Abstract
The disposal of olive mill wastewater (OMWW) poses significant environmental challenges due to its high content of phytotoxic and pollutant compounds. This study aims to explore the chemical composition of OMWW derived from various olive varieties (Buža, Buža puntoža, Istarska bjelica, Leccino, and Rosinjola) and assess its antifungal potential against phytopathogenic fungi from the Botryosphaeriaceae family. OMWW samples were analyzed for their physicochemical properties, phenolic composition via LC-MS/MS, and antifungal activity against Botryosphaeria dothidea (Moug. ex Fr.) Ces. & De Not., Diplodia mutila (Fr.) Fr., D. seriata De Not., Dothiorella iberica A.J.L. Phillips, J. Luque & A. Alves, Do. sarmentorum (Fr.) A.J.L. Phillips, Alves & Luque, and Neofusicoccum parvum (Pennycook & Samuels) Crous, Slippers & A.J.L. Phillips. Antifungal efficacy was tested at varying concentrations, alongside the phenolic compounds hydroxytyrosol and vanillic acid. Antifungal activity varied across fungal species and OMWW concentrations. Lower OMWW concentrations inhibited mycelial growth in some pathogens, while higher concentrations often had a stimulatory effect. Among the OMWW treatments, Leccino and Buža showed the most significant antifungal activity against species from the Botryosphaeriaceae family. The results demonstrated significant variability in OMWW composition, with Istarska bjelica exhibiting the highest concentrations of phenolic compounds, sugars, dry matter, and carbon and nitrogen content. The results also highlight the impact of acidification on the phenolic profile of OMWW. Treatment with HCl significantly altered the concentration of individual phenolic compounds, either enhancing their release or contributing to their degradation. Among the two compounds, vanillic acid showed greater efficacy than hydroxytyrosol. In addition, microorganisms isolated from OMWW, including Bacillus velezensis Ruiz-Garcia et al., Rhodotorula mucilaginosa (A. Jörg.) F.C. Harrison, Nakazawaea molendiniolei (N. Cadez, B. Turchetti & G. Peter) C. P. Kurtzman & C. J. Robnett, and Penicillium crustosum Thom, demonstrated antagonistic potential against fungal pathogens, with B. velezensis showing the strongest inhibitory effect. The greatest antagonistic effect against fungi was observed with the species Do. Iberica. The findings highlight the potential of OMWW as a sustainable alternative to chemical fungicides, simultaneously contributing to the management of waste and protection of plants through circular economy principles.
1. Introduction
The olive tree has been cultivated in all Mediterranean regions for over 2500 years [1]. It is of great economic value in this area. According to the latest data from 2023, olives are grown on approximately 11.1 million ha, yielding an average of 20.2 million t of olives annually [2]. The most important product of olive processing is olive oil. According to the latest data from 2022, global olive oil production amounted to 2.7 million t [2].
Plant pathogenic fungi are among the leading causes of yield loss in agricultural production, and the primary challenge in their management lies in the continued reliance on chemical agents for plant protection. Most of these pesticides are classified as toxic substances, posing a risk to ecosystems and contributing to environmental degradation. Although the number of studies focusing on alternative protection methods, such as plant-based formulations, composts, and similar approaches, is increasing, they remain insufficiently researched to enable widespread practical application. Utilizing agricultural waste as a natural solution for plant protection could facilitate a circular economy and potentially reduce the release of harmful chemicals into the environment. Olive mill wastewater (OMWW) is a byproduct generated during olive oil extraction using two-phase or three-phase systems. OMWW contains high concentrations of salts, organic matter, and chemicals (mainly phenols), which are phytotoxic and can adversely affect the physical, chemical, and biological properties of the soil [3]. A significant environmental concern with OMWW lies in its disposal, as it is considered a major environmental pollutant [4]. Typically, OMWW is discarded directly in landfills without any prior treatment [5].
However, due to its high content of mineral and organic matter, OMWW can also have positive effects on plants if properly treated (e.g., filtration, centrifugation, thermal treatment, etc.) and applied correctly [3]. Studies have also shown that OMWW exhibits antimicrobial effects against phytopathogenic fungi and bacteria [6,7,8]. The antimicrobial activity of OMWW has been found to depend on the olive variety from which it is derived, as phenolic concentrations and chemical composition vary between varieties. Differences in the chemical composition of OMWW are attributed to geographic, agronomic, seasonal, and other factors [8].
OMWW components also have potential applications in other industries, such as the food industry [9] and cosmetics [10]. Among the most prominent components of OMWW is hydroxytyrosol, a phenolic compound predominantly found in olive leaves and pulp, with smaller amounts present in olive oil, that has been shown to be effective in combating bacterial and fungal pathogens [11,12,13].
Given the growing awareness of the dangers associated with chemical pesticides, such as their impact on human and animal health, and the emergence of resistant microorganisms, significant efforts are being made to replace chemical pesticides with alternative and less toxic agents. The development of new plant protection strategies is particularly important in the context of the European Union’s (EU) efforts to transition from conventional agriculture to environmentally friendly practices with minimal environmental impact [14]. To this end, the EU has introduced two strategic documents: the “European Green Deal”, which outlines strategies for achieving sustainable economic growth, and the “Biodiversity Strategy”, aimed at promoting the sustainable use of plant protection agents and achieving a 50% reduction in their use by 2030. The antimicrobial properties of olive mill waste could be utilized to combat plant pathogens, opening up new possibilities for recycling these distinctive bioactive byproducts [15]. The amount of research regarding the effects of OMWW on pathogens remains limited, with experiments being carried out on only a narrow range of fungal species.
The aim of this study was to determine the chemical composition of OMWW derived from various olive varieties, analyze the microbial population present in OMWW, and assess the antifungal potential of these OMWW along with two phenolic compounds—one identified as the most studied in previous research and the other as the most abundant in the analyzed OMWW. Specifically, this study focused on their application in controlling phytopathogenic fungi from the Botryosphaeriaceae family and evaluating the antagonistic interactions between these pathogens and microorganisms isolated from OMWW, with the aim of exploring potential utilization strategies for OMWW as an industrial byproduct. Species from the Botryosphaeriaceae family are among the most aggressive olive pathogens, causing branch and twig dieback, fruit and leaf drop, and, consequently, yield reduction and economic losses for producers [16,17]. Given their high aggressiveness, limited treatment options, and significant impact on olive productivity, effective and sustainable control strategies against Botryosphaeriaceae are urgently needed.
2. Materials and Methods
2.1. OMWW Collection
The OMWW was collected directly from olive processors in Istria County, Croatia, in 2021. The interval between harvest and oil processing was four hours, with the paste processing temperature maintained at 24 °C. Malaxation was performed with continuous cooling, using water at 12 °C. The olive oil was extracted through centrifugation, employing a two-phase Pieralisi system with a hammer mill (Pieralisi Group, Ancona, Italy). The OMWW was derived from the Croatian olive cultivars Buža, Buža Puntoža, Istarska Bjelica, and Rosinjola, as well as the Italian olive cultivar Leccino.
2.2. Pretreatment of the Sample
After collecting the OMWW samples from the olive mill, they were stored under refrigeration at 4 °C for nine days [18]. The samples’ color was initially determined, followed by filtration through a vacuum filtration system using high-flow-rate 21/N-grade filter paper, 80 g/m2, a thickness of 0.28 mm, a filtration speed of 10 s/10 mL, and a pore size of 20–25 µm (Munktell, Fisher Scientific, Göteborg, Sweden). After filtration, the samples were subjected to centrifugation at 4000 rpm for 10 min at +4 °C, utilizing a Hettich 320 R centrifuge (Merck, Darmstadt, Germany). The pH of the OMWW samples was determined at room temperature using a pH meter (MP220 Basic pH/mV/°C Meter, Mettler-Toledo GmbH, Giessen, Germany) that had been calibrated with certified pH buffers (Mettler-Toledo GmbH, Greifensee, Switzerland) as reference materials.
Subsequently, the samples were divided into two fractions. Hydrochloric acid (HCl) was added to one fraction to lower the final pH to 2, aiming to prevent oxidation and thus preserve phenolic compounds [8,19,20], while the other fraction was left in its natural form without any additives. Each fraction was then further divided, with one aliquot stored at −20 °C and the other at room temperature.
2.3. Determination of Physical and Chemical Parameters
2.3.1. Sample Preparation and LC-MS/MS Analysis of Phenolic Compounds
OMWW (300 µL) was combined with 1200 µL of methanol to achieve a 1:5 dilution ratio. The mixture was thoroughly vortexed and centrifuged at 16,000× g for five minutes at 25 °C using a Domel Centric 350 centrifuge (Železniki, Slovenia). After centrifugation, 300 µL of the supernatant was freeze-dried using a Labogene Coolsafe 95-15 Pro system (Allerød, Denmark) and reconstituted in 600 µL of the initial mobile phase, consisting of water with 2% methanol and 0.1% acetic acid. The reconstituted solution was vortexed for 30 s and transferred to HPLC vials for analysis. The phenolic profile was determined using LC-MS/MS, comprising an autosampler (Shimadzu Nexera SIL-40CX3, Kyoto, Japan), two solvent delivery units (Shimadzu Nexera LC-40DX3, Kyoto, Japan), a thermostatic column compartment (Shimadzu Nexera CTO-40C, Kyoto, Japan), and a triple quadrupole mass spectrometer (Shimadzu LCMS8045, Kyoto, Japan). Separation was carried out on a C18 core-shell column (2.1 mm × 150 mm, 2.7 µm, Advanced Materials Technology, Wilmington, DE, USA) maintained at 37 °C. A 1 µL sample was injected, and separation was achieved using a linear gradient elution of mobile phase A (water/0.1% acetic acid) and mobile phase B (methanol/0.1% acetic acid) at a flow rate of 0.35 mL/min. The gradient conditions were as follows: 0–0.75 min, 98% A; 0.75–15 min, 98% A to 50% A; 15–15.1 min, 50% A to 0% A; 15.1–20 min, 0% A; 20–20.1 min, 0% A to 98% A; and 20.1–25 min, 98% A. Polyphenolic compounds were identified and quantified using analytical standards. Each sample was analyzed in quadruplicate, and the phenolic content was expressed as the mean of these four measurements ± standard error.
2.3.2. Spectrophotometric Determination of Sugar
The determination of sugar concentration was performed according to the method of DuBois et al. [21], using a spectrophotometer (PerkinElmer, Lambda 45, Waltham, MA, USA) at wavelengths of 480 nm and 490 nm. The absorbance of the samples was measured in 1 cm quartz cuvettes after the addition of the DuBois reagent. The measurements were performed at room temperature, and the concentration of sugar in each sample was calculated from a calibration curve generated using glucose standards in the range of 0 to 100 mg/L. Three replicates were performed for each OMWW sample. The concentration of sugar is expressed as the mean value of these replicates ± standard error.
2.3.3. Determination of Dry Matter and Water Content
To determine the dry matter content and water volume fraction, 3 mL of each sample was pipetted into a glass beaker. The samples were placed in a drying chamber (Binder, FD 56, Tuttlingen, Germany) at 103 °C for 24 h. The mass of the empty glass beaker, as well as the mass of the sample before and after drying, was measured using an analytical balance (Mettler Toledo, XP205, Switzerland). Each measurement was conducted in three replicates. The dry matter mass was calculated using the following formula: mdm = m3 − m1, where mdm is the mass of the dry matter (in mg), m1 is the mass of the empty glass beaker, m2 is the mass of the sample and glass beaker before drying, and m3 is the mass of the sample and glass beaker after 24 h of drying. The initial (wet) mass of the sample was determined as: mwet = m2 − m1, where mwet represents the mass of the sample before drying (excluding the beaker). The water content (WH2O) was then calculated using the following formula: WH2O = 100% − Wdm, where Wdm represents the percentage of dry matter, calculated as: Wdm= (mdm/mwet) × 100%. The amount of dry matter and the percentage of water content were expressed as the mean value of three replicates ± standard error.
2.3.4. Determination of Carbon and Nitrogen Content
The total carbon and nitrogen content was simultaneously analyzed in the sample using a TOC-L-CPH/TNM-L-ROHS analyzer (Shimadzu Corporation, Kyoto, Japan). A 3 mL sample was mixed with 27 mL of a 0.2% HCl solution. The sample was homogenized for one min and subsequently analyzed using the instrument. The concentration of carbon and nitrogen is expressed as the mean value of three replicates.
2.4. Antifungal Efficacy of OMWW and Components
2.4.1. Utilized Isolates
To evaluate the effects of OMWW and its components, representative isolates of phytopathogenic fungi sourced from olive trees were employed. The list of species used is presented in Table 1. Fungal isolates were cultured on potato dextrose agar (PDA) and maintained at 25 °C for five days in darkness.

Table 1.
List of used fungal species, isolate names, and GenBank IDs, with references.
2.4.2. Experimental Setup
To evaluate antifungal efficacy, five untreated OMWWs were used, along with the compound vanillic acid, which was either the most abundant or among the most abundant components of the OMWWs examined in this study. Additionally, hydroxytyrosol, identified in previous studies as a dominant OMWW component with potent antimicrobial activity [8,12], was included. Vanillic acid and hydroxytyrosol were sourced from Sigma Aldrich (Merck KGaA, Darmstadt, Germany).
Antifungal testing was conducted using OMWW at five concentrations, corresponding to volume ratios of 0.2%, 0.5%, 2%, 6%, and 10% in the substrate, while hydroxytyrosol and vanillic acid were tested at two concentrations, corresponding to volume ratios of 0.1% and 0.5% in the substrate [6,12]. Pure PDA served as the negative control, while Nativo 75WG (Bayer d.o.o., Zagreb, Croatia), commonly used against phytopathogenic fungi in olive cultivation, was the positive control. Nativo 75WG is a fungicide formulated as a water-dispersible granule. It contains two active ingredients: trifloxystrobin (250 g/kg) and tebuconazole (500 g/kg). The fungicide was diluted to the recommended concentration for olive tree treatment, according to the manufacturer’s guidelines, of 20 g/100 l. PDA was used instead of water [22].
The temperature of PDA was monitored until it cooled to approximately 45 °C [6]. Once cooled, 10 mL of PDA was transferred into a sterile Falcon tube. The appropriate quantity of the OMWW, compound, or fungicide was pipetted into the tube, stirred with a glass rod, and gently vortexed to achieve a homogeneous mixture. The solution was then poured into Petri dishes, and a 4 mm diameter plug of an actively growing fungal culture was inoculated onto the center of the substrate, with the plug’s upper surface facing the medium. The Petri dishes were sealed with parafilm and incubated in darkness at 25 °C. Each treatment and concentration were conducted in triplicate.
Fungal mycelial growth was assessed at two and seven days post-inoculation. For isolates showing no fungal growth at the final measurement, half of the mycelial plug was transferred with a sterile needle onto fresh PDA in sterile Petri dishes. The samples were incubated under the same conditions. Treatments were classified as fungistatic if fungal growth resumed or fungicidal if no growth was observed. The minimum inhibitory concentration (MIC) was recorded as the lowest concentration that completely inhibited mycelial growth, while the minimum fungicidal concentration (MFC) was the lowest concentration resulting in fungicidal activity.
2.5. Antagonistic Activity of Microorganisms Isolated from OMWW
2.5.1. Isolation of Microorganisms from OMWW
The OMWW samples were shaken and vortexed. Subsequently, 100 µL of untreated OMWW was pipetted onto three different media: PDA, malt dextrose agar (MEA), and nutrient agar (NA). Three replicates were prepared for each medium. After seven days of incubation at room temperature (23 °C), microorganisms that developed on the media were subcultured onto pure media. For fungi, a PDA medium supplemented with the antibiotic streptomycin was used. For bacterial purification, the samples were placed in sterile PBS (phosphate-buffered saline), vortexed, and centrifuged at 1000× g for 5 min to separate bacteria and yeast, after which they were inoculated onto pure NA. These procedures were repeated until pure cultures were obtained. In total, three yeast isolates, one bacterial isolate, and three fungal isolates were obtained.
2.5.2. Identification of Microorganisms from OMWW
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) was used for the identification of yeast and bacterial isolates. Briefly, the yeast strains were grown on PDA + streptomycin agar, and bacterial strains were grown aerobically on NA agar plates for four days at room temperature (23 °C). A small proportion of yeast/bacterial colonies was subsequently analyzed using a Bruker Microflex LT MS machine (Bruker Daltonics, Manning Park Billerica, MA, USA). Rapid on-plate formic acid (FA) treatment, followed by the application of α-cyano-4-hydroxycinnamic acid (HCCA), was used to extract protein from bacterial/yeast cells, according to the manufacturer’s instructions. For each spot, 40 sub-spectra for each of the 40 randomized positions within the spot were collected and assembled into one main spectrum. The mass spectra profiles were assessed, visualized, and analyzed/compared to reference spectra from the Bruker Biotyper version 3.1 (Build 65) database using Bruker flexControl 3.4 software. A mass range of 2000 to 20,000 Da was used for the analysis.
For the identification of filamentous fungi, the PCR method was used. The fungal isolates were cultured on PDA for five days at 25 °C in darkness. A small portion of mycelium from the colony margins was aseptically collected using a sterile laboratory needle for genomic DNA extraction. Total genomic DNA was extracted using the Maxwell® RSC Instrument (Promega, Madison, WI, USA) and the Maxwell® RSC Plant DNA Kit (Promega). The genomic DNA concentration was quantified post-isolation using a Maxwell Promega Quantus fluorometer (Promega). The internal transcribed spacer (ITS) regions were amplified and sequenced using the primer pairs ITS1 (5’ TCCGTAGGTGAACCTGCGG 3’) and ITS4 (5’ TCCTCCGCTTATTGATATGC 3’) [23]. Each PCR mixture had a final volume of 25 µL, containing 12.5 µL of EmeraldAmp® GT PCR Master Mix, 0.5 µL of each primer (10 µM), 6.5 µL of nuclease-free water, and 5 µL of template DNA at a concentration of 5 ng/µL. PCR amplification was conducted using a SureCycler 8800 Thermal Cycler (Agilent Technologies, Santa Clara, CA, USA) according to a slightly modified program described by [24]. In the case of the yeast isolate R_BB, MALDI-TOF did not yield results; therefore, PCR analysis was also performed using the same equipment and chemicals. The difference lay in the PCR amplification conditions. The program included an initial denaturation step at 95 °C for 2 min, followed by 30 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, elongation at 72 °C for 1 min, and a final extension step at 72 °C for 5 min [23]. Gel electrophoresis was performed using a 1% agarose gel at 110 V for 20 min in 1x TAE buffer, powered by an omniPAC Midi CS-300V electrophoresis power supply (Cleaver Scientific, Rugby, Warwickshire, UK). The PCR products were visualized with an iBright CL1000 Imaging System (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA), purified using the GenElute™ PCR Clean-Up Kit (Sigma-Aldrich, Burlington, MA, USA), and subsequently sent to Macrogen Europe for sequencing. The nucleotide sequences were examined and refined using Sequencher 5.0 software (Gene Codes Corporation, Ann Arbor, MI, USA). A comparative analysis was performed using relevant sequences from species in the GenBank database, National Center for Biotechnology Information (NCBI). The consensus sequences obtained in this study were submitted to GenBank. Both the sequence data from the isolates analyzed in this study and additional relevant sequences from GenBank were incorporated into the phylogenetic analysis. The evolutionary relationships were inferred using the neighbor-joining method [25], with the resulting optimal phylogenetic tree illustrated. Bootstrap analysis, based on 1000 replicates, was conducted to assess the reliability of clustering, with the bootstrap values displayed adjacent to the branches [26]. Evolutionary distances were computed using the maximum composite likelihood approach, expressed as the number of nucleotide substitutions per site [27]. The phylogenetic analyses were carried out using MEGA11 software (Pennsylvania State University, State College, PA, USA) [28].
2.5.3. Antagonistic Assay
The antagonistic assay was conducted following the dual-culture method [29], as described in Živković et al. [30]. Isolates were pre-incubated in darkness at 25 °C for seven days. Sterile Petri dishes were filled with 10 mL of PDA. Using a 4 mm diameter cork borer, a mycelial disk of the pathogen was cut and placed on one side of the Petri dish, ensuring that the top side of the plug faced the medium. On the opposite side of the Petri dish, a loopful of bacteria or yeast (1 µL) was placed 3 cm away from the mycelial plug using a sterile inoculation loop. A pure PDA plate with a pathogen mycelial plug served as the control. The assay was performed in triplicate. For the antagonistic assay with Penicillium sp., the same procedure was followed, except that a 4 mm mycelial disk of the antagonist was used instead of the loopful.
The percentage of growth inhibition (PGI) was determined using the formula described in Živković et al. [30]: PGI (%) = (KR − R1)/KR × 100, where KR represents the distance (in mm) from the point of inoculation to the colony margin on the control plates, and R1 denotes the distance from the point of inoculation to the colony margin on the treated plates, specifically in the direction of the antagonist [31]. The PGI values were categorized using a growth inhibition category (GIC) scale ranging from 0 to 4 as follows: 0 = no growth inhibition, 1 = 1–25% growth inhibition, 2 = 26–50% growth inhibition, 3 = 51–75% growth inhibition, and 4 = 76–100% growth inhibition. The zone of inhibition was measured as the distance between the fungal pathogen and the area of antagonist growth after seven days of incubation [30].
2.6. Statistical Analysis
Statistical analysis of the chemical profile of the OMWW, calculations related to the antagonistic effect, percentage inhibition calculations, MIC and MFC values, and bar chart creation for antagonism were performed using Microsoft Office Excel. Graphical representations (HeatMaps) were generated using Python 3.10.12. The antifungal efficacy of OMWW, its components, and the fungicide were assessed using SAS Enterprise Guide 8.4. Data were expressed as arithmetic means, standard deviations, and 95% confidence intervals for the mean.
3. Results
3.1. Physicochemical Properties of the OMWW
The color of the OMWW varied among olive varieties, ranging from yellow to brown (Table 2). The darkest color was observed in OMWW from Istarska bjelica, while the lightest was found in OMWW from Buža puntoža. Regarding pH values, they tended to lean towards acidic, except for OMWW from Buža puntoža, which had a neutral pH of 7.17. The highest levels of dry matter and sugars were recorded in OMWW from Istarska Bjelica (35.72 mg/mL and 4.05 mg/mL, respectively), while the lowest levels were observed in OMWW from Buža Puntoža (1.54 mg/mL and 0.17 mg/mL, respectively).

Table 2.
Results of the physicochemical analysis of OMWW.
Following HCl treatment, OMWW from Buža puntoža also exhibited the lowest concentration of dry matter and sugars (3.07 mg/mL and 0.25 mg/mL, respectively), although these levels were higher compared to the untreated sample. Surprisingly, OMWW from Istarska bjelica, which had the highest dry matter and sugar content without HCl, demonstrated a sharp reduction in dry matter to 4.1 mg post-HCl treatment, while retaining the highest sugar concentration among all varieties and treatments (5.0 mg/mL). The highest concentration of dry matter after HCl treatment was recorded in OMWW from Leccino.
For all OMWW samples, except those from Leccino, the sugar concentrations were higher in HCl-treated samples compared to untreated samples.
Regarding the concentrations of carbon and nitrogen in the OMWW samples, the highest concentrations were observed in OMWW from Istarska bjelica, while the lowest were found in OMWW from Buža puntoža. Concerning the HCl treatments and carbon content, lower carbon concentrations were recorded in OMWW treated with HCl from Buža, Buža puntoža, Istarska bjelica, and Leccino. However, the opposite trend was observed for Rosinjola, where higher carbon concentrations were found in HCl-treated OMWW compared to untreated samples. Regarding nitrogen content, higher nitrogen concentrations were observed in HCl-treated OMWW from Buža, Istarska bjelica, and Rosinjola compared to untreated samples, while lower nitrogen concentrations were found in HCl-treated OMWW from Buža puntoža and Leccino.
3.2. HPLC Analysis of Phenolic Compounds
The data presented indicate a significant impact of acidification with HCl on the phenolic profiles of OMWW. The effects can be categorized into three main trends: an increase in phenolic concentrations, a decrease in certain compounds, and the complete absence of specific phenolics following acidification (Figure 1). For instance, HCl treatment increased the concentrations of the most abundant phenolics in OMWW from Buža, Buža puntoža, and Istarska bjelica, while a decrease was observed in OMWW from Leccino and Rosinjola. In some cases, such as in OMWW from Buža puntoža, caffeic acid was not detected in the sample without HCl treatment but was present following HCl treatment. Conversely, quercetin-3,4′-diglucoside was detected without HCl treatment but was absent after HCl treatment.
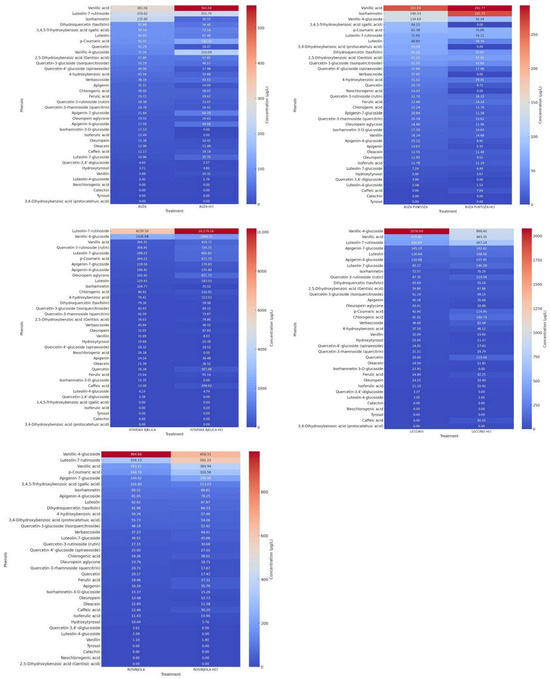
Figure 1.
The heatmap presents the relative concentrations of individual phenolic compounds identified in OMWW from five olive varieties: Buža, Buža puntoža, Istarska bjelica, Leccino, and Rosinjola. For each variety, untreated samples (left in each pair) are compared to acidified samples treated with HCl (right in each pair). Rows represent specific phenolic compounds, while columns correspond to individual samples. Within each OMWW sample, phenolic compounds are ordered from the most to the least abundant based on the untreated condition. The color gradient indicates relative concentration levels, with red representing higher values and blue indicating lower concentrations.
Significant variations in isorhamnetin concentrations were observed across all four measurements for each OMWW, as indicated by the standard deviation. Furthermore, larger deviations in concentration between measurements were recorded for other phenolics, such as luteolin and quercetin, particularly in OMWW from Buža and Buža puntoža.
The most abundant components in OMWW from Buža without HCl treatment were vanillic acid, luteolin-7-rutinoside, isorhamnetin, dihydroquercetin (taxifolin), and 3,4,5-trihydroxy benzoic acid (gallic acid). In contrast, with HCl treatment, the most abundant phenolics were luteolin-7-rutinoside, vanillic acid, vanillic-4-glucoside, p-coumaric acid, and 3,4,5-trihydroxybenzoic acid (gallic acid).
For OMWW from Buža puntoža, the most abundant components without HCl treatment were vanillic acid, isorhamnetin, vanillic-4-glucoside, 3,4,5-trihydroxybenzoic acid (gallic acid), and p-coumaric acid. Following HCl treatment, the most abundant phenolics were 3,4-dihydroxybenzoic acid (protocatechuic acid), vanillic acid, vanillic-4-glucoside, p-coumaric acid, and luteolin-7-rutinoside.
In OMWW from Istarska bjelica, the most abundant phenolics without HCl treatment were luteolin-7-rutinoside, vanillic-4-glucoside, vanillic acid, quercetin-3-rutinoside (rutin), and luteolin-7-glucoside. After HCl treatment, the most abundant phenolics were luteolin-7-rutinoside, verbascoside, vanillic-4-glucoside, quercetin-3-rutinoside (rutin), and luteolin-7-glucoside.
For OMWW from Leccino, the most abundant phenolics without HCl treatment were vanillic-4-glucoside, vanillic acid, luteolin-7-rutinoside, apigenin-7-glucoside, and luteolin. After HCl treatment, the most abundant components were luteolin-7-rutinoside, vanillic-4-glucoside, vanillic acid, luteolin-7-glucoside, and luteolin.
In OMWW from Rosinjola, the most abundant phenolics without HCl treatment were vanillic-4-glucoside, luteolin-7-rutinoside, vanillic acid, p-coumaric acid, and apigenin-7-glucoside. With HCl treatment, the most abundant were luteolin-7-rutinoside, vanillic-4-glucoside, vanillic acid, 3,4-dihydroxybenzoic acid (protocatechuic acid), and p-coumaric acid.
Overall, vanillic acid, vanillic-4-glucoside, and luteolin-7-rutinoside were the most abundant phenolics in OMWW samples collected in this study. The highest phenolic concentration was recorded in OMWW from Istarska bjelica, while the lowest was observed in OMWW from Buža puntoža. This is consistent with the findings for total dry matter content, which was also highest in Istarska bjelica and lowest in Buža puntoža. Acidification with HCl generally resulted in higher total phenolic concentrations across all OMWW samples, except for Buža puntoža.
3.3. Antifungal Activity of OMWW
The results of the ANOVA analysis showed significant differences between the applied treatments and their effects on the growth of phytopathogenic fungi mycelia. The results of the ANOVA analysis and the inhibition percentage calculations are presented in the Supplementary File in Tables S1–S12. For clarity, the text discusses the results of the inhibition percentage calculations.
Overall, among the tested fungi, N. parvum proved to be the most resistant species, where only the vanillic acid component and fungicide had an inhibitory effect on fungal mycelial growth (Figure 2). Conversely, Do. iberica was the most susceptible species to all treatments. Inhibitory effects of OMWW on fungal mycelial growth on the seventh day of measurement were observed exclusively for Do. sarmentorum. For D. seriata, all OMWW treatments stimulated mycelial growth. In D. mutila, only the lowest concentration of all OMWWs had an inhibitory effect, whereas higher concentrations exhibited a stimulatory effect across all treatments. A similar pattern was observed for B. dothidea, where lower OMWW concentrations inhibited mycelial growth, while higher concentrations stimulated growth. Vanillic acid was more effective compared to the component hydroxytyrosol, and the fungicide Nativo 75WG also demonstrated high efficacy in inhibiting fungal mycelial growth. Among the OMWW treatments, the most significant effects were observed with OMWW from Leccino, followed by Buža. Considering that the treatments’ effects were more pronounced on the second day of measurement and that most treatments acted fungistatically, the percentage inhibition of fungal mycelial growth by fungi and treatments on the second day was graphically represented.

Figure 2.
The heatmap presents the percentage of fungal mycelial growth inhibition measured on day two for six species of the Botryosphaeriaceae family: Botryosphaeria dothidea, Diplodia mutila, Diplodia seriata, Dothiorella iberica, Dothiorella sarmentorum, and Neofusicoccum parvum. Each panel represents one fungal species. Treatments include OMWW from five olive varieties, two phenolic compounds (hydroxytyrosol and vanillic acid), and a commercial fungicide (Nativo 75WG). The x-axis displays treatment concentrations, while the y-axis lists the applied treatments with inhibition percentages. The color gradient reflects the intensity of fungal inhibition, from blue (lowest or negative/stimulatory effect) to red (highest inhibition, up to 100%). Negative values indicate a stimulatory effect on fungal growth.
When analyzing each treatment individually and its effects on fungi, OMWW from Buža at lower concentrations (0.2%, 0.5%, and 2%) inhibited the mycelial growth of B. dothidea; however, at higher concentrations (6% and 10%), greater growth than the control was recorded. For D. mutila, the inhibitory effects of this OMWW from Buža were observed at a concentration of 0.2%, whereas at concentrations ≥0.5%, significantly higher mycelial growth compared to the control was recorded, indicating a stimulatory effect. For Do. iberica, 100% inhibition was observed at all concentrations of this OMWW on the second day of measurement. For Do. sarmentorum, no inhibition was observed at the lowest concentration. At higher concentrations, mycelial growth was inhibited by 100% on both the second and seventh days of measurement.
OMWW from Buža puntoža inhibited the growth of B. dothidea at lower concentrations (0.2% and 0.5%) by 31.95% and 22.19%, respectively. At a concentration of 2%, it had no effect, while at 6% and 10%, it stimulated mycelial growth. For D. mutila, only a minimal inhibitory effect (3.11%) was observed at the lowest concentration, with higher concentrations resulting in a stimulatory effect. For Do. iberica, the greatest inhibition (62.65%) was recorded at the lowest concentration, with inhibitory effects diminishing at higher concentrations. For Do. sarmentorum, inhibitory effects were recorded at concentrations of 0.2% and 0.5% (22.71% and 21.12%), but higher concentrations had no significant effect on mycelial growth.
OMWW from Istarska bjelica showed negligible effects on mycelial inhibition at a concentration of 0.2%, while higher concentrations exhibited a stimulatory effect. For D. mutila and B. dothidea, inhibitory effects were recorded only at 0.2% (2.7% and 1.87%). For Do. iberica, the greatest inhibitory effects were observed at the lowest concentrations (48.19% and 46.18%), diminishing with higher concentrations. For Do. sarmentorum, 100% inhibition was recorded at the lowest concentration; at 0.5%, inhibition was mild (8.76%), and at higher concentrations, the effects ranged from neutral to slightly stimulatory.
OMWW from Leccino inhibited the growth of B. dothidea at a concentration of 0.2% by 12.45%, with higher concentrations showing stimulatory effects. For D. mutila, a similar pattern was observed, with a 9.78% inhibition at 0.2%. For Do. iberica, 100% inhibition was recorded at concentrations of 0.2% and 0.5%, while inhibition diminished with higher concentrations. For Do. sarmentorum, 100% inhibition was observed at concentrations of 0.2–6% on both the second and seventh days of measurement. Only this species showed mycelial inhibition on the seventh day (with OMWW from Leccino and Buža showing 100% inhibition).
OMWW from Rosinjola inhibited the growth of B. dothidea and D. mutila only at the lowest concentration (10.79% and 11.11%, respectively), while higher concentrations showed a stimulatory effect. For Do. iberica, higher concentrations resulted in greater inhibition, with the highest inhibition (42.97%) at 10%. For Do. sarmentorum, growth inhibition was observed only at the lowest concentration (14.34%).
Regarding hydroxytyrosol, this component stimulated the growth of B. dothidea. For D. mutila and D. seriata, inhibitory effects on mycelial growth were observed only on the second day of measurement (15.11% and 16.89%; and 11.25 and 12.50). No inhibitory effects were observed on Do. iberica, Do. sarmentorum, or N. parvum. In contrast, vanillic acid exhibited inhibitory effects on all fungi, with greater inhibition at higher concentrations. For B. dothidea, inhibition at 0.1% was 70.12% and 12.02% on the second and seventh days, respectively, and 96.68% and 77.13% at 0.5%. For D. mutila, inhibition at 0.1% was observed only on the second day (40.44%), while at 0.5%, inhibition was 100% on both days. For D. seriata, inhibition was observed on both days at both concentrations, while higher concentrations resulted in 100% inhibition. For Do. iberica and Do. sarmentorum, inhibition at lower concentrations occurred on both days, while at higher concentrations, inhibition reached 100% for both species on both days. For N. parvum, inhibition at the lower concentration occurred only on the second day (48.45%), while at the higher concentration, inhibition was 100% on both days.
The fungicide Nativo 75WG was more effective than all other treatments for B. dothidea, D. mutila, and D. seriata. For Do. Iberica, the fungicide outperformed all other treatments, except vanillic acid at 0.5%. For Do. sarmentorum, the fungicide was effective (100%) on both days, as were OMWW from Buža at concentrations of 0.5–10% and OMWW from Leccino at 0.2–6%. For N. parvum, Nativo 75WG was also highly effective (100% and 93.41%), but vanillic acid at a concentration of 0.5% was more effective, achieving 100% inhibition on both days.
Regarding the MIC and MFC values (Table S13), the fungicide, as expected, showed the best results, exhibiting a fungicidal effect on all tested fungi except Diplodia seriata, where only a fungistatic effect was observed. Among the other treatments, MFC values were recorded only for certain species in the Buža, Leccino, and vanillic acid treatments.
3.4. Antagonistic Assay
3.4.1. Identification of Microorganisms
The list of all species isolated from the OMWW is presented in Table 3. A total of one bacterium, three yeasts, and three filamentous fungi were isolated. The bacterium was found only in OMWW from Buža. From the OMWW of Istarska bjelica and Leccino, only Penicillium crustosum Thom was isolated. The ITS region sequences of the isolates were deposited in GenBank under accession numbers PV092539 for isolate R_BB, PQ826427 for isolate BJ_P, PQ826435 for isolate L_P, and PQ826436 for isolate BP_P. The phylogenetic analysis confirms the identification of this species (Figure S1).

Table 3.
The list of species isolated from each OMWW.
3.4.2. Antagonistic Assay Results
The greatest antagonistic effect against fungi was observed with the species Do. iberica, where four out of seven tested isolates exhibited a strong antagonistic impact, with a GIC value of 4 (Table S14). The highest antagonistic effect on B. dothidea was shown by P. crustosum from OMWW Istarska bjelica (BJ_P) (70.54%), while P. crustosum from OMWW Buža puntoža (BP_P) (67.86%) was also effective (Figure 3). In contrast, N. molendiniolei (R_BB) demonstrated the weakest effect (11.76%). For D. mutila, the highest inhibition percentage was observed with P. crustosum from OMWW Leccino (L_P) (96.67%), and P. crustosum from OMWW Istarska bjelica (BJ_P) also showed significant effectiveness (85%). Against D. seriata, P. crustosum from OMWW Leccino (L_P) (82.22%) and P. crustosum from OMWW Buža puntoža (BP_P) (81.67%) exhibited the highest inhibition. In comparison, B. velezensis (B_BB) was substantially less effective.
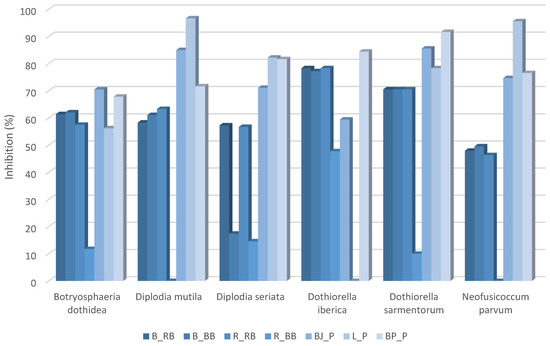
Figure 3.
Results of the statistical analysis of the antagonistic activity of microorganisms isolated from OMWWs against phytopathogenic fungi. The graph represents the calculated percentage of inhibition of fungal mycelial growth by microorganisms isolated from OMWWs. Different isolates were tested against six phytopathogenic fungi: Botryosphaeria dothidea, Diplodia mutila, Diplodia seriata, Dothiorella iberica, Dothiorella sarmentorum, and Neofusicoccum parvum. Higher inhibition percentages indicate stronger antagonistic effects.
The best effect on Do. iberica was observed with P. crustosum from OMWW Buža puntoža (BP_P) (84.44%), while P. crustosum from OMWW Istarska bjelica (BJ_P) (59.44%) showed moderate effectiveness. For Do. sarmentorum, P. crustosum from OMWW Buža puntoža (BP_P) (91.67%) and Istarska bjelica (BJ_P) (85.56%) demonstrated notably high inhibition, while N. molendiniolei (R_BB) exhibited a very weak effect (10%). For N. parvum, P. crustosum from OMWW Leccino (L/P) almost completely inhibited pathogen growth (95.57%), while those from Istarska bjelica (BJ_P) (74.69%) and Buža puntoža (BP_P) (76.54%) were also effective.
Among the tested Penicillium isolates, L_P emerged as the most efficient antagonistic organism, particularly against D. mutila and N. parvum (Figure S2). The weakest antagonistic effect among the treatments was observed with the R_BB isolate, N. molendiniolei, as no antagonistic interaction was recorded between this isolate and D. mutila or N. parvum. Additionally, no antagonistic effect was observed between the L_P isolate and Do. iberica.
4. Discussion
As previously mentioned, OMWW and its management pose a significant challenge in agriculture. However, some OMWWs have demonstrated effectiveness in inhibiting the growth of phytopathogenic fungi, as well as the potential to stimulate plant growth due to their organic content. Moreover, OMWWs can serve as a resource for extracting phenols or microorganisms that have proven effective in suppressing the growth of phytopathogenic fungi.
Among the OMWW treatments in this study, the most significant effects were observed with OMWWs from the Leccino variety, followed by Buža. While these olive varieties are highly praised and considered among the favorites of olive oil enthusiasts, the Botryosphaeriaceae species seem to “lose the will to live”, indicating that the OMWW from these olive varieties inhibits the growth of fungi as effectively as their oils delight gourmets. A varied effect of olive mill wastes on phytopathogenic fungi was reported in the study by Cayuela et al. [15]. The authors noted that the diverse effects observed in certain cases, such as with Globisporangium ultimum (Trow) Uzuhashi, Tojo & Kakish (syn. Pythium ultimum) and Botrytis cinerea Pers., showed no correlation with the measured chemical properties of the residues but were likely linked to specific compounds present in varying concentrations within the residues. Cayuela et al. [15] emphasize that the limited research examining the antifungal potential of olive mill wastes often yield conflicting results. This variability arises from the numerous factors that can influence the effectiveness of these wastes in suppressing pathogens. For instance, Bonanomi et al. [32] observed that dry olive mill residue exhibited phytotoxic effects on various crop species, which, in certain cases, increased the number of fungal diseases. Similarly, in our research, it was confirmed that treatments had a stimulative effect on certain fungi, while in some cases, the effect depended on the concentration. At lower concentrations, the impact was inhibitory, whereas at higher concentrations, it became stimulative, and vice versa.
Istrian olive oils are known to contain a relatively high amount of polyphenols compared to values reported in the literature [33]. Among these, olive oils from the Istarska bjelica variety stand out for their exceptionally high polyphenol content [33]. Similarly, in our research, the highest phenolic concentration was recorded in OMWWs derived from Istarska bjelica. This can also be linked to the fact that this OMWW had the highest dry matter content, which corresponded with elevated levels of sugars, nitrogen, and carbon. Although the phenolic concentration was the highest in this OMWW, and the antimicrobial activity of OMWW is often associated with the presence of phenols, it did not demonstrate the strongest inhibition of fungal mycelium growth. The antifungal properties of phenolic compounds against pathogenic fungi have been documented in several studies. These studies describe the effects of phenolics on fungi such as Verticillium dahliae Klebahn [34], Phytophthora sp., Alternaria sp., Fusarium sp., and others [35,36]. Additionally, Krid et al. [12] identified hydroxytyrosol as the main antimicrobial compound in OMWW. In our research, vanillic acid, vanillic-4-glucoside, and luteolin-7-rutinoside were identified as the most abundant phenolic compounds in OMWW. Regarding hydroxytyrosol, which is widely cited as the phenol with the strongest antimicrobial activity, its effectiveness was limited to D. mutila and D. seriata. It did not affect N. parvum, and its impact on B. dothidea, Do. iberica, and Do. sarmentorum was mildly stimulatory. However, this stimulatory effect should be interpreted with caution, given the sensitivity and variability of biological assays. In the case of vanillic acid, an increase in its concentration was associated with greater inhibitory effects. At a concentration of 0.5%, it completely inhibited the growth of tested fungal species, demonstrating its significant antifungal potential.
Regarding the acidification of OMWW with HCl and its impact on phenols, it significantly influences the phenolic composition of OMWW, with both positive and negative effects. In many cases, acidification with HCl resulted in either an increase or decrease in specific phenols. This suggests that acidification may enhance the stability or release of these phenols, possibly by breaking down glycosylated forms into their free phenolic counterparts. Conversely, reductions in phenolic content could be attributed to the degradation of sensitive phenols under acidic conditions or their transformation into other phenolic derivatives that were not quantified in this dataset. Certain phenols are particularly susceptible to degradation under specific conditions [37,38].
In addition to phenols, other agents responsible for the antimicrobial activity of OMWW are mentioned in the literature. Yangui et al. [6] suggested the potential application of OMWW and bacteria isolated from OMWW (Bacillus subtilis (Ehrenberg 1835) Cohn, Trinickia caryophylli (Burkholder 1942) Estrada-de los Santos (syn. Burkholderia caryophylli), and Pseudomonas fluorescens Migula for controlling the pathogenic fungus Armillaria mellea (Vahl) P. Kumm). In the work of Alfano et al. [35], the antifungal potential of olive waste compost was examined. The compost was found to contain significant populations of active microbes capable of degrading chitin and cellulose. Plate inhibition assays demonstrated that extracts from compost water strongly inhibited the growth of several pathogens, including Fusarium oxysporum f.sp. lycopersici, Globisporangium ultimum (Trow) Uzuhashi, Tojo & Kakish (syn. Pythium ultimum), Phytophthora infestans (Mont.) de Bary, Sclerotinia sclerotiorum (Lib.) de Baryand, and V. dahliae. The inhibitory effects were attributed to the antagonistic activities of microorganisms present in the compost, including large populations of aerobic spore-forming bacteria and actinomycetes. Muzzalupo et al. (2020) [36] reported the great effectiveness of olive leaf extracts in controlling fungal pathogens, either in their free form or encapsulated in chitosan-tripolyphosphate nanoparticles. Their study documented high inhibition rates for the germination and growth of Fusarium proliferatum (Matsush.) Nirenberg ex Gerlach & Nirenberg.
Several microorganisms were isolated from the OMWW used in this study, including P. crustosum, B. velezensis, R. mucilaginosa, and N. molendiniolei.
P. crustosum is a common fungal species frequently associated with food contamination, leading to the spoilage of various foods. This species has been previously reported on olives and their byproducts in Spain [39]. It has shown significant potential for future industrial applications due to its pronounced enzymatic activities [39]. Gharsallah et al. [40] also identified P. crustosum from insects collected in olive orchards. The authors demonstrated the pathogenicity of P. crustosum through assays performed on excised shoots, where the isolate P. crustosum F14 caused browning in the cortex. Additionally, the study documented antagonistic interactions between this isolate and fungal species such as Aspergillus calidoustus Varga, Houbraken & Samson, Penicillium chrysogenum Thom, and Alternaria consortialis (Thm.) J.W. Groves & S. Hughes. In contrast, no antagonistic effects were observed with the isolate P. crustosum F33, which was also collected from insects in olive orchards. This study also confirmed differences in the antagonistic potential among Penicillium sp. isolates, as well as variations in the antagonistic effects of the same isolate on different fungal species. The strongest antagonistic effect was observed between the L/P isolate and the species N. parvum and D. mutila, while the weakest interaction was noted between L/P and Do. iberica, where no antagonistic effect of the isolate on the pathogen was recorded.
B. velezensis Ruiz-Garcia et al. is an aerobic, Gram-positive bacterium capable of forming endospores and enhancing plant growth. Various strains of this species have been documented for their ability to inhibit the growth of microbial pathogens, including fungi, bacteria, and nematodes [41]. B. velezensis OEE1, isolated from the endogenous root tissue of olive trees, exhibited antifungal activity under in vitro conditions against V. dahliae, with an inhibition rate exceeding 92%. Under in vivo conditions, B. velezensis OEE1 significantly reduced the final mean disease severity index, the percentage of dead plants, the area under the disease progress curve, and the microsclerotia density in naturally infested soil [42]. In the study by Castro et al. [43], under in vitro conditions, strain XT1 demonstrated the ability to reduce fungal mycelium by 34–100%. When applied directly to young olive trees, it decreased the incidence rate of Verticillium wilt and the severity of symptoms. Additionally, it increased polyphenol oxidase (PPO) activity by 395%, indicating the enhanced resistance of plant tissues to the disease, and reduced the number of fungal microsclerotia in the soil. The B. velezensis isolate in this study exhibited a strong antagonistic effect on pathogens, with the greatest impact observed on Do. iberica and the least on D. seriata.
R. mucilaginosa is a biotechnologically significant yeast that has garnered considerable attention as a potential platform strain due to its ability to utilize a wide range of substrates, exceptional stress tolerance, and other advantageous traits. R. mucilaginosa is considered a highly suitable candidate for producing carotenoids, lipids, enzymes, and other valuable bioproducts, particularly through the biorefining of low-cost agricultural waste materials [44]. Ghilardi et al. [45] demonstrated that substrates derived from olive mill waste can be effectively utilized for carotenoid production by R. mucilaginosa. Interestingly, Jarboui et al. [46,47] identified that R. mucilaginosa CH4 can play a significant role in the purification of OMWW by removing polyphenolic compounds, including catechol, gallic acid, p-coumaric acid, protocatechuic acid, tyrosol, vanillic acid, etc. In this study, the yeast mentioned was isolated from the OMWW of Buža and Rosinjola, with the R_RB isolated from the OMWW of Rosinjola showing a stronger impact on pathogens.
N. molendiniolei (syn. Nakazawaea molendini-olei or Candida molendinolei) has been recognized for its resistance to phenolic compounds and its ability to convert oleuropein into hydroxytyrosol [48]. It has also been utilized as a starter culture for controlled olive fermentation, as demonstrated in the study by Ciafardini and Zullo [49]. Furthermore, N. molendiniolei exhibits significant enzymatic activities, such as β-glucosidase and peroxidase. These activities contribute to limiting the increase in the acidity of olive oil during storage; however, they are also associated with an increase in oxidative parameters, which ultimately result in a decline in olive oil quality over time [50].
5. Conclusions
This study confirms the potential of OMWW as a sustainable alternative to chemical fungicides. Rich in bioactive compounds such as phenols, OMWW represents an interesting and environmentally friendly solution for crop protection in the Mediterranean region while simultaneously reducing environmental burdens. Among the OMWW treatments, Leccino and Buža showed the most significant antifungal activity against aggressive pathogens from the Botryosphaeriaceae family, which are known for causing substantial yield losses in woody crops.
The results also highlight the impact of acidification on the phenolic profile of OMWW. Treatment with HCl significantly altered the concentration of individual phenolic compounds, either enhancing their release or contributing to their degradation, suggesting that pH manipulation could be a tool for optimizing OMWW bioactivity. Furthermore, among the two phenols tested, vanillic acid demonstrated exceptional antifungal activity, while microorganisms isolated from OMWW, such as B. velezensis, further emphasized the biological potential of this waste. These findings open up possibilities for integrating OMWW into sustainable crop protection systems.
Future studies should focus on the standardization of production processes, the optimization of concentrations, and the combination of OMWW with other bioactive compounds or microorganisms. This could make OMWW a key component in integrated management systems, offering the dual benefit of crop protection and the promotion of sustainable agricultural practices. In addition, OMWW serves as a valuable source for the isolation of microorganisms that can be used for various purposes, including pigment production, in the food industry, and others.
In conclusion, OMWW exemplifies the principles of the circular economy by transforming agricultural waste into a valuable product. While the results are promising, additional research is essential to address challenges, such as the stimulatory effects of higher OMWW concentrations on certain pathogens. This study lays the foundation for the further development of eco-friendly crop protection methods aligned with the goals of the European Green Deal and the target of reducing pesticide use by 50% by 2030.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae11070819/s1, Table S1. The results of the one-way ANOVA (mean ± standard deviation, in mm) for the mycelial growth of Botryosphaeria dothidea under different treatments. Table S2. Inhibitory effect (%) of treatment on the mycelial growth of Botryosphaeria dothidea. Table S3. The results of the one-way ANOVA (mean ± standard deviation, in mm) for the mycelial growth of Diplodia mutila under different treatments. Table S4. Inhibitory effect (%) of treatment on the mycelial growth of Diplodia mutila. Table S5. The results of the one-way ANOVA (mean ± standard deviation, in mm) for the mycelial growth of Diplodia seriata under different treatments. Table S6. Inhibitory effect (%) of treatment on the mycelial growth of Diplodia seriata. Table S7. The results of the one-way ANOVA (mean ± standard deviation, in mm) for the mycelial growth of Dothiorella iberica under different treatments. Table S8. Inhibitory effect (%) of treatment on the mycelial growth of Dothiorella iberica. Table S9. The results of the one-way ANOVA (mean ± standard deviation, in mm) for the mycelial growth of Dothiorella sarmentorum under different treatments. Table S10. Inhibitory effect (%) of treatment on the mycelial growth of Dothiorella sarmentorum. Table S11. The results of the one-way ANOVA (mean ± standard deviation, in mm) for the mycelial growth of Neofusicoccum parvum under different treatments. Table S12. Inhibitory effect (%) of treatment on the mycelial growth of Neofusicoccum parvum. Table S13. MIC and MFC values of treatments. Table S14. Growth Inhibition Category (GIC) scale results from the antagonism test, where a higher number indicates a greater level of inhibition. Figure S1. The evolutionary relationships were determined using the Neighbor-Joining method, and the optimal phylogenetic tree is presented. Figure S2. Results of the conducted tests.
Author Contributions
Conceptualization—E.P., K.V., J.Ć., and S.G.; Data curation—E.P., A.A., and Z.U., Formal analysis—E.P., Funding acquisition—S.G., A.A., I.G., K.V., S.G.B., N.M., and Z.U. Methodology—E.P., K.V., A.A., I.G., N.M., J.Ć., Z.U., and S.G.; Investigation—E.P., A.A., I.G., N.M., and Z.U., Project administration—S.G. and A.A., Resources—S.G., N.M., S.G.B., Z.U., A.A., and I.G., Supervision—K.V. and S.G., Visualization—E.P., Writing—original draft preparation, E.P.; Writing—review and editing—E.P., K.V., A.A., I.G., N.M., J.Ć., Z.U., S.G.B., and S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Croatian Science Foundation under the project numbers HRZZ-UIP-2020-02-7413 and HRZZ-DOK-2021-02-2882, and the Slovenian Research and Innovation Agency (research program P1-0005 and P4-0092).
Data Availability Statement
Data is contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Žužić, I. Buđenje Istarskog Maslinarstva 1960-ih Godina; Propaganda d.o.o.: Poreč, Croatia, 2023. [Google Scholar]
- FAO, Food and Agriculture Organization of the United Nations. FAOSTAT: Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 19 February 2025).
- Shabir, S.; Ilyas, N.; Saeed, M.; Bibi, F.; Sayyed, R.Z.; Almaki, W.H. Treatment technologies for olive mill wastewater with impacts on plants. Environ. Res. 2023, 216, 114399. [Google Scholar] [CrossRef]
- Ghilardi, C.; Negrete, P.S.; Gutierrez, G.R.; Monetta, P.; Arroyo-Lopez, F.N.; Hornero-Mendez, D.; Carelli, A.A.; Borroni, V. Influence of olive mill waste phenolic compounds levels on carotenoid production by Rhodotorula spp. Process Biochem. 2022, 120, 275–286. [Google Scholar] [CrossRef]
- Bouhia, Y.; Hafidi, M.; Ouhdouch, Y.; El Boukhari, M.E.; El Fels, L.; Zeroual, Y.; Lyamlouli, K. Microbial community succession and organic pollutants removal during olive mill waste sludge and green waste co-composting. Front. Microbiol. 2022, 12, 814553. [Google Scholar] [CrossRef] [PubMed]
- Yangui, T.; Rhouma, A.; Ali Triki, M.; Gargouri, K.; Bouzid, J. Control of damping-off caused by Rhizoctonia solani and Fusarium solani using olive mill waste water and some of its indigenous bacterial strains. Crop Prot. 2008, 27, 189–197. [Google Scholar] [CrossRef]
- Cibelli, F.; Bevilacqua, A.; Raimondo, M.L.; Campaniello, D.; Carlucci, A.; Ciccarone, C.; Sinigaglia, M.; Corbo, M.R. Evaluation of fungal growth on olive-mill wastewaters treated at high temperature and by high-pressure homogenization. Front. Microbiol. 2017, 8, 2515. [Google Scholar] [CrossRef]
- Yakhlef, W.; Arhab, R.; Romero, C.; Brenes, M.; de Castro, A.; Medina, E. Phenolic composition and antimicrobial activity of Algerian olive products and by-products. LWT 2018, 93, 323–328. [Google Scholar] [CrossRef]
- Galanakis, C.M. Phenols recovered from olive mill wastewater as additives in meat products. Trends Food Sci. Technol. 2018, 79, 98–105. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Tsatalas, P.; Galanakis, I.M. Implementation of phenols recovered from olive mill wastewater as UV booster in cosmetics. Ind. Crops Prod. 2018, 111, 30–37. [Google Scholar] [CrossRef]
- Medina, E.; Romero, C.; de los Santos, B.; de Castro, A.; García, A.; Romero, F.; Brenes, M. Antimicrobial activity of olive solutions from stored alpeorujo against plant pathogenic microorganisms. J. Agric. Food Chem. 2011, 59, 6921–6929. [Google Scholar] [CrossRef]
- Krid, S.; Bouaziz, M.; Ali Triki, M.; Gargouri, A.; Rhouma, A. Inhibition of olive knot disease by polyphenols extracted from olive mill waste water. J. Plant Pathol. 2011, 93, 561–568. [Google Scholar]
- Bleve, G.; Gallo, A.; Altomare, C.; Vurro, M.; Maiorano, G.; Cardinali, A.; D’Antuono, I.; Marchi, G.; Mita, G. In vitro activity of antimicrobial compounds against Xylella fastidiosa, the causal agent of the olive quick decline syndrome in Apulia (Italy). FEMS Microbiol. Lett. 2018, 365, fnx281. [Google Scholar] [CrossRef] [PubMed]
- Bažok, R. Je li održiva uporaba pesticida doista održiva? Glas. Biljn. Zaštite 2020, 20, 384–389. [Google Scholar]
- Cayuela, M.L.; Millner, P.D.; Meyer, S.L.; Roig, A. Potential of olive mill waste and compost as biobased pesticides against weeds, fungi, and nematodes. Sci. Total Environ. 2008, 399, 11–18. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R.; Peduto, F.; Vossen, P.M.; Krueger, W.H.; Gubler, W.D. Olive twig and branch dieback: Etiology, incidence, and distribution in California. Plant Dis. 2013, 97, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Petrović, E.; Vrandečić, K.; Belušić Vozila, A.; Ćosić, J.; Godena, S. Diversity and pathogenicity of Botryosphaeriaceae species isolated from olives in Istria, Croatia, and evaluation of varietal resistance. Plants 2024, 13, 1813. [Google Scholar] [CrossRef]
- Russo, E.; Spallarossa, A.; Comite, A.; Pagliero, M.; Guida, V.; Belotti, V.; Caviglia, D.; Schito, A.M. Valorization and potential antimicrobial use of olive mill wastewater (OMW) from Italian olive oil production. Antioxidants 2022, 11, 903. [Google Scholar] [CrossRef]
- Klen, T.J.; Vodopivec, B.M. Ultrasonic extraction of phenols from olive mill wastewater: Comparison with conventional methods. J. Agric. Food Chem. 2011, 59, 12725–12731. [Google Scholar] [CrossRef]
- Klen, T.J.; Wondra, A.G.; Vrhovšek, U.; Vodopivec, B.M. Phenolic profiling of olives and olive oil process-derived matrices using UPLC-DAD-ESI-QTOF-HRMS analysis. J. Agric. Food Chem. 2015, 63, 3859–3872. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Palfi, M. Antifungal Activity of Essential Oils and Their Components Against Phytopathogenic Fungi Under In Vitro Conditions. Ph.D. Thesis, Josip Juraj Strossmayer University of Osijek, Ruđer Bošković Institute, Zagreb, Croatia, 2017. [Google Scholar]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications, 1st ed.; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press Inc.: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- Demjanová, S.; Jevinová, P.; Pipová, M.; Regecová, I. Identification of Penicillium verrucosum, Penicillium commune, and Penicillium crustosum isolated from chicken eggs. Processes 2021, 9, 53. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Fokkema, N.J. Fungal antagonism in the phyllosphere. Ann. Appl. Biol. 1978, 89, 115–117. [Google Scholar] [CrossRef]
- Živković, S.; Stojanović, S.; Ivanović, Ž.; Gavrilović, V.; Popović, T.; Balaž, J. Screening of antagonistic activity of microorganisms against Colletotrichum acutatum and Colletotrichum gloeosporioides. Arch. Biol. Sci. Belgrade 2010, 62, 611–623. [Google Scholar] [CrossRef]
- Korsten, L.; De Jager, E.S. Mode of action of Bacillus subtilis for control of avocado postharvest pathogens. S. Afr. Avocado Grow. Assoc. Yearb. 1995, 18, 124–130. [Google Scholar]
- Bonanomi, G.; Giorgi, V.; Del Sorbo, G.; Neri, D.; Scala, F. Olive mill residues affect saprophytic growth and disease incidence of foliar and soilborne plant fungal pathogens. Agric. Ecosyst. Environ. 2006, 115, 194–200. [Google Scholar] [CrossRef]
- Bertoša, M.; Matijašić, R. (Eds.) Istarska Enciklopedija; Leksikografski zavod Miroslav Krleža: Zagreb, Croatia, 2005. [Google Scholar]
- Markakis, E.A.; Tjamos, S.E.; Antoniou, P.P.; Roussos, P.A.; Paplomatas, E.J.; Tjamos, E.C. Phenolic responses of resistant and susceptible olive cultivars induced by defoliating and nondefoliating Verticillium dahliae pathotypes. Plant Dis. 2010, 94, 1156–1162. [Google Scholar] [CrossRef]
- Alfano, G.; Lustrato, G.; Lima, G.; Vitullo, D.; Ranalli, G. Characterization of composted olive mill wastes to predict potential plant disease suppressiveness. Biol. Control 2011, 58, 199–207. [Google Scholar] [CrossRef]
- Muzzalupo, I.; Badolati, G.; Chiappetta, A.; Picci, N.; Muzzalupo, R. In vitro antifungal activity of olive (Olea europaea) leaf extracts loaded in chitosan nanoparticles. Front. Bioeng. Biotechnol. 2020, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Brenes, M.; García, A.; García, P.; Garrido, A. Acid hydrolysis of secoiridoid aglycons during storage of virgin olive oil. J. Agric. Food Chem. 2001, 49, 5609–5614. [Google Scholar] [CrossRef]
- Romero, C.; Brenes, M.; García, P.; García, A.; Garrido, A. Polyphenol changes during fermentation of naturally black olives. J. Agric. Food Chem. 2004, 52, 1973–1979. [Google Scholar] [CrossRef]
- Baffi, M.A.; Romo-Sánchez, S.; Úbeda-Iranzo, J.; Briones-Pérez, A.I. Fungi isolated from olive ecosystems and screening of their potential biotechnological use. New Biotechnol. 2012, 29, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Gharsallah, H.; Ksentini, I.; Naayma, S.; Taieb, K.H.; Abdelhedi, N.; Schuster, C.; Triki, M.A.; Ksantini, M.; Leclerque, A. Identification of fungi in Tunisian olive orchards: Characterization and biological control potential. BMC Microbiol. 2020, 20, 307. [Google Scholar] [CrossRef]
- Rabbee, M.F.; Ali, M.S.; Choi, J.; Hwang, B.S.; Jeong, S.C.; Baek, K.H. Bacillus velezensis: A valuable member of bioactive molecules within plant microbiomes. Molecules 2019, 24, 1046. [Google Scholar] [CrossRef] [PubMed]
- Cheffi Azabou, M.; Gharbi, Y.; Medhioub, I.; Ennouri, K.; Barham, H.; Tounsi, S.; Triki, M.A. The endophytic strain Bacillus velezensis OEE1: An efficient biocontrol agent against Verticillium wilt of olive and a potential plant growth-promoting bacteria. Biol. Control 2020, 142, 104168. [Google Scholar] [CrossRef]
- Castro, D.; Torres, M.; Sampedro, I.; Martínez-Checa, F.; Torres, B.; Béjar, V. Biological control of Verticillium wilt on olive trees by the salt-tolerant strain Bacillus velezensis XT1. Microorganisms 2020, 8, 1080. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Cheng, P.; Yu, G. Rhodotorula mucilaginosa—Alternative sources of natural carotenoids, lipids, and enzymes for industrial use. Heliyon 2022, 8, e11505. [Google Scholar] [CrossRef]
- Ghilardi, C.; Sanmartin Negrete, P.; Carelli, A.A.; Borroni, V. Evaluation of olive mill waste as substrate for carotenoid production by Rhodotorula mucilaginosa. Bioresour. Bioprocess. 2020, 7, 52. [Google Scholar] [CrossRef]
- Jarboui, R.; Baati, H.; Fetoui, F.; Gargouri, A.; Gharsallah, N.; Ammar, E. Yeast performance in wastewater treatment: Case study of Rhodotorula mucilaginosa. Environ. Technol. 2012, 33, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Jarboui, R.; Magdich, S.; Ayadi, R.J.; Gargouri, A.; Gharsallah, N.; Ammar, E. Aspergillus niger P6 and Rhodotorula mucilaginosa CH4 used for olive mill wastewater (OMW) biological treatment in single pure and successive cultures. Environ. Technol. 2013, 34, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Ghomari, O.; Merzouki, M.; Benlemlih, M. Optimization of bioconversion of oleuropein, of olive leaf extract, to hydroxytyrosol by Nakazawaea molendini-olei using HPLC-UV and a method of experimental design. J. Microbiol. Methods 2020, 176, 106010. [Google Scholar] [CrossRef] [PubMed]
- Ciafardini, G.; Zullo, B.A. Use of selected yeast starter cultures in industrial-scale processing of brined Taggiasca black table olives. Food Microbiol. 2019, 84, 103250. [Google Scholar] [CrossRef]
- Giavalisco, M.; Zotta, T.; Parente, E.; Siesto, G.; Capece, A.; Ricciardi, A. Effect of oil-borne yeasts on the quality of extra-virgin olive oils of Basilicata region. Int. J. Food Microbiol. 2023, 386, 110041. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).