Exogenous 24-Epibrassinolide Alleviated Selenium Stress in Peach Seedling
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Condition
2.2. Experimental Treatments
2.3. Plant Biomass Determination
2.4. Total Selenium Content Determination
2.5. Inorganic Se and Organic Se Contents Determination
2.6. Selenium Content Determination in Plant Subcellular Distribution
2.7. Determination of Selenium Chemical Forms
2.8. Gene Expression Analysis Using Quantitative Real-Time PCR (qRT-PCR)
2.9. Statistical Analysis
3. Result
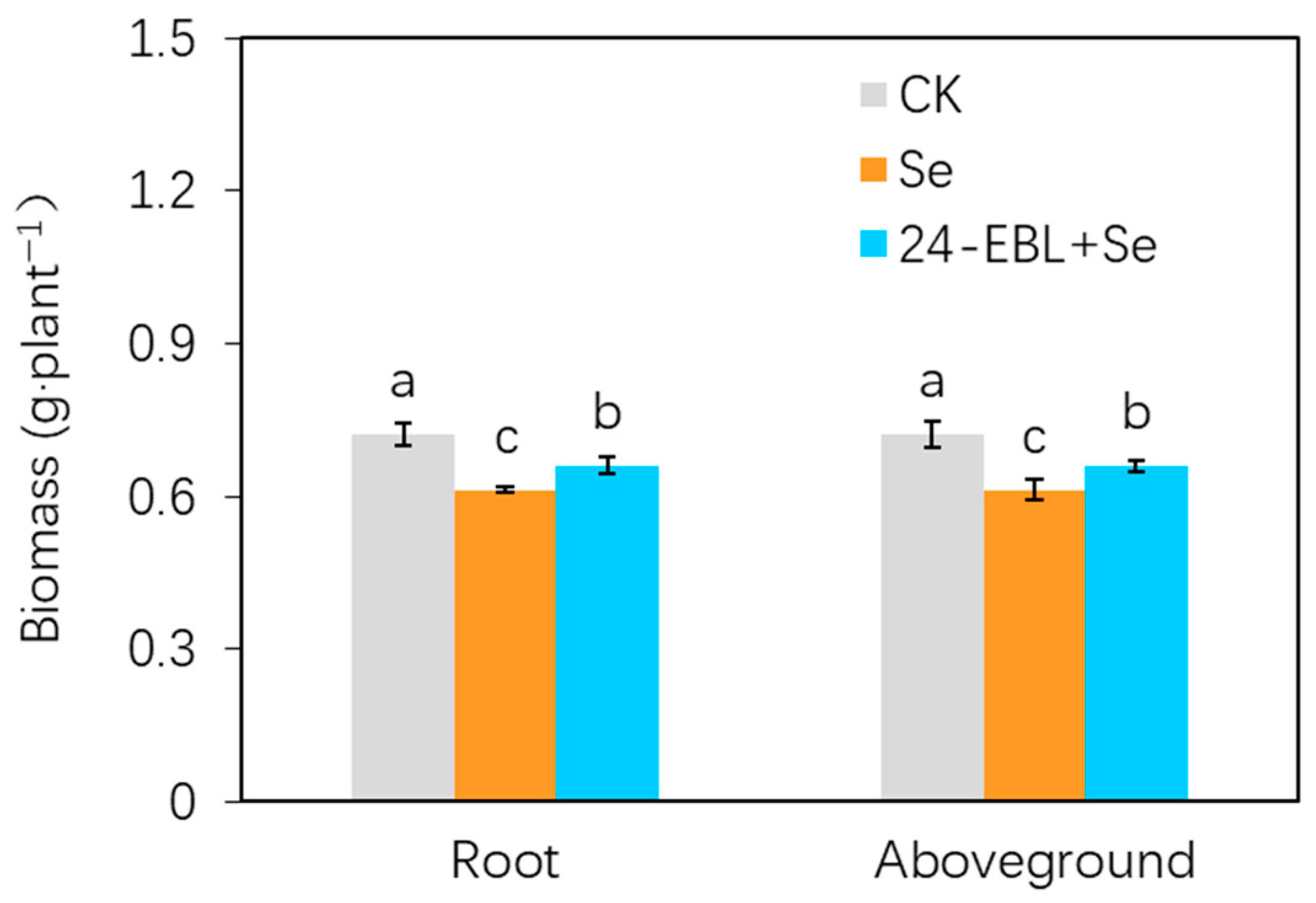
3.1. Biomass of Peach Seedlings
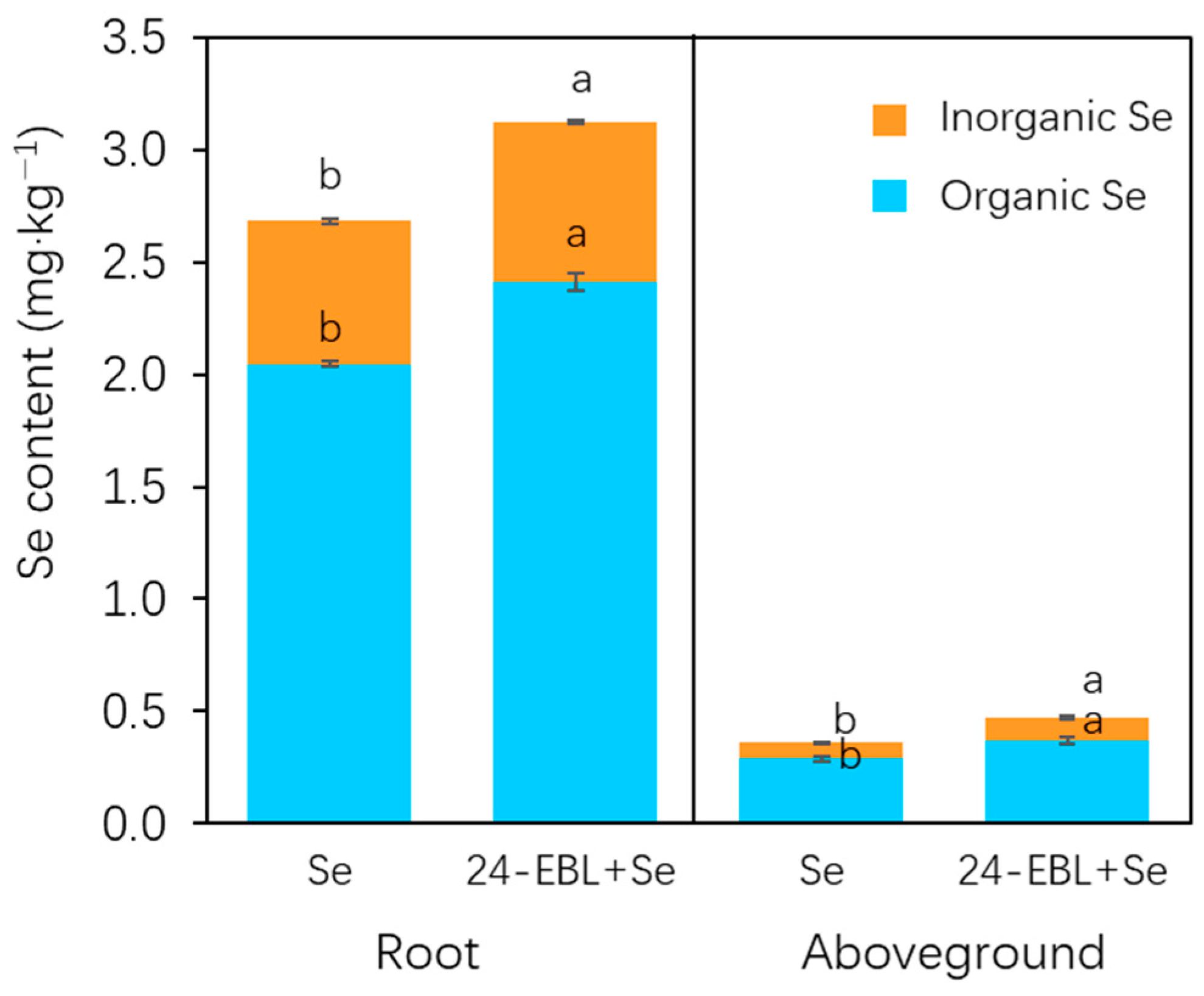
3.2. Selenium Content in Peach Seedling
3.3. Subcellular Distribution of Selenium in Peach Seedlings
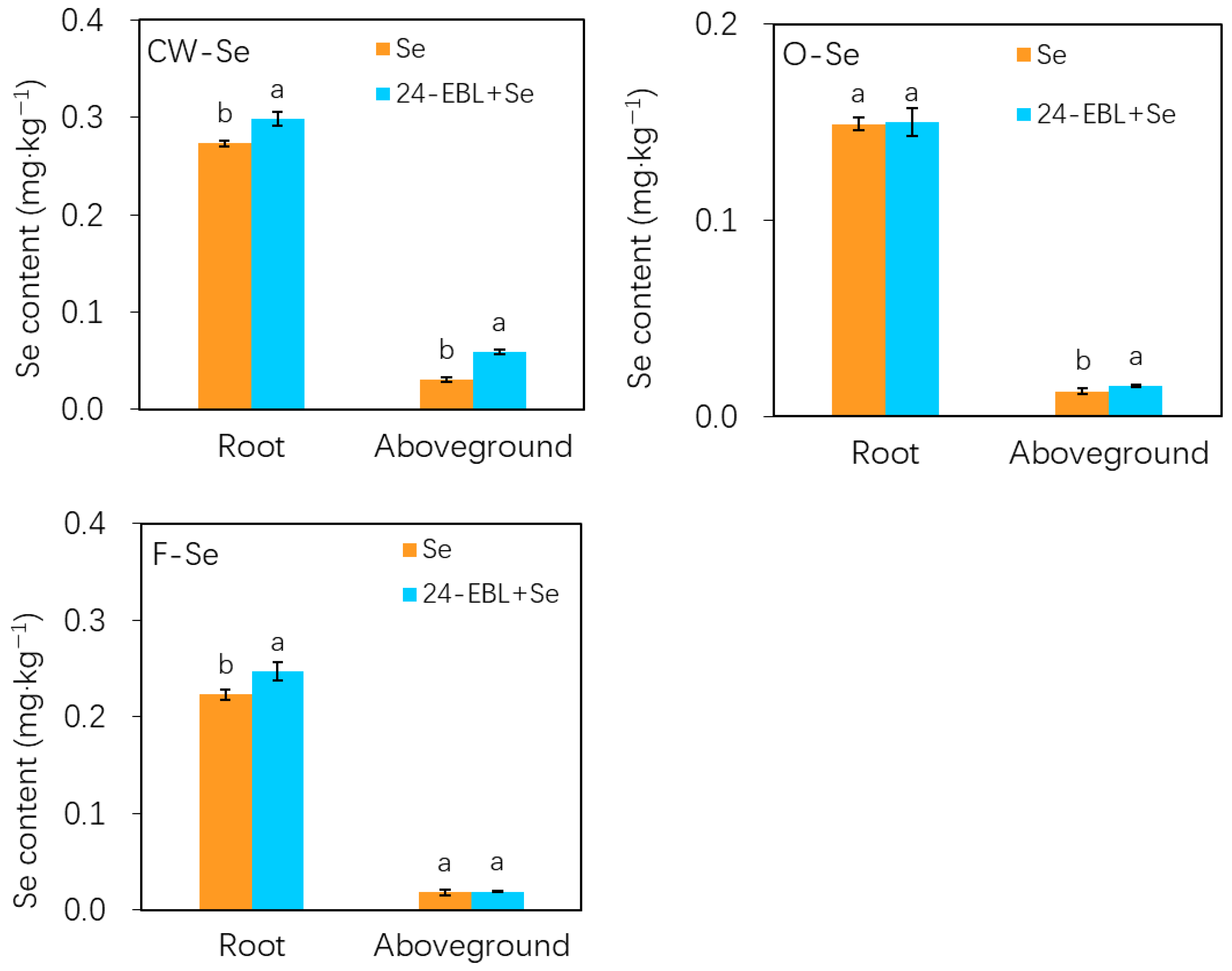
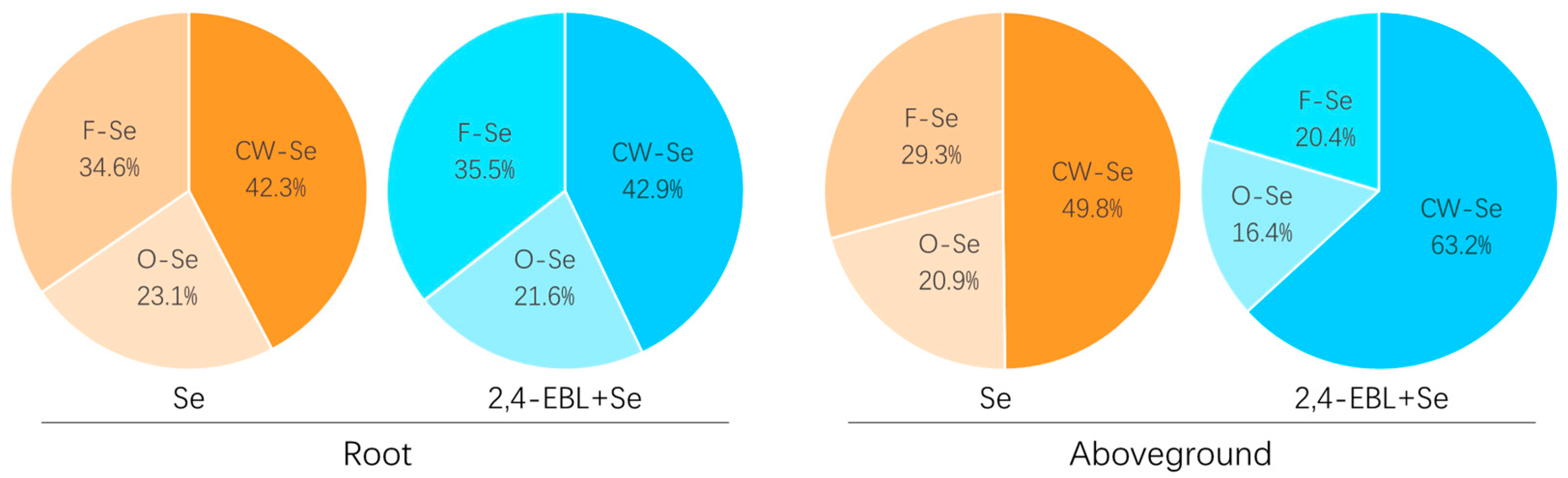
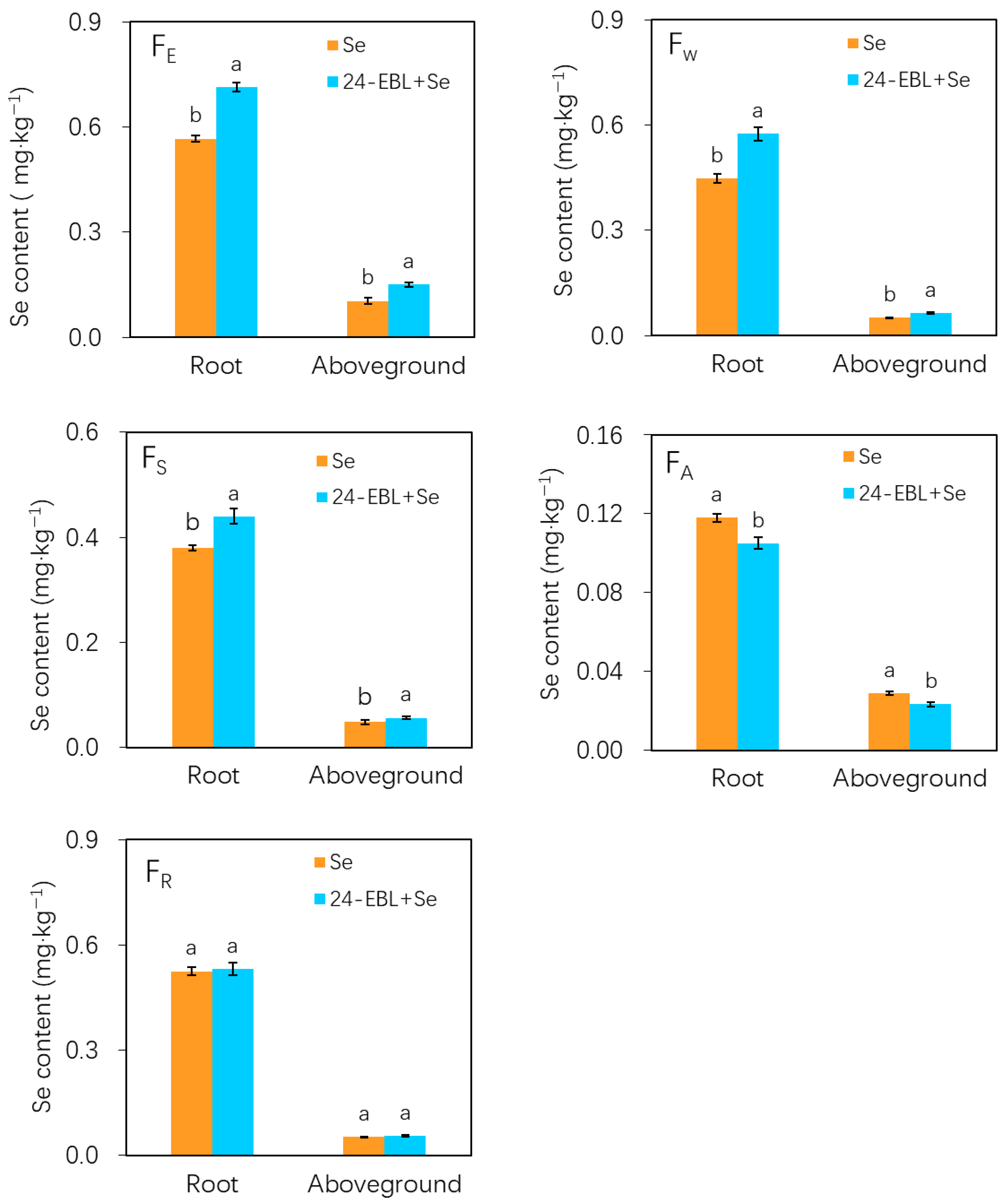
3.4. Chemical Forms of Selenium in Peach Seedlings
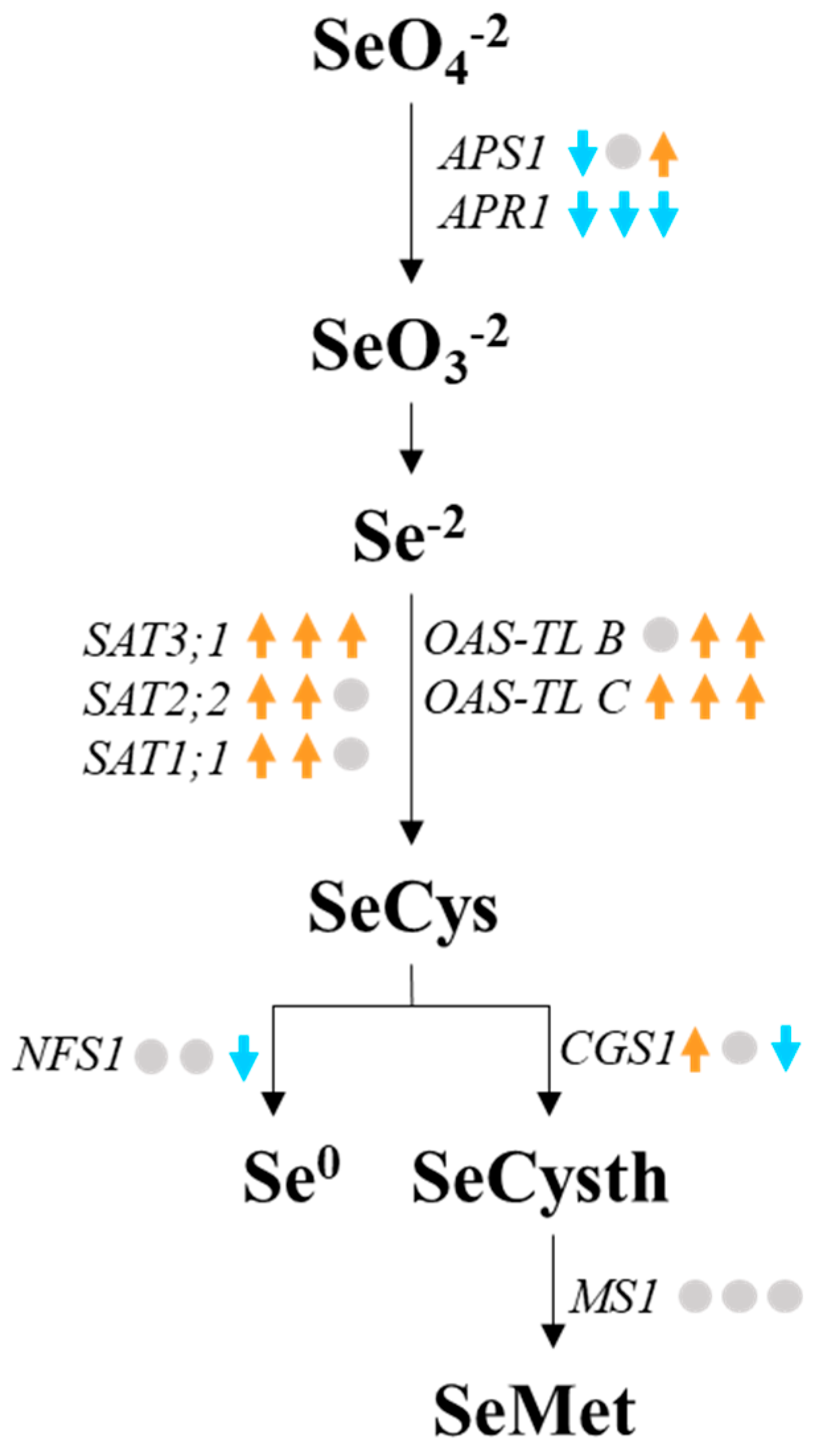
3.5. Expression Analysis of Selenium Metabolism-Related Genes in Peach Seedlings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hartikainen, H. Biogeochemistry of selenium and its impact on food chain quality and human health. J. Trace Elem. Med. Biol. 2005, 18, 309–318. [Google Scholar] [CrossRef]
- Lv, Q.; Liang, X.; Nong, K.; Gong, Z.; Qin, T.; Qin, X.; Wang, D.; Zhu, Y. Advances in research on the toxicological effects of selenium. Bull. Environ. Contam. Toxicol. 2021, 106, 715–726. [Google Scholar] [CrossRef]
- Rayman, M.P.; Infante, H.G.; Sargent, M. Food-chain selenium and human health: Spotlight on speciation. Br. J. Nutr. 2008, 100, 238–253. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Z.; Luo, L.Y.; Fu, P.N.; Wang, Q.; Li, H.F. Selenium uptake and biotransformation in brassica rapa supplied with selenite and selenate: A hydroponic work with HPLC speciation and RNA-sequencing. J. Agric. Food Chem. 2019, 67, 12408–12418. [Google Scholar] [CrossRef]
- Song, J.; Yu, S.; Yang, R.; Xiao, J.; Liu, J. Opportunities for the use of selenium nanoparticles in agriculture. NanoImpact 2023, 31, 100478. [Google Scholar] [CrossRef]
- Fordyce, F.M. Selenium deficiency and toxicity in the environment. In Essentials of Medical Geology, 1st ed.; Selinus, O., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 375–416. [Google Scholar]
- Thiry, C.; Ruttens, A.; Temmerman, L.D.; Schneider, Y.J.; Pussemier, L. Current knowledge in species-related bioavailability of selenium in food. Food Chem. 2012, 130, 767–784. [Google Scholar] [CrossRef]
- Winkel, L.H.; Johnson, C.A.; Lenz, M.; Grundl, T.; Leupin, O.X.; Amini, M.; Charlet, L. Environmental selenium research: From microscopic processes to global understanding. Environ. Sci. Technol. 2012, 46, 571–579. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, J.; Li, F.; Zeng, X.; Wu, Z.; Huang, Y.; Xue, Y.; Wang, Y. High concentrations of Se inhibited the growth of rice seedlings. Plants 2024, 13, 1580. [Google Scholar] [CrossRef]
- Xue, T.; Hartikainen, H.; Piironen, V. Antioxidative and growth-promoting effect of selenium on senescing lettuce. Plant Soil 2001, 237, 55–61. [Google Scholar] [CrossRef]
- Li, J.; Liang, D.; Qin, S.; Feng, P.; Wu, X. Effects of selenite and selenate application on growth and shoot selenium accumulation of pak choi (Brassica chinensis L.) during successive planting conditions. Sci. Pollut. Res. 2015, 22, 11076–11086. [Google Scholar] [CrossRef]
- Reynolds, R.J.B.; Pilon-Smits, E.A.H. Plant selenium hyperaccumulation-Ecological effects and potential implications for selenium cycling and community structure. Biochim. Biophys. Acta (BBA) Gen. Subj. 2018, 1862, 2372–2382. [Google Scholar] [CrossRef]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Wani, A.S.; Ahmad, A.; Hayat, S.; Tahir, I. Epibrassinolide and proline alleviate the photosynthetic and yield inhibition under salt stress by acting on antioxidant system in mustard. Plant Physiol. Biochem. 2019, 135, 385–394. [Google Scholar] [CrossRef]
- Ali, M.M.; Anwar, R.; Malik, A.U.; Khan, A.S.; Ahmad, S.; Hussain, Z.; Hasan, M.U.; Nasir, M.; Chen, F. Plant Growth and Fruit Quality Response of Strawberry is Improved After Exogenous Application of 24-Epibrassinolide. J. Plant Growth Regul. 2021, 41, 1786–1799. [Google Scholar] [CrossRef]
- Krishna, P.; Prasad, B.D.; Rahman, T. Brassinosteroid action in plant abiotic stress tolerance. Methods Mol. Biol. 2017, 1564, 193–202. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, A.; Bhardwaj, R. Plant steroidal hormone epibrassinolide regulate-heavy metal stress tolerance in Oryza sativa L. by modulating antioxidant defense expression. Environ. Exp. Bot. 2016, 122, 1–9. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Choudhary, S.P.; Chen, S.; Xia, X.J.; Shi, K.; Zhou, Y.; Yu, J. Role of brassinosteroids in alleviation of phenanthrene–cadmium co-contamination-induced photosynthetic inhibition and oxidative stress in tomato. J. Exp. Bot. 2013, 64, 199–213. [Google Scholar] [CrossRef]
- Kumar, N.; Sharma, V.; Kaur, G.; Lata, C.; Dasila, H.; Perveen, K.; Khan, F.; Gupta, V.K.; Khanam, M.N. Brassinosteroids as promoters of seedling growth and antioxidant activity under heavy metal zinc stress in mung bean (Vigna radiata L.). Front. Microbiol. 2023, 14, 1259103. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, D.; Li, Z.; Liu, X.; Liu, H.; Yan, K.; Liu, B.; Zhang, J.; Lin, L. Melatonin and 24-epibrassinolide promote the growth and selenium uptake of wild peach seedlings under selenium stress. Environ. Prog. Sustain. Energy 2025, 44, e14615. [Google Scholar] [CrossRef]
- Lin, L.J.; Li, Z.Y.; Wang, J.; Liang, D.; Xia, H.; Lv, X.L.; Tang, Y.; Wang, X.; Deng, Q.X.; Liao, M. 24-epibrassinolide promotes selenium uptake in grapevine under selenium stress. Sci. Hortic. 2023, 308, 111564. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Wang, J.; Liang, D.; Xia, H.; Lv, X.; Deng, Q.; Wang, X.; Luo, X.; Liao, M.; et al. Gibberellic acid promotes selenium accumulation in Cyphomandra betacea under selenium stress. Front. Plant Sci. 2022, 13, 968768. [Google Scholar] [CrossRef]
- Wirtz, M.; Hell, R. Functional analysis of the cysteine synthase protein complex from plants: Structural, biochemical and regulatory properties. J. Plant Physiol. 2006, 163, 273–286. [Google Scholar] [CrossRef]
- Wirtz, M.; Beard, K.F.M.; Lee, C.P.; Boltz, A.; Schwarzländer, M.; Fuchs, C.; Meyer, A.J.; Heeg, C.; Sweetlove, L.J.; Ratcliffe, R.G.; et al. Mitochondrial cysteine synthase complex regulates O-acetylserine biosynthesis in plants. J. Biol. Chem. 2012, 287, 27941–27947. [Google Scholar] [CrossRef]
- Li, H.; Luo, N.; Zhang, L.J.; Zhao, H.M.; Li, Y.W.; Cai, Q.Y.; Wong, M.H.; Mo, C.H. Do arbuscular mycorrhizal fungi affect cadmium uptake kinetics, subcellular distribution and chemical forms in rice? Sci. Total Environ. 2016, 571, 1183–1190. [Google Scholar] [CrossRef]
- Hall, J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Li, K.; Wan, Y.; Wang, Q.; Zhuang, Z.; Guo, Y.; Li, H. Uptake, translocation and biotransformation of selenium nanoparticles in rice seedlings (Oryza sativa L.). J. Nanobiotechnol. 2020, 18, 103. [Google Scholar] [CrossRef]
- Tangjaidee, P.; Swedlund, P.; Xiang, J.; Yin, H.; Quek, S.Y. Selenium-enriched plant foods: Selenium accumulation, speciation, and health functionality. Front. Nutr. 2023, 9, 962312. [Google Scholar] [CrossRef]
- Liu, H.; Shi, Z.; Li, J.; Zhao, P.; Qin, S.; Nie, Z. The impact of phosphorus supply on selenium uptake during hydroponics experiment of winter wheat (Triticum aestivum) in China. Front. Plant Sci. 2018, 9, 373. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.H.; Quinn, C.F.; Hell, R.; Mendel, R.R. Selenium metabolism in plants. In Cell Biology of Metals and Nutrients, 1st ed.; Hell, R., Mendel, R.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 225–241. [Google Scholar] [CrossRef]
- Freeman, J.L.; Zhang, L.H.; Marcus, M.A.; Fakra, S.; McGrath, S.P.; Pilon-Smits, E.A. Spatial imaging, speciation, and quantification of selenium in the hyperaccumulator plants Astragalus bisulcatus and Stanleya pinnata. Plant Physiol. 2006, 142, 124–134. [Google Scholar] [CrossRef]
- Both, E.B.; Shao, S.; Xiang, J.; Jókai, Z.; Yin, H.; Liu, Y.; Magyar, A.; Dernovics, M. Selenolanthionine is the major water-soluble selenium compound in the selenium tolerant plant Cardamine violifolia. Biochim. Biophys. Acta (BBA) Gen. Subj. 2018, 1862, 2354–2362. [Google Scholar] [CrossRef]
- Freeman, J.L.; Tamaoki, M.; Stushnoff, C.; Quinn, C.F.; Cappa, J.J.; Devonshire, J.; Fakra, S.C.; Marcus, M.A.; McGrath, S.P.; Van Hoewyk, D.; et al. Molecular mechanisms of selenium tolerance and hyperaccumulation in Stanleya pinnata. Plant Physiol. 2010, 153, 1630–1652. [Google Scholar] [CrossRef] [PubMed]
- Shibagaki, N.; Rose, A.; McDermott, J.P.; Fujiwara, T.; Hayashi, H.; Yoneyama, T.; Davies, J.P. Selenate-resistant mutants of Arabidopsis thaliana identify Sultr1;2, a sulfate transporter required for efficient transport of sulfate into roots. Plant J. 2002, 29, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Trippe, R.C.; Elizabeth, A.H.; Smits, P. Selenium transport and metabolism in plants: Phytoremediation and biofortification implications. J. Hazard. Mater. 2021, 404, 124178. [Google Scholar] [CrossRef] [PubMed]
- Revilleza, M.J.; Galvez, A.F.; Krenz, D.C.; Lumen, B.O.D. An 8 kDa methionine-rich protein from soybean (Glycine max) cotyledon: Identification, purification, and n-terminal sequence. J. Agric. Food Chem. 1996, 44, 2930–2935. [Google Scholar] [CrossRef]
- White, P.J.; Bowen, H.C.; Marshall, B.; Broadley, M.R. Extraordinarily high leaf selenium to sulfur ratios define ‘Se-accumulator’ plants. Ann. Bot.-Lond. 2007, 100, 111–118. [Google Scholar] [CrossRef]
- Finley, J.W.; Sigrid-Keck, A.; Robbins, R.J.; Hintze, K.J. Selenium enrichment of broccoli: Interactions between selenium and secondary plant compounds. J. Nutr. 2005, 135, 1236–1238. [Google Scholar] [CrossRef]
- Kaur, M.; Sharma, S. Selenium affects protein accumulation and its distribution in different protein fractions in developing wheat grains. Cereal Res. Commun. 2021, 49, 109–117. [Google Scholar] [CrossRef]
- Dumont, E.; Vanhaecke, F.; Cornelis, R. Selenium speciation from food source to metabolites: A critical review. Anal. Bioanal. Chem. 2006, 385, 1304–1323. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hang, Z.; Cao, Q.; Du, Y.; Zhang, J.; Lin, L.; Zhang, M.; Wang, X. Exogenous 24-Epibrassinolide Alleviated Selenium Stress in Peach Seedling. Horticulturae 2025, 11, 909. https://doi.org/10.3390/horticulturae11080909
Hang Z, Cao Q, Du Y, Zhang J, Lin L, Zhang M, Wang X. Exogenous 24-Epibrassinolide Alleviated Selenium Stress in Peach Seedling. Horticulturae. 2025; 11(8):909. https://doi.org/10.3390/horticulturae11080909
Chicago/Turabian StyleHang, Zhiyu, Qizhe Cao, Yunyao Du, Jinrong Zhang, Lijin Lin, Mingfei Zhang, and Xun Wang. 2025. "Exogenous 24-Epibrassinolide Alleviated Selenium Stress in Peach Seedling" Horticulturae 11, no. 8: 909. https://doi.org/10.3390/horticulturae11080909
APA StyleHang, Z., Cao, Q., Du, Y., Zhang, J., Lin, L., Zhang, M., & Wang, X. (2025). Exogenous 24-Epibrassinolide Alleviated Selenium Stress in Peach Seedling. Horticulturae, 11(8), 909. https://doi.org/10.3390/horticulturae11080909





