Recent Advances in Postharvest Physiology and Preservation Technology of Peach Fruit: A Systematic Review
Abstract
1. Introduction
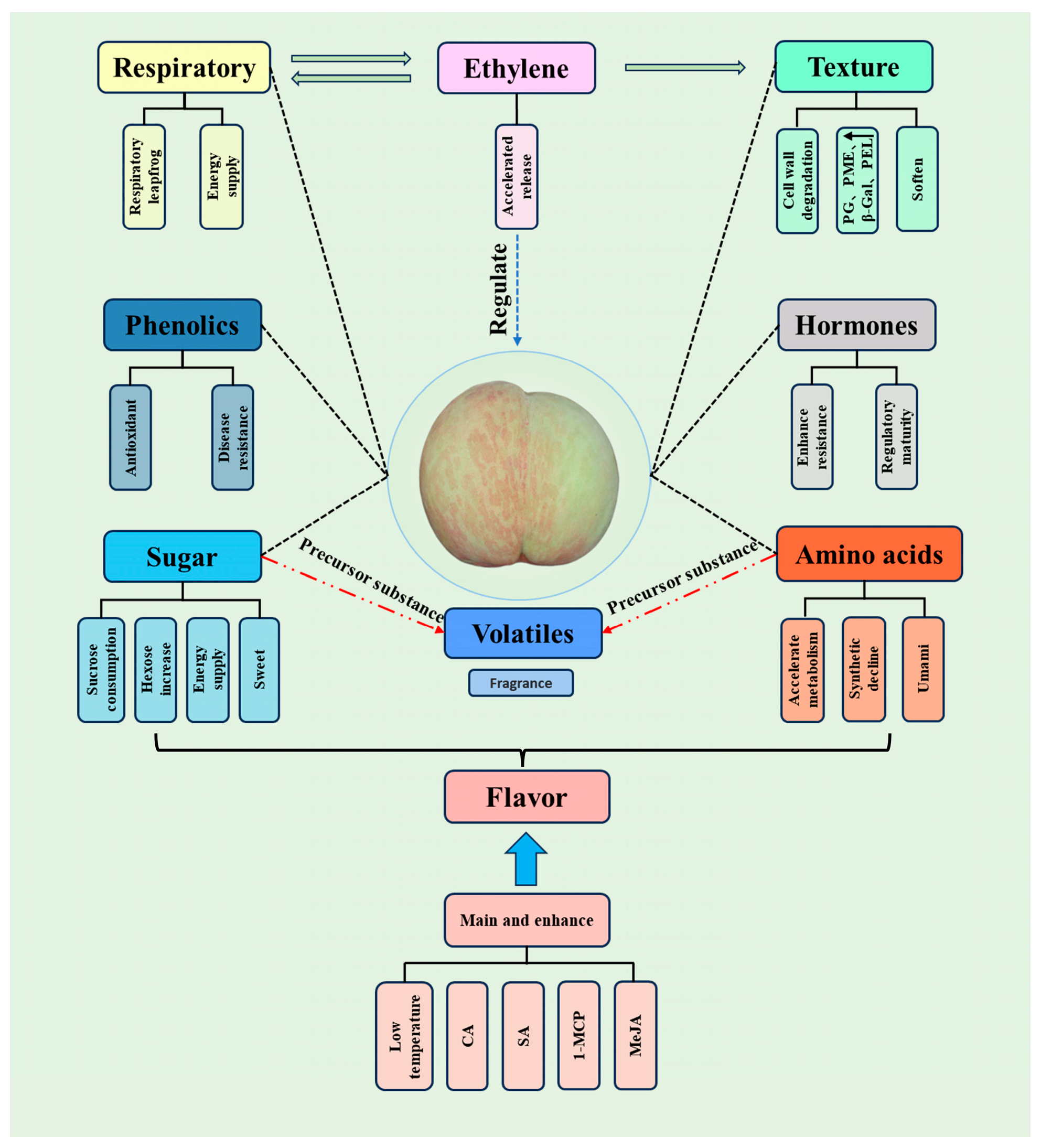
2. Physiological
2.1. Respiration and Ethylene
2.2. Hormone
2.3. Texture
2.4. Nutrition
2.4.1. Sugar
2.4.2. Amino Acid
2.4.3. Phenolics
2.4.4. Volatile
2.5. Flavor
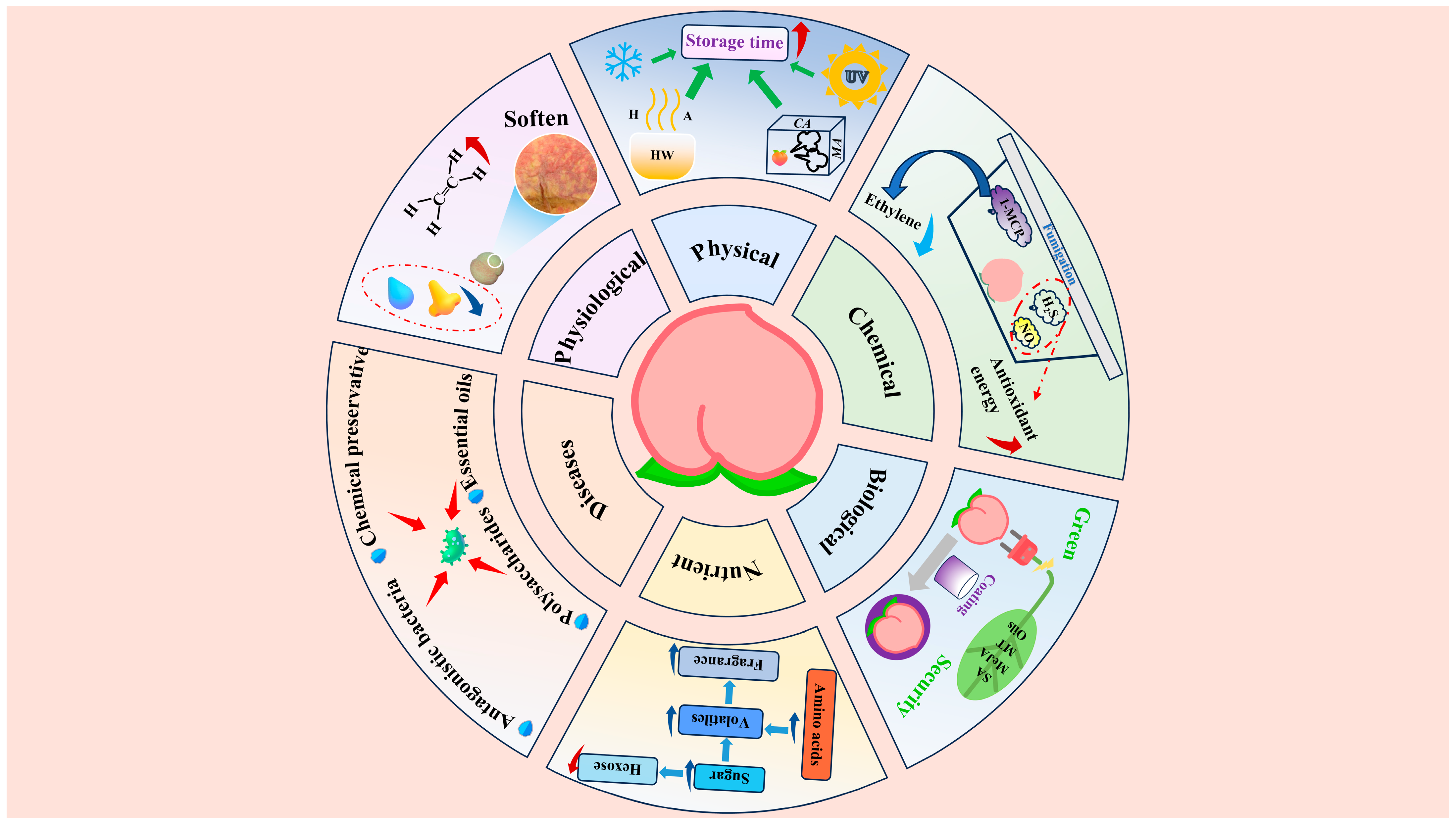
3. Diseases and Control
3.1. Brown Rot
3.2. Soft Rot
3.3. Gray Mold
4. Physical Preservation
4.1. Low-Temperature Storage
4.2. Heat Treatment
4.3. Controlled Atmosphere Storage
4.4. UV Treatment
5. Chemical Preservation
5.1. 1-MCP
5.2. NO
5.3. H2S
6. Biological Preservation
6.1. Endogenous Plant Hormones
6.1.1. Salicylic Acid
6.1.2. Jasmonic Acid
6.1.3. Melatonin
6.2. Essential Oils
6.3. Coating Preservation
7. Summary and Prospects
- Intelligent dynamic monitoring and control: Sensor technology will be deeply integrated into the preservation process, collecting real-time data on temperature, humidity, ethylene concentration, and fruit physiological indicators. Combined with AI predictions of ripeness and disease risk, this will enable automatic optimization of the storage environment. Controlled atmosphere (CA) storage technology will focus on precise control of the O2/CO2 ratio to effectively slow down fruit respiration metabolism.
- Synergistic effects of green preservation technologies: The utilization of natural plant hormones, essential oils, and coating technologies will employ nano-microcapsule encapsulation technology to enhance the sustained release performance of active ingredients. This will reduce material volatilization while extending preservation time. The synergistic application of physical and biological preservation technologies will help further reduce the risk of chemical residues.
- Molecular mechanism analysis and development of storage-resistant varieties: The utilization of multi-omics technologies, encompassing transcriptomics and metabolomics, will facilitate an exhaustive examination of the molecular regulatory networks implicated in postharvest softening, browning, and cold damage in peach fruits. The findings of this research will provide a theoretical basis for the development of storage-resistant varieties and the mitigation of cell wall degradation and membrane lipid peroxidation processes.
- The integration of whole-chain preservation technology is a subject that has been the focus of much recent research. The integration of preharvest cultivation management, harvest maturity grading, pretreatment technology, and low-temperature logistics is imperative to establish an integrated preservation technology system encompassing the entire field–storage–transportation–retail process.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, H.; Huang, X.; Chen, T.; Zhou, P.; Huang, X.; Jin, W.; Liu, D.; Zhang, H.; Zhou, J.; Wang, Z.; et al. Fruit quality prediction based on soil mineral element content in peach orchard. Food Sci. Nutr. 2022, 10, 1756–1767. [Google Scholar] [CrossRef] [PubMed]
- FAO. 2023. Available online: https://www.fao.org/faostat/zh/#data (accessed on 25 March 2025).
- Mihaylova, D.; Popova, A.; Desseva, I.; Manolov, I.; Petkova, N.; Vrancheva, R.; Peltekov, A.; Slavov, A.; Zhivondov, A. Comprehensive Evaluation of Late Season Peach Varieties (Prunus persica L.): Fruit Nutritional Quality and Phytochemicals. Molecules 2021, 26, 2818. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Y.; Kou, X.; Li, J.; Luo, D.; Huang, T.; Wang, X.; Cao, S. Salicylic acid mitigates chilling injury to peaches by improving antioxidant capacity and energy metabolism. Sci. Hortic. 2024, 338, 113841. [Google Scholar] [CrossRef]
- An, X.; Xu, Y.; Jiang, L.; Huan, C.; Yu, Z. Effects of postharvest temperature on apoptosis-related enzyme activity and gene expression in peach fruits (Prunus persica L. cv. Xiahui 8). Sci. Hortic. 2019, 245, 178–184. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, J.; Wei, H.; Ding, Z.; Li, X.; Liu, Z.; Wang, H.; Wang, Y. Biological control efficacy of Bacillus licheniformis HG03 against soft rot disease of postharvest peach. Food Control 2023, 145, 109402. [Google Scholar] [CrossRef]
- Abdipour, M.; Hosseinifarahi, M.; Naseri, N. Combination method of UV-B and UV-C prevents post-harvest decay and improves organoleptic quality of peach fruit. Sci. Hortic. 2019, 256, 108564. [Google Scholar] [CrossRef]
- Hanif, M.; Azam, M.; Khan, M.A.; Zahid, T.; Ali, B.; Akram, M.T.; Naveed, K.; Ilahy, R.; Hussai, I.; Liu, H. Investigating the effect of marigold flower extract on bioactive compounds, antioxidants and postharvest quality and shelf life of peach during storage. J. Food Meas. Charact. 2025, 19, 1837–1850. [Google Scholar] [CrossRef]
- Huan, C.; Han, S.; Jiang, L.; An, X.; Yu, Z. Postharvest hot air and hot water treatments affect the antioxidant system in peach fruit during refrigerated storage. Postharvest Biol. Technol. 2017, 126, 1–14. [Google Scholar] [CrossRef]
- Dong, X.; He, Y.; Yuan, C.; Cheng, X.; Li, G.; Shan, Y.; Zhu, X. Controlled Atmosphere Improves the Quality, Antioxidant Activity and Phenolic Content of Yellow Peach during the Shelf Life. Antioxidants 2022, 11, 2278. [Google Scholar] [CrossRef]
- Han, S.; Wang, X.; Cong, H.; Wu, Y.; Cai, H. Assessment of quality and antioxidant capacity of peach in response to different UV-C dose irradiation. J. Food Sci. 2024, 89, 8900–8909. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.; Ge, S.; Xiao, G.; Chen, F.; Ding, S.; Wang, R. 1-MCP treatment improves the postharvest quality of Jinxiu yellow peach by regulating cuticular wax composition and gene expression during cold storage. J. Food Sci. 2024, 89, 2787–2802. [Google Scholar] [CrossRef]
- Liu, S.; Jing, G.; Zhu, S. Nitric oxide (no) involved in antioxidant enzyme gene regulation to delay mitochondrial damage in peach fruit. Postharvest Biol. Technol. 2022, 192, 111993. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, D.; Zhao, L.; Luo, Y.; Li, J.; Xie, B. Hydrogen sulfide retards fruit softening and prevents flesh browning in cold-stored peaches by regulating cell wall-modifying enzymes, phenolic, and proline metabolism. Postharvest Biol. Technol. 2024, 207, 13. [Google Scholar] [CrossRef]
- Chen, M.; Guo, H.; Chen, S.; Li, T.; Li, M.; Rashid, A.; Xu, C.; Wang, K. Methyl Jasmonate Promotes Phospholipid Remodeling and Jasmonic Acid Signaling to Alleviate Chilling Injury in Peach Fruit. J. Agric. Food Chem. 2019, 67, 9958–9966. [Google Scholar] [CrossRef]
- Bao, Z.; Zhou, Q.; Yu, Y.; Chen, W.; Yang, Z.; Cao, S. Melatonin treatment induces dna methylation to alleviate chilling induced-browning in cold stored peach fruit. Postharvest Biol. Technol. 2024, 208, 112686. [Google Scholar] [CrossRef]
- Wang, X.; Pan, L.; Wang, Y.; Meng, J.; Deng, L.; Niu, L.; Liu, H.; Ding, Y.; Yao, J.; Nieuwenhuizen, N. PpIAA1 and PpERF4 form a positive feedback loop to regulate peach fruit ripening by integrating auxin and ethylene signals. Plant Sci. 2021, 313, 111084. [Google Scholar] [CrossRef]
- Qin, Q.; Wang, L.; Wang, Q.; Wang, R.; Li, C.; Qiao, Y.; Liu, H. Postharvest Flavor Quality Changes and Preservation Strategies for Peach Fruits: A Comprehensive Review. Plants 2025, 14, 1310. [Google Scholar] [CrossRef]
- Abeles, F.B.; Morgan, P.W.; Saltveit, M.E. Ethylene in Plant Biology; Academic Press: Cambridge, MA, USA, 1992. [Google Scholar]
- Wang, X.; Wen, H.; Suprun, A.; Zhu, H. Ethylene Signaling in Regulating Plant Growth, Development, and Stress Responses. Plants 2025, 14, 309. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Liu, C.J.; Zhang, C.; Shi, K.; Wang, M.; Wang, Y.; Song, Q.; Chen, X.; Jin, P. Hydrogen sulfide alleviates chilling injury by modulating respiration and energy metabolisms in cold-stored peach fruit. Postharvest Biol. Technol. 2023, 199, 112291. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, H.; Xu, X.; Yu, Z.F. Postharvest application of methyl jasmonate inhibited ethylene biosynthesis and signaling in peach through activating the negative feedback of JA-signaling pathway. Postharvest Biol. Technol. 2024, 202, 112965. [Google Scholar] [CrossRef]
- Onik, J.C.; Wai, S.C.; Li, A.; Lin, Q.; Sun, Q.; Wang, Z.; Duan, Y. Melatonin treatment reduces ethylene production and maintains fruit quality in apple during postharvest storage. Food Chem. 2021, 337, 127753. [Google Scholar] [CrossRef]
- Li, T.; Jiang, Z.; Zhang, L.; Tan, D.; Wei, Y.; Yuan, H.; Li, T.; Wang, A. Apple (Malus domestica) MdERF2 negatively affects ethylene biosynthesis during fruit ripening by suppressing MdACS1 transcription. Plant J. 2016, 88, 735–748. [Google Scholar] [CrossRef]
- Gu, C.; Guo, Z.H.; Cheng, H.Y.; Zhou, Y.H.; Qi, K.J.; Wang, G.M.; Zhang, S.L. A HD-ZIP II HOMEBOX transcription factor, PpHB.G7, mediates ethylene biosynthesis during fruit ripening in peach. Plant Sci. 2019, 278, 12–19. [Google Scholar] [CrossRef]
- Wu, X.; Yuan, J.; Wang, X.; Yu, M.; Ma, R.; Yu, Z. Synergy of Nitric Oxide and 1-Methylcyclopropene Treatment in Prolong Ripening and Senescence of Peach Fruit. Foods 2021, 10, 2956. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, H.; Dai, X.; Yu, M.; Yu, Z. Effect of methyl jasmonate on the quality and antioxidant capacity by modulating ascorbate-glutathione cycle in peach fruit. Sci. Hortic. 2022, 303, 111216. [Google Scholar] [CrossRef]
- Wolters, H.; Jürgens, G. Survival of the flexible: Hormonal growth control and adaptation in plant development. Nat. Rev. Genet. 2009, 10, 305–317. [Google Scholar] [CrossRef]
- Seo, D.H.; Jeong, H.; Choi, Y.D.; Jang, G. Auxin controls the division of root endodermal cells. Plant Physiol. 2021, 187, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Yamamuro, C. Interplays between auxin and GA signaling coordinate early fruit development. Hortic. Res. 2022, 9, uhab078. [Google Scholar] [CrossRef] [PubMed]
- Leyser, O. Auxin Signaling. Plant Physiol. 2018, 176, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Bao, Z.; Yu, Y.; Chen, W.; Yang, Z.; Cao, S. Iaa regulated levels of endogenous phytohormones in relation to chilling tolerance in cold-stored peaches after harvest. Postharvest Biol. Technol. 2023, 205, 112490. [Google Scholar] [CrossRef]
- Dong, N.G.; Qi, J.X.; Li, Y.F.; Chen, Y.H.; Hao, Y.B. Effects of Abscisic Acid and Nitric Oxide on Chilling Resistance and Activation of the Antioxidant System in Walnut Shoots In Vitro. J. Am. Soc. Hortic. Sci. 2017, 142, 376–384. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Falagán, N.; Giné-Bordonaba, J.; Wójcik, D.A.; Terry, L.A.; Alamar, M.C. Cultivar and tissue-specific changes of abscisic acid, its catabolites and individual sugars during postharvest handling of flat peaches (Prunus persica cv. Platycarpa). Postharvest Biol. Technol. 2021, 181, 111688. [Google Scholar] [CrossRef]
- Wang, P.; Lu, S.; Zhang, X.; Hyden, B.; Qin, L.; Liu, L.; Bai, Y.; Han, Y.; Wen, Z.; Xu, J.; et al. Double NCED isozymes control ABA biosynthesis for ripening and senescent regulation in peach fruits. Plant Sci. 2021, 304, 110739. [Google Scholar] [CrossRef]
- Lu, D.; Xu, M.; Li, Y.; He, X.; Cao, J.; Zhu, C.; Sun, C.; Jia, H.; Li, S. PpGATA4 mediates fruit softening and transcriptionally regulates PpEXPA1 in peach (Prunus persica). Plant Sci. 2025, 352, 112341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.H.; Zhang, Y.; Yang, T.M.; Luo, D.L.; Zhao, Z.B.; Ba, L.J.; Xu, S.; Li, J.; Cao, S. Physiological and transcriptomic analysis of salicylic acid on membrane lipid metabolism and cell wall metabolism in postharvest peach fruit. Postharvest Biol. Technol. 2025, 229, 113683. [Google Scholar] [CrossRef]
- Tatsuki, M.; Sawamura, Y.; Yaegaki, H.; Suesada, Y.; Nakajima, N. The storage temperature affects flesh firmness and gene expression patterns of cell wall-modifying enzymes in stony hard peaches. Postharvest Biol. Technol. 2021, 181, 111658. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, J.; Wang, X.; Wang, Y.; Yuan, Y.; Yang, S. PpERF/ABR1 functions as an activator to regulate PpPG expression resulting in fruit softening during storage inpeach (Prunus persica). Postharvest Biol. Technol. 2022, 189, 111919. [Google Scholar] [CrossRef]
- Xu, Z.; Dai, J.Y.; Liang, L.P.; Shi, P.; Shah, K.; Liu, H.; Ma, J.; Xing, L.; Hu, Y.; Zhang, D.; et al. A peach ethylene response factor PpERF61 is involved in fruit ripening by modulating ripening-related genes and PpSEP1. Postharvest Biol. Technol. 2023, 206, 112584. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Wang, X.J.; Xu, Z.; Shi, P.; Gu, M.J.; Kang, T.Y.; Li, Q.; Zhang, D.; Zhao, C.P. PrupeFUL4 regulates ripening and softening of peach fruits through ethylene biosynthesis. Acta Physiol. Plant. 2022, 44, 23. [Google Scholar] [CrossRef]
- Li, J.; Li, F.; Qian, M.; Han, M.; Liu, H.; Zhang, D.; Ma, J.; Zhao, C. Characteristics and regulatory pathway of the PrupeSEP1 SEPALLATA gene during ripening and softening in peach fruits. Plant Sci. 2017, 257, 63–73. [Google Scholar] [CrossRef]
- Rothkegel, K.; Espinoza, A.; Sanhueza, D.; Lillo-Carmona, V.; Riveros, A.; Campos-Vargas, R.; Meneses, C. Identification of DNA Methylation and Transcriptomic Profiles Associated with Fruit Mealiness in Prunus persica (L.) Batsch. Front. Plant Sci. 2021, 12, 684130. [Google Scholar] [CrossRef]
- Xian, M.; Bi, J.; Xie, Y.; Jin, X. Modulating pectin structure and enhancing texture of frozen yellow peaches: The impact of low-temperature blanching. Int. J. Biol. Macromol. 2024, 271, 132618. [Google Scholar] [CrossRef]
- Qian, M.; Xu, Z.; Zhang, Z.; Li, Q.; Yan, X.; Liu, H.; Han, M.; Li, F.; Zheng, J.; Zhang, D.; et al. The downregulation of PpPG21 and PpPG22 influences peach fruit texture and softening. Planta 2021, 254, 22. [Google Scholar] [CrossRef]
- Zhou, H.; Su, M.; Du, J.; Zhang, X.; Li, X.; Zhang, M.; Hu, Y.; Huan, C.; Ye, Z. Crucial roles of sorbitol metabolism and energy status in the chilling tolerance of yellow peach. Plant Physiol. Biochem. 2023, 204, 108092. [Google Scholar] [CrossRef]
- Vimolmangkang, S.; Zheng, H.; Peng, Q.; Jiang, Q.; Wang, H.; Fang, T.; Liao, L.; Wang, L.; He, H.; Han, Y. Assessment of Sugar Components and Genes Involved in the Regulation of Sucrose Accumulation in Peach Fruit. J. Agric. Food Chem. 2016, 64, 6723–6729. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, X.; Ye, Z.; Su, M.; Zhang, X.; Du, J.; Li, X.; Zhou, H.; Huan, C. Transcriptome Co-Expression Network Analysis of Peach Fruit with Different Sugar Concentrations Reveals Key Regulators in Sugar Metabolism Involved in Cold Tolerance. Foods 2023, 12, 2244. [Google Scholar] [CrossRef]
- Liu, G.; Bi, J.; Chen, Q. Impact of postharvest ripening on peach quality: Aroma release and formation mechanism. Food Chem. 2025, 479, 143743. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhao, B.; Cai, Z.; Yan, J.; Ma, R.; Yu, M. Amino Acid Profiles in Peach (Prunus persica L.) Fruit. Foods 2022, 11, 1718. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Luo, H.; Chen, Y.; Shen, H.; Shi, P.; Yang, F.; Li, H.; Yu, L.J. Elucidating Softening Mechanism of Honey Peach (Prunus persica L.) Stored at Ambient Temperature Using Untargeted Metabolomics Based on Liquid Chromatography-Mass Spectrometry. Horticulturae 2023, 9, 1210. [Google Scholar] [CrossRef]
- Manzoor, M.; Anwar, F.; Mahmood, Z.; Rashid, U.; Ashraf, M. Variation in minerals, phenolics and antioxidant activity of peel and pulp of different varieties of peach (Prunus persica L.) fruit from Pakistan. Molecules 2012, 17, 6491–6506. [Google Scholar] [CrossRef]
- Saidani, F.; Giménez, R.; Aubert, C.; Chalot, G.; Betran, J.A.; Gogorcena, Y. Phenolic, sugar and acid profiles and the antioxidant composition in the peel and pulp of peach fruits. J. Food Compos. Anal. 2017, 62, 126–133. [Google Scholar] [CrossRef]
- Mihaylova, D.; Popova, A.; Dincheva, I. Pattern Recognition of Varieties of Peach Fruit and Pulp from Their Volatile Components and Metabolic Profile Using HS-SPME-GC/MS Combined with Multivariable Statistical Analysis. Plants 2022, 11, 3219. [Google Scholar] [CrossRef]
- Farcuh, M.; Hopfer, H. Aroma volatiles as predictors of chilling injury development during peach (Prunus persica (L) Batsch) cold storage and subsequent shelf-life. Postharvest Biol. Technol. 2023, 195, 112137. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, B.; Zhang, Y.; Song, H.; Shen, Z.; Ma, R.; Yu, M. Deciphering aroma complexity between melting flesh and stony hard peach (Prunus persica L.) fruit through integrative analysis of volatile contributions. LWT 2024, 209, 116780. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, Y.; Jiang, S.; Wang, X.; Xu, F.; Wang, H.; Shao, X. Flavor development in peach fruit treated with 1-methylcyclopropene during shelf storage. Food Res. Int. 2020, 137, 109653. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, S.; Ye, Z.; Zhou, H.; Shen, S.; Zheng, X.; Chen, H. The effects of chilling (4 °C) and non-chilling (12 °C) temperatures on storage quality and flavor development of yellow peach fruit. J. Food Compos. Anal. 2025, 139, 107094. [Google Scholar] [CrossRef]
- Liu, H.; He, H.; Liu, C.; Wang, C.; Qiao, Y.; Zhang, B. Changes of Sensory Quality, Flavor-Related Metabolites and Gene Expression in Peach Fruit Treated by Controlled Atmosphere (CA) under Cold Storage. Int. J. Mol. Sci. 2022, 23, 7141. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Duan, W.; Xie, K.; Ren, C.; Zhu, C.; Chen, K. Effect of salicylic acid treatment on sensory quality, flavor-related chemicals and gene expression in peach fruit after cold storage. Postharvest Biol. Technol. 2019, 161, 111089. [Google Scholar] [CrossRef]
- Cai, H.; Han, S.; Yu, M.; Ma, R.; Yu, Z. The alleviation of methyl jasmonate on loss of aroma lactones correlated with ethylene biosynthesis in peaches. J. Food Sci. 2020, 85, 2389–2397. [Google Scholar] [CrossRef]
- Baró-Montel, N.; Eduardo, I.; Usall, J.; Casals, C.; Arús, P.; Teixidó, N.; Torres, R. Exploring sources of resistance to brown rot in an interspecific almond × peach population. J. Sci. Food Agric. 2019, 99, 4105–4113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, W.; Han, X.; Wu, B.; Song, Z.; Shi, J. Plant glycerol suppresses brown rot of peach fruit by enhancing disease resistance. Physiol. Mol. Plant Pathol. 2024, 129, 8. [Google Scholar] [CrossRef]
- Li, J.; Wei, Y.; Chen, Y.; Ye, J.; Jiang, S.; Xu, F.; Shao, X. Combined application of alginate oligosaccharide and marine yeast sporidiobolus pararoseus to control brown rot of peach fruit. Postharvest Biol. Technol. 2024, 208, 10. [Google Scholar] [CrossRef]
- Chen, M.; Jia, F.; Chen, S.; Zheng, Y.; Hu, Y.; Liu, W.; Liu, C.; Sun, X.; Lu, J.; Chen, G.; et al. Streptomyces virginiae XDS1-5, an antagonistic actinomycete, as a biocontrol to peach brown rot caused by Monilinia fructicola. J. Sci. Food Agric. 2024, 104, 7514–7523. [Google Scholar] [CrossRef]
- Xu, Y.; Wei, Y.; Jiang, S.; Xu, F.; Wang, H.; Shao, X. Preparation and characterization of tea tree oil solid liposomes to control brown rot and improve quality in peach fruit. LWT 2022, 162, 113442. [Google Scholar] [CrossRef]
- Jiang, S.; Zhao, T.; Wei, Y.; Cao, Z.; Shao, X. Preparation and characterization of tea tree oil/hydroxypropyl-β-cyclodextrin inclusion complex and its application to control brown rot in peach fruit. Food Hydrocoll. 2021, 121, 107037. [Google Scholar] [CrossRef]
- Li, Q.; Wei, Y.; Chen, Y.; Jiang, S.; Ye, J.; Xu, F.; Lou, Y.j.; Ding, P.b.; Ouaziz, M.; Shao, X.f. Agaro-oligosaccharides enhanced the monilinia fructicola resistance of peach fruit by regulating antioxidative and phenylpropanoid metabolism. Postharvest Biol. Technol. 2024, 217, 113126. [Google Scholar] [CrossRef]
- Wang, X.; Huang, M.; Li, W.; Shi, Y.; Tang, Y.; Zhang, H.; Wu, B.; Zhang, B.; Song, Z.; Shi, J. Antifungal activity of 2-decanone against monilinia fructicola and its application in combination with boscalid in mitigating brown rot disease in peach fruit. Physiol. Mol. Plant Pathol. 2025, 138, 102665. [Google Scholar] [CrossRef]
- Zhou, D.; Wei, Y.; Peng, J.; Tu, S.; Wang, Z.; Tu, K. Carvacrol and eugenol inhibit postharvest soft rot disease by enhancing defense response in peaches during storage. J. Food Process. Preserv. 2019, 43, e14086.1–e14086.11. [Google Scholar] [CrossRef]
- Wang, X.; Xu, F.; Wang, J.; Jin, P.; Zheng, Y. Bacillus cereus AR156 induces resistance against Rhizopus rot through priming of defense responses in peach fruit. Food Chem. 2013, 136, 400–406. [Google Scholar] [CrossRef]
- Taheri, P. Alternative approach to management of rhizopus rot of peach (Prunus persica L.) using the essential oil of Thymus vulgaris (L.). Mycosphere 2018, 9, 510–517. [Google Scholar] [CrossRef]
- Dai, B.; Wang, Y.; Zhou, H.; Wang, L.; Zhou, L.; Mao, J.; Zhang, S.y.; Shen, S.l.; Zheng, X.l.; Huan, C. Control efficiency and potential mechanisms of chlorogenic acid against postharvest gray mold caused by botrytis cinerea on peach fruit. Postharvest Biol. Technol. 2024, 218, 113134. [Google Scholar] [CrossRef]
- Fan, L.; Wei, Y.; Chen, Y.; Jiang, S.; Xu, F.; Zhang, C.; Wang, H.; Shao, X. Epinecidin-1, a marine antifungal peptide, inhibits Botrytis cinerea and delays gray mold in postharvest peaches. Food Chem. 2023, 403, 134419. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Z.; Chen, Y.; Li, B.; Tian, S. Botrytis cinerea. Curr. Biol. 2023, 33, R460–R462. [Google Scholar] [CrossRef]
- Song, C.; Wang, K.; Xiao, X.; Liu, Q.; Yang, M.; Li, X.; Feng, Y.; Li, S.; Shi, L.; Chen, W.; et al. Membrane lipid metabolism influences chilling injury during cold storage of peach fruit. Food Res. Int. 2022, 157, 111249. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, W.; Cao, J.; Li, Y. Effect of chilling temperatures on physiological properties, phenolic metabolism and antioxidant level accompanying pulp browning of peach during cold storage. Sci. Hortic. 2019, 255, 175–182. [Google Scholar] [CrossRef]
- Zhao, H.; Meng, S.; Fu, M.; Chen, Q. Near-freezing temperature storage improves peach fruit chilling tolerance by regulating the antioxidant and proline metabolism. Horticulturae 2024, 10, 337. [Google Scholar] [CrossRef]
- Guo, T.; Li, J.; Guo, M.; Yang, Q.; Dai, X.; Qiao, X.; Song, Z.; Tian, C.y.; Li, Y.j.; Ge, H.; et al. Low temperature inhibits pectin degradation by PpCBFs to prolong peach storage time. J. Food Sci. 2023, 88, 3725–3736. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Yi, J.; Zhang, X.; Tu, K. Integrated omics analysis reveals the interactions among ROS metabolism and malate metabolism associated with chilling tolerance in peach fruit treated with hot air. Food Res. Int. 2025, 200, 115435. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, Q.; Peng, J.; Tu, S.; Tu, K. Metabolic analysis of phenolic profiles reveals the enhancements of anthocyanins and procyanidins in postharvest peach as affected by hot air and ultraviolet c. Postharvest Biol. Technol. 2020, 167, 111227. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, Y.; Wang, L.; Hou, Y.; Bao, Y.; Jia, Z.; Zheng, Y.; Jin, P. Hot water treatment improves peach fruit cold resistance through pphsfa4c-mediated hsf-hsp and ros pathways. Postharvest Biol. Technol. 2023, 199, 112272. [Google Scholar] [CrossRef]
- Spadoni, A.; Guidarelli, M.; Sanzani, S.M.; Ippolito, A.; Mari, M. Influence of hot water treatment on brown rot of peach and rapid fruit response to heat stress. Postharvest Biol. Technol. 2014, 94, 66–73. [Google Scholar] [CrossRef]
- Ai, S.; Xu, S.; Wu, C.; Grierson, D.; Chen, K.; Xu, C. Novel insights into modified atmosphere mediated cold tolerance in peach fruit during postharvest storage. Postharvest Biol. Technol. 2024, 218, 113187. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, X.; Su, M.; Du, J.; Li, X.; Zhang, M.; Hu, Y.; Huan, C.; Ye, Z. Controlled atmosphere storage alleviates internal browning in flat peach fruit by regulating energy and sugar metabolisms. Plant Physiol. Biochem. 2022, 186, 107–120. [Google Scholar] [CrossRef]
- Santin, M.; Giordani, T.; Cavallini, A.; Bernardi, R.; Castagna, A.; Hauser, M.T.; Ranieri, A. UV-B exposure reduces the activity of several cell wall-dismantling enzymes and affects the expression of their biosynthetic genes in peach fruit (Prunus persica L.; cv. Fairtime, melting phenotype). Photochem. Photobiol. Sci. 2019, 18, 1280–1289. [Google Scholar] [CrossRef]
- Santin, M.; Lucini, L.; Castagna, A.; Chiodelli, G.; Hauser, M.T.; Ranieri, A. Post-harvest uv-b radiation modulates metabolite profile in peach fruit. Postharvest Biol. Technol. 2018, 139, 127–134. [Google Scholar] [CrossRef]
- Santin, M.; Ranieri, A.; Hauser, M.T.; Miras-Moreno, B.; Rocchetti, G.; Lucini, L.; Strid, Å.; Castagna, A. The outer influences the inner: Postharvest UV-B irradiation modulates peach flesh metabolome although shielded by the skin. Food Chem. 2021, 338, 127782. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Liu, Q.; Zhu, T.; Li, T.; Fan, G.; Li, X.; Wu, C. Effects of ultraviolet C on the quality and aroma volatile in peach fruit during postharvest storage. Food Chem. 2024, 456, 139906. [Google Scholar] [CrossRef] [PubMed]
- Kan, J.; Hui, Y.; Lin, X.; Liu, Y.; Jin, C. Postharvest ultraviolet-C treatment of peach fruit: Changes in transcriptome profile focusing on genes involved in softening and senescence. J. Food Process. Preserv. 2021, 45, e15813. [Google Scholar] [CrossRef]
- Zhou, H.J.; Zhang, X.N.; Su, M.S.; Du, J.H.; Li, X.W.; Ye, Z.W. Effects of ultraviolet-c pretreatment on sugar metabolism in yellow peaches during shelf life. HortScience 2020, 55, 416–423. [Google Scholar] [CrossRef]
- Schreiner, M.; Mewis, I.; Huyskens-Keil, S.; Jansen, M.A.K.; Zrenner, R.; Winkler, J.B.; Brien, N.; Krumbein, A. UV-B-induced secondary plant metabolites—Potential benefits for plant and human health. Crit. Rev. Plant Sci. 2012, 31, 229–240. [Google Scholar] [CrossRef]
- Araque, L.C.O.; Rodoni, L.M.; Darré, M.; Ortiz, C.M.; Civello, P.M.; Vicente, A.R. Cyclic low dose UV-C treatments retain strawberry fruit quality more effectively than conventional pre-storage single high fluence applications. LWT 2018, 92, 304–311. [Google Scholar] [CrossRef]
- Zhou, H.; Ye, Z.; Wang, L.; Zhang, S.; Yuan, Z.; Su, M.; Zhang, X.; Du, D.; Li, X.; Zhang, M.; et al. 1-MCP regulates taste development in cold-stored peach fruit through modulation of sugar, organic acid, and polyphenolic metabolism. Postharvest Biol. Technol. 2025, 225, 113518. [Google Scholar] [CrossRef]
- Qian, C.; Ji, Z.; Zhu, Q.; Qi, X.; Li, Q.; Yin, J. Effects of 1-MCP on proline, polyamine, and nitric oxide metabolism in postharvest peach fruit under chilling stress. Hortic. Plant J. 2021, 7, 188–196. [Google Scholar] [CrossRef]
- Cai, H.; Han, S.; Jiang, L.; Yu, M.; Ma, R.; Yu, Z. 1-MCP treatment affects peach fruit aroma metabolism as revealed by transcriptomics and metabolite analyses. Food Res. Int. 2019, 122, 573–584. [Google Scholar] [CrossRef]
- Zhang, Y.; Ling, J.; Zhou, H.; Tian, M.; Huang, W.; Luo, S.; Hu, H.; Li, P. 1-methylcyclopropene counteracts ethylene inhibition of anthocyanin accumulation in peach skin after harvest. Postharvest Biol. Technol. 2022, 183, 111737. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, J.; Song, C.; Qi, S.; Lin, Q.; Cui, Y.; Ling, J.; Duan, Y. Nitric oxide alleviates chilling injury by regulating the metabolism of lipid and cell wall in cold-storage peach fruit. Plant Physiol. Biochem. 2021, 169, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Shi, Y.; Zhu, S.; Song, Z.; Shi, J. Enhancing the resistance of peach fruit against monilinia fructicola using exogenous nitric oxide by activating the gamma-aminobutyric acid shunt. Postharvest Biol. Technol. 2023, 200, 112314. [Google Scholar] [CrossRef]
- Guo, X.; Huang, D.; Jing, G.; Feng, J.; Zhu, S. Nitric oxide-mediated DNA methylation enhances cold resistance in postharvest peach fruit. Food Chem. 2023, 404, 134660. [Google Scholar] [CrossRef]
- Cai, H.; Han, S.; Yu, M.; Ma, R.; Yu, Z. Exogenous nitric oxide fumigation promoted the emission of volatile organic compounds in peach fruit during shelf life after long-term cold storage. Food Res. Int. 2020, 133, 109135. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, L.; Liu, Z.; Wang, K.; Wu, Z.; Zheng, Y.; Jin, P. Combining molecular docking and experimental approaches to uncover how Ca2+/PpCaM1-PpCAMTA3 regulated PpLCD2 expression and hydrogen sulfide production contributing to chilling tolerance in peach fruit. Postharvest Biol. Technol. 2024, 216, 113074. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Song, Q.; Wu, T.; Shi, K.; Qiu, T.; Jiang, J.; Wang, Z.; Liu, Z.; Jin, P.; et al. Hydrogen sulfide enhances ppbhlh3-controlled sucrose accumulation in peach chilling tolerance. Postharvest Biol. Technol. 2025, 219, 113259. [Google Scholar] [CrossRef]
- Watkins, C.B. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef]
- Zhu, L.Q.; Du, H.Y.; Wang, W.; Zhang, W.; Shen, Y.G.; Wan, C.P.; Chen, J. Synergistic effect of nitric oxide with hydrogen sulfide on inhibition of ripening and softening of peach fruits during storage. Sci. Hortic. 2019, 256, 108591. [Google Scholar] [CrossRef]
- Han, S.; Cai, H.; An, X.; Huan, C.; Wu, X.; Jiang, L.; Yu, M.; Ma, R.; Yu, Z. Effect of nitric oxide on sugar metabolism in peach fruit (cv. xiahui 6) during cold storage. Postharvest Biol. Technol. 2018, 142, 72–80. [Google Scholar] [CrossRef]
- Yu, M.; Chen, Y.; Zhu, Q.; Wu, X.; Jiang, S.; Wei, Y.; Ye, J.; Xu, F.; Shao, X. Hydrogen sulfide mediated methyl jasmonate -induced cold resistance in peach fruit. Postharvest Biol. Technol. 2024, 210, 112779. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Shao, J.; Zhang, C.; Mei, L.; Wang, K.; Jin, P.; Zheng, Y. Hydrogen sulfide alleviates chilling injury in peach fruit by maintaining cell structure integrity via regulating endogenous H2S, antioxidant and cell wall metabolisms. Food Chem. 2022, 391, 133283. [Google Scholar] [CrossRef]
- Gomes da Silva, K.; Costa, I.H.D.L.; Fonseca, L.M.; Saraiva, M.M.T.; Antunes, B.D.F.; Borges, C.D. Food biopreservation, global trends and applications: A bibliometric approach. Food Control 2025, 168, 110901. [Google Scholar] [CrossRef]
- Gacnik, S.; Munda, A.; Veberic, R.; Hudina, M.; Mikulic-Petkovsek, M. The use of preharvest and postharvest treatments with salicylic acid to control brown rot and the response to its infection with the synthesis of phenolic compounds in peach fruit. Hortic. Environ. Biotechnol. 2024, 65, 939–955. [Google Scholar] [CrossRef]
- Ji, N.; Wang, J.; Zuo, X.; Li, Y.; Zheng, Y. Ppwrky45 is involved in methyl jasmonate primed disease resistance by enhancing the expression of jasmonate acid biosynthetic and pathogenesis-related genes of peach fruit. Postharvest Biol. Technol. 2021, 172, 111390. [Google Scholar] [CrossRef]
- Duan, W.Y.; Yang, C.; Cao, X.M.; Zhang, C.; Liu, H.R.; Chen, K.S.; Li, X.; Zhang, B. Transcriptome and DNA methylome analysis reveal new insights into methyl jasmonate-alleviated chilling injury of peach fruit after cold storage. Postharvest Biol. Technol. 2022, 189, 111915. [Google Scholar] [CrossRef]
- Huan, C.; Yang, X.; Wang, L.; Kebbeh, M.; Wang, Y.; Dai, B.; Shen, S.; Zheng, X.; Zhou, H. Methyl jasmonate treatment regulates α-linolenic acid metabolism and jasmonate acid signaling pathway to improve chilling tolerance in both stony hard and melting flesh peaches. Postharvest Biol. Technol. 2022, 190, 111960. [Google Scholar] [CrossRef]
- Cao, S.; Song, C.; Shao, J.; Bian, K.; Chen, W.; Yang, Z. Exogenous Melatonin Treatment Increases Chilling Tolerance and Induces Defense Response in Harvested Peach Fruit during Cold Storage. J. Agric. Food Chem. 2016, 64, 5215–5222. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Hao, W.; Yan, L.; Zhang, H.; Zhang, J.; Liu, C.; Zheng, L. Postharvest melatonin treatment enhanced antioxidant activity and promoted GABA biosynthesis in yellow-flesh peach. Food Chem. 2023, 419, 136088. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yan, B.; Wang, L.; Wang, Z.; Shan, Z.; Yu, Z. Melatonin application inhibits anthocyanin biosynthesis in postharvest peach fruit as revealed by transcriptional and liquid chromatographic-tandem mass spectrometric analysis. J. Sci. Food Agric. 2025, 105, 5227–5238. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ma, R.; Xu, J.; Shen, Z.; Yu, M. Effect of melatonin treatment on improving jasmonates content and cell wall stability involved in enhanced chilling tolerance of peach fruit during cold storage. Postharvest Biol. Technol. 2024, 213, 112957. [Google Scholar] [CrossRef]
- Yang, W.; Wang, L.; Ban, Z.; Yan, J.; Lu, H.; Zhang, X. Efficient microencapsulation of Syringa essential oil; The valuable potential on quality maintenance and storage behavior of peach. Food Hydrocoll. 2019, 95, 177–185. [Google Scholar] [CrossRef]
- Min, D.; Wu, H.; Xu, M.; Leng, P.; Sun, J.; Liu, Y.G. Antifungal and mechanism of rose essential oil against monilinia fructicola caused brown rot of peach fruit. Postharvest Biol. Technol. 2025, 222, 113398. [Google Scholar] [CrossRef]
- Sultan, M.; Hafez, O.M.; Saleh, M.A. Active coating film based on gelatin/trans-cinnamic acid for cold preservation of peach fruits (Prunus persica L. Bastch). J. Stored Prod. Res. 2024, 106, 102286. [Google Scholar] [CrossRef]
- Gan, Z.; Zhang, Y.; Jin, Z.; Wang, Y.; Li, J.; Yang, C.; Cao, Q.; Chen, J.; Rong, Z.; Lu, X.; et al. Gum arabic coating alleviates chilling injury of cold-stored peach by regulating reactive oxygen species, phenolic, and sugar metabolism. Food Chem. 2024, 455, 139899. [Google Scholar] [CrossRef]
- Jiao, W.X.; Shu, C.; Li, X.X.; Cao, J.K.; Fan, X.G.; Jiang, W.B. Preparation of a chitosan-chlorogenic acid conjugate and its application as edible coating in postharvest preservation of peach fruit. Postharvest Biol. Technol. 2019, 154, 129–136. [Google Scholar] [CrossRef]
- Li, X.Y.; Du, X.L.; Liu, Y.; Tong, L.J.; Wang, Q.; Li, J.L. Rhubarb extract incorporated into an alginate-based edible coating for peach preservation. Sci. Hortic. 2019, 257, 108685. [Google Scholar] [CrossRef]
- Sortino, G.; Saletta, F.; Puccio, S.; Scuderi, D.; Allegra, A.; Inglese, P.; Farina, V. Extending the shelf life of white peach fruit with 1-methylcyclopropene and aloe arborescens edible coating. Agriculture 2020, 10, 151. [Google Scholar] [CrossRef]
- Tang, T.; Zhou, H.; Wang, L.; Zhao, J.; Ma, L.; Ling, J.; Li, G.; Huang, W.; Li, P.; Zhang, Y. Post-harvest Application of Methyl Jasmonate or Prohydrojasmon Affects Color Development and Anthocyanins Biosynthesis in Peach by Regulation of Sucrose Metabolism. Front. Nutr. 2022, 9, 871467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Zhou, D.; Ge, X.; Luo, Y.; Su, J. The role of essential oils in maintaining the postharvest quality and preservation of peach and other fruits. J. Food Biochem. 2022, 46, e14513. [Google Scholar] [CrossRef] [PubMed]
- Aaqil, M.; Peng, C.; Kamal, A.; Nawaz, T.; Gong, J. Recent Approaches to the Formulation, Uses, and Impact of Edible Coatings on Fresh Peach Fruit. Foods 2024, 13, 267. [Google Scholar] [CrossRef]
| Disease | Postharvest Treatment | Main Findings | Refs. |
|---|---|---|---|
| Brown rot | Plant glycerol combined with Silwet L-77 surfactant treatment | Increases antioxidant enzyme activity and SA content, activating the G3P pathway. | [63] |
| Marine yeast (Sporidiobolus pararoseus) and alginate oligosaccharide | Increases the activity and gene expression of catalase, phenylalanine ammonia lyase, chalcone isomerase, and other resistant enzymes. | [64] | |
| Streptomyces virginiae (XDS1-5) | Destroys the cell membrane structure of M. fructicola. | [65] | |
| Tea tree oil | Increases fruit antibacterial properties. | [66,67] | |
| Agaro-oligosaccharides | Increases antioxidant capacity and phenylalanine metabolism. | [68] | |
| 2-decanone | Downregulation of MfBmp1 and MfPls1 expression reduces spore germination and adhesion formation, inhibiting the growth of M. fructicola mycelium. | [69] | |
| Soft rot | Bacillus licheniformis HG03 | Increases free radical scavenging capacity, activates MAPK signaling pathways and WRKY transcription factor expression. | [6] |
| Carvacrol and eugenol | Increases the activity of defense-related enzymes and improves the content of phenols, flavonoids, lignin, and glycoproteins rich in hydroxyproline. | [70] | |
| Bacillus cereus AR156 | Increases the activity and antioxidant properties of chitinase and β-1,3-glucanase. | [71] | |
| Thymus vulgaris | Maintains anthocyanin and carbohydrate content. | [72] | |
| Gray mold | Chlorogenic acid | Reduces ergosterol content and synthetic enzyme gene expression, thereby inhibiting the activity of B. cinerea proteins. | [73] |
| Natural peptide (Epinecidin-1) | Destroys the B. cinerea structure. | [74] |
| Postharvest Treatment | Main Findings | Ref. |
|---|---|---|
| Low-temperature | Storage at 0 °C maintains higher levels of phosphatidylcholine and phosphatidylethanolamine than storage at 4 °C. | [76] |
| 4 °C delays fruit softening and preserves sweetness, but can cause cold damage and bitterness. | [58] | |
| 4–6 °C is conducive to the accumulation of phenolic substances, while 0–2 °C delays browning. | [77] | |
| −1 °C improves antioxidant capacity and enhances proline accumulation | [78] | |
| Inhibition of pectinase gene expression. | [79] | |
| Hot air | Increases antioxidant enzyme activity and reduces reactive oxygen species accumulation and malic acid degradation. | [80] |
| Increase the metabolism of phenylpropane and promote the synthesis of anthocyanins and proanthocyanidins. | [81] | |
| Hot water | Activates the transcription of heat shock proteins PpHSPs and PpAPXs to reduce ROS accumulation and alleviate CI. | [82] |
| Reduces the expression levels of genes encoding cell wall degradation enzymes and increases the expression levels of phenylalanine ammonia lyase, chalcone isomerase, heat shock protein 7, and reactive oxygen species scavenging genes. | [83] | |
| Modified atmosphere | PpERF61 can activate jasmonic acid and gamma-aminobutyric acid biosynthesis gene expression. | [84] |
| Controlled atmosphere | Increases the levels of total phenols, total flavonoids, epicatechin, neochlorogenic acid, and chlorogenic acid. | [10] |
| Maintains higher energy levels and sucrose content. | [85] | |
| Increases the accumulation of highly aromatic volatile compounds, raises the double bound index values of fatty acids and sucrose, and enhances flavor. | [59] | |
| UV-B | Reduces cell wall degradation enzyme activity and maintain fruit hardness. | [86] |
| Affects the metabolism of biochemical substances such as phenols, terpenoids, lipids, and alkaloids. | [87] | |
| Increases levels of terpenoids, phenylpropanoids, phytochemicals, and fatty acid metabolites in peach flesh. | [88] | |
| UV-C | Promotes ester and lactone synthesis to enhance fruit aroma. | [89] |
| Upregulate genes related to antioxidant and defense responses, while downregulating the expression of genes related to cell wall degradation, membrane lipid oxidation, ethylene synthesis, and oxidative stress. | [90] | |
| Reduces ethylene production rate and increase sucrose accumulation. | [91] |
| Postharvest Treatment | Main Findings | Ref. |
| 1-MCP | Upregulates epidermal wax crystal formation genes to improve fruit resistance. | [12] |
| Reduces sweetness and bitterness, enhances sourness and umami, and lowers CI. | [94] | |
| Increases proline and polyamine content to enhance cold resistance. | [95] | |
| Reduces ethylene production and delays the synthesis of volatile substances. | [96] | |
| Enhances anthocyanin synthase activity and the expression of related genes and transcription factors to improve fruit skin coloration. | [97] | |
| NO | Enhances the antioxidant system and ascorbic acid glutathione cycle. | [13] |
| Inhibits cell wall hydrolase activity and related gene expression, upregulates key lipid metabolism gene expression, maintains hardness, and alleviates CI. | [98] | |
| Increases endogenous NO and gamma-aminobutyric acid content, upregulates key enzymes and gene expression involved in gamma-aminobutyric acid shunting, increases ATP levels and energy charge, and enhances resistance to M. fructicola. | [99] | |
| Maintains DNA methyltransferase activity and transcription levels, mediates methylation of cold-resistant genes, and enhances cold resistance. | [100] | |
| By regulating fatty acid metabolism, promotes the synthesis and release of volatile organic compounds, thereby alleviating loss. | [101] | |
| H2S | Inhibits cell wall degradation enzyme activity, increases proline and total phenol content, and alleviates IB. | [14] |
| Increasing cellular calcium ion concentration and expression of calmodulin genes PpCaM and PpLCD2, as well as increasing H2S synthesis, enhances cold resistance. | [102] | |
| Activates the activity and gene expression of sucrose phosphate synthase (SPS) and sucrose synthase (SS-s) to promote sucrose accumulation and enhance the cold tolerance of refrigerated peach fruit. | [103] |
| Postharvest Treatment | Main Findings | Ref. |
|---|---|---|
| Salicylic acid | Increases antioxidant capacity and raises energy levels. | [4] |
| Delays the decline in volatile ester and lactone content and promotes sucrose accumulation. | [60] | |
| Inhibits the activity of M. fructicola spores and increases the content of chlorogenic acid, anthocyanin-3-glucoside, and anthocyanin-3-rutinoside. | [110] | |
| Inhibits the activity and gene expression of enzymes related to membrane lipid and cell wall degradation | [37] | |
| Methyl jasmonate | Maintains membrane integrity, increases phospholipid enrichment, and activates the JA signaling pathway. | [15] |
| Activates JA biosynthesis, enhances the expression levels of PpCHI, PpGLU, PpPR-like, PpLOX, PpAOS, and PpOPR3, and improves resistance to R. stolonifer. | [111] | |
| Increases antioxidant enzyme activity and the ascorbic acid glutathione cycle. | [27] | |
| Increases the transcriptional level of PpNAC1 and PpMYC2.2, which reduces the degree of DNA methylation in fruits during cold storage and shelf life. | [112] | |
| Maintains fatty acid unsaturation, increases α-linolenic acid accumulation, and promote jasmonic acid and jasmonic acid-isoleucine synthesis. | [113] | |
| Melatonin | Increases the accumulation of polyamines, gamma-aminobutyric acid, and proline. | [114] |
| Upregulates PpGAD1 and PpGAD4 expression and downregulates PpGABA-T expression, leading to gamma-aminobutyric acid accumulation. | [115] | |
| Enhances the activity of methylesterase and demethylase, mediates DNA methylation of browning genes, and increases the content of phenolic substances. | [16] | |
| Inhibits ethylene production and reduces anthocyanin content. | [116] | |
| Increases α-linolenic acid metabolism, enhances JA and MeJA accumulation, strengthens glutathione metabolism, and increases the proportion of unsaturated fatty acids. | [117] | |
| Syringa essential oil | Promotes the synthesis of esters and terpenoids while inhibiting the production of aldehydes. | [118] |
| Rose essential oil | Damages the morphology and structure of M. fructicola, affecting the respiration process and inhibiting the decrease in total phenolic content. | [119] |
| Trans-cinnamic acid gelatin | Enhances antioxidant activity, inhibits weight loss, and increases total soluble solids. | [120] |
| Gum arabic | Maintains phenolic compounds and sucrose content, enhances antioxidant activity, and reduces ROS levels. | [121] |
| Chitosan–chlorogenic acid conjugate | Enhances ROS scavenging capacity and antioxidant activity. | [122] |
| Rhubarb extract and sodium alginate | Reducing the respiration rate has a significant inhibitory effect on Penicillium expansum. | [123] |
| 1-MCP and Aloe Arborescens | Reduces transpiration and respiration. | [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, S.; Zhang, G.; Luo, Y.; Qiu, J.; Ba, L.; Xu, S.; Zhao, Z.; Luo, D.; Dong, G.; Ren, Y. Recent Advances in Postharvest Physiology and Preservation Technology of Peach Fruit: A Systematic Review. Horticulturae 2025, 11, 1007. https://doi.org/10.3390/horticulturae11091007
Cao S, Zhang G, Luo Y, Qiu J, Ba L, Xu S, Zhao Z, Luo D, Dong G, Ren Y. Recent Advances in Postharvest Physiology and Preservation Technology of Peach Fruit: A Systematic Review. Horticulturae. 2025; 11(9):1007. https://doi.org/10.3390/horticulturae11091007
Chicago/Turabian StyleCao, Sen, Guohe Zhang, Yinmei Luo, Jingshi Qiu, Liangjie Ba, Su Xu, Zhibing Zhao, Donglan Luo, Guoliang Dong, and Yanling Ren. 2025. "Recent Advances in Postharvest Physiology and Preservation Technology of Peach Fruit: A Systematic Review" Horticulturae 11, no. 9: 1007. https://doi.org/10.3390/horticulturae11091007
APA StyleCao, S., Zhang, G., Luo, Y., Qiu, J., Ba, L., Xu, S., Zhao, Z., Luo, D., Dong, G., & Ren, Y. (2025). Recent Advances in Postharvest Physiology and Preservation Technology of Peach Fruit: A Systematic Review. Horticulturae, 11(9), 1007. https://doi.org/10.3390/horticulturae11091007





