The Influence of Bud Positions on the Changes in Carbohydrates and Nitrogen in Response to Hydrogen Cyanamide During Budbreak in Low-Chill Kiwifruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Treatment, and Sampling
2.2. Phenological Observation
2.3. Weather and Chilling Calculation
2.4. Biochemical Analysis
2.4.1. Non-Structural Carbohydrate Concentrations
2.4.2. Total Nitrogen Content
2.5. Statistical Analysis
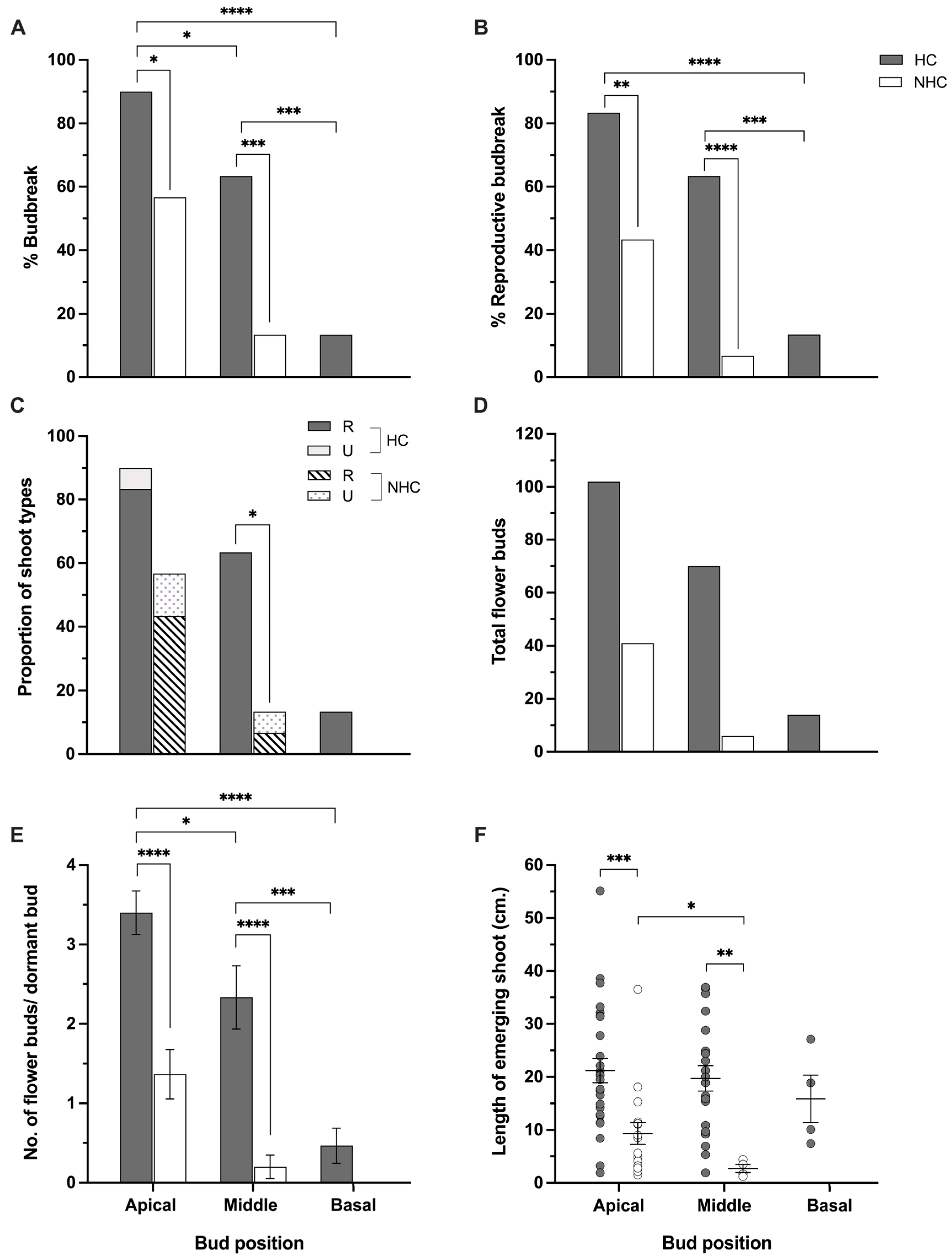
3. Results
3.1. Budbreak and Phenological Development
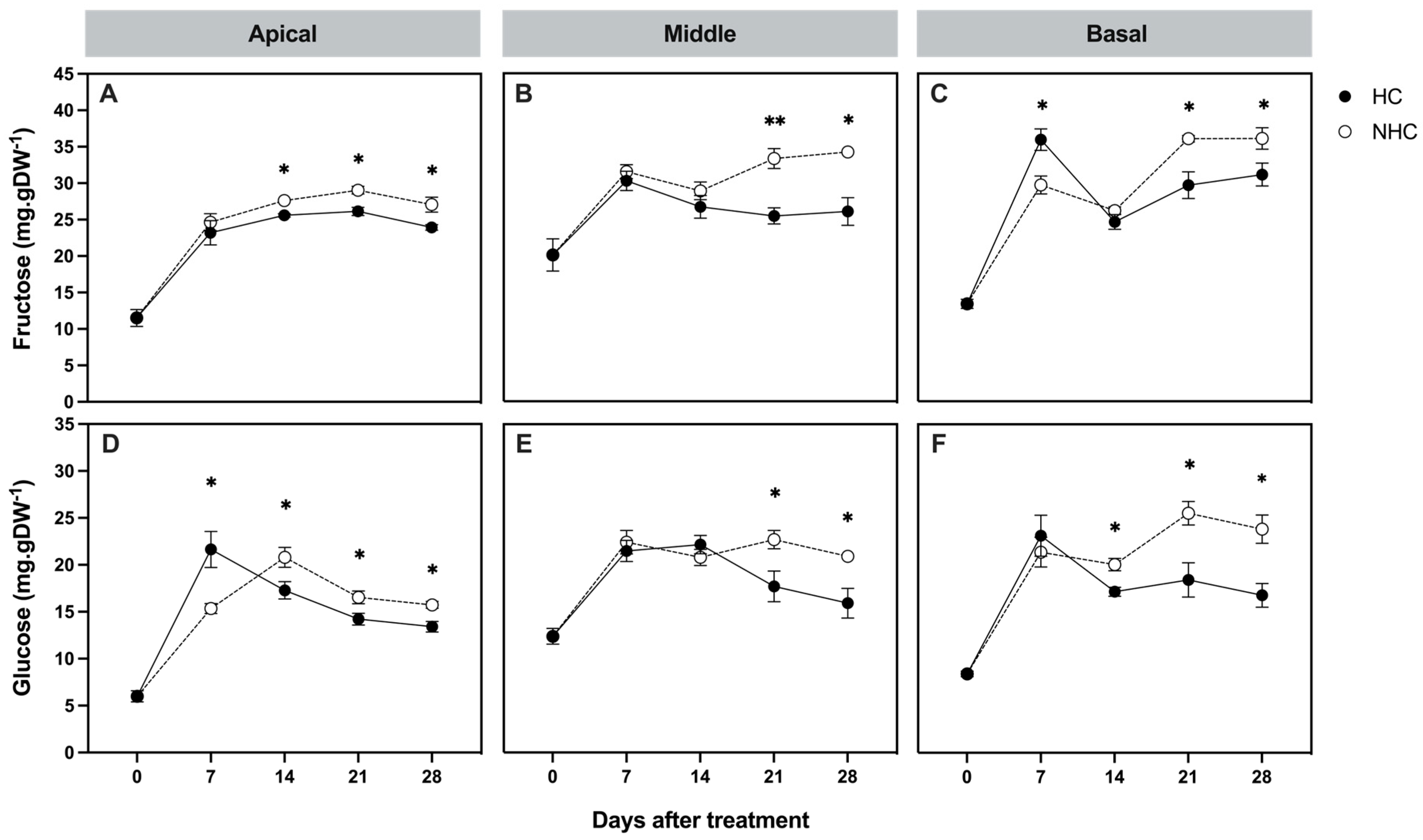
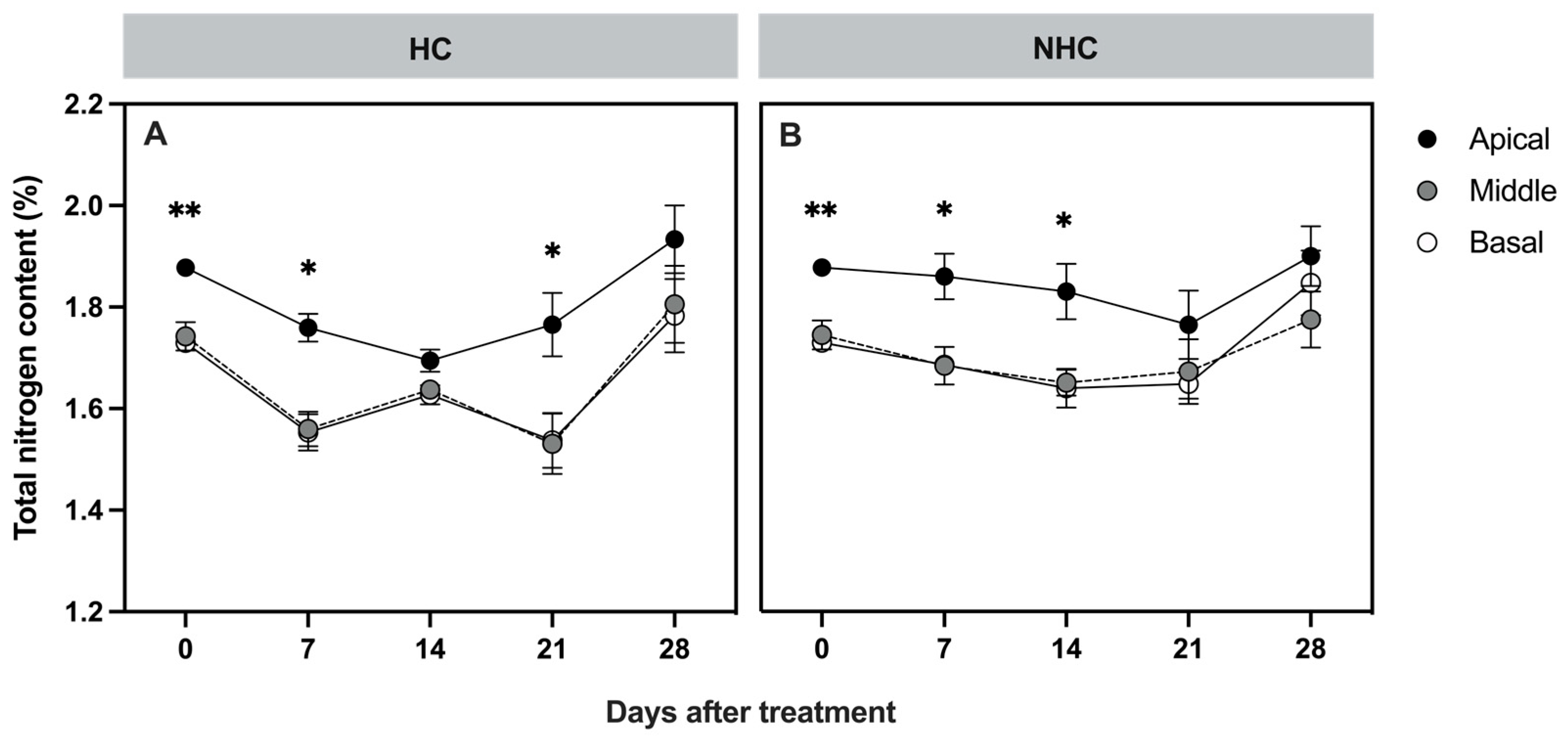
3.2. Biochemical Analysis
3.2.1. Non-Structural Carbohydrate Concentrations
3.2.2. Total Nitrogen Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HC | Hydrogen cyanamide |
| NSCs | Non-structural carbohydrates |
| N | Nitrogen |
| CH | Chilling hours |
| PGO | Peroxidase—glucose oxidase |
| RI | Refractive index |
| DAT | Days after treatment |
| DBT | Days before treatment |
References
- Beauvieux, R.; Wenden, B.; Dirlewanger, E. Bud dormancy in perennial fruit tree species: A pivotal role for oxidative cues. Front. Plant Sci. 2018, 9, 657. [Google Scholar] [CrossRef] [PubMed]
- McPherson, H.G.; Stanley, C.J.; Warrington, I.J. The response of bud break and flowering to cool winter temperatures in kiwifruit (Actinidia deliciosa). J. Hortic. Sci. 1995, 70, 737–747. [Google Scholar]
- Morley-Bunker, M.J.; Salinger, M.J. Kiwifruit development: The effect of temperature on budburst and flowering. Weather Clim. 1987, 7, 26–30. [Google Scholar] [CrossRef]
- Salinger, M.J.; Kenny, G.J.; Morley-Bunker, M.J. Climate and kiwifruit cv. Hayward 1. Influences on development and growth. N. Z. J. Crop Hortic. Sci. 1993, 21, 235–245. [Google Scholar] [CrossRef]
- Nanninga, C.; Buyarski, C.R.; Pretorius, A.M.; Montgomery, R.A. Increased exposure to chilling advances the time to budburst in North American tree species. Tree Physiol. 2017, 37, 1727–1738. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Li, D.; Li, L.; Han, F.; Liu, X.; Zhang, P.; Zhong, C. The Differentiation of Chilling Requirements of Kiwifruit Cultivars Related to Ploidy Variation. HortScience 2017, 52, 1676–1679. [Google Scholar] [CrossRef]
- Kamyun, N.; Pichakum, A.; Bunwong, K.; Tiensuwan, M.; Moore, E.J. Development of Chill Unit Calculation for Peach ‘Jade’ Fruit Trees Grown in Northern Thailand. Acta Hortic. 2013, 1059, 147–154. [Google Scholar] [CrossRef]
- Pichakum, A.; Chaiwimol, W.; Meetam, M.; Songnuan, W. Responses of green kiwifruit grown in low-chill area to hydrogen cyanamide application. Acta Hortic. 2018, 1206, 97–103. [Google Scholar] [CrossRef]
- McPherson, H.G.; Hall, A.J.; Stanley, C.J. Seasonal and Regional Variation in Budbreak and Flowering of Kiwifruit Vines in New Zealand. N. Z. J. Crop Hortic. Sci. 1994, 22, 263–276. [Google Scholar] [CrossRef]
- Inglese, P.; Gullo, G.; Pace, L.S. Effect of cyanamide on budbreak and cane fruitfulness for ‘Hayward’ kiwifruit in relation to cane length and time of application. N. Z. J. Crop Hortic. Sci. 1998, 26, 45–53. [Google Scholar] [CrossRef]
- McPherson, H.G.; Richardson, A.C.; Snelgar, W.P.; Currie, M.B. Effects of hydrogen cyanamide on budbreak and flowering in kiwifruit (Actinidia deliciosa ’Hayward’). N. Z. J. Crop Hortic. Sci. 2001, 29, 277–285. [Google Scholar] [CrossRef]
- Powell, A.A.; David, G.H.; Tunnell, E. Effect of hydrogen cyanamide (Dormext) on replacing lack of chilling in kiwifruit (Actinidia deliciosa). Small Fruits Rev. 2000, 1, 79–92. [Google Scholar] [CrossRef]
- Richardson, A.C.; Dawson, T.E.; Hampton, R.E.; Blank, R.H. The effects of hydrogen cyanamide and mineral oil on kiwifruit performance. Proc. N. Z. Plant Prot. Conf. 1994, 47, 314–319. [Google Scholar] [CrossRef]
- Schuck, E.; Petri, J.L. The effect of concentrations and application of hydrogen cyanamide on kiwifruit dormancy breaking. Acta Hortic. 1995, 395, 177–184. [Google Scholar] [CrossRef]
- Smith, G.S.; Clark, C.J.; Boldingh, H.L. Seasonal accumulation of starch by components of the kiwifruit vine. Ann. Bot. 1992, 70, 19–25. [Google Scholar] [CrossRef]
- Ben Mohamed, H.; Vadel, A.M.; Geuns, J.M.C.; Khemira, H. Biochemical changes in dormant grapevine shoot tissues in response to chilling: Possible role in dormancy release. Sci. Hortic. 2010, 124, 440–447. [Google Scholar] [CrossRef]
- Bonhomme, M.; Peuch, M.; Ameglio, T.; Rageau, R.; Guilliot, A.; Decourteix, M.; Alves, G.; Sakr, S.; Lacointe, A. Carbohydrate uptake from xylem vessels and its distribution among stem tissues and buds in walnut (Juglans regia L.). Tree Physiol. 2009, 30, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Chantuma, P.; Lacointe, A.; Kasemsap, P.; Thanisawanyangkura, S.; Gohet, E.; Clément, A.; Guilliot, A.; Ameglio, T.; Thaler, P. Carbohydrate storage in wood and bark of rubber trees submitted to different level of C demand induced by latex tapping. Tree Physiol. 2009, 29, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, H.; Blanke, M. Changes in carbohydrate levels and relative water content (RWC) to distinguish dormancy phases in sweet cherry. J. Plant Physiol. 2017, 218, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.G.; Ross, J.J.; Babst, B.A.; Wienclaw, B.N.; Beveridge, C.A. Sugar demand, not auxin, is the initial regulator of apical dominance. Proc. Natl. Acad. Sci. USA 2014, 111, 6092–6097. [Google Scholar] [CrossRef] [PubMed]
- Kebrom, T.H. A growing stem inhibits bud outgrowth—The overlooked theory of apical dominance. Front. Plant Sci. 2017, 8, 1874. [Google Scholar] [CrossRef] [PubMed]
- Bertheloot, J.; Barbier, F.; Boudon, F.; Perez-Garcia, M.D.; Péron, T.; Citerne, S.; Dun, E.; Beveridge, C.; Godin, C.; Sakr, S. Sugar availability suppresses the auxin-induced strigolactone pathway to promote bud outgrowth. New Phytol. 2019, 225, 866–879. [Google Scholar] [CrossRef] [PubMed]
- Coleman, G.D. Physiology and regulation of seasonal nitrogen cycling in woody plants. J. Crop Improv. 2004, 10, 237–259. [Google Scholar] [CrossRef]
- Ledgard, S.F.; Smith, G.S. Fate of 15N-labelled nitrogen fertilizer applied to kiwifruit (Actinidia deliciosa) vines. Plant Soil 1992, 147, 59–68. [Google Scholar] [CrossRef]
- Walton, E.F.; Clark, C.J.; Boldingh, H.L. Effect of hydrogen cyanamide on amino acid profiles in kiwifruit buds during budbreak. Plant Physiol. 1991, 97, 1256–1259. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Fuchigami, L.H. Growth of young apple trees in relation to reserve nitrogen and carbohydrates. Tree Physiol. 2002, 22, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.J.; Ledgard, S.F. Uptake of 15N by kiwifruit vines from applications of nitrogen fertilizer prior to budbreak. Ann. Bot. 1993, 71, 311–316. [Google Scholar] [CrossRef]
- O’Kennedy, B.T.; Hennerty, M.J.; Titus, J.S. Changes in the nitrogen reserves of apple shoots during the dormant season. J. Hortic. Sci. 1975, 50, 321–329. [Google Scholar] [CrossRef]
- Li, G.; Lin, R.; Egekwu, C.; Blakeslee, J.; Lin, J.; Pettengill, E.; Coleman, G.D. Seasonal Nitrogen Remobilization and the Role of Auxin Transport in Poplar Trees. J. Exp. Bot. 2020, 71, 4512–4530. [Google Scholar] [CrossRef] [PubMed]
- Gomez, L.; Vercambre, G.; Jordan, M.O. Spatial-Temporal Management of Nitrogen and Carbon on the Peach Tree (Prunus persicae L. Batsch.). Sci. Hortic. 2020, 273, 109613. [Google Scholar] [CrossRef]
- Richardson, A.C.; Walton, E.F.; Meekings, J.S.; Boldingh, H.L. Carbohydrate changes in kiwifruit buds during the onset and release from dormancy. Sci. Hortic. 2010, 124, 463–468. [Google Scholar] [CrossRef]
- Brundell, D.J. Flower development of the Chinese gooseberry (Actinidia chinensis Planch.): I. Development of the flowering shoot. N. Z. J. Bot. 1975, 13, 473–483. [Google Scholar] [CrossRef]
- Weinberger, J. Chilling requirements of peach varieties. Proc. Am. Soc. Hortic. Sci. 1950, 56, 122–128. [Google Scholar]
- Bellasio, C.; Fini, A.; Ferrini, F. Evaluation of a high throughput starch analysis optimised for wood. PLoS ONE 2014, 9, e86645. [Google Scholar] [CrossRef] [PubMed]
- Erez, A. Bud Dormancy; Phenomenon, Problems and Solutions in the Tropics and Subtropics. In Temperate Fruit Crops in Warm Climates; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 17–48. [Google Scholar] [CrossRef]
- Linsley-Noakes, G.C. Improving flowering of kiwifruit in climatically marginal areas using hydrogen cyanamide. Sci. Hortic. 1989, 38, 247–259. [Google Scholar] [CrossRef]
- Walton, E.F.; Fowke, P.J. Effect of hydrogen cyanamide on kiwifruit shoot flower number and position. J. Hortic. Sci. 1993, 68, 529–534. [Google Scholar] [CrossRef]
- Hartmann, T.P.; Spiers, J.D.; Stein, L.A.; Scheiner, J.J. Effect of warm temperature interruption on the accumulation of winter chilling in kiwifruit (Actinidia chinensis Planch. and A. deliciosa A. Chev.). HortScience 2024, 59, 691–698. [Google Scholar] [CrossRef]
- Jackson, J.E.; Bepete, M. The effect of hydrogen cyanamide (Dormex) on flowering and cropping of different apple cultivars under tropical conditions of sub-optimal winter chilling. Sci. Hortic. 1995, 60, 293–304. [Google Scholar] [CrossRef]
- Manson, P.J.; Snelgar, W.P.; Seelye, R.J. Relationships between timing of cane tipping and yield of kiwifruit (Actinidia deliciosa). J. Hortic. Sci. 1991, 66, 415–422. [Google Scholar] [CrossRef]
- Ongaro, V.; Leyser, O. Hormonal Control of Shoot Branching. J. Exp. Bot. 2008, 59, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Chabikwa, T.; Barbier, F.; Dun, E.A.; Fichtner, F.; Dong, L.; Kerr, S.C.; Beveridge, C.A. Auxin-Independent Effects of Apical Dominance Induce Changes in Phytohormones Correlated with Bud Outgrowth. Plant Physiol. 2023, 192, 1420–1434. [Google Scholar] [CrossRef] [PubMed]
- Ben Mohamed, H.; Vadel, A.M.; Geuns, J.M.C.; Khemira, H. Carbohydrate changes during dormancy release in Superior Seedless grapevine cuttings following hydrogen cyanamide treatment. Sci. Hortic. 2012, 140, 19–25. [Google Scholar] [CrossRef]
- Liang, D.; Huang, X.; Shen, Y.; Shen, T.; Zhang, H.; Lin, L.; Xia, H. Hydrogen Cyanamide Induces Grape Bud Endodormancy Release through Carbohydrate Metabolism and Plant Hormone Signaling. BMC Genom. 2019, 20, 1034. [Google Scholar] [CrossRef] [PubMed]
- Maurel, K.; Leite, G.B.; Bonhomme, M.; Guilliot, A.; Rageau, R.; Pétel, G.; Sakr, S. Trophic control of bud break in peach (Prunus persica) trees: A possible role of hexoses. Tree Physiol. 2004, 24, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Roitsch, T.; González, M.C. Function and regulation of plant invertases: Sweet sensations. Trends Plant Sci. 2004, 9, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Hubmann, A.M.; Roth, A.; Monschein, S. Breaking Buds—Stop and Go? Acid Invertase Activities in Apple Leaf Buds during Dormancy Release until Bud Break. Agronomy 2023, 13, 2659. [Google Scholar] [CrossRef]
- González-Rossia, D.; Reig, C.; Dovis, V.; Gariglio, N.; Agustí, M. Changes on carbohydrates and nitrogen content in the bark tissues induced by artificial chilling and its relationship with dormancy bud break in Prunus sp. Sci. Hortic. 2008, 118, 275–281. [Google Scholar] [CrossRef]
- Smith, G.S.; Buwalda, J.G.; Clark, C.J. Nutrient dynamics of a kiwifruit ecosystem. Sci. Hortic. 1988, 37, 87–109. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaiwimol, W.; Songnuan, W.; Ohara, H.; Juprasong, Y.; Pichakum, A. The Influence of Bud Positions on the Changes in Carbohydrates and Nitrogen in Response to Hydrogen Cyanamide During Budbreak in Low-Chill Kiwifruit. Horticulturae 2025, 11, 847. https://doi.org/10.3390/horticulturae11070847
Chaiwimol W, Songnuan W, Ohara H, Juprasong Y, Pichakum A. The Influence of Bud Positions on the Changes in Carbohydrates and Nitrogen in Response to Hydrogen Cyanamide During Budbreak in Low-Chill Kiwifruit. Horticulturae. 2025; 11(7):847. https://doi.org/10.3390/horticulturae11070847
Chicago/Turabian StyleChaiwimol, Wanichaya, Wisuwat Songnuan, Hitoshi Ohara, Yotin Juprasong, and Aussanee Pichakum. 2025. "The Influence of Bud Positions on the Changes in Carbohydrates and Nitrogen in Response to Hydrogen Cyanamide During Budbreak in Low-Chill Kiwifruit" Horticulturae 11, no. 7: 847. https://doi.org/10.3390/horticulturae11070847
APA StyleChaiwimol, W., Songnuan, W., Ohara, H., Juprasong, Y., & Pichakum, A. (2025). The Influence of Bud Positions on the Changes in Carbohydrates and Nitrogen in Response to Hydrogen Cyanamide During Budbreak in Low-Chill Kiwifruit. Horticulturae, 11(7), 847. https://doi.org/10.3390/horticulturae11070847







