Functional Analysis of Penicillium expansum Glucose Oxidase-Encoding Gene, GOX2, and Its Expression Responses to Multiple Environmental Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Fungal Strains
2.2. GOX2 Gene Sequence Acquisition and Retrieval in P. expansum
2.3. Composition and Physicochemical Properties of GOX Amino Acids
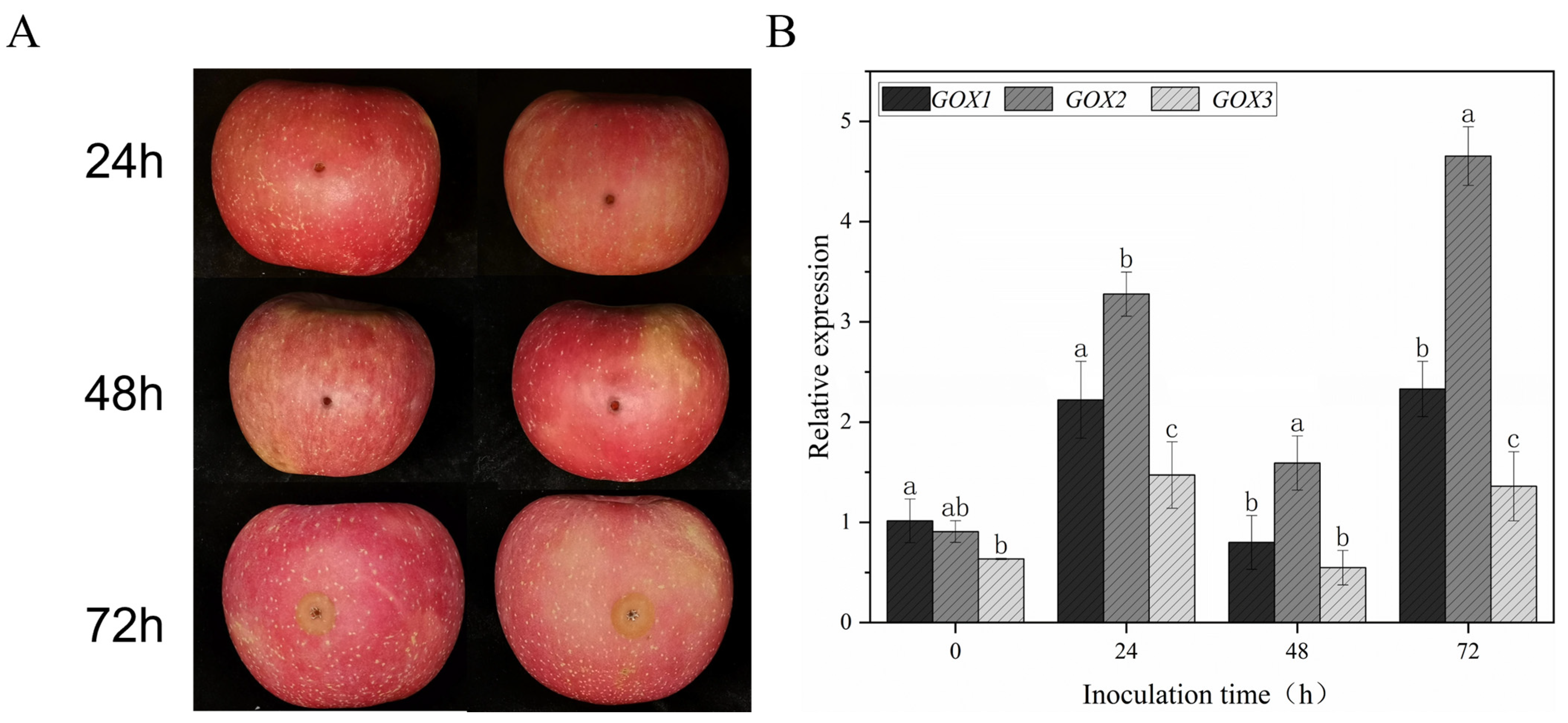
2.4. Relative Expression Analysis of GOX Protein Expression Genes GOX1, GOX2 and GOX3
2.5. Phylogenetic and Conserved Domains Analysis of the GOX2 Gene
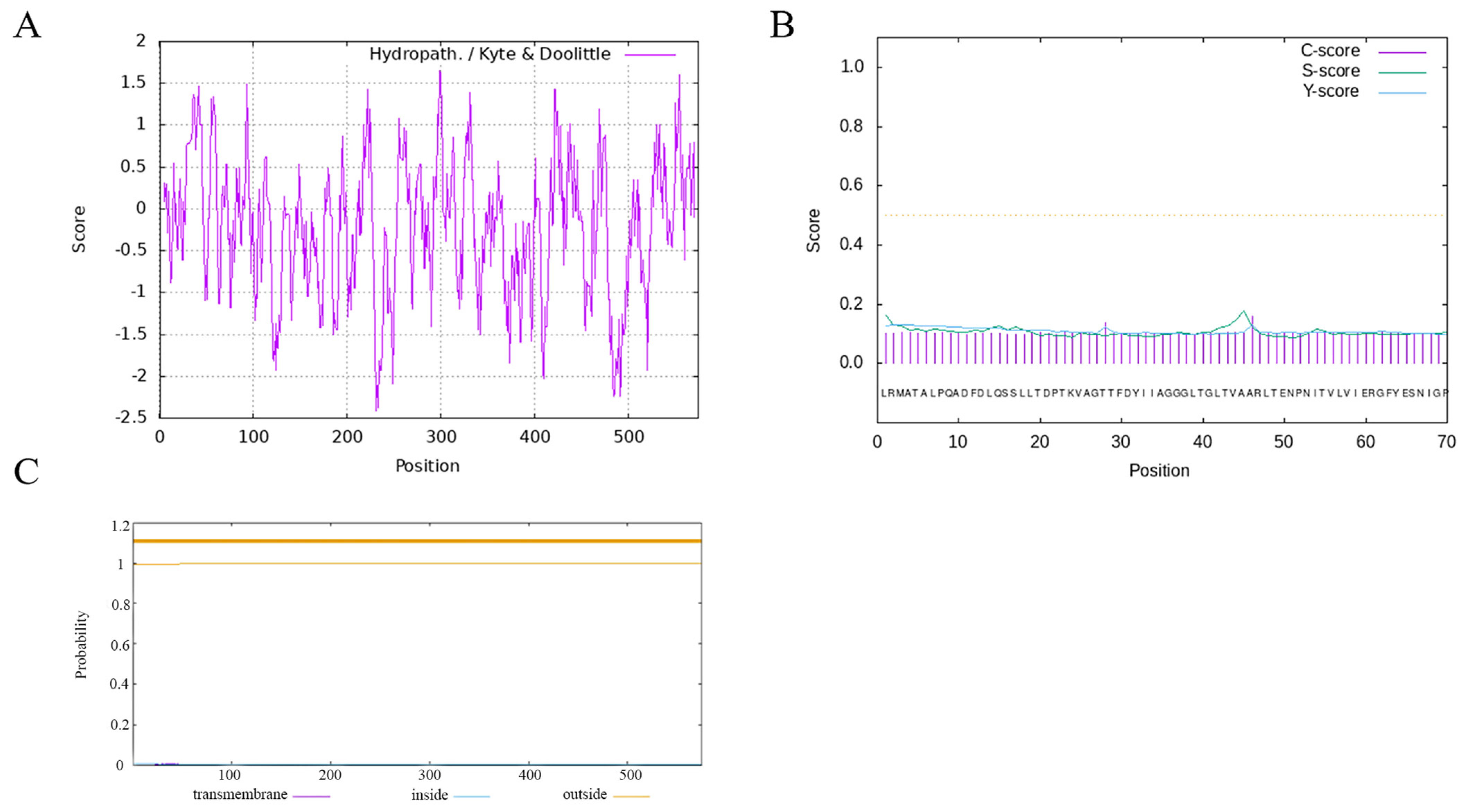
2.6. Hydrophobicity, Transmembrane Helix Structure, and Signal Peptide Prediction Analysis of GOX Protein
2.7. Prediction and Analysis of GOX Secondary Structure
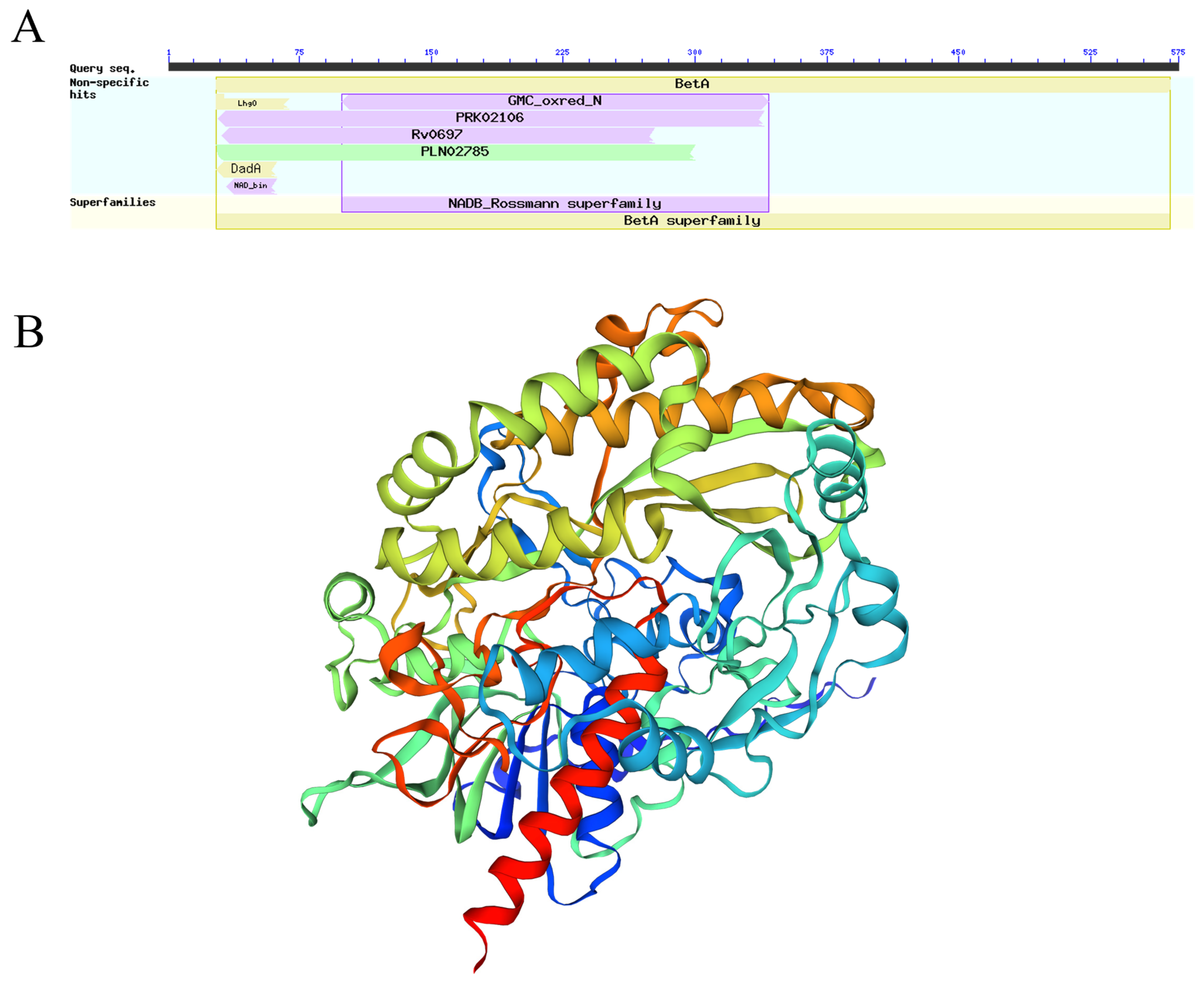
2.8. GOX Conserved Domain Analysis and GOX Tertiary Structure Prediction Analysis
2.9. Analysis of Relative Expression Quantities of GOX2 Gene Under Different Temperature, pH, Carbon Source and Antifungal Agent Conditions
2.10. Statistical Analysis
3. Results
3.1. Sequence Comparison of GOX Gene and Analysis of Physicochemical Properties of GOX Protein
3.2. Gene Expression Analysis and RT-qPCR Validation
3.3. Phylogenetic and Conserved Structural Domain Analysis of the P. expansum GOX2 Gene
3.4. Extended Analysis of Hydrophilicity, Transmembrane Helical Structure and Signal Peptide Prediction of the P. expansum GOX
3.5. GOX Secondary Structure Forecast Analysis
3.6. Analysis of Conserved Structural Domains and Tertiary Structure Prediction Analysis of P. expansum GOX
3.7. Analysis of Relative Expression Levels of GOX2 Under Different Conditions of Temperature, pH, Carbon Substrate and Bacteriostatic Agents
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| GOX | Glucose oxidase |
| GLA | Gluconic acid |
| PAT | Patulin |
| FAD | Flavin Adenine Dinucleotide |
References
- Prusky, D.; Lichter, A. Mechanisms Modulating Fungal Attack in Post-Harvest Pathogen Interactions and Their Control. Eur. J. Plant Pathol. 2008, 121, 281–289. [Google Scholar] [CrossRef]
- Van Zeebroeck, M.; Van Linden, V.; Ramon, H.; De Baerdemaeker, J.; Nicolaï, B.M.; Tijskens, E. Impact Damage of Apples during Transport and Handling. Postharvest Biol. Technol. 2007, 45, 157–167. [Google Scholar] [CrossRef]
- Li, Y.; Jiao, W.; Du, Y.; Wang, M.; Fu, M. Involvement of Organic Acid in the Control Mechanism of ε-Poly-L-Lysine (ε-PL) on Blue Mold Caused by Penicillium expansum in Apple Fruits. Horticulturae 2022, 8, 468. [Google Scholar] [CrossRef]
- Vico, I.; Duduk, N.; Vasic, M.; Nikolic, M. Identification of Penicillium expansum Causing Postharvest Blue Mold Decay of Apple Fruit. Pestic. I Fitomedicina 2014, 29, 257–266. [Google Scholar] [CrossRef]
- Ballester, A.-R.; Marcet-Houben, M.; Levin, E.; Sela, N.; Selma-Lázaro, C.; Carmona, L.; Wisniewski, M.; Droby, S.; González-Candelas, L.; Gabaldón, T. Genome, Transcriptome, and Functional Analyses of Penicillium expansum Provide New Insights Into Secondary Metabolism and Pathogenicity. MPMI 2015, 28, 232–248. [Google Scholar] [CrossRef]
- Wang, K.; Zheng, X.; Zhang, X.; Zhao, L.; Yang, Q.; Boateng, N.A.S.; Ahima, J.; Liu, J.; Zhang, H. Comparative Transcriptomic Analysis of the Interaction between Penicillium expansum and Apple Fruit (Malus pumila Mill.) during Early Stages of Infection. Microorganisms 2019, 7, 495. [Google Scholar] [CrossRef]
- Luciano-Rosario, D.; Keller, N.P.; Jurick, W.M. Penicillium expansum: Biology, Omics, and Management Tools for a Global Postharvest Pathogen Causing Blue Mould of Pome Fruit. Mol. Plant Pathol. 2020, 21, 1391–1404. [Google Scholar] [CrossRef]
- Mahato, D.K.; Kamle, M.; Sharma, B.; Pandhi, S.; Devi, S.; Dhawan, K.; Selvakumar, R.; Mishra, D.; Kumar, A.; Arora, S.; et al. Patulin in Food: A Mycotoxin Concern for Human Health and Its Management Strategies. Toxicon 2021, 198, 12–23. [Google Scholar] [CrossRef]
- Prusky, D.; McEvoy, J.L.; Saftner, R.; Conway, W.S.; Jones, R. Relationship Between Host Acidification and Virulence of Penicillium spp. on Apple and Citrus Fruit. Phytopathology 2004, 94, 44–51. [Google Scholar] [CrossRef]
- Barad, S.; Horowitz, S.B.; Kobiler, I.; Sherman, A.; Prusky, D. Accumulation of the Mycotoxin Patulin in the Presence of Gluconic Acid Contributes to Pathogenicity of Penicillium expansum. MPMI 2014, 27, 66–77. [Google Scholar] [CrossRef]
- Cunningham, J.E.; Kuiack, C. Production of Citric and Oxalic Acids and Solubilization of Calcium Phosphate by Penicillium bilaii. Appl. Environ. Microbiol. 1992, 58, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Jimdjio Kouasseu, C.; Yang, X.; Xue, H.; Bi, Y.; Liu, Z.; Xi, J.; Nan, M.; Prusky, D. Reactive Oxygen Species Metabolism Modulation on the Quality of Apple Fruits Inoculated with Penicillium expansum under Different Ambient pHs. Horticulturae 2023, 9, 538. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; Tian, S. Function of pH-dependent Transcription Factor PacC in Regulating Development, Pathogenicity, and Mycotoxin Biosynthesis of Phytopathogenic Fungi. FEBS J. 2022, 289, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Selvig, K.; Alspaugh, J.A. pH Response Pathways in Fungi: Adapting to Host-Derived and Environmental Signals. Mycobiology 2011, 39, 249–256. [Google Scholar] [CrossRef]
- Lucena-Agell, D.; Galindo, A.; Arst, H.N.; Peñalva, M.A. Aspergillus Nidulans Ambient pH Signaling Does Not Require Endocytosis. Eukaryot. Cell 2015, 14, 545–553. [Google Scholar] [CrossRef]
- Hadas, Y.; Goldberg, I.; Pines, O.; Prusky, D. Involvement of Gluconic Acid and Glucose Oxidase in the Pathogenicity of Penicillium expansum in Apples. Phytopathology 2007, 97, 384–390. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, J.; Du, G.; Chen, J. Multivariate Modular Engineering of the Protein Secretory Pathway for Production of Heterologous Glucose Oxidase in Pichia Pastoris. Enzym. Microb. Technol. 2015, 68, 33–42. [Google Scholar] [CrossRef]
- Barad, S.; Horowitz, S.B.; Moscovitz, O.; Lichter, A.; Sherman, A.; Prusky, D. A Penicillium expansum Glucose Oxidase–Encoding Gene, GOX2, Is Essential for Gluconic Acid Production and Acidification During Colonization of Deciduous Fruit. MPMI 2012, 25, 779–788. [Google Scholar] [CrossRef]
- Qianqian, L.; Qingmin, C.; Hu, L.; Yamin, D.; Wenxiao, J.; Fei, S.; Maorun, F.; Subo, T.; Xiaofei, X. Direct Antifungal Activity of Methyl Salicylate on Soft Rot Caused by Rhizopus Stolonifer and Its Membrane Targeting Mechanism. Postharvest Biol. Technol. 2024, 218, 113169. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, L.; Wang, Y.; Lang, J.; Ye, M.; Liu, Q.; Ma, Q.; Zhou, N. Phosphorus-Solubilizing Fungi Promote the Growth of Fritillaria taipaiensis P. Y. Li by Regulating Physiological and Biochemical Reactions and Protecting Enzyme System–Related Gene Expression. Front. Genet. 2025, 15, 1459191. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Barad, S.; Sela, N.; Kumar, D.; Kumar-Dubey, A.; Glam-Matana, N.; Sherman, A.; Prusky, D. Fungal and Host Transcriptome Analysis of pH-Regulated Genes during Colonization of Apple Fruits by Penicillium expansum. BMC Genom. 2016, 17, 330. [Google Scholar] [CrossRef]
- Lai, T.; Yu, Q.; Pan, J.; Wang, J.; Tang, Z.; Bai, X.; Shi, L.; Zhou, T. The Identification and Comparative Analysis of Non-Coding RNAs in Spores and Mycelia of Penicillium expansum. JoF 2023, 9, 999. [Google Scholar] [CrossRef]
- Jiao, W.; Liu, X.; Li, Y.; Li, B.; Du, Y.; Zhang, Z.; Chen, Q.; Fu, M. Organic Acid, a Virulence Factor for Pathogenic Fungi, Causing Postharvest Decay in Fruits. Mol. Plant Pathol. 2022, 23, 304–312. [Google Scholar] [CrossRef]
- Alkan, N.; Meng, X.; Friedlander, G.; Reuveni, E.; Sukno, S.; Sherman, A.; Thon, M.; Fluhr, R.; Prusky, D. Global Aspects of pacC Regulation of Pathogenicity Genes in Colletotrichum Gloeosporioides as Revealed by Transcriptome Analysis. MPMI 2013, 26, 1345–1358. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Khan, A.; Hashem, A.; Abd_Allah, E.F.; Al-Harrasi, A. The Molecular Mass and Isoelectric Point of Plant Proteomes. BMC Genom. 2019, 20, 631. [Google Scholar] [CrossRef]
- Ono, K. Signal Peptides and Their Fragments in Post-Translation: Novel Insights of Signal Peptides. Int. J. Mol. Sci. 2024, 25, 13534. [Google Scholar] [CrossRef]
- Owji, H.; Nezafat, N.; Negahdaripour, M.; Hajiebrahimi, A.; Ghasemi, Y. A Comprehensive Review of Signal Peptides: Structure, Roles, and Applications. Eur. J. Cell Biol. 2018, 97, 422–441. [Google Scholar] [CrossRef]
- Zeng, X.; Wu, P.; Yao, C.; Liang, J.; Zhang, S.; Yin, H. Small Molecule and Peptide Recognition of Protein Transmembrane Domains. Biochemistry 2017, 56, 2076–2085. [Google Scholar] [CrossRef]
- Kang, Q.; Zhang, D. Principle and Potential Applications of the Non-Classical Protein Secretory Pathway in Bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 953–965. [Google Scholar] [CrossRef]
- Jiang, Q.; Jin, X.; Lee, S.-J.; Yao, S. Protein Secondary Structure Prediction: A Survey of the State of the Art. J. Mol. Graph. Model. 2017, 76, 379–402. [Google Scholar] [CrossRef] [PubMed]
- Yatsyshyn, V.Y.; Fedorovych, D.V.; Sibirny, A.A. Metabolic and Bioprocess Engineering of the Yeast Candida Famata for FAD Production. J. Ind. Microbiol. Biotechnol. 2014, 41, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.T.; Harper, J.C.; Dolan, P.L.; Manginell, M.M.; Arango, D.C.; Rawlings, J.A.; Apblett, C.A.; Brozik, S.M. Rational Redesign of Glucose Oxidase for Improved Catalytic Function and Stability. PLoS ONE 2012, 7, e37924. [Google Scholar] [CrossRef]
- Witt, S.; Singh, M.; Kalisz, H.M. Structural and Kinetic Properties of Nonglycosylated Recombinant Penicillium amagasakiense Glucose Oxidase Expressed in Escherichia Coli. Appl. Environ. Microbiol. 1998, 64, 1405–1411. [Google Scholar] [CrossRef]
- Peñalva, M.A.; Arst, H.N. Regulation of Gene Expression by Ambient pH in Filamentous Fungi and Yeasts. Microbiol. Mol. Biol. Rev. 2002, 66, 426–446. [Google Scholar] [CrossRef]
- Prusky, D.; Yakoby, N. Pathogenic Fungi: Leading or Led by Ambient pH? Mol. Plant Pathol. 2003, 4, 509–516. [Google Scholar] [CrossRef]
- Serrano, C.Z. Development and Secondary Metabolism in Penicillium expansum: Implication of Global Transcription Factors VeA and BrlA. Doctoral Dissertation, Institut National Polytechnique de Toulouse-INPT, Toulouse, France, 2020. [Google Scholar]
- Chen, Y.; Li, B.Q.; Xu, X.D.; Zhang, Z.Q.; Tian, S.P. The pH-responsive PacC transcription factor plays pivotal roles in virulence and patulin biosynthesis in Penicillium expansum. Environ. Microbiol. 2018, 20, 4063–4078. [Google Scholar]
- He, X.; Chen, Y.; Tian, S.; Li, B. PePalA/B/C Are Required for Virulence and Patulin Biosynthesis by Regulating the PePacC-Processing Proteolytic Activity in Penicillium expansum. Food Qual. Saf. 2025, 9, fyae045. [Google Scholar] [CrossRef]
- Yin, X.; Shin, H.-D.; Li, J.; Du, G.; Liu, L.; Chen, J. P Gas, a Low-pH-Induced Promoter, as a Tool for Dynamic Control of Gene Expression for Metabolic Engineering of Aspergillus niger. Appl. Environ. Microbiol. 2017, 83, e03222-16. [Google Scholar] [CrossRef]
- Orejas, M.; Espeso, E.A.; Tilburn, J.; Sarkar, S.; Arst, H.N.; Penalva, M.A. Activation of the Aspergillus PacC Transcription Factor in Response to Alkaline Ambient pH Requires Proteolysis of the Carboxy-Terminal Mmety. Genes Dev. 1995, 9, 1622–1632. [Google Scholar]
- Barad, S.; Sela, N.; Dubey, A.K.; Kumar, D.; Luria, N.; Ment, D.; Cohen, S.; Schaffer, A.A.; Prusky, D. Differential Gene Expression in Tomato Fruit and Colletotrichum Gloeosporioides during Colonization of the RNAi–SlPH Tomato Line with Reduced Fruit Acidity and Higher pH. BMC Genomics 2017, 18, 579. [Google Scholar] [CrossRef]
- Yang, J.; Fan, Y.; Liu, F.; Ding, Y.; Yu, L.; Wang, Y.; Wu, A.; Jiao, Z.; Wang, C. Temperature-Induced Metabolic Changes of Apples Infected with Penicillium expansum Characterized by Non-Targeted High-Resolution Mass Spectrometry. Postharvest Biol. Technol. 2024, 209, 112700. [Google Scholar] [CrossRef]
- Zong, Y.; Li, B.; Tian, S. Effects of Carbon, Nitrogen and Ambient pH on Patulin Production and Related Gene Expression in Penicillium expansum. Int. J. Food Microbiol. 2015, 206, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Gould, G.W. Methods for Preservation and Extension of Shelf Life. Int. J. Food Microbiol. 1996, 33, 51–64. [Google Scholar] [CrossRef]
- O’Connell, C.A.; Dollimore, D. A Study of the Decomposition of Calcium Propionate, Using Simultaneous TG-DTA. Thermochim. Acta 2000, 357–358, 79–87. [Google Scholar] [CrossRef]
- Sequeira, S.O.; Phillips, A.J.L.; Cabrita, E.J.; Macedo, M.F. Antifungal Treatment of Paper with Calcium Propionate and Parabens: Short-Term and Long-Term Effects. Int. Biodeterior. Biodegrad. 2017, 120, 203–215. [Google Scholar] [CrossRef]



| Primer | Primer Sequences (5′–3′) |
|---|---|
| 28S-F | GGAACGGGACGTCATAGAGG |
| 28S-R | AGAGCTGCATTCCCAAACAAC |
| GOX1-F | CACCACTGTTGACCACGCCTATG |
| GOX1-R | CCAAGACCATTGCCAGATCGGATG |
| GOX2-F | CCGCACCGAGATTGTTAGATCAGG |
| GOX2-R | TCCCAGTTCCACCCTTCCATTCC |
| GOX3-F | GGCACTTGGAGGAACATCGA |
| GOX3-R | TGGTCTCCCATGCGTCAAT |
| Protein Name | Molecular Weigh (kDa) | Theoretica (PI) | Instability Index | Grand Average of Hydropathicity (GRAVY) | (Arg + Lys) | (Asp + Glu) | Aliphatic Inde |
|---|---|---|---|---|---|---|---|
| GOX | 63.88 | 5.72 | 42.16 | −0.225 | 60 | 47 | 82.77 |
| Amino Acid Composition | GOX (%) | Amino Acid Composition | GOX (%) |
|---|---|---|---|
| Ala | 7.3 | Lys | 3.3 |
| Arg | 5.2 | Met | 1.0 |
| Asn | 6.1 | Phe | 4.4 |
| Asp | 5.8 | Pro | 5.0 |
| Cys | 1.0 | Ser | 5.8 |
| Gln | 4.2 | Thr | 7.1 |
| Glu | 4.6 | Trp | 2.1 |
| Gly | 9.4 | Tyr | 2.9 |
| His | 3.1 | Val | 6.7 |
| Ile | 5.4 | Pyl | 0.0 |
| Leu | 9.4 | Sec | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Ru, Y.; Yuan, X.; Huang, S.; Yuan, D.; Fu, M.; Jiao, W. Functional Analysis of Penicillium expansum Glucose Oxidase-Encoding Gene, GOX2, and Its Expression Responses to Multiple Environmental Factors. Horticulturae 2025, 11, 860. https://doi.org/10.3390/horticulturae11070860
Yuan Y, Ru Y, Yuan X, Huang S, Yuan D, Fu M, Jiao W. Functional Analysis of Penicillium expansum Glucose Oxidase-Encoding Gene, GOX2, and Its Expression Responses to Multiple Environmental Factors. Horticulturae. 2025; 11(7):860. https://doi.org/10.3390/horticulturae11070860
Chicago/Turabian StyleYuan, Yongcheng, Yutong Ru, Xiaohe Yuan, Shuqi Huang, Dan Yuan, Maorun Fu, and Wenxiao Jiao. 2025. "Functional Analysis of Penicillium expansum Glucose Oxidase-Encoding Gene, GOX2, and Its Expression Responses to Multiple Environmental Factors" Horticulturae 11, no. 7: 860. https://doi.org/10.3390/horticulturae11070860
APA StyleYuan, Y., Ru, Y., Yuan, X., Huang, S., Yuan, D., Fu, M., & Jiao, W. (2025). Functional Analysis of Penicillium expansum Glucose Oxidase-Encoding Gene, GOX2, and Its Expression Responses to Multiple Environmental Factors. Horticulturae, 11(7), 860. https://doi.org/10.3390/horticulturae11070860





