Impact of Substrate Amount and Fruiting Induction Methods in Lentinula edodes Cultivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate Production
2.2. Spawn Production
2.3. Production and Harvesting
2.4. Fruiting Induction
- Submersion method: The substrate (rectangular cube and cylindrical blocks) was transferred to tanks filled with water until they were completely submerged, for 8 h. The tank has a volume measurement of 2.05 × 0.57 × 0.84 m. After this period, the blocks were removed and relocated to the cultivation chamber, maintaining the previous environmental conditions.
- Injection method: A device with a fine tip, approximately 2 cm in diameter, was introduced into the center of the substrate (rectangular and cylindrical blocks) to a depth of 19 cm. This water injection equipment was designed to facilitate the process of water stress induction. During the procedure, water was injected at a flow rate of 0.045 L per second. The injection rod remained inserted in the block for a duration proportional to the block’s weight: 34 s for 2 kg blocks and 60 s for 3.5 kg blocks. To assemble the water injection system for the cultivation blocks, the following materials and configurations were employed, as exemplified in Figure 2:
- The perforation rod was made of stainless steel with a diameter of 6 mm, featuring a 3 cm pointed tip at its end. A depth limiter, also made of stainless steel, was installed to regulate the insertion length of the rod into the cultivation block. This limiter had a circular shape with a 3 cm diameter. A ½” threaded fitting was adapted at the opposite end to connect the rod to the elbow joint.
- The 90° elbow joint was made of galvanized steel with a ½” thickness. One end received the perforation rod, and the other was fitted with a straight male quick-connect fitting (6 mm x ½” threaded) to allow the attachment of the water supply hose.
- A 6 mm hose (3 m in length) was connected through a hose reducer fitting (6 mm to ½”), enabling the transition to a larger diameter hose.
- The ½” hose (10 m in length) was connected to a water tap using a quick-connect coupling system with a ¾” threaded fitting, which served as the water source.
- The average pressure exerted by the injection rod during the water application process was 70 kPa.
- The water used was untreated and sourced directly from an artesian well. It was stored in a conical water tank with a total capacity of 10,000 L, with the following specifications: column height of 6.0 m, cone height of 0.8 m, upper section (“bowl”) height of 3.2 m, total height of 10.0 m, column diameter of 0.95 m, and bowl diameter of 1.91 m.
2.5. Analyzed Variables and Experimental Design
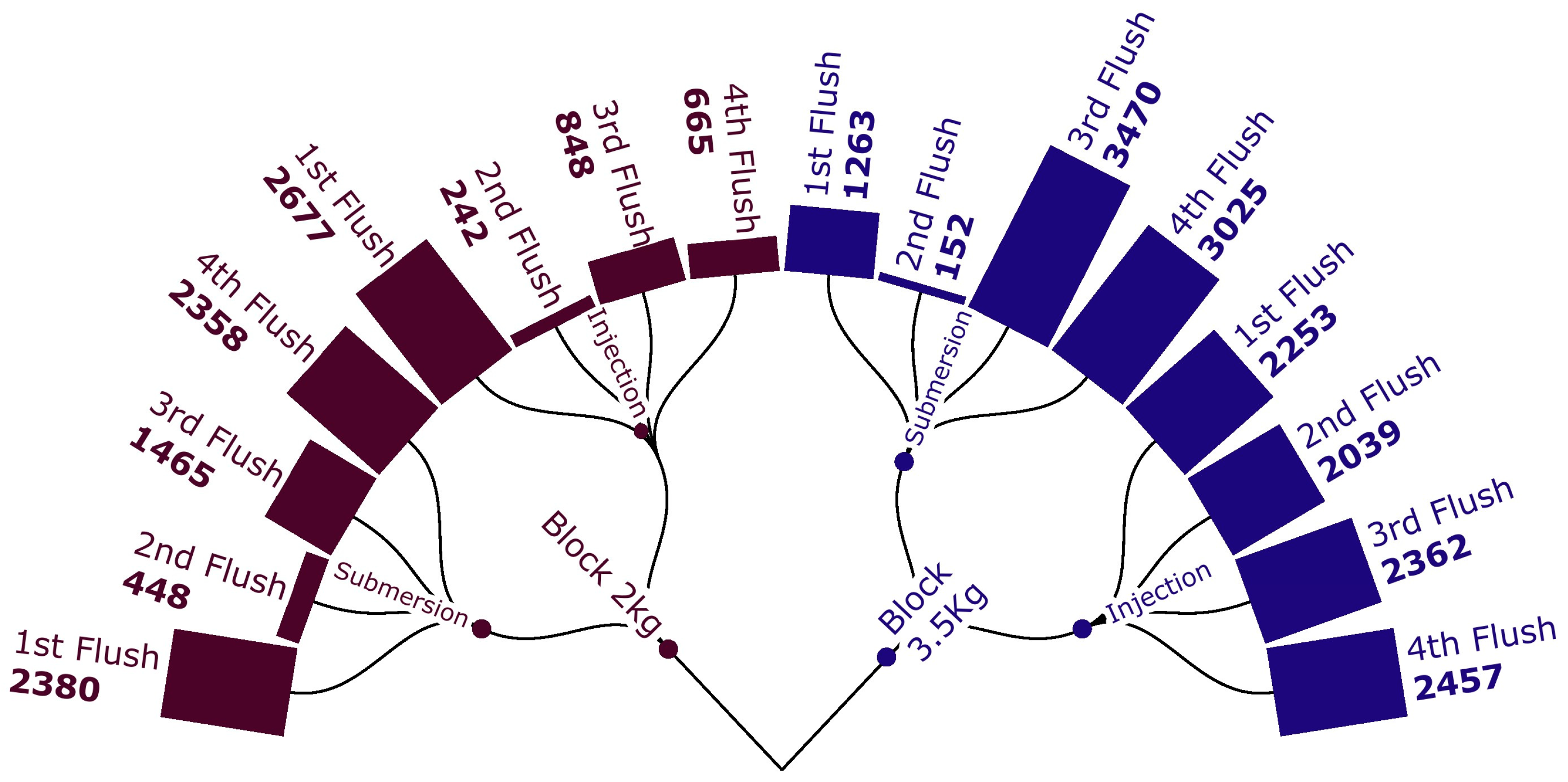
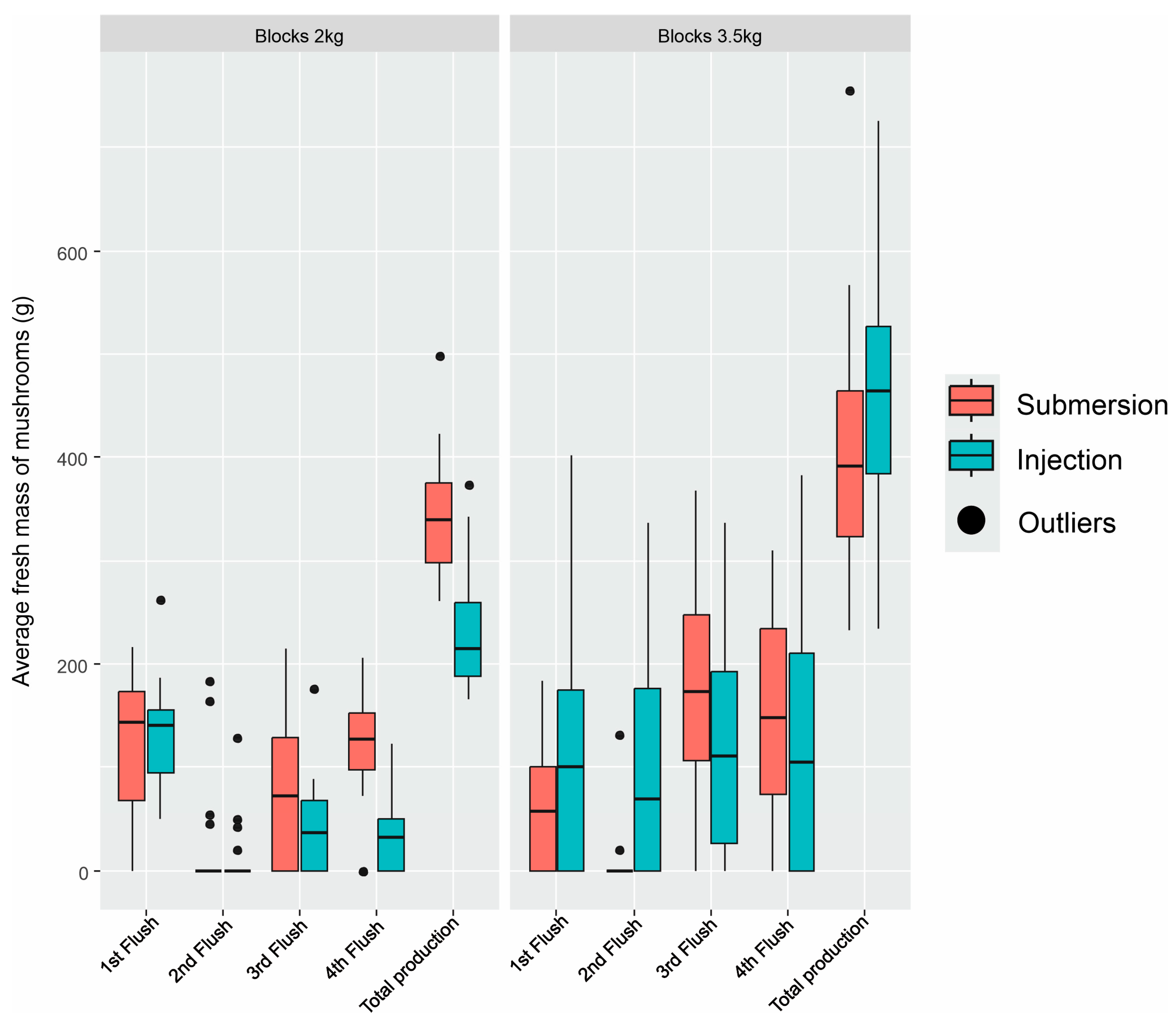
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmad, I.; Arif, M.; Xu, M.; Zhang, J.; Ding, Y.; Lyu, F. Therapeutic values and nutraceutical properties of shiitake mushroom (Lentinula edodes): A review. Trends Food Sci. Technol. 2023, 134, 123–135. [Google Scholar] [CrossRef]
- Fukushima-Sakuno, E. Bioactive small secondary metabolites from the mushrooms Lentinula edodes and Flammulina velutipes. J. Antibiot. 2020, 73, 687–696. [Google Scholar] [CrossRef]
- Roszczyk, A.; Turło, J.; Zagożdżon, R.; Kaleta, B. Immunomodulatory properties of polysaccharides from Lentinula edodes. Int. J. Mol. Sci. 2022, 23, 8980. [Google Scholar] [CrossRef]
- Royse, D.J.; Baars, J.; Tan, Q. Current overview of mushroom production in the world. In Edible and Medicinal Mushrooms: Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 5–13. [Google Scholar] [CrossRef]
- Sánchez, J.E.; Zied, D.C.; Albertó, E. Edible mushroom production in the Americas. In Proceedings of the 9th International Conference on Mushroom Biology and Mushroom Products, Shanghai, China, 12–19 November 2018; pp. 2–11. [Google Scholar]
- Atila, F. Compositional changes in lignocellulosic content of some agro-wastes during the production cycle of shiitake mushroom. Sci. Hortic. 2019, 245, 263–268. [Google Scholar] [CrossRef]
- Zied, D.C.; Maciel, W.P.; Marques, S.C.; da Silveira e Santos, D.M.; Rinker, D.L.; Dias, E.S. Selection of strains for shiitake production in axenic substrate. World J. Microbiol. Biotechnol. 2016, 32, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, F.; Fu, Y.; Li, D.; Sun, X.; Li, C.; Song, B.; Li, Y. Effects of mixed agro-residues (corn crop waste) on lignin-degrading enzyme activities, growth, and quality of Lentinula edodes. RSC Adv. 2020, 10, 9798–9807. [Google Scholar] [CrossRef] [PubMed]
- Sarhadi, H.R.; Ramezan, D.; Zarabi, M.M.; Pirnia, M.; Dehsorkhi, A.N.; Yousefshahi, B. Evaluation of physical and chemical properties of substrate components in the production process of shiitake mushroom (Lentinula edodes (Berk.) Pegler). J. Plant Prod. 2021, 28, 131–146. [Google Scholar] [CrossRef]
- MAPA, Ministério da Agricultura, Pecuária e Abastecimento, Brasil. Instrução Normativa SDA Nº 17. Métodos Analíticos Oficiais Para Análise de Substratos Para Plantas e Condicionadores de Solo; Secretaria de Defesa Agropecuária: Brasília, Brazil, 2017; p. 8.
- MAPA, Ministério da Agricultura, Pecuária e Abastecimento, Brasil. Manual de Métodos Analíticos Oficiais Para Fertilizantes e Corretivos; Secretaria de Defesa Agropecuária: Brasília, Brazil, 2017; p. 240.
- Moreaux, K. Spawn Production. In Edible and Medicinal Mushrooms: Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 89–128. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://www.R-project.org (accessed on 23 May 2024).
- Flourish, a Canva UK Operations Limited Brand. Data Visualization and Storytelling, London, UK, 2024. Available online: https://flourish.studio/ (accessed on 25 March 2025).
- Yu, G.; Li, X.; Zhao, S.; Sun, S.; Yu, Y.; Chen, J.; Cheng, X.H.; Li, W. Waste apple wood: A safe and economical alternative substrate for the cultivation of Pleurotus ostreatus and Lentinula edodes. Folia Hortic. 2022, 34, 1–13. [Google Scholar] [CrossRef]
- Sassine, Y.N.; Nabhan, S.; Rachkidy, E.; El Sebaaly, Z. Valorization of agro-forest wastes (oak acorns, vineyard pruning, and olive pruning) through the cultivation of shiitake (Lentinula edodes) mushrooms. Heliyon 2024, 10, e32562. [Google Scholar] [CrossRef] [PubMed]
- Zied, D.C.; Silva, B.D.; Caitano, C.E.C.; Vieira Junior, W.G.; da Silva Freitas, M.A.; Teixeira, P.A.G.; Pardo-Giménez, A. Use of Eucalyptus Charcoal Waste in the Formulation of Substrate for the Cultivation of Two Strains (LED 20/11 and LED 20/12) of Lentinula edodes. Agronomy 2024, 14, 811. [Google Scholar] [CrossRef]
- Vieira Junior, W.G.; Caitano, C.E.C.; da Silva Alves, L.; Teixeira, P.A.G.; Noble, R.; Pardo, J.E.; Zied, D.C. From waste to resource: Sustainable reuse of spent shiitake mushroom substrate in subsequent production cycles. Int. Biodeterior. Biodegrad. 2025, 200, 106034. [Google Scholar] [CrossRef]
- Abilio, D.P.; de Oliveira, G.L.P.; Martins, O.G.; da Silva Motta, S.; Siqueira, O.A.P.A.; de Andrade, M.C.N. Produção de Lentinula edodes em toras de híbridos de Eucalyptus grandis e Eucalyptus urophylla. Rev. Em Agronegócio E Meio Ambiente 2020, 13, 1433–1446. [Google Scholar] [CrossRef]
- Urben, A.F. Produção de Cogumelos Por Meio de Tecnologia Chinesa Modificada: Biotecnologia e Aplicações na Agricultura e na Saúde, 3rd ed.; Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA): Brasilia, Brazil, 2017; p. 274.
- Castro, C.P.D.; Abreu, C.G.D.; Moraes, T.S.J.D.; Zied, D.C.; Siqueira, F.G.D.; Dias, E.S. Shiitake mushroom cultivation in composted substrate: Is it possible? Ciênc. Agrotec. 2024, 48, e013224. [Google Scholar] [CrossRef]
- Avila, I.A.F.; da Silva Alves, L.; Zied, D.C. Bioconversion of rice straw by Lentinula edodes under different spawn formulations. Braz. J. Microbiol. 2023, 54, 3137–3146. [Google Scholar] [CrossRef]
- Kim, I.Y.; Kim, S.C.; Noh, J.H.; Choi, S.G.; Lee, W.H.; Ko, H.G.; Park, H.S.; Koo, C.D. Effects of productivity of Lentinula edodes according to the control of high-temperature environment in summer. J. Mushroom 2015, 13, 288–293. [Google Scholar] [CrossRef]
- Kobayashi, N.; Wada, N.; Yokoyama, H.; Tanaka, Y.; Suzuki, T.; Habu, N.; Konno, N. Extracellular enzymes secreted in the mycelial block of Lentinula edodes during hyphal growth. AMB Express 2023, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Shang, X.; Zhang, M.; Yu, H.; Zhang, D.; Tan, Q.; Song, C. Cultivation methods and biology of Lentinula edodes. Appl. Microbiol. Biotechnol. 2025, 109, 63. [Google Scholar] [CrossRef]
- Shah, T.D. Analysis of consumer perceptions regarding mushroom consumption in their regular diet: A case of Western-India (Gujarat). Sarhad J. Agric. 2021, 37, 613–621. [Google Scholar]
- Cianni, R.; Pippinato, L.; Mancuso, T. A systematic review on drivers influencing consumption of edible mushrooms and innovative mushroom-containing products. Appetite 2023, 182, 106454. [Google Scholar] [CrossRef]
- Li, C.; Xu, S. Edible mushroom industry in China: Current state and perspectives. Appl. Microbiol. Biotechnol. 2022, 106, 3949–3955. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Hong, S.C.; Rinker, D.L.; Choi, M.S.; Lee, B.H.; Yang, J.K. Effects of nutrient composition on yield and quality of mushroom in Lentinula edodes cultivation using softwood sawdust. J. Korean Wood Sci. Technol. 2010, 38, 124–134. [Google Scholar] [CrossRef][Green Version]
- Frankl, J. Changes in swelling and water absorption of wood degraded by brown rot fungi. Adv. Mater. Res. 2013, 778, 818–822. [Google Scholar] [CrossRef]
- Ogawa, K.; Yashima, T. Enhanced water uptake in the longitudinal direction by shiitake mycelium in shiitake cultivation logs: Increase in effective diffusion coefficient based on mass of liquid water uptake. Wood Sci. Technol. 2021, 55, 1237–1267. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Eid, E.M.; Al-Huqail, A.A.; Širić, I.; Adelodun, B.; Abou Fayssal, S.; Valadez-Blanco, R.; Goala, M.; Ajibade, F.O.; Choi, K.S.; et al. Kinetic studies on delignification and heavy metals uptake by shiitake (Lentinula edodes) mushroom cultivated on agro-industrial wastes. Horticulturae 2022, 8, 316. [Google Scholar] [CrossRef]
- El Sebaaly, Z.; Assadi, F.; Sassine, Y.N.; Shaban, N. Substrate types effect on nutritional composition of button mushroom (Agaricus bisporus). J. Agric. For. 2019, 65, 73–80. [Google Scholar] [CrossRef]
- Shiomi, H.F.; Minhoni, M.T.D.A.; Machado, J.O.; Cargnelutti Filho, A. Thermal and mechanical shocks affecting the first flush of production of Lentinula edodes on Eucalyptus saligna logs. Braz. J. Microbiol. 2007, 38, 200–203. [Google Scholar] [CrossRef][Green Version]
- Mao, L.; Cone, J.W.; Hendriks, W.H.; Sonnenberg, A.S. Wheat bran addition improves Ceriporiopsis subvermispora and Lentinula edodes growth on wheat straw, but not delignification. Anim. Feed Sci. Technol. 2020, 259, 114361. [Google Scholar] [CrossRef]
- Raut, J.K. Current status, challenges and prospects of mushroom industry in Nepal. Int. J. Agric. Econ. 2019, 4, 154–160. [Google Scholar] [CrossRef]
- Sexsmith, K.; Huerta-Arredondo, I.; Gorgo-Simcox, M.; Palacios, E.; Griffin, M.A. Farm Labor Shortage in the Pennsylvania Mushroom Industry: A Gender-Sensitive Comparison of Farmer and Farmworker Perspectives. Rural Sociol. 2024, 89, 404–430. [Google Scholar] [CrossRef]
- Kim, M.J.; Jeong, Y.S.; Kim, E.; Jang, Y.; Ka, K.H. Selection of Lentinula edodes sawdust cultivation cultivars suitable for sawdust block medium cultivation. Korea J. Mycol. 2023, 51, 81–90. [Google Scholar] [CrossRef]
- Robinson, B.; Winans, K.; Kendall, A.; Dlott, J.; Dlott, F. A life cycle assessment of Agaricus bisporus mushroom production in the USA. Int. J. LCA 2019, 24, 456–467. [Google Scholar] [CrossRef]
- Silva, S.T.D.; Atapattu, N.S.B.M.; Kumara, K.L.W. The Water Footprint of Oyster mushroom (Pleurotus ostreatus) Cultivation under Small-scale Polybag Farming Conditions in Sri Lanka. J. Agric. Sci.–Sri Lanka 2023, 18, 172–182. [Google Scholar] [CrossRef]
- Mekonnen, M.M.; Hoekstra, A.Y. A global assessment of the water footprint of farm animal products. Ecosystems 2012, 15, 401–415. [Google Scholar] [CrossRef]
- Governo do Brasil, Tabelas de Preços Referencias Para o Segmentos Específicos de Obras. Available online: https://www.gov.br/mdr/pt-br/assuntos/seguranca-hidrica/projeto-sao-francisco/Tabelas_de_Precos_Referenciais___PISF_e_Segmentos_especificos_de_obras___RL.pdf (accessed on 23 April 2025).
- Agência IBGE notícias, Instituto Brasileiro de Geografia e Estatística. Em 2020, Para Cada R$ 1,00 Gerado Pela Economia Foram Consumidos 6,2 Litros de Água. Available online: https://agenciadenoticias.ibge.gov.br/agencia-noticias/2012-agencia-de-noticias/noticias/37054-em-2020-para-cada-r-1-00-gerado-pela-economia-foram-consumidos-6-2-litros-de-agua (accessed on 23 April 2025).

| Variables Analyzed | Unit of Measurement | Results |
|---|---|---|
| N | 0.703 ± 0.01 | |
| Ca | % | 0.629 ± 0.01 |
| Mg | 0.321 ± 0.01 | |
| S | 0 ± 0.01 | |
| Na | mg/kg | 226.124 ± 3.85 |
| C/N ratio | 75 ± 1.37 | |
| Electrical conductivity | mS/cm | 1.768 ± 0.06 |
| pH | 5.89 ± 0.09 | |
| Primordia Induction | Block of 2 kg | Block of 3.5 kg |
|---|---|---|
| Yield, % | ||
| Submersion | 16.62 ± 0.67 a A | 11.30 ± 0.81 b |
| Injection | 11.08 ± 0.70 b B | 13.01 ± 0.82 a |
| CV, % | 25.96 | |
| Biological efficiency, % | ||
| Submersion | 41.55 ± 1.67 a A | 28.25 ± 2.03 b |
| Injection | 27.7 ± 1.75 b B | 32.52 ± 2.05 a |
| CV, % | 25.96 | |
| Number of mushrooms, u | ||
| Submersion | 10.15 ± 0.67 a A | 7.95 ± 0.68 b |
| Injection | 6.90 ± 0.68 b B | 9.20 ± 0.87 a |
| CV, % | 38.24 | |
| Mushroom weight, g | ||
| Submersion | 34.63 ± 1.83 b | 51.93 ± 2.26 a |
| Injection | 34.20 ± 2.02 b | 54.66 ± 3.92 a |
| CV, % | 26.95 | |
| Primordia Induction | Block of 2 kg | Block of 3.5 kg |
|---|---|---|
| Weight gain before 2nd flush, % | ||
| Submersion | 33.1 ± 2.20 a A | 21.1 ± 1.85 b A |
| Injection | 18.7 ± 2.11 B | 13.0 ± 2.10 B |
| CV, % | 33.12 | |
| Weight gain before 3rd flush, % | ||
| Submersion | 20.4 ± 1.43 | 18.5 ± 1.54 A |
| Injection | 21.6 ± 0.92 a | 9.0 ± 1.26 b B |
| CV, % | 33.61 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, B.d.S.; Vieira Junior, W.G.; de Paula, A.T.C.; Santana, A.B.; Freitas, M.A.d.S.; Hirai, M.M.; Alves, L.d.S.; Zied, D.C. Impact of Substrate Amount and Fruiting Induction Methods in Lentinula edodes Cultivation. Horticulturae 2025, 11, 915. https://doi.org/10.3390/horticulturae11080915
Rocha BdS, Vieira Junior WG, de Paula ATC, Santana AB, Freitas MAdS, Hirai MM, Alves LdS, Zied DC. Impact of Substrate Amount and Fruiting Induction Methods in Lentinula edodes Cultivation. Horticulturae. 2025; 11(8):915. https://doi.org/10.3390/horticulturae11080915
Chicago/Turabian StyleRocha, Bruno de Souza, Wagner Gonçalves Vieira Junior, Adriano Taffarel Camargo de Paula, Asser Botelho Santana, Marcos Antônio da Silva Freitas, Milton Mineo Hirai, Lucas da Silva Alves, and Diego Cunha Zied. 2025. "Impact of Substrate Amount and Fruiting Induction Methods in Lentinula edodes Cultivation" Horticulturae 11, no. 8: 915. https://doi.org/10.3390/horticulturae11080915
APA StyleRocha, B. d. S., Vieira Junior, W. G., de Paula, A. T. C., Santana, A. B., Freitas, M. A. d. S., Hirai, M. M., Alves, L. d. S., & Zied, D. C. (2025). Impact of Substrate Amount and Fruiting Induction Methods in Lentinula edodes Cultivation. Horticulturae, 11(8), 915. https://doi.org/10.3390/horticulturae11080915






