Dynamic Coupling Mechanism of Soil Microbial Community Shifts and Nutrient Fluxes During the Life Cycle of Dictyophora rubrovolvata
Abstract
1. Introduction
2. Materials and Methods
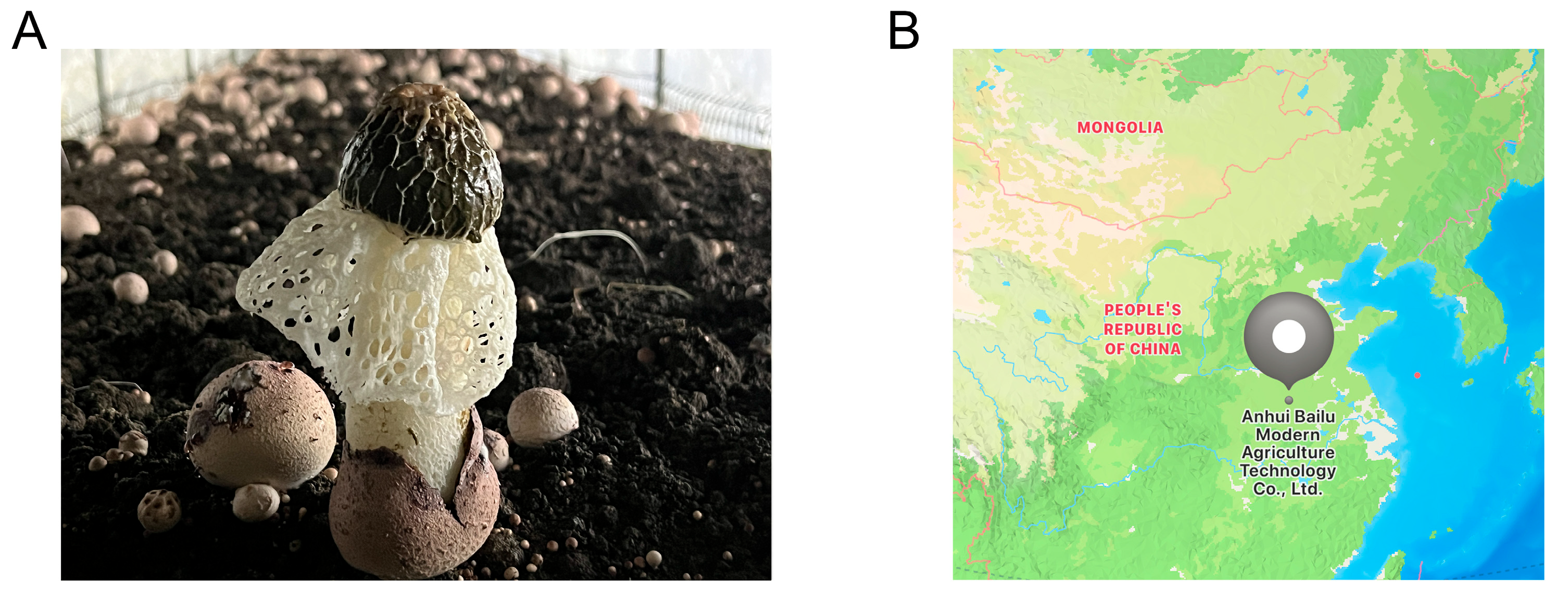
2.1. Experimental Site and Experimental Design
2.2. Cultivation Conditions
2.3. Experimental Process
2.4. Determination of Soil Physicochemical Properties
2.5. High-Throughput Sequencing Analysis
2.6. Statistical Analyses
3. Results
3.1. Physical and Chemical Properties of Soil in Different Growth Stages
3.2. Diversity of Soil Microorganisms in Different Growth Stages
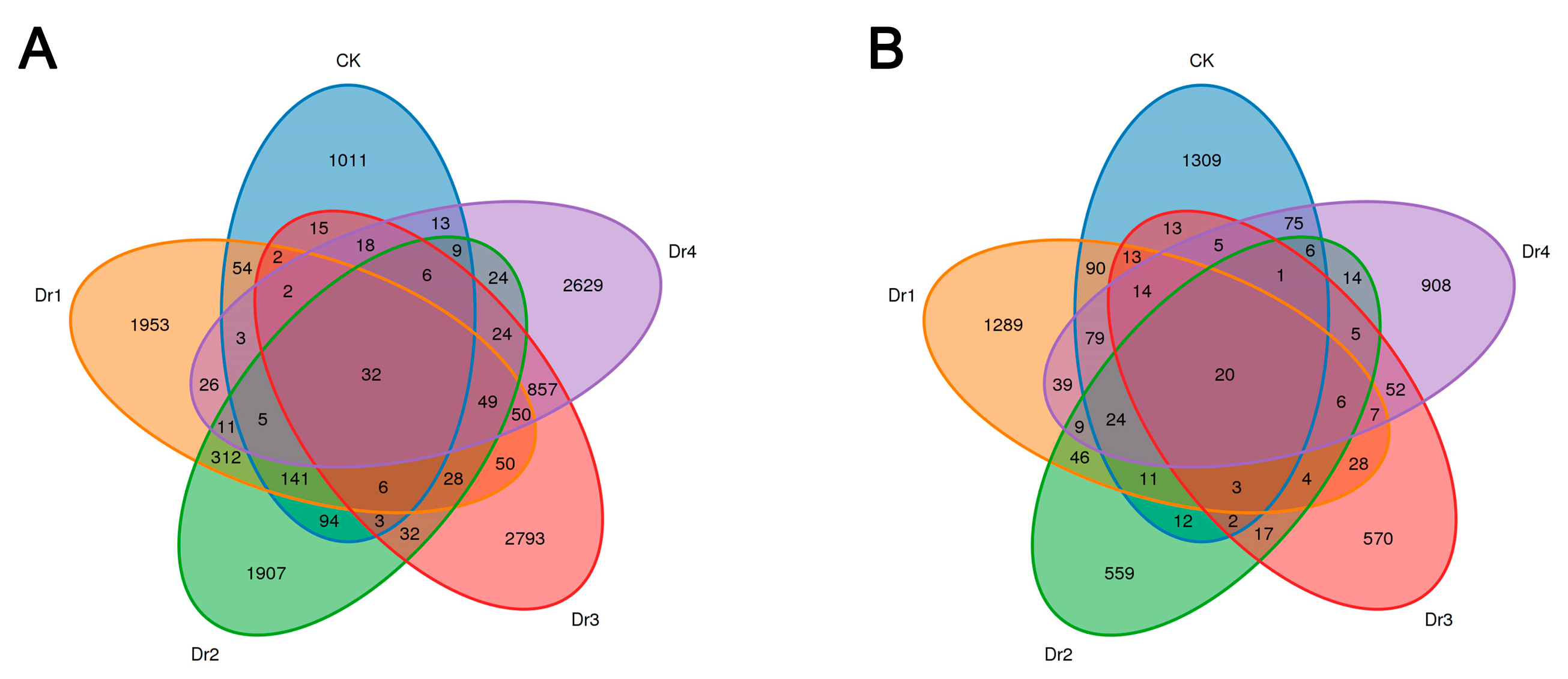
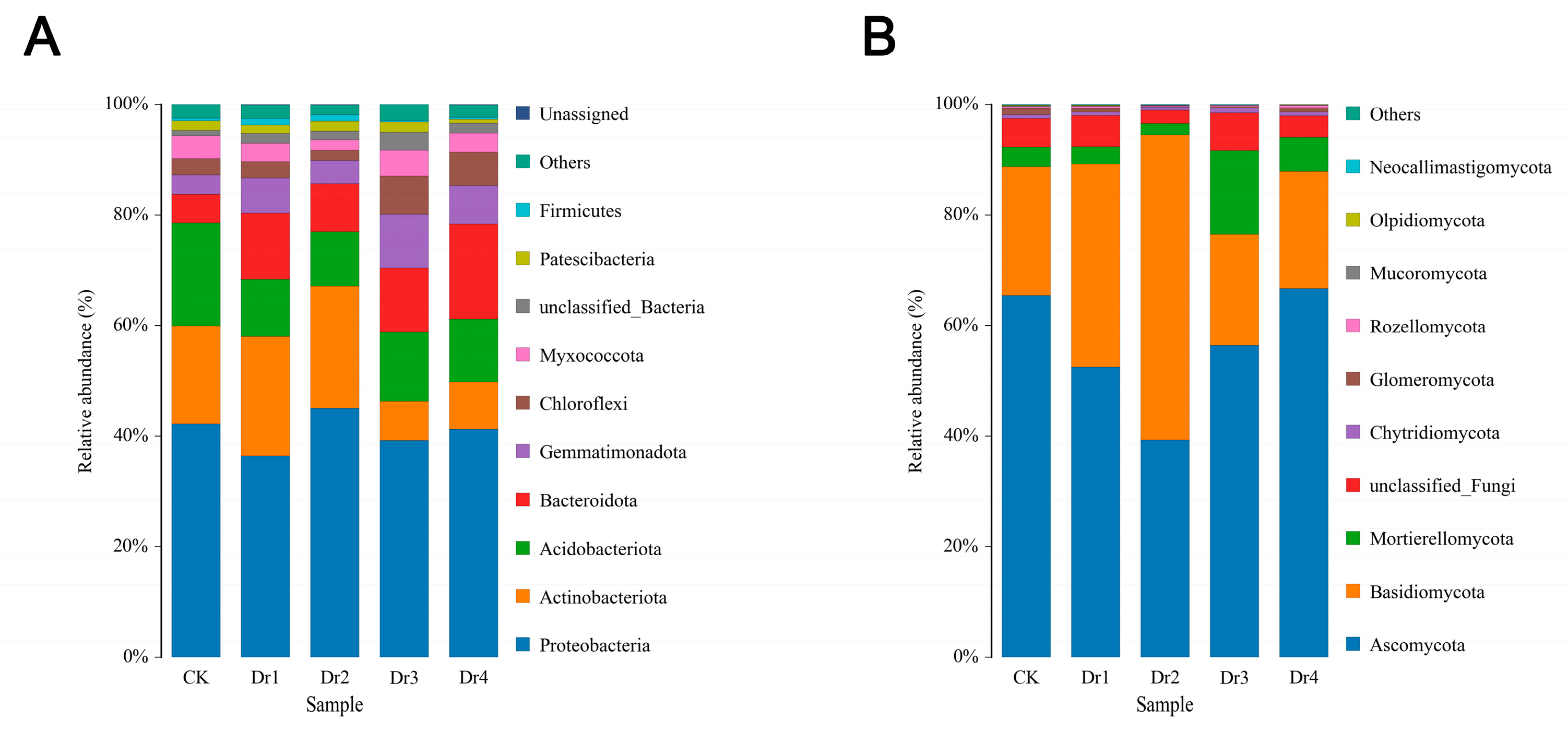
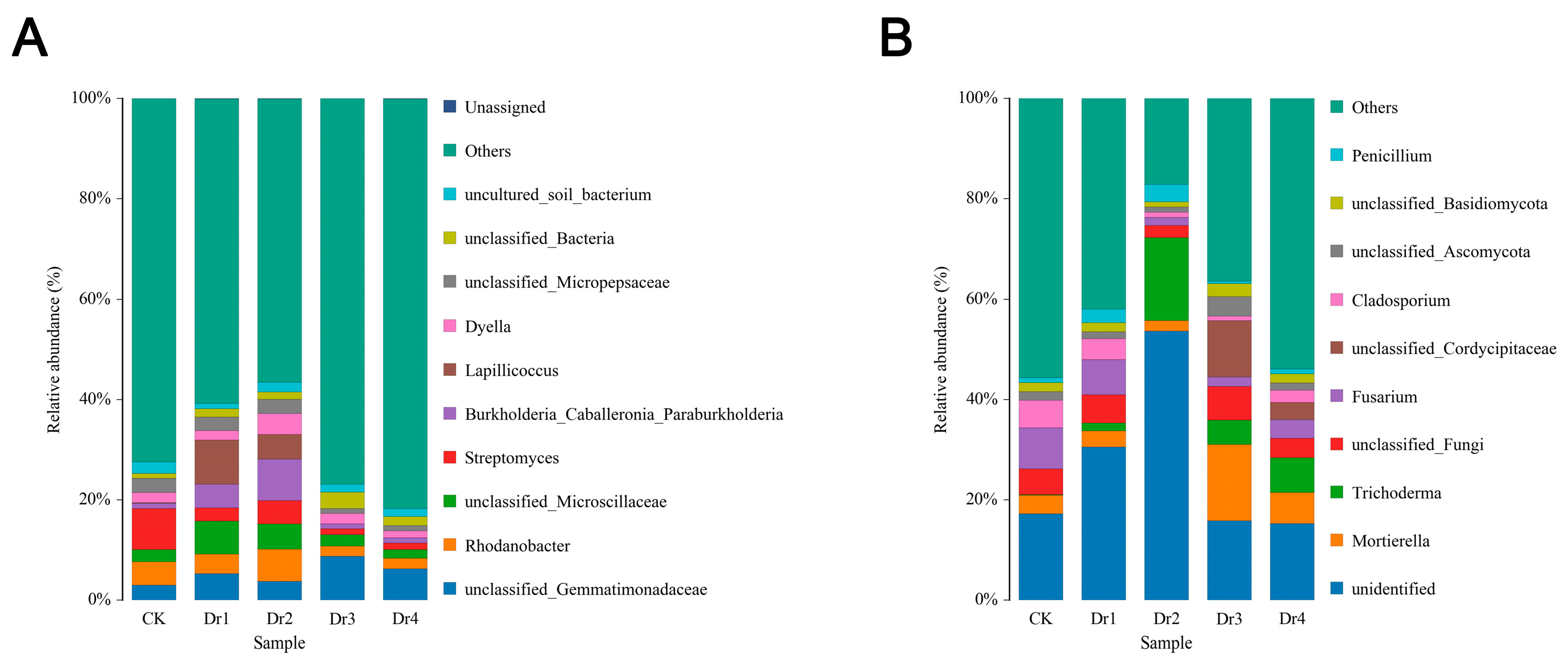
3.2.1. Analysis of Soil Bacteria and Fungi in Different Stages
3.2.2. Diversity of Soil Microbial Communities in Different Stages
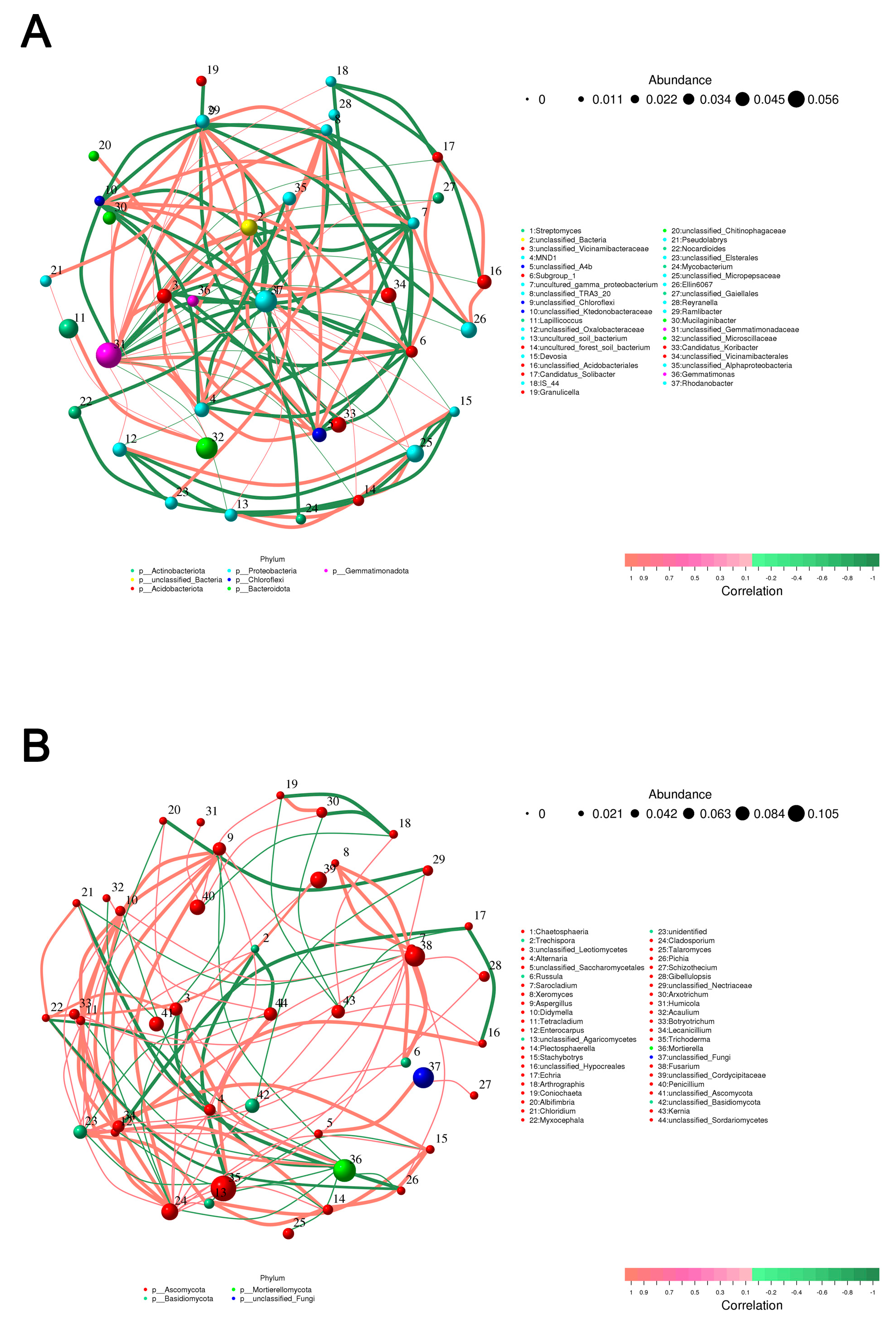
3.2.3. Co-Occurrence Network Analysis
3.2.4. Analysis of Correlation Between Soil Factors and Microbial Communities
3.2.5. Comparison of Predicted Bacteria Functional Genes During Bare Soil and Harvest Stage
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, H.; Yang, J.; Li, S.; Cui, S.W.; Tan, H.; Nie, S. Effects of different fractions of polysaccharides from Dictyophora indusiata on high-fat diet-induced metabolic syndrome in mice. Int. J. Biol. Macromol. 2024, 272, 132744. [Google Scholar] [CrossRef]
- Sun, L.; Bao, C.; Chang, W.; Zhuang, Y. Preparation, characterisation, antioxidant and antiglycation activities of the novel polysaccharides from the pileus of Dictyophora rubrovolvata. Int. J. Food Sci. Technol. 2017, 52, 161–170. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, L.; Teng, L.; Li, Y.; Cheng, J.; Chen, J.; Deng, C. Mechanism of the anti-inflammatory activity by a polysaccharide from Dictyophora indusiata in lipopolysaccharide-stimulated macrophages. Int. J. Biol. Macromol. 2019, 126, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Wang, L.; Zhang, F.; Zhang, Y.; Zheng, B.-D. Structural characterization and bifidogenic activity of polysaccharide from Dictyophora indusiata. Food Biosci. 2023, 51, 102297. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, X.; Zhao, J.; Tang, X.; Pasquali, M.; Migheli, Q.; Berg, G.; Cernava, T. Occurrence of green mold disease on Dictyophora rubrovolvata caused by Trichoderma koningiopsis. J. Plant Pathol. 2021, 103, 981–984. [Google Scholar] [CrossRef]
- Kalberer, P.P. Water relations of the mushroom culture Agaricus bisporus: Study of a single break. Sci. Hortic. 1990, 41, 277–283. [Google Scholar] [CrossRef]
- Nuralykyzy, B.; Nie, J.; Mei, H.Y.; Zhang, Y.Z.; Rogers, K.M.; Li, C.L.; Yuan, Y.W. Synergies between carbon sequestration, nitrogen utilization, a mushroom quality: A comprehensive review of substrate, fungi, and soil Interactions. J. Agric. Food Chem. 2025, 73, 14144–14157. [Google Scholar] [CrossRef]
- Yu, F.M.; Jayawardena, R.S.; Thongklang, N.; Lv, M.L.; Zhu, X.T.; Zhao, Q. Morel production associated with soil nitrogen-fixing and nitrifying microorganisms. J. Fungi. 2022, 8, 299. [Google Scholar] [CrossRef]
- Liu, W.-Y.; Guo, H.-B.; Bi, K.-X.; Alekseevna, S.L.; Qi, X.-J.; Yu, X.-D. Determining why continuous cropping reduces the production of the morel Morchella sextelata. Front. Microbiol. 2022, 13, 903983. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, H.; Zhang, Y.; Dong, C. Artificial cultivation of true morels: Current state, issues and perspectives. Crit. Rev. Biotechnol. 2017, 38, 259–271. [Google Scholar] [CrossRef]
- Chen, B.; Shao, G.; Zhou, T.; Fan, Q.; Yang, N.; Cui, M.; Zhang, J.; Wu, X.; Zhang, B.; Zhang, R. Dazomet changes microbial communities and improves morel mushroom yield under continuous cropping. Front. Microbiol. 2023, 14, 1200226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, S.; Luo, D.; Mao, P.; Rosazlina, R.; Martin, F.; Xu, L. Decline in morel production upon continuous cropping is related to changes in soil mycobiome. J. Fungi. 2023, 9, 492. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, A.; Tang, C.; Yang, Y.; Zhang, J.; Zhou, S. The changes of microbial diversity and isolation of microorganism in soil for alleviating the production decreasing after continuouscultivation of Ganoderma lucidum. Curr. Microbiol. 2024, 81, 321. [Google Scholar] [CrossRef]
- Ke, L.-Q.; Li, P.-D.; Xu, J.-P.; Wang, Q.-S.; Wang, L.-L.; Wen, H.-P. Microbial communities and soil chemical features associated with commercial production of the medicinal mushroom Ganoderma lingzhi in soil. Sci. Rep. 2019, 9, 15839. [Google Scholar] [CrossRef]
- Ji, W.; Zhang, N.; Su, W.; Wang, X.; Liu, X.; Wang, Y.; Chen, K.; Ren, L. The impact of continuous cultivation of Ganoderma lucidum on soil nutrients, enzyme activity, and fruiting body metabolites. Sci. Rep. 2024, 14, 10097. [Google Scholar] [CrossRef]
- Tong, X.-Q.; Jiang, H.; Liang, Y.; Rao, Y.-X.; Mei, L.; Wang, Y.-J. Waterlogging reduces soil colonization by antagonistic fungi and restores production in Ganoderma lucidum continuous cultivation. Crop Prot. 2020, 137, 105314. [Google Scholar] [CrossRef]
- Ren, F.; Zhang, Y.; Yu, H.; Zhang, Y.A. Ganoderma lucidum cultivation affect microbial community structure of soil, wood segments and tree roots. Sci. Rep. 2020, 10, 3435. [Google Scholar] [CrossRef]
- Fu, T.; Li, X.; Lei, F.; Li, W.; Zhang, Y.; Zhang, B.; Tang, L.; Wang, X.; Zhang, Q.; Yang, Z. Impact of biochars fabricated with typical feedstocks on soil environment and crop productivity: A case study with D. rubrovolvata. Soil Use Manage. 2024, 40, e12965. [Google Scholar] [CrossRef]
- Qin, S.; Wang, J.; Liu, S.; Tang, X.; Liu, Z.; Liu, R.; Wang, Y.; Song, L.; Chen, X.; Cernava, T. First report of green mold disease caused by Penicillium citrinum on Dictyophora rubrovolvata in China. Plant Dis. 2023, 107, 966. [Google Scholar] [CrossRef]
- Bolger, A.M.; Marc, L.; Bjoern, U. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. Embnet. J. 2011, 17, 200. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Srivastava, A.; Akhter, Y.; Verma, D. A step-by-step procedure for analysing the 16S rRNA-based microbiome diversity using QIIME 2 and comprehensive PICRUSt2 illustration for functional prediction. Arch. Microbiol. 2024, 206, 467. [Google Scholar] [CrossRef]
- Duan, M.; Li, Y.; Zhu, G.; Wu, X.; Huang, H.; Qin, J.; Long, S.; Li, X.; Feng, B.; Qin, S. Soil chemistry, metabarcoding, and metabolome analyses reveal that a sugarcane Dictyophora indusiata intercropping system can enhance soil health by reducing soil nitrogen loss. Front. Microbiol. 2023, 14, 1193990. [Google Scholar] [CrossRef]
- Lei, Y.; Jiang, F.; Di, X.; Lin, D.; Ling, P.; Zhang, Y.; Sun, Y.; Lin, Z.; Li, J. Cultivating Dictyophora indusiata with Cenchrus fungigraminus and seafood mushroom spent substrate enhanced soil nutrient accumulation and microbial community stabilisation. Environ. Technol. Innov. 2025, 38, 104200. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Pan, W.; Tang, S.; Zhou, J.; Wanek, W.; Gregory, A.S.; Ge, T.; Marsden, K.A.; Chadwick, D.R.; Liang, Y.; Wu, L.; et al. Long-term manure and mineral fertilisation drive distinct pathways of soil organic nitrogen decomposition: Insights from a 180-year-old study. Soil Biol. Biochem. 2025, 207, 109840. [Google Scholar] [CrossRef]
- Ge, Z.-W.; Brenneman, T.; Bonito, G.; Smith, M.E. Soil pH and mineral nutrients strongly influence truffles and other ectomycorrhizal fungi associated with commercial pecans (Carya illinoinensis). Plant Soil. 2017, 418, 493–505. [Google Scholar] [CrossRef]
- Chen, S.; Qi, G.; Luo, T.; Zhang, H.; Jiang, Q.; Wang, R.; Zhao, X. Continuous-cropping tobacco caused variance of chemical properties and structure of bacterial network in soils. Land Degrad. Dev. 2018, 29, 4106–4120. [Google Scholar] [CrossRef]
- Tong, L.; Li, H.; Liu, X.; Li, B.; Chen, L.; Chen, G.; Zeng, X.; Geng, Y. Effects of continuous cropping of Dictyophora on soil physical and chemical properties, microbial biomass and enzyme activity. J. Innov. Soc. Sci. Res. 2021, 8, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, J.; Liu, Q.; Zhou, Z.; Chen, F.; Xiang, D. Effects of consecutive monoculture of sweet potato on soil bacterial community as determined by pyrosequencing. J. Basic Microbiol. 2018, 59, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Penton, C.R.; Lv, N.; Xue, C.; Yuan, X.; Ruan, Y.; Li, R.; Shen, Q. Banana Fusarium wilt disease incidence is influenced by shifts of soil microbial communities under different monoculture spans. Microb. Ecol. 2017, 75, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zu, C.; Wang, C.; Yang, J.; Yu, H.; Wu, H. Different responses of rhizosphere and non-rhizosphere soil microbial communities to consecutive Piper nigrum L. monoculture. Sci. Rep. 2016, 6, 35825. [Google Scholar] [CrossRef]
- Xiong, W.; Li, Z.; Liu, H.; Xue, C.; Zhang, R.; Wu, H.; Li, R.; Shen, Q. The effect of long-term continuous cropping of black pepper on soil bacterial communities as determined by 454 pyrosequencing. PLoS ONE 2015, 10, 0136946. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Ru, H.; He, T.; Sun, N. Study on microbial community succession and functional analysis during biodegradation of mushroom residue. Biomed Res. Int. 2021, 2021, 6620574. [Google Scholar] [CrossRef]
- Pan, X.; Raaijmakers, J.M.; Carrión, V.J. Importance of Bacteroidetes in host–microbe interactions and ecosystem functioning. Trends Microbiol. 2023, 31, 959–971. [Google Scholar] [CrossRef]
- Pang, F.; Solanki, M.K.; Wang, Z. Streptomyces can be an excellent plant growth manager. World J. Microbiol. Biotechnol. 2022, 38, 193. [Google Scholar] [CrossRef]
- Shepherdson, E.M.F.; Baglio, C.R.; Elliot, M.A. Streptomyces behavior and competition in the natural environment. Curr. Opin. Microbiol. 2022, 71, 102257. [Google Scholar] [CrossRef]
- Wang, M.; Li, H.; Li, J.; Zhang, W.; Zhang, J. Streptomyces Strains and Their Metabolites for Biocontrol of Phytopathogens in Agriculture. J. Agric. Food Chem. 2024, 72, 2077–2088. [Google Scholar] [CrossRef]
- Alam, K.; Mazumder, A.; Sikdar, S.; Zhao, Y.-M.; Hao, J.; Song, C.; Wang, Y.; Sarkar, R.; Islam, S.; Zhang, Y.; et al. Streptomyces: The biofactory of secondary metabolites. Front. Microbiol. 2022, 13, 968053. [Google Scholar] [CrossRef] [PubMed]
- Dorchenkova, Y.A.; Gracheva, T.A.; Babich, T.L.; Sokolova, D.S.; Alexandrova, A.V.; Pham, G.T.H.; Lysak, L.V.; Golovchenko, A.V.; Manucharova, N.A. Soil Actinomycetes of Vietnam tropical forests. Forests 2022, 13, 1863. [Google Scholar] [CrossRef]
- Sugden, A.M. A global map of soil bacteria. Science 2018, 359, 320–325. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, P.; Liu, Z.; Yu, Y.; Tan, X.; Huang, X.; Wen, J.; Zhang, W. High-Throughput sequencing uncovers fungal community succession during Morchella sextelata development. J. Fungi 2025, 11, 364. [Google Scholar] [CrossRef]
- Tan, H.; Liu, T.; Yu, Y.; Tang, J.; Jiang, L.; Martin, F.M.; Peng, W. Morel production related to soil microbial diversity and evenness. Microbiol. Spectrum. 2021, 9, 00229-21. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The current status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef]
- Demjanová, S.; Jevinová, P.; Pipová, M.; Regecová, I. Identification of Penicillium verrucosum, Penicillium commune, and Penicillium crustosum isolated from chicken eggs. Processes 2020, 9, 53. [Google Scholar] [CrossRef]
- Gordon, T.R. Fusarium oxysporum and the Fusarium Wilt Syndrome. Annu. Rev. Phytopathol. 2017, 55, 23–39. [Google Scholar] [CrossRef]
- Colavolpe, M.B.; Mejía, S.J.; Albertó, E. Efficiency of treatments for controlling Trichoderma spp during spawning in cultivation of lignicolous mushrooms. Braz. J. Microbiol. 2014, 45, 1263–1270. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Z.; Li, X.; Xie, M.; Lu, J.; Bao, D.; Jiang, S. Dynamic Coupling Mechanism of Soil Microbial Community Shifts and Nutrient Fluxes During the Life Cycle of Dictyophora rubrovolvata. Horticulturae 2025, 11, 989. https://doi.org/10.3390/horticulturae11080989
Song Z, Li X, Xie M, Lu J, Bao D, Jiang S. Dynamic Coupling Mechanism of Soil Microbial Community Shifts and Nutrient Fluxes During the Life Cycle of Dictyophora rubrovolvata. Horticulturae. 2025; 11(8):989. https://doi.org/10.3390/horticulturae11080989
Chicago/Turabian StyleSong, Zilin, Xueli Li, Mengdi Xie, Juan Lu, Dapeng Bao, and Shengjuan Jiang. 2025. "Dynamic Coupling Mechanism of Soil Microbial Community Shifts and Nutrient Fluxes During the Life Cycle of Dictyophora rubrovolvata" Horticulturae 11, no. 8: 989. https://doi.org/10.3390/horticulturae11080989
APA StyleSong, Z., Li, X., Xie, M., Lu, J., Bao, D., & Jiang, S. (2025). Dynamic Coupling Mechanism of Soil Microbial Community Shifts and Nutrient Fluxes During the Life Cycle of Dictyophora rubrovolvata. Horticulturae, 11(8), 989. https://doi.org/10.3390/horticulturae11080989




