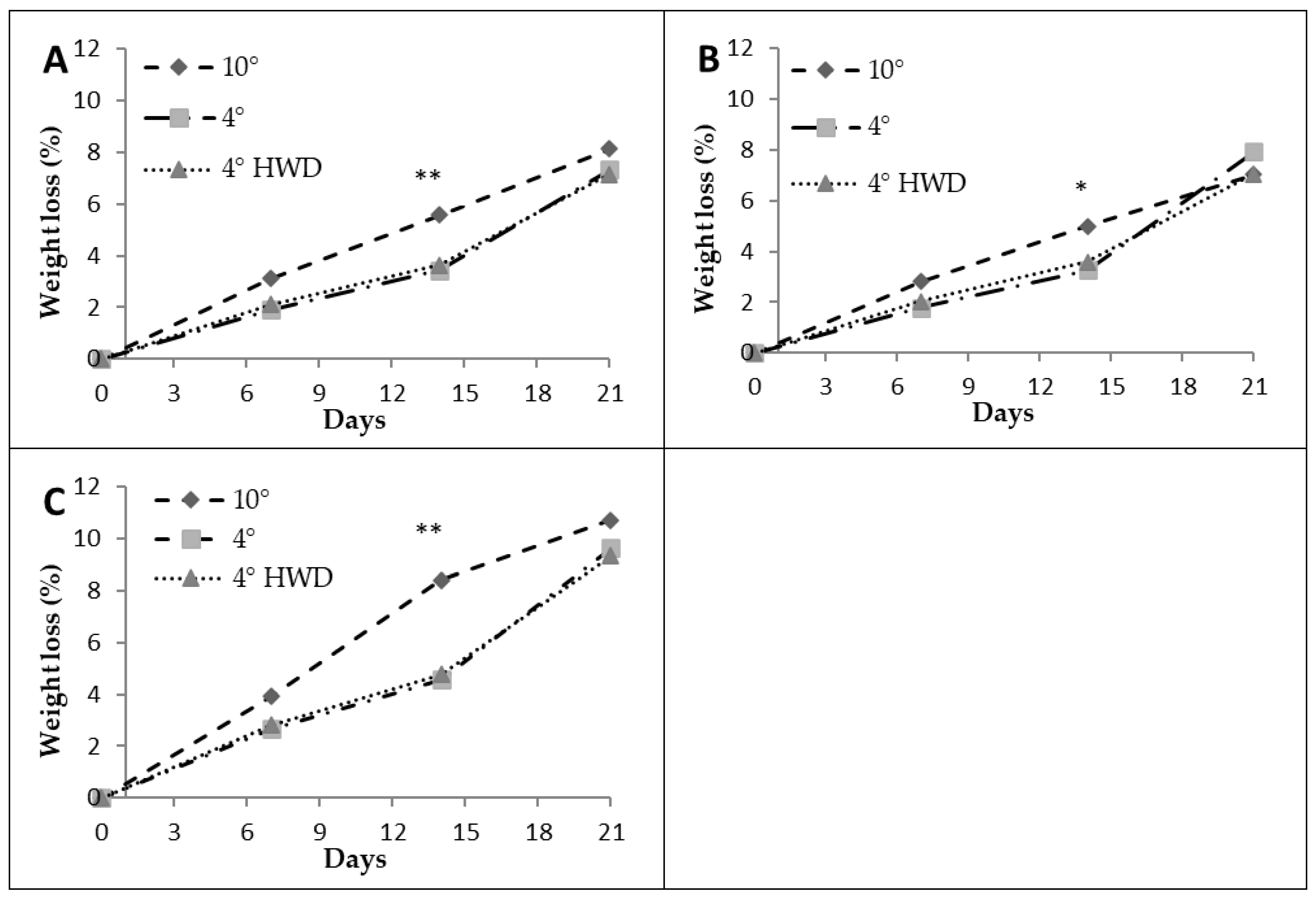
3.1. Weight Loss
3.2. Visual Quality Assessment
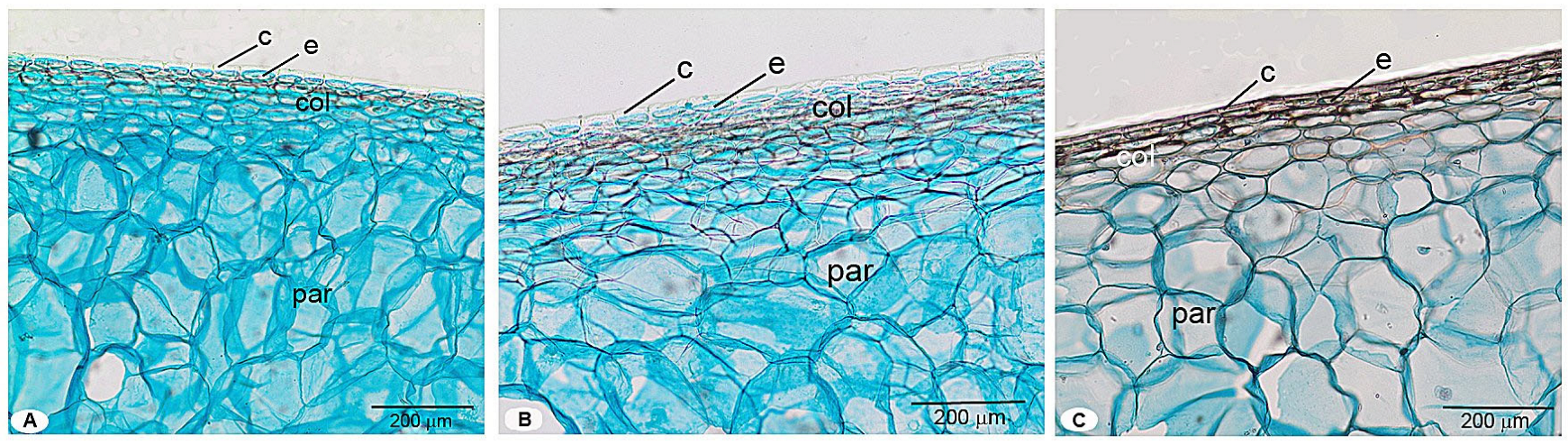
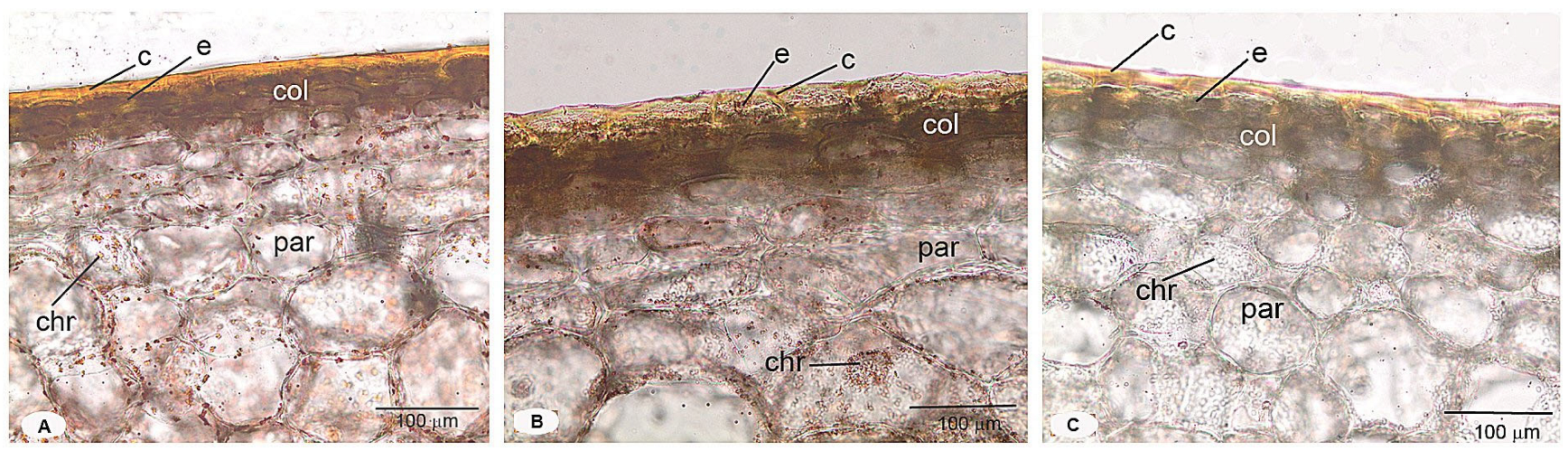
3.3. Comparative Histological Analysis of Pericarp
3.4. Color Changes
3.5. Fruit Composition
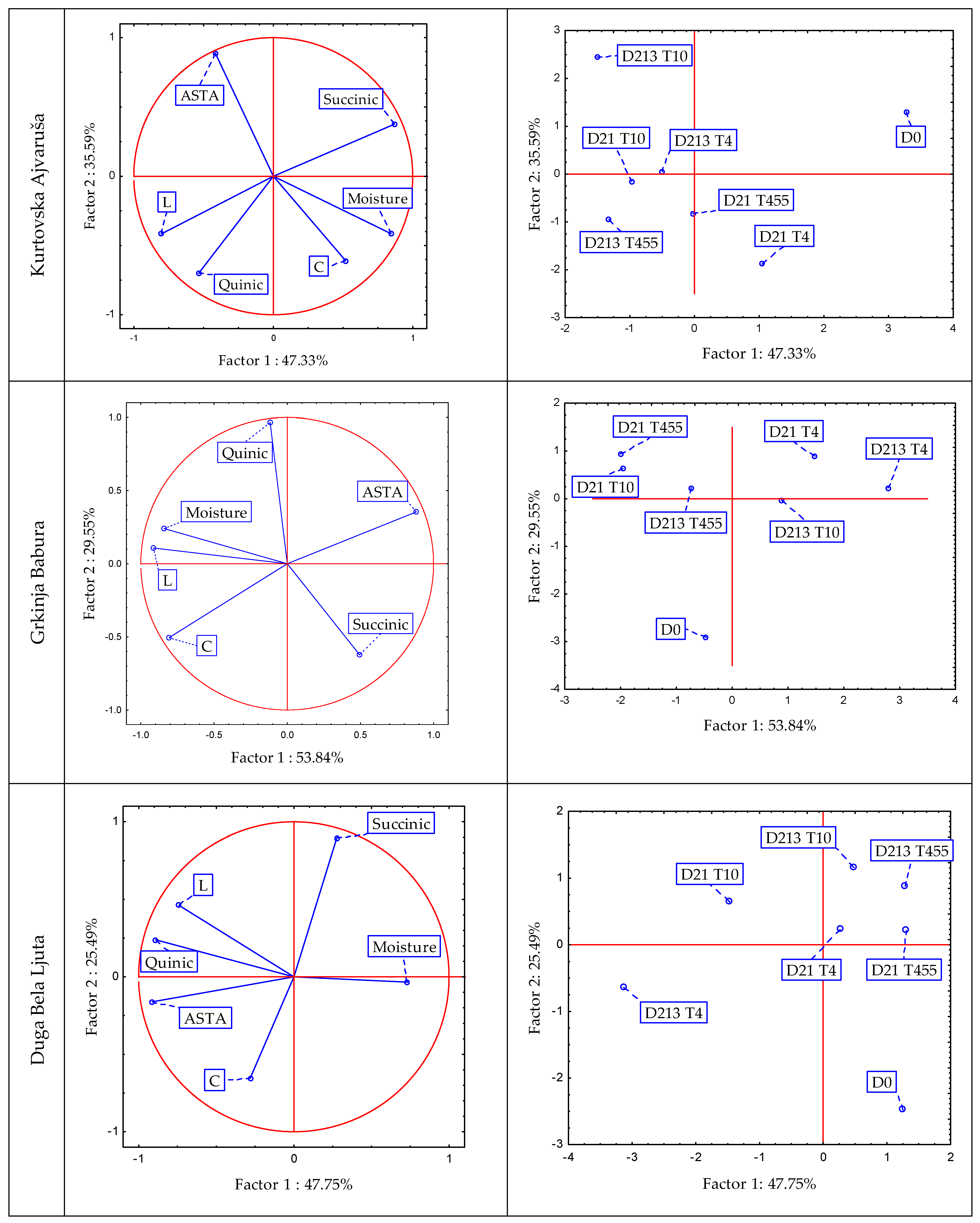
3.6. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bosland, P.W.; Votava, E.J. Peppers: Vegetable and Spice Capsicums, 2nd ed.; CABI: Ascot, UK, 2012. [Google Scholar] [CrossRef]
- Hernández-Pérez, T.; Gómez-García, M.D.R.; Valverde, M.E.; Paredes-López, O. Capsicum annuum (hot pepper): An ancient Latin-American crop with outstanding bioactive compounds and nutraceutical potential. A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2972–2993. [Google Scholar] [CrossRef]
- Lazo, C.J.; Jerusalem, E.; Conejos, G.A.; Malabanan, J.A.; Gallego, M. Consumer quality preferences: Parameters to accelerate bell pepper imaging and classification technology. BIO Web Conf. 2024, 144, 01003. [Google Scholar] [CrossRef]
- Cheema, A.; Padmanabhan, P.; Amer, A.; Parry, M.J.; Lim, L.T.; Subramanian, J.; Paliyath, G. Postharvest hexanal vapor treatment delays ripening and enhances shelf life of greenhouse grown sweet bell pepper (Capsicum annum L.). Postharvest Biol. Technol. 2018, 136, 80–89. [Google Scholar] [CrossRef]
- Tripodi, P.; Rabanus-Wallace, M.T.; Barchi, L.; Kale, S.; Esposito, S.; Acquadro, A.; Schafleitner, R.; van Zonneveld, M.; Prohens, J.; Diez, M.J.; et al. Global range expansion history of pepper (Capsicum spp.) revealed by over 10,000 genebank accessions. Proc. Natl. Acad. Sci. USA 2021, 118, e2104315118. [Google Scholar] [CrossRef]
- Mladenović, J.; Pavlović, N.; Marjanović, M.; Tomić, D.; Grubišić, M.; Zornić, V.G.; Zdravković, J. Breeding potential of morphological and phytochemical characteristics of landraces and autochthone varieties of of landraces and autochthone varieties of of landraces and autochthone varieties of of landraces and autochthone varieties of Capsicum annuum L. in Republic of Serbia. Not. Bot. Horti Agrobot. Cluj-Napoca 2024, 52, 13435. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Kevrešan, Ž.; Mastilović, J.; Zorić, L.; Tomšik, A.; Belović, M.; Pestorić, M.; Karanović, D.; Luković, J. Evaluation of mineral profile, texture, sensory and structural characteristics of old pepper landraces. J. Food Process. Preserv. 2017, 41, e13141. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Milenković, L.; Vasić, M.; Girek, Z.; Zdravković, M.; Zdravković, J. Old cultivars and populations from traditional pepper-growing regions of Serbia as breeding potential. J. Agric. Sci. 2013, 5, 132–140. [Google Scholar] [CrossRef]
- Eggink, P.M.; Maliepaard, C.; Tikunov, Y.; Haanstra, J.P.W.; Bovy, A.G.; Visser, R.G.F. A taste of sweet pepper: Volatile and non-volatile chemical composition of fresh sweet pepper (Capsicum annuum) in relation to sensory evaluation of taste. Food Chem. 2012, 132, 301–310. [Google Scholar] [CrossRef]
- García-Vásquez, R.; Vera-Guzmán, A.M.; Carrillo-Rodríguez, J.C.; Pérez-Ochoa, M.L.; Aquino-Bolaños, E.N.; Alba-Jiménez, J.E.; Chávez-Servia, J.L. Bioactive and nutritional compounds in fruits of pepper (Capsicum annuum L.) landraces conserved among indigenous communities from Mexico. AIMS Agric. Food 2023, 8, 832–850. [Google Scholar] [CrossRef]
- Kader, A.A. Postharvest Technology of Horticultural Crops, 3rd ed.; Agriculture and Natural Resources, University of California: Oakland, CA, USA, 2002; Volume 3311. [Google Scholar]
- Bar-Yosef, A.; Alkalai-Tuvia, S.; Perzelan, Y.; Aharon, Z.; Ilić, Z.; Lurie, S.; Fallik, E. Effect of shrink packaging in combination with rinsing and brushing treatment on chilling injury and decay of sweet pepper during storage. Adv. Hortic. Sci. 2009, 23, 225–230. [Google Scholar]
- Fallik, E.; Ilić, Z. Hot water treatments. In Novel Postharvest Treatments of Fresh Produce, 1st ed.; Pareek, S., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 241–258. [Google Scholar] [CrossRef]
- Fallik, E.; Ilic, Z. Positive and negative effects of heat treatment on the incidence of physiological disorders in fresh produce. In Postharvest Physiological Disorders in Fruits and Vegetables, 1st ed.; Tonetto de Freitas, S., Pareek, S., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 111–126. [Google Scholar] [CrossRef]
- Fallik, E.; Ilic, Z. Pre- and postharvest treatments affecting flavor quality of fruits and vegetables. In Preharvest Modulation of Postharvest Fruit and Vegetable Quality, 1st ed.; Siddiqui, M.W., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 139–168. [Google Scholar] [CrossRef]
- Endo, H.; Miyazaki, K.; Ose, K.; Imahori, Y. Hot water treatment to alleviate chilling injury and enhance ascorbate-glutathione cycle in sweet pepper fruit during postharvest cold storage. Sci. Hortic. 2019, 257, 108715. [Google Scholar] [CrossRef]
- Fallik, E.; Grinberg, S.; Alkalai, S.; Yekutieli, O.; Wiseblum, A.; Regev, R.; Beres, H.; Bar-Lev, E. A unique rapid hot water treatment to improve storage quality of sweet pepper. Postharvest Biol. Technol. 1999, 15, 25–32. [Google Scholar] [CrossRef]
- Kantakhoo, J.; Imahori, Y. Antioxidative responses to pre-storage hot water treatment of red sweet pepper (Capsicum annuum L.) fruit during cold storage. Foods 2021, 10, 3031. [Google Scholar] [CrossRef]
- Kovač, R.; Kevrešan, Ž.; Ilić, Z.; Milenković, L.; Tubić, L.; Ubibarip Samek, D.; Đerić, M. Preserving traditional cultivar “Duga Bela” pepper: The efficacy of hot water dipping. In Proceedings of the e-Proceedings: 5th International Congress: Food Technology, Quality and Safety, Novi Sad, Serbia, 16–18 October 2024; pp. 145–151. [Google Scholar]
- Fallik, E.; Grinberg, S.; Alkalai, S.; Lurie, S. The effectiveness of postharvest hot water dipping on the control of grey and black moulds in sweet red pepper (Capsicum annuum). Plant Pathol. 1996, 45, 644–649. [Google Scholar] [CrossRef]
- Sakaldas, M.; Kaynas, K. Biochemical and quality parameters changes of green sweet bell peppers as affected by different postharvest treatments. Afr. J. Biotechnol. 2010, 9, 8174–8181. [Google Scholar] [CrossRef]
- Melgarejo, P.; Calín-Sánchez, Á.; Carbonell-Barrachina, Á.A.; Martínez-Nicolás, J.J.; Legua, P.; Martínez, R.; Hernández, F. Antioxidant activity, volatile composition and sensory profile of four new very-early apricots (Prunus armeniaca L.). J. Sci. Food Agric. 2014, 94, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Milović, M.; Kevrešan, Ž.; Mastilović, J.; Kovač, R.; Kalajdžić, J.; Magazin, N.; Bajić, A.; Milić, B.; Barać, G.; Keserović, Z. Could an early treatment with GA and BA impact prolonged cold storage and shelf life of apricot? Horticulturae 2022, 8, 1220. [Google Scholar] [CrossRef]
- Nunes, N.; Cecilia, M.; Jean-Pierre, E. Relationship between weight loss and visual quality of fruits and vegetables. Proc. Fla. State Hortic. Soc. 2007, 120, 235–245. [Google Scholar]
- Lakušić, B.; Stevanović, B.; Jančić, R.; Lakušić, D. Habitat-related adaptations in morphology and anatomy of Teucrium (Lamiaceae) species from the Balkan peninsula (Serbia and Montenegro). Flora 2010, 205, 633–646. [Google Scholar] [CrossRef]
- Parsons, E.P.; Popopvsky, S.; Lohrey, G.T.; Lü, S.; Alkalai-Tuvia, S.; Perzelan, Y.; Paran, I.; Fallik, E.; Jenks, M.A. Fruit cuticle lipid composition and fruit post-harvest water loss in an advanced backcross generation of pepper (Capsicum sp.). Physiol. Plant. 2012, 146, 15–25. [Google Scholar] [CrossRef]
- Lara, I.; Belge, B.; Goulao, L.F. The fruit cuticle as a modulator of postharvest quality. Postharvest Biol. Technol. 2014, 87, 103–112. [Google Scholar] [CrossRef]
- Konishi, A.; Terabayashi, S.; Itai, A. Relationship of cuticle development with water loss and texture of pepper fruit. Can. J. Plant Sci. 2021, 102, 103–111. [Google Scholar] [CrossRef]
- Usman, M.G.; Rafii, M.Y.; Ismail, M.R.; Malek, M.A.; Latif, M.A. Expression of target gene Hsp70 and membrane stability determine heat tolerance in chili pepper. J. Am. Soc. Hort. Sci. 2015, 140, 144–150. [Google Scholar] [CrossRef]
- Gonzalez, M.E.; Barrett, D.M. Thermal, high pressure, and electric field processing effects on plant cell membrane integrity and relevance to fruit and vegetable quality. J. Food Sci. 2010, 75, 121–130. [Google Scholar] [CrossRef]
- Fallik, E.; Aharoni, Y.; Grinberg, S.; Copel, A.; Klein, J.D. Postharvest hydrogen peroxide treatment inhibits decay in eggplant and sweet red pepper. Crop Prot. 1994, 13, 451–454. [Google Scholar] [CrossRef]
- Fallik, E.; Grinberg, S.; Ziv, O. Potassium bicarbonate reduces postharvest decay development on bell pepper fruits. J. Hortic. Sci. 1997, 72, 35–41. [Google Scholar] [CrossRef]
- Han, Y.; Floros, J.D.; Linton, R.H.; Nielsen, S.S.; Nelson, P.E. Response Surface Modeling for the Inactivation of Escherichia coli O157:H7 on Green Peppers (Capsicum annuum L.) by Chlorine Dioxide Gas Treatments. J. Food Prot. 2001, 64, 1128–1133. [Google Scholar] [CrossRef]
- Yuk, H.G.; Bartz, J.A.; Schneider, K.R. The effectiveness of sanitizer treatments in inactivation of Salmonella spp. from bell pepper, cucumber, and strawberry. J. Food Sci. 2006, 71, M95–M99. [Google Scholar] [CrossRef]
- Alvarado-Casillas, S.; Ibarra-Sánchez, S.; Rodríguez-García, O.; Martínez-González, N.; Castillo, A. Comparison of rinsing and sanitizing procedures for reducing bacterial pathogens on fresh cantaloupes and bell peppers. J. Food Prot. 2007, 70, 655–660. [Google Scholar] [CrossRef] [PubMed][Green Version]
- González-Aguilar, G.A.; Gayosso, L.; Cruz, R.; Fortiz, J.; Baez, R.; Wang, C.Y. Polyamines induced by hot water treatments reduce chilling injury and decay in pepper fruit. Postharvest Biol. Technol. 2000, 18, 19–26. [Google Scholar] [CrossRef]
- Raffo, A.; Baiamonte, I.; Nardo, N.; Paoletti, F. Internal quality and antioxidants content of cold-stored red sweet peppers as affected by polyethylene bag packaging and hot water treatment. Eur. Food Res. Technol. 2007, 225, 395–405. [Google Scholar] [CrossRef]
- Feng, Y.; Zhao, L.; Xia, Y.; Dai, L. Unveiling the dual impacts of the aesthetic deficiency of foods on consumers’ purchase intentions. Sci. Rep. 2025, 15, 11218. [Google Scholar] [CrossRef]
- Grzegorzewska, M.; Machlanska, A. The Post-Cutting Hot Water Treatment of Pepper Fruit: Impact on Quality During Short-Term Storage. Agronomy 2025, 15, 1406. [Google Scholar] [CrossRef]
- Tiamiyu, Q.O.; Adebayo, S.E.; Ibrahim, N. Recent advances on postharvest technologies of bell pepper: A review. Heliyon 2023, 9, e15302. [Google Scholar] [CrossRef]
- Maalekuu, K.; Elkind, Y.; Tuvia-Alkalai, S.; Shalom, Y.; Fallik, E. The influence of harvest season and cultivar type on several quality traits and quality stability of three commercial sweet bell peppers during the harvest period. Adv. Hortic. Sci. 2004, 18, 21–25. [Google Scholar]
- Ilić, Z.S.; Šunić, L.; Fallik, E. Quality evaluation and antioxidant activity of mini sweet pepper cultivars during storage in modified atmosphere packaging (MAP). Rom. Biotechnol. Lett. 2017, 22, 12214–12223. [Google Scholar]
- Majomot, A.M.C.C.; Bayogan, E.R.V. Effect of Hot Water Treatment and Evaporative Cooling on Some Postharvest Characteristics of Sweet Pepper (Capsicum annuum cv. ‘Sweet Cayenne’). Mindanao J. Sci. Technol. 2019, 17, 71–83. [Google Scholar]
- Abdullah, M.A. Enhancement of Sweet Pepper Fruits Quality and Storability by Some Postharvest. Ann. Agric. Sci. Moshtohor. 2019, 57, 447–454. [Google Scholar] [CrossRef]
- Selahle, K.M.; Sivakumar, D.; Jifon, J.; Soundy, P. Postharvest responses of red and yellow sweet peppers grown under photo-selective nets. Food Chem. 2015, 173, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Luning, P.A.; Yuksel, D.; de Vries, R.V.; Roozen, J.P. Aroma changes in fresh bell peppers (Capsicum annuum) after hot-air drying. J. Food Sci. 1994, 59, 1048–1053. [Google Scholar] [CrossRef]
- Estrada, B.; Pomar, F.; Díaz, J.; Merino, F.; Bernal, M.A. Pungency level in fruits of the Padrón pepper with different water supply. Sci. Hortic. 1999, 81, 385–396. [Google Scholar] [CrossRef]


| Traditional Cultivar | Storage Condition | Storage Duration | ||||||
|---|---|---|---|---|---|---|---|---|
| 14 Days | 21 Days | |||||||
| Chilling Injury (%) | Decay Incidence (%) | Weight Loss (%) | Chilling Injury (%) | Decay Incidence (%) | Weight Loss (%) | General Appearance | ||
| Kurtovska Ajvaruša | 10 °C | 0 | 2 | 3.9 | 0 | 10.5 | 6.5 | 2.3 |
| 4 °C | 0.5 | 0 | 2.2 | 5.0 | 3.0 | 2.6 | 3.0 | |
| 4 °C + HWD | 0 | 0 | 2.5 | 1.2 | 0 | 2.9 | 3.4 | |
| Grkinja Babura | 10 °C | 0 | 2.3 | 5.0 | 0 | 20.0 | 8.0 | 1.5 |
| 4 °C | 0.5 | 0 | 2.5 | 8.0 | 7.2 | 3.0 | 2.6 | |
| 4 °C + HWD | 0 | 0 | 2.8 | 2.0 | 2 | 3.4 | 2.8 | |
| Duga Bela Ljuta | 10 °C | 0 | 1.8 | 4.2 | 0 | 16.7 | 7.5 | 1.7 * |
| 4 °C | 1.0 | 0 | 2.1 | 7.0 | 5.0 | 2.8 | 3.0 | |
| 4 °C + HWD | 0 | 0 | 2.4 | 1.5 | 1.1 | 3.0 | 3.1 | |
| L* | C | ΔE* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Days | Kurtovska Ajvaruša | ||||||||
| 0 | 35.6 ab | 32.7 a | 36.6 b | 34.9 abcde | 34.4 abcd | 30.6 a | N.D. | N.D. | N.D. |
| 10 °C | 4 °C | 4 °C HWD | 10 °C | 4 °C | 4 °C HWD | 10 °C | 4 °C | 4 °C HWD | |
| 21 | 36.3 b | 36.3 b | 36.9 bc | 33.8 abc | 34.2 bcde | 32.5 ab | 4.5 | 1.0 | 3.2 |
| 21 + 3 | 36.2 bcd | 37.1 b | 36.3 ab | 30.6 a | 33.1 abc | 32.0 ab | 6.7 | 2.6 | 3.7 |
| Grkinja Babura | |||||||||
| 0 | 40.5 cdef | 42.7 efg | 43.7 fg | 47.8 k | 43.5 ij | 44.5 k | N.D. | N.D. | N.D. |
| 10 °C | 4 °C | 4 °C HWD | 10 °C | 4 °C | 4 °C HWD | 10 °C | 4 °C | 4 °C HWD | |
| 21 | 40.9 ef | 44.2 fg | 45.5 g | 39.3 efghi | 42.7 hij | 42.7 hij | 10.6 | 3.2 | 3.8 |
| 21 + 3 | 40.5 cdef | 43.3 fg | 44.3 fg | 36.1 bcde | 38.4 defgh | 40.8 fghij | 13.7 | 6.8 | 6.1 |
| DugaBela Ljuta | |||||||||
| 0 | 40.4 cde | 38.9 bcde | 40.7 cde | 41.1 ghij | 35.7 bcde | 38.5 defgh | N.D. | N.D. | N.D. |
| 10 °C | 4 °C | 4 °C HWD | 10 °C | 4 °C | 4 °C HWD | 10 °C | 4 °C | 4 °C HWD | |
| 21 | 43.6 ef | 40.9 ef | 41.4 ef | 38.4 defgh | 36.5 bcdef | 37.3 cdefg | 5.1 | 2.4 | 2.4 |
| 21 + 3 | 41.1 de | 42.3 efg | 41.5 ef | 35.0 abcde | 37.2 cdefg | 35.3 bcde | 8.2 | 3.9 | 4.1 |
| Treatment (T) | * | NS | |||||||
| Day (D) | * | ** | |||||||
| Variety (V) | ** | ** | |||||||
| T × D | NS | ** | |||||||
| T × V | * | ** | |||||||
| D × V | NS | NS | |||||||
| T × D × V | NS | NS | |||||||
| Water Content (%) | ASTA Units | |||||
|---|---|---|---|---|---|---|
| Days | Kurtovska Ajvaruša | |||||
| 0 | 92.3 ghi | 18.4 ij | ||||
| 10 °C | 4 °C | 4°C HWD | 10 °C | 4 °C | 4°C HWD | |
| 21 | 91.8 bcd | 92.3 hi | 92.2 fghi | 21.2 k | 12.5 cd | 15.7 fg |
| 21 + 3 | 91.7 bc | 91.9 cde | 91.8 cde | 28.2 l | 19.6 jk | 16.2 fgh |
| Grkinja Babura | ||||||
| 0 | 92.8 j | 10.0 ab | ||||
| 10 °C | 4 °C | 4°C HWD | 10 °C | 4 °C | 4°C HWD | |
| 21 | 92.4 hi | 92.4 hi | 92.7 j | 17.1 ghi | 9.3 a | 11.3 bc |
| 21 + 3 | 92.3 fghi | 92.2 fghi | 92.8 j | 18.0 hij | 12.7 cd | 12.9 cd |
| DugaBela Ljuta | ||||||
| 0 | 92.8 j | 12.9 cde | ||||
| 10 °C | 4 °C | 4°C HWD | 10 °C | 4 °C | 4°C HWD | |
| 21 | 92.1 efgh | 92.0 def | 92.2 fghi | 11.6 bc | 17.9 hij | 14.8 ef |
| 21 + 3 | 91.1 a | 91.6 b | 92.0 defg | 28.6 l | 13.7 de | 11.2 bc |
| Treatment (T) | ** | ** | ||||
| Day (D) | ** | ** | ||||
| Variety (V) | ** | ** | ||||
| T × D | ** | ** | ||||
| T × V | ** | ** | ||||
| D ×V | ** | ** | ||||
| T × D × V | ** | ** | ||||
| Quinic (mg/100 g FW) | Succinic (mg/100 g FW) | |||||
|---|---|---|---|---|---|---|
| Days | Kurtovska Ajvaruša | |||||
| 0 | 13.9 b | 43.7 i | ||||
| 10 °C | 4 °C | 4 °C HWD | 10 °C | 4 °C | 4 °C HWD | |
| 21 | 16.5 def | 17.8 gh | 16.8 ef | 18.5 a | 29.3 gh | 25.0 cdefg |
| 21 + 3 | 15.6 cd | 16.1 de | 20.0 k | 27.8 fgh | 27.6 fgh | 23.2 bcdef |
| Grkinja Babura | ||||||
| 0 | 11.6 a | 29.7 gh | ||||
| 10 °C | 4 °C | 4 °C HWD | 10 °C | 4 °C | 4 °C HWD | |
| 21 | 18.6 hij | 19.2 jk | 19.0 ij | 22.8 abcde | 21.3 abcd | 19.5 ab |
| 21 + 3 | 17.1 fg | 18.2 hij | 18.8 hij | 28.8 gh | 28.3 gh | 29.3 gh |
| DugaBela Ljuta | ||||||
| 0 | 14.6 b | 19.5 ab | ||||
| 10 °C | 4 °C | 4 °C HWD | 10 °C | 4 °C | 4 °C HWD | |
| 21 | 21.5 l | 22.4 l | 18.0 ghi | 23.7 bcdef | 25.6 defgh | 26.5 efgh |
| 21 + 3 | 24.5 m | 18.6 hij | 15.6 cd | 20.9 abc | 26.0 efgh | 25.4 defgh |
| Treatment (T) | ** | * | ||||
| Day (D) | ** | ** | ||||
| Variety (V) | ** | ** | ||||
| T × D | ** | NS | ||||
| T × V | ** | NS | ||||
| D × V | ** | ** | ||||
| T × D × V | ** | ** | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milenković, L.; Ilić, Z.S.; Kevrešan, Ž.; Tubić, L.; Ubiparip Samek, D.; Đerić, M.; Kovač, R. Postharvest Quality Maintenance of Traditional Serbian Peppers: The Impact of Heat Treatment and Storage Temperature. Horticulturae 2025, 11, 1048. https://doi.org/10.3390/horticulturae11091048
Milenković L, Ilić ZS, Kevrešan Ž, Tubić L, Ubiparip Samek D, Đerić M, Kovač R. Postharvest Quality Maintenance of Traditional Serbian Peppers: The Impact of Heat Treatment and Storage Temperature. Horticulturae. 2025; 11(9):1048. https://doi.org/10.3390/horticulturae11091048
Chicago/Turabian StyleMilenković, Lidija, Zoran S. Ilić, Žarko Kevrešan, Ljiljana Tubić, Dragana Ubiparip Samek, Marina Đerić, and Renata Kovač. 2025. "Postharvest Quality Maintenance of Traditional Serbian Peppers: The Impact of Heat Treatment and Storage Temperature" Horticulturae 11, no. 9: 1048. https://doi.org/10.3390/horticulturae11091048
APA StyleMilenković, L., Ilić, Z. S., Kevrešan, Ž., Tubić, L., Ubiparip Samek, D., Đerić, M., & Kovač, R. (2025). Postharvest Quality Maintenance of Traditional Serbian Peppers: The Impact of Heat Treatment and Storage Temperature. Horticulturae, 11(9), 1048. https://doi.org/10.3390/horticulturae11091048








