Algae Extracts and Zeolite Modulate Plant Growth and Enhance the Yield of Tomato Solanum lycopersicum L. Under Suboptimum and Deficient Soil Water Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Geographic Location
2.2. Chemical Features of Organic and Inorganic Products
2.2.1. Algae Extract
2.2.2. Zeolite
2.3. Experimental Design
2.4. Application of Algae Extract and Zeolite
2.5. Irrigation System
2.6. Variables
2.6.1. Climate
2.6.2. Plant Growth (Plant Height, Stem Thickness, and Plant Vigor)
2.6.3. Physiological Indicators
2.6.4. Yield Components and Tomato Yield
2.7. Data Analysis
3. Results
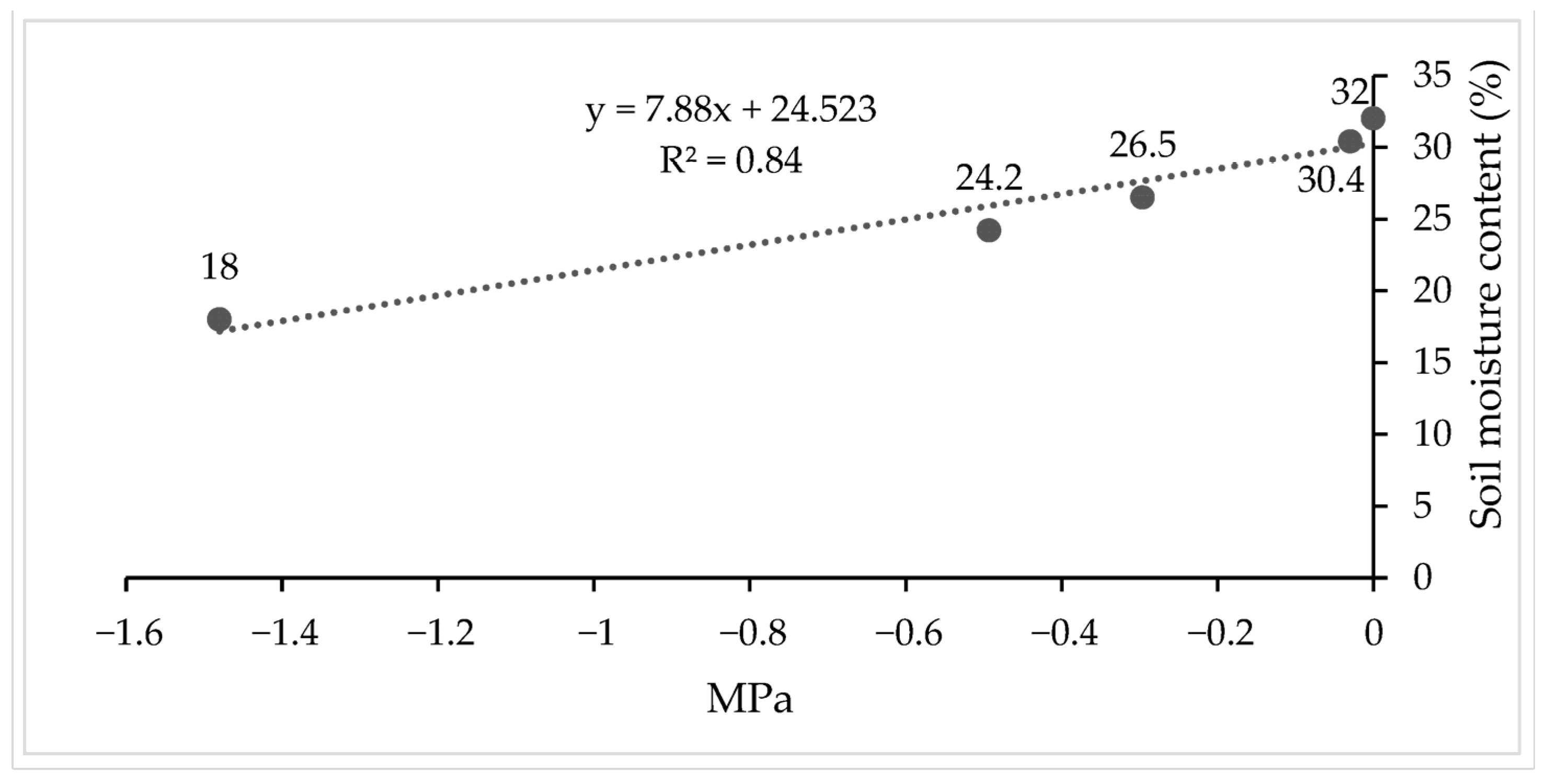
3.1. Climatic Behavior in the Study
3.2. Analyis of Variance
3.3. Plant Growth (Plant Height, Stem Thickness, and Plant Vigor)
3.4. Physiological Indicators
3.5. Productivity Indicators and Tomato Yield
4. Discussion
4.1. Plant Growth
4.2. Physiological Indicators
4.3. Productivity Indicators and Tomato Yield
4.4. Tomato Yield
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muhammad, M.; Majeed, M.; Nazim, M.; Liu, Y.-H.; Li, L.; Li, W.-J. Soil salinity and drought tolerance: An evaluation of plant growth, productivity, microbial diversity, and amelioration strategies. Plant Stress 2024, 11, 100319. [Google Scholar] [CrossRef]
- Mancosu, N.; Snyder, R.L.; Kyriakakis, G.; Spano, D. Water Scarcity and Future Challenges for Food Production. Water 2015, 7, 975–992. [Google Scholar] [CrossRef]
- Nasir, M.W.; Toth, Z. Effect of Drought Stress on Potato Production: A Review. Agronomy 2022, 12, 635. [Google Scholar] [CrossRef]
- Wahab, A.; Muhammad, M.; Munir, A.; Abdi, G.; Zaman, W.; Ayaz, A.; Khizar, C.; Reddy, S.P.P. Role of Arbuscular Mycorrhizal Fungi in Regulating Growth, Enhancing Productivity, and Potentially Influencing Ecosystems under Abiotic and Biotic Stresses. Plants 2023, 12, 3102. [Google Scholar] [CrossRef]
- Suliman, M.; Scaini, A.; Manzoni, S.; Vico, G. Soil properties modulate actual evapotranspiration, and precipitation impacts on crop yields in the USA. Sci. Total. Environ. 2024, 949, 175172. [Google Scholar] [CrossRef] [PubMed]
- Xolocotzi-Acoltzi, S.; Pedroza-Sandoval, A.; García-De los Santos, G.; Álvarez-Vázquez, P.; Gramillo-Ávila, I. Growth, Produc-tivity, Yield Components and Seasonality of Different Genotypes of Forage Clover Lotus corniculatus L. under Varied Soil Moisture Contents. Plants 2024, 13, 1407. [Google Scholar] [CrossRef]
- Yáñez-Chávez, L.G.; Pedroza-Sandoval, A.; Trejo-Calzada, R.; Sánchez-Cohen, I.; Velásquez-Valle, M.A. Growth, Physiology, and Productivity of Bouteloua gracilis and Cenchrus ciliaris Using Moisture Retainers under Different Planting Methods. Agriculture 2023, 13, 1134. [Google Scholar] [CrossRef]
- Pedroza-Parga, E.; Pedroza-Sandoval, A.; Velasquez-Valle, M.A.; Sánchez-Cohen, I.; Trejo-Calzada, R.; Samaniego-Gaxiola, J.A. Effect of soil cover on the growth and productivity of buffel grass (Cenchrus ciliaris L.) in degraded soils of arid zones. Rev. Mex. Cienc. Pecu. 2022, 13, 866–878. [Google Scholar] [CrossRef]
- Rai, G.K.; Magotra, I.; Khanday, D.M.; Choudhary, S.M.; Bhatt, A.; Gupta, V.; Rai, P.K.; Kumar, P. Boosting Drought Tolerance in Tomatoes through Stimulatory Action of Salicylic Acid Imparted Antioxidant Defense Mechanisms. Agronomy 2024, 14, 1227. [Google Scholar] [CrossRef]
- Núñez-Gómez, D.; Legua, P.; Lidón, V.; Conesa, A.; Martínez-Nicolás, J.J.; Melgarejo, P. Evaluation of Agricultural Soil-Improving Zeolite for Improving Irrigation Water Quality. Appl. Sci. 2024, 14, 418. [Google Scholar] [CrossRef]
- Wahab, A.; Abdi, G.; Saleem, M.H.; Ali, B.; Ullah, S.; Shah, W.; Mumtaz, S.; Yasin, G.; Muresan, C.C.; Marc, R.A. Plants’ Physio-Biochemical and Phyto-Hormonal Responses to Alleviate the Adverse Effects of Drought Stress: A Comprehensive Review. Plants 2022, 11, 1620. [Google Scholar] [CrossRef]
- Morales-Meléndez, R.; Arroyo-Ramírez, A.; Camposeco-Montejo, N.; Méndez-López, A.; López-Pérez, M.C. Ascophyllum Nodosum y Nitrato de Calcio como Bioestimulantes en el Desarrollo y Rendimiento de Cultivo de Tomate. Cienc. Lat. Rev. Científica Multidiscip. 2024, 8, 1574–1589. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant Properties of Seaweed Extracts in Plants: Implications towards Sustainable Crop Production. Plants 2021, 10, 531. [Google Scholar] [CrossRef]
- Cardarelli, M.; El Chami, A.; Rouphael, Y.; Ciriello, M.; Bonini, P.; Erice, G.; Cirino, V.; Basile, B.; Corrado, G.; Choi, S.; et al. Plant biostimulants as natural alternatives to synthetic auxins in strawberry production: Physiological and metabolic in-sights. Front. Plant Sci. 2024, 14, 1337926. [Google Scholar] [CrossRef]
- Almaroai, Y.A.; Eissa, M.A. Role of Marine Algae Extracts in Water Stress Resistance of Onion Under Semiarid Conditions. J. Soil Sci. Plant Nutr. 2020, 20, 1092–1101. [Google Scholar] [CrossRef]
- Hassan, R.H.; El-Said, N.A.; El-Sayed, A.B. Effect of Algae Extracts on Growth, Yield, and Essential Oil of Fennel (Foeniculum vulgare Mill.) Plant. Sci. J. Flowers Ornam. Plants 2022, 9, 363–372. [Google Scholar] [CrossRef]
- Othman, S.A.; Soliman, M.S.S.; Ramadan, A.I. Application of algae and zeolite in wastewater treatment: Effects on maize properties and soil fertility. Water Sci. 2024, 38, 391–403. [Google Scholar] [CrossRef]
- Liu, H.; Xiao, C.; Qiu, T.; Deng, J.; Cheng, H.; Cong, X.; Cheng, S.; Rao, S.; Zhang, Y. Selenium Regulates Antioxidant, Photosynthesis, and Cell Permeability in Plants under Various Abiotic Stresses: A Review. Plants 2022, 12, 44. [Google Scholar] [CrossRef]
- Azpilcueta-Pérez, M.E.; Pedroza-Sandoval, A.; Sánchez-Cohen, I.; Trejo-Calzada, R.; Jacobo-Salcedo, M.R. Effect of Selenium supply on some attributes and mineral content in bean seed (Phaseolus vulgaris L.). Rev. Bio Cienc. 2022, 9, e1270. [Google Scholar]
- Khalvandi, M.; Siosemardeh, A.; Roohi, E.; Keramati, S. Salicylic acid alleviated the effect of drought stress on photosynthetic characteristics and leaf protein pattern in winter wheat. Heliyon 2021, 7, e05908. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Olvera, M.; Martin-Vasquez, C.; Mayordomo, C.; Illescas-Miranda, J.; Bono, M.; Coego, A.; Alonso, J.; Hernández-González, M.; Jiménez-Arias, D.; Forment, J.; et al. ABA-receptor agonist iSB09 decreases soil water consumption and increases tomato CO2 assimilation and water use efficiency under drought stress. Environ. Exp. Bot. 2024, 225, 105847. [Google Scholar] [CrossRef]
- Cataldo, E.; Salvi, L.; Paoli, F.; Fucile, M.; Masciandaro, G.; Manzi, D.; Masini, C.M.; Mattii, G.B. Application of Zeolites in Agriculture and Other Potential Uses: A Review. Agronomy 2021, 11, 1547. [Google Scholar] [CrossRef]
- Méndez-Argüello, B.; Saldivar, R.H.L. Uso potencial de la zeolita en la agricultura sustentable de la nueva revolución verde. Ecosistemas y Recur. Agropecu. 2019, 6, 191–193. [Google Scholar] [CrossRef]
- Murnane, J.G.; Brennan, R.B.; Fenton, O.; Healy, M.G. Zeolite Combined with Alum and Polyaluminum Chloride Mixed with Agricultural Slurries Reduces Carbon Losses in Runoff from Grassed Soil Boxes. J. Environ. Qual. 2016, 45, 1941–1948. [Google Scholar] [CrossRef]
- Villalba, M.d.L.; Rubio, A.H.O.; Colmenero, S.L.H.; Ochoa, R.J.M.; Mireles, G.F. Utilización y Aplicación de Zeolitas Naturales su Importancia en México; Universidad Autónoma de Chihuahua, Chih. - CONACYT: Chihuahua, México, 2019; p. 160. [Google Scholar]
- Guaya, D.; Valderrama, C.; Farran, A.; Cortina, J.L. Modification of a natural zeolite with Fe(III) for simultaneous phosphate and ammonium removal from aqueous solutions. J. Chem. Technol. Biotechnol. 2015, 91, 1737–1746. [Google Scholar] [CrossRef]
- Bhat, J.A.; Shivaraj, S.M.; Singh, P.; Navadagi, D.B.; Tripathi, D.K.; Dash, P.K.; Solanke, A.U.; Sonah, H.; Deshmukh, R. Role of Silicon in Mitigation of Heavy Metal Stresses in Crop Plants. Plants 2019, 8, 71. [Google Scholar] [CrossRef]
- Khan, A.; Khan, A.L.; Muneer, S.; Kim, Y.-H.; Al-Rawahi, A.; Al-Harrasi, A. Silicon and salinity: Crosstalk in crop-mediated stress tol-erance mechanisms. Front. Plant Sci. 2019, 10, 1429. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Bhat, J.A.; Siddiqui, M.H.; Rinklebe, J.; Ahmad, P.; Singh, V. Integration of silicon and secondary metabolites in plants: A significant association in stress tolerance. J. Exp. Bot. 2020, 71, 6758–6774. [Google Scholar] [CrossRef]
- Bojórquez-Quintal, E.; Escalante-Magaña, C.; Echevarría-Machado, I.; Martínez-Estévez, M. Aluminum, a Friend or Foe of Higher Plants in Acid Soils. Front. Plant Sci. 2017, 8, 1767. [Google Scholar] [CrossRef]
- Ofoe, R.; Thomas, R.H.; Asiedu, S.K.; Wang-Pruski, G.; Fofana, B.; Abbey, L. Aluminum in plant: Benefits, toxicity and tol-erance mechanisms. Front. Plant Sci. 2023, 13, 1085998. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, X.Q.; Hu, Z.M.; Shao, J.F.; Che, J.; Chen, R.F.; Dong, X.Y.; Shen, R.F. Aluminium alleviates manganese toxicity to rice by decreasing root symplastic Mn uptake and reducing availability to shoots of Mn stored in roots. Ann. Bot. 2015, 116, 237–246. [Google Scholar] [CrossRef]
- Sun, L.L.; Zhang, M.S.; Liu, X.M.; Mao, Q.Z.; Shi, C.; Kochian, L.V.; Liao, H. Aluminium is essential for root growth and de-velopment of tea plants (Camellia sinensis). J. Integr. Plant Biol. 2020, 2, 984–997. [Google Scholar] [CrossRef]
- Hazrati, S.; Tahmasebi-Sarvestani, Z.; Mokhtassi-Bidgoli, A.; Modarres-Sanavy, S.A.M.; Mohammadi, H.; Nicola, S. Effects of zeolite and water stress on growth, yield and chemical compositions of Aloe vera L. Agric. Water Manag. 2017, 181, 66–72. [Google Scholar] [CrossRef]
- Selahvarzi, Y.; Kamali, M.; Sarfaraz, S.; Zabiha, M. Effect of natural zeolite and drought stress on some physiological and morphological traits of Festuca arundinacea grass. In Proceedings of the XI International Scientific Agricultural Symposium, Jahorina, Bosnia and Herzegovina, 8–9 October 2020; pp. 357–362. [Google Scholar]
- Azpilcueta-Pérez, M.E.; Pedroza-Sandoval, A.; Trejo-Calzada, R.; Sánchez-Cohen, I. y Jacobo-Salcedo, Ma. Del, R. Chemical residuality in maize (Zea mays L.) fields irrigated with deep well water. Rev. Ecosistemas Y Recur. Agropecu. 2018, 5, 111–117. [Google Scholar] [CrossRef]
- Teweldebrihan, M.D.; Dinka, M.O. Sustainable Water Management Practices in Agriculture: The Case of East Africa. Encyclopedia 2025, 5, 7. [Google Scholar] [CrossRef]
- Mota-Ituarte, M.; Pedroza-Sandoval, A.; Minjares-Fuentes, R.; Trejo-Calzada, R.A.; Zegbe, J.; Quezada-Rivera, J.J. Water deficit and salinity modify some morphometric, physiological, and productive attributes of Aloe vera (L.). Bot. Sci. 2023, 101, 463–475. [Google Scholar] [CrossRef]
- Egea, I.; Estrada, Y.; Flores, F.B.; Bolarín, M.C. Improving production and fruit quality of tomato under abiotic stress: Genes for the future of tomato breeding for a sustainable agriculture. Environ. Exp. Bot. 2022, 204, 105086. [Google Scholar] [CrossRef]
- FAO. El Cultivo de Tomate Con Buenas Prácticas Agrícolas en La Agricultura Urbana y Periurbana; Ñemity; FAO: Rome, Italy, 2013; p. 1. [Google Scholar]
- Medina-García, G.; Díaz-Padilla, G.; López-Hernández, J.; Ruiz-Corral, J.A.; Marín-Silva, M. Estadísticas Climatológicas Básicas del Estado de Durango. (Periodo 1961–2003). Campo Experimental Valle del Guadiana. Centro de Investigación Regional Norte Centro-Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias. Man. Técnico 2005, 1, 240. Available online: https://es.scribd.com/document/375829218/Estadisticas-Climatologicas-Basicas-Para-El-Estado-de-Durango (accessed on 8 October 2024).
- Drygaś, B.; Piechowiak, T.; Balawejder, M.; Matłok, N.; Kreczko, J.; Puchalski, C. The Eliciting Effect of Aqueous Extracts from 549 Ascophyllum nodosum Algae on the Cultivation of Arugula (Eruca sativa Mill.) Microgreens. Sustainability 2024, 16, 7436. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, H.; Kaur, H.; Srivastava, S. The beneficial roles of trace and ultratrace elements in plants. Plant Growth Regul. 2023, 100, 219–236. [Google Scholar] [CrossRef]
- Richards, L.A. Porous plate apparatus for measuring moisture retention and transmission by soil. Soil Sci. 1948, 66, 105–110. [Google Scholar] [CrossRef]
- Horsfall, J.G.; Barratt, R.W. An Improved Grading System for Measuring Plant Disease. Phytopathology 1945, 35, 655. Available online: https://en.wikipedia.org/wiki/Horsfall%E2%80%93Barratt_scale (accessed on 12 April 2024).
- De Lima, V.R.; de Mello Prado, R.; Reyes, H.A.; Caione, G. Efecto del horario de medición, posición y porción de la hoja en los índices de clorofila en la papa. Idesia 2014, 32, 23–28. [Google Scholar] [CrossRef]
- Browne, M.; Yardimci, N.T.; Scoffoni, C.; Jarrahi, M.; Sack, L. Prediction of leaf water potential and relative water content using terahertz radiation spectroscopy. Plant Direct 2020, 4, e00197. [Google Scholar] [CrossRef]
- Arminjon, L.; Lefort, F. Quick In Vitro Screening of PGPMs for Salt Tolerance and Evaluation of Induced Tolerance to Saline Stress in Tomato Culture. Microorganisms 2025, 13, 246. [Google Scholar] [CrossRef]
- Pedroza-Sandoval, A.; González-Espíndola, L.A.; Gramillo-Ávila, I.; Miranda-Rojas, J.A. Certain Physiological and Chemical Indicators Drive the Yield and Quality of Cladode Mucilage in Three Fodder Nopal Morphotypes (Opuntia spp.) Under Different Soil Water Content Conditions. Agriculture 2025, 15, 593. [Google Scholar] [CrossRef]
- Chojnacka, K.; Michalak, I.; Dmytryk, A.; Gramza, M.; Słowiński, A.; Górecki, H. Algal Extracts as Plant Growth Biostimulants. In Marine Algae Extracts: Processes, Products, and Applications; Kim, S.-K., Chojnacka, K., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2015; Chapter 11. [Google Scholar] [CrossRef]
- Espinosa-Antón, A.A.; Hernández-Herrera, R.M.; González-González, M. Extractos bioactivos de algas marinas como bio-estimulantes del crecimiento y la protección de las plantas. Biotecnol. Veg. 2020, 20, 257–282. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S2074-86472020000400257&lng=es&tlng=es (accessed on 23 March 2023).
- Bjerring, J.; Ottosen, C.O.; Sindbjerg, F.I.; Zhou, R. Elevated CO2 induce alterations in the hormonal regulation of stomata in drought stressed tomato seedlings. Plant Physiol. Biochem. 2024, 212, 108762. [Google Scholar] [CrossRef]
- Ntanasi, T.; Karavidas, I.; Spyrou, G.P.; Giannothanasis, E.; Aliferis, K.A.; Saitanis, C.; Fotopoulos, V.; Sabatino, L.; Savvas, D.; Ntatsi, G. Plant Biostimulants Enhance Tomato Resilience to Salinity Stress: Insights from Two Greek Landraces. Plants 2024, 13, 1404. [Google Scholar] [CrossRef] [PubMed]
- Sfechis, S.; Vidican, R.; Sandor, M.; Stolen, V.; Sandor, V.; Muste, B. Using Assessment of Zeolite Amendments in Agriculture. ProEnvironment 2015, 8, 85–88. Available online: http://journals.usamvcluj.ro/index.php/promediu (accessed on 1 August 2024).
- Chávez, E.; Díaz, D.; Rangel, E.; Legorreta, F.; López, A.; Trujillo, L.; Reyes, V.; Fuentes, D. Characterization and suitability of Mexican rocks as natural fertilizers for preharvest tomato plant growth. Pol. J. Environ. Stud. 2025, 34, 2049–2061. [Google Scholar] [CrossRef]
- Zhang, C.; Li, W.; Wu, Y.; Li, S.; Hua, B.; Sun, H. Chloroplast Functionality at the Interface of Growth, Defense, and Genetic Innovation: A Multi-Omics and Technological Perspective. Plants 2025, 14, 978. [Google Scholar] [CrossRef]
- Muhammad, N.; Zvobgo, G.; Guo-ping, Z. A review: The beneficial effect of aluminum on plant growth in acid soil and the possible mechanisms. J. Integr. Agric. 2018, 17, 60345–60347. [Google Scholar] [CrossRef]
- Sazali, N.; Harun, Z.; Sazali, N. Effectiveness of Zeolite [LTA] amendment in improving the growth of cherry tomato (Solanum lycopersicum) and its physicochemical properties of soil. J. Aust. Ceram. Soc. 2024, 61, 869–886. [Google Scholar] [CrossRef]
- Méndez, A.B.; Vera, R.I.; Cárdenas, F.A.; Villarreal GDe los, S.; Ibarra, J.L.; Lira, S.R.H. Water holding capacity of substrates containing zeolite and its effect on growth, biomass production and chlorophyll content of Solanum lycopersicum Mill. Nova Sci. 2018, 10, 45–60. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Ma, J.F.; Bélanger, R.R. Editorial: Role of Silicon in Plants. Front. Plant Sci. 2017, 8, 1858. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Téllez, L.I.; Gómez-Merino, F.C. Editorial: Beneficial elements: Novel players in plant biology for innovative crop production, volume II. Front. Plant Sci. 2023, 14, 1303462. [Google Scholar] [CrossRef]
- Boyer, S.J. Relationship of Water Potential to Growth of Leaves. Plant Physiol. 1968, 43, 1056–1062. [Google Scholar] [CrossRef]
- Arcoverde, G.B.; Rodrigues, B.M.; Pompelli, M.F.; Santos, M.G. Water relations and some aspects of leaf metabolism of Jatropha curcas young plants under two water deficit levels and recovery. Braz. J. Plant Physiol. 2011, 23, 123–130. [Google Scholar] [CrossRef]
- González-Espíndola, L.A.; Pedroza-Sandoval, A.; Trejo-Calzada, R.; Jacobo-Salcedo, M.d.R.; García de los Santos, G.; Quezada-Rivera, J.J. Relative Water Content, Chlorophyll Index, and Photosynthetic Pigments on Lotus corniculatus L. in Response to Water Deficit. Plants 2024, 13, 961. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, L.; Valdés, R.; Verdecia, J.; Arias, L.; Medina, R.; Velasco, E. Growth, relative water content, transpiration and pho-tosynthetic pigment content in coffee trees (Coffea arabica L.) growing at different sunlight regimes. Cultiv. Trop. 2001, 22, 37–41. [Google Scholar]
- Florido, B.M.; Bao, F.L. Tolerancia a estrés por déficit hídrico en tomate (Solanum lycopersicum L.). Cultiv. Trop. 2014, 35, 70–88. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0258-59362014000300008&lng=es&tlng=es (accessed on 17 October 2023).
- Górka, B.; Korzeniowska, K.; Lipok, J.; Wieczorek, P.P. The Biomass of Algae and Algal Extracts in Agricultural Production. In Characteristics and Applications; Developments in Applied Phycology; Springer: Cham, Switzerland, 2018; Volume 8. [Google Scholar]
- Muhammad, I.; Shalmani, A.; Ali, M.; Yang, Q.-H.; Ahmad, H.; Li, F.B. Mechanisms Regulating the Dynamics of Photosynthesis Under Abiotic Stresses. Front. Plant Sci. 2021, 11, 615942. [Google Scholar] [CrossRef]
- Li, J.; Van Gerrewey, T.; Geelen, D. A Meta-Analysis of Biostimulant Yield Effectiveness in Field Trials. Front. Plant Sci. 2022, 14, 836702. [Google Scholar] [CrossRef]
- Majid, K.; Gholamin, R.; Jamaati-e-Somarin, I.; Zabihi-eMahmoodabad, R. The leaf chlorophyll content and stress resistance relationship considering in Corn cultivars (Zea. Mays). Adv. Environ. Biol. 2011, 5, 118–122. [Google Scholar]
- Soca, M.; Lorente, E. Efecto de las zeolitas naturales y activadas en la producción de tomate. Agrotec. De Cuba 2015, 39, 67–78. Available online: https://www.grupoagricoladecuba.gag.cu/media/Agrotecnia/pdf/39_2015/No_1/69-80.pdf (accessed on 7 March 2024).
- NORMA Oficial Mexicana. NOM-127-SSA1-2021, Agua Para Uso y Consumo Humano; Límites Permisibles de la Calzidad del Agua; 2021. Available online: https://www.dof.gob.mx/nota_detalle_popup.php?codigo=5650705 (accessed on 12 April 2024).
- Mesa, T.; Polo, J.; Arabia, A.; Caselles, V.; Munné-Bosch, S. Differential physiological response to heat and cold stress of tomato plants and its implication on fruit quality. J. Plant Physiol. 2022, 268, 153581. [Google Scholar] [CrossRef]
- Biglia, A.; Gresta, F.; Patono, D.L.; Comba, L.; Lovisolo, C.; Gay, P.; Schubert, A. Identification of drought-salinity combined stress in tomato plants by vegetation indices. J. Agric. Eng. 2024, 55, 1–9. [Google Scholar] [CrossRef]
- Kipp, J. Optimal Climate Regions in Mexico for Greenhouse Crop Production; Wageningen UR Greenhouse Horticulture: Bleiswijk, The Netherlands, 2020; p. 20. Available online: https://edepot.wur.nl/144644 (accessed on 15 July 2024).
- Moon, J.; Park, Y.J.; Bin Choi, Y.; Truong, T.Q.; Huynh, P.K.; Kim, Y.B.; Kim, S.M. Physiological Effects and Mechanisms of Chlorella vulgaris as a Biostimulant on the Growth and Drought Tolerance of Arabidopsis thaliana. Plants 2024, 13, 3012. [Google Scholar] [CrossRef] [PubMed]
- Murshed, R.; Lopez-Lauri, F.; Sallanon, H. Effect of water stress on antioxidant systems and oxidative parameters in fruits of tomato (Solanum lycopersicon L., cv. Micro-tom). Physiol. Mol. Biol. Plants 2013, 19, 363–378. [Google Scholar] [CrossRef]
- Fan, X.-X.; Xu, Z.-G.; Liu, X.-Y.; Tang, C.-M.; Wang, L.-W.; Han, X.-L. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Al-aghabary, K.; Zhu, Z.; Shi, Q. Influence of Silicon Supply on Chlorophyll Content, Chlorophyll Fluorescence, and Anti-oxidative Enzyme Activities in Tomato Plants Under Salt Stress. J. Plant Nutr. 2005, 27, 2101–2115. [Google Scholar] [CrossRef]
- Pompeiano, A.; Moles, T.M.; Mariotti l Santaniello, A.; Di Baccio, D.; Scartazza, T.; Reyes, H.; Guglielminetti, L. Tomato bio-diversity reveals landrace enhanced drought-adaptive strategy. Plant Physiol. Biochem. 2025, 220, 109495. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S. Response of plants to water stress. Front. Plant Sci. 2014, 13, 86. [Google Scholar] [CrossRef] [PubMed]




| Physical Characteristics | Chemical and Biological Characteristics | ||
|---|---|---|---|
| Macronutrients (ppm) | Micronutrients (ppm) | Phytohormones and Vitamins (ppm) | |
| Humidity: 97.02 | N: 5500 | Zn: 2005 Mg: 1204.5 Fe: 932.6 Si: 3800 Bo 1:00 | Cytokinin: 2900 |
| Proteins: (Nx6.25) 2.27 | P: 67,300 | Ascorbic acid: 129 | |
| Total solids: 2.98 | K < 43,700 | Carotenes: 60 | |
| Biochar: 0.406 | Ca: 1660 | Niacin: 30 | |
| Organic matter: 0.700 | Mg: 882.5 | Riboflavin: 10 | |
| Carbohydrates > 40.00 | S: 1200 | Thiamin: 5 | |
| Physical Characteristics | Chemical Characteristics |
|---|---|
| Color: Greenish pink | Al2O3 (11.99%) |
| Odor: Odorless–earthy | SiO2 (53.45%) |
| pH of 5% aqueous solution: 7.9 | CaO (8.06%) |
| Element adsorption: Rapid | Na2O (1.16%) |
| Selectivity: N > P > K > Ca | Fe2O (33.96%) |
| Element release: Slow | K2O (6.11%) |
| MgO (4.76%) | |
| MnO (0.09%) | |
| P2O (50.23%) |
| VS | DF | Variable | Prob. > F | Significance |
|---|---|---|---|---|
| Blocks | 2 | Height plant | <0.0173 | * |
| Treatments | 7 | <0.0001 | ** | |
| Blocks | 2 | Stem thickness | <0.0142 | * |
| Treatments | 7 | <0.0001 | ** | |
| Blocks | 2 | Plant vigor | <0.0195 | * |
| Treatments | 7 | <0.0001 | ** | |
| Blocks | 2 | Relative water content | <0.0001 | ** |
| Treatments | 7 | <0.0001 | ** | |
| Blocks | 2 | Chlorophyll index | <0.0001 | ** |
| Treatments | 7 | <0.0001 | ** | |
| Blocks | 2 | Photosynthesis | <0.0001 | ** |
| Treatments | 7 | <0.0001 | ** | |
| Blocks | 2 | Number of fruits harvested per plant−1 | <0.2907 | * |
| Treatments | 7 | <0.0001 | ** | |
| Blocks | 2 | Fruit weight per plant−1 | <0.0001 | ** |
| Treatments | 7 | <0.0001 | ** | |
| Blocks | 2 | Yield m−2 | <0.0001 | ** |
| Treatments | 7 | <0.0001 | ** |
| Soil Moisture Content (%) | Organic and Inorganic Products | NFHPP | Fruit Weight (kg Plant−1) | Yield (kg m−2) |
|---|---|---|---|---|
| 25% ± 2 | Control | 2.5 bc ± 0.35 | 0.04 ab ± 0.009 | 0.27 ab ± 0.01 |
| Algae extract (AE) | 6.62 a ± 0.17 | 0.16 ab ± 0.02 | 0.90 a ± 0.04 | |
| Zeolite (Z) | 5.62 a ± 1.23 | 0.17 a ± 0.1 | 0.3 ab ± 0.2 | |
| AE + Z | 6.41 a ± 0.38 | 0.15 ab ± 0.09 | 0.63 ab ± 0.31 | |
| 20% ± 2 | Control | 1.85 bc ± 0.17 | 0.03 b ± 0.01 | 0.18 b ± 0.09 |
| Algae extract (AE) | 2.00 bc ± 0.35 | 0.03 b ± 0.01 | 0.16 b ± 0.1 | |
| Zeolite (Z) | 1.75 c ± 1.41 | 0.03 b ± 0.02 | 0.1 b ± 0.06 | |
| AE + Z | 4.5 ab ± 0.7 | 0.04 ab ± 0.02 | 0.20 b ± 0.12 |
| CCI | PH | ST | VIG | NFHPP | WFHPP | YIELD | |
|---|---|---|---|---|---|---|---|
| CCI | 1.00000 | 0.54398 < 0.0001 | 0.36819 0.0002 | 0.52466 < 0.0001 | 0.50271 < 0.0001 | 0.53735 < 0.0001 | 0.53735 < 0.0001 |
| PH | 1.00000 | 0.42651 < 0.0001 | 0.53038 < 0.0001 | 0.3890 < 0.0001 | 0.50671 < 0.0001 | 0.50671 < 0.0001 | |
| ST | 1.00000 | 0.42944 < 0.0001 | 0.31766 0.0016 | 0.29390 0.0037 | 0.29390 0.0037 | ||
| VIG | 1.00000 | 0.57788 < 0.0001 | 0.71558 < 0.0001 | 0.71558 < 0.0001 | |||
| NFHPP | 1.00000 | 0.73931 < 0.0001 | 0.73931 < 0.0001 | ||||
| WFHPP | 1.00000 | 1.00000 < 0.0001 | |||||
| YIELD | 1.00000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda-Rojas, J.A.; Pedroza-Sandoval, A.; Gramillo-Ávila, I.; Trejo-Calzada, R.; Sánchez-Cohen, I.; Yáñez-Chávez, L.G. Algae Extracts and Zeolite Modulate Plant Growth and Enhance the Yield of Tomato Solanum lycopersicum L. Under Suboptimum and Deficient Soil Water Content. Horticulturae 2025, 11, 902. https://doi.org/10.3390/horticulturae11080902
Miranda-Rojas JA, Pedroza-Sandoval A, Gramillo-Ávila I, Trejo-Calzada R, Sánchez-Cohen I, Yáñez-Chávez LG. Algae Extracts and Zeolite Modulate Plant Growth and Enhance the Yield of Tomato Solanum lycopersicum L. Under Suboptimum and Deficient Soil Water Content. Horticulturae. 2025; 11(8):902. https://doi.org/10.3390/horticulturae11080902
Chicago/Turabian StyleMiranda-Rojas, José Antonio, Aurelio Pedroza-Sandoval, Isaac Gramillo-Ávila, Ricardo Trejo-Calzada, Ignacio Sánchez-Cohen, and Luis Gerardo Yáñez-Chávez. 2025. "Algae Extracts and Zeolite Modulate Plant Growth and Enhance the Yield of Tomato Solanum lycopersicum L. Under Suboptimum and Deficient Soil Water Content" Horticulturae 11, no. 8: 902. https://doi.org/10.3390/horticulturae11080902
APA StyleMiranda-Rojas, J. A., Pedroza-Sandoval, A., Gramillo-Ávila, I., Trejo-Calzada, R., Sánchez-Cohen, I., & Yáñez-Chávez, L. G. (2025). Algae Extracts and Zeolite Modulate Plant Growth and Enhance the Yield of Tomato Solanum lycopersicum L. Under Suboptimum and Deficient Soil Water Content. Horticulturae, 11(8), 902. https://doi.org/10.3390/horticulturae11080902








