Graft Compatibility of Local Grapevine Varieties with Grapevine Rootstocks in Yozgat Province
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location and Year
2.2. Plant Materials
2.3. Production of Grafted Grapevine Saplingss
2.4. Planting and Cultivation of Grafted Grapevine Saplings
2.5. Transfer of Grafted Grapevine Saplings to the Vineyard
2.6. Data Collection
2.6.1. Callusing Chamber Performance
2.6.2. Field Performance
2.7. Data Analysis
3. Results
General Evaluation
4. Discussion
General Evaluation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Çelik, S. Bağcılık (Ampeloloji), 1st ed.; Anadolu Printing Industry and Trade Ltd., Co.: Tekirdağ, Türkiye, 2011; pp. 1–428. [Google Scholar]
- Bozkurt, A.; Yağcı, A. Grafted grapevine saplings yields of Karaerik and Narince grape cultivars grafted on different rootstocks in potted grafted grapevine saplings production. Bahçe 2024, 53, 1–8. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; van der Heijden, M.G.A. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef]
- Pisciotta, A.; Barone, E.; Di Lorenzo, R. Table-grape cultivation in soil-less systems: A review. Horticulturae 2022, 8, 553. [Google Scholar] [CrossRef]
- Tedesco, S.; Fevereiro, P.; Kragler, F.; Pina, A. Plant grafting and graft incompatibility: A review from the grapevine perspective. Sci. Hortic. 2022, 299, 111019. [Google Scholar] [CrossRef]
- Darikova, J.A.; Savva, Y.V.; Vaganov, E.A.; Grachev, A.M.; Kuznetsova, G.V. Grafts of woody plants and the problem of incompatibility between scion and rootstock (a review). J. Siberian Fed. Univ. Biol. 2011, 1, 54–63. [Google Scholar]
- Bester, A.J. Factors Influencing the Success or Failure of Graft Unions in Grapevine. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2020. [Google Scholar]
- Deshmukh, M.R.; Patil, S.G. Grafting compatibility and vine vigour in grapes. J. Maharashtra Agric. Univ. 2009, 34, 30–32. [Google Scholar]
- Berg, G.; Smalla, K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere: Plant species, soil type and rhizosphere communities. FEMS Microbiol. Ecol. 2009, 68, 1–13. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, S.; Li, R.; Zhang, J.; Liu, Y.; Lv, L.; Zhu, H.; Wu, W.; Li, W. Plant cultivars imprint the rhizosphere bacterial community composition and association networks. Soil Biol. Biochem. 2017, 109, 145–155. [Google Scholar] [CrossRef]
- Arrigo, N.; Arnold, C. Naturalised Vitis rootstocks in Europe and consequences to native wild grapevine. PLoS ONE 2007, 2, e521. [Google Scholar] [CrossRef]
- Turkish Statistical Institute. Data Portal for Statistics—Agriculture-Crop Production Statistics. 2024. Available online: https://biruni.tuik.gov.tr/medas/?kn=92&locale=tr (accessed on 17 May 2025).
- Zhang, X.; Walker, R.R.; Stevens, R.M.; Prior, L.D. Yield-salinity relationships of different grapevine (Vitis vinifera L.) scion–rootstock combinations. Aust. J. Grape Wine Res. 2002, 8, 150–156. [Google Scholar] [CrossRef]
- Serra, I.; Strever, A.; Myburgh, P.A.; Deloire, A. Review: The interaction between rootstocks and cultivars (Vitis vinifera L.) to enhance drought tolerance in grapevine. Aust. J. Grape Wine Res. 2014, 20, 1–14. [Google Scholar] [CrossRef]
- Ollat, N.; Bordenave, L.; Tandonnet, J.P.; Boursiquot, J.M.; Marguerit, E. Grapevine rootstocks: Origins and perspectives. Acta Hortic. 2016, 1136, 11–22. [Google Scholar] [CrossRef]
- Çelik, H.; Çelik, M.; Kadıoğlu, R.; Çelik, S.; Kocamaz, E.; Yalçın, R.; Özkaya, M.T. Use and production of fruit and grapevine grafted grapevine saplingss in Turkey. In Proceedings of the IV. Türkiye Ziraat Mühendisliği Teknik Kongresi, Ankara, Türkiye, 26 January 1995; Volume 2, pp. 941–964. [Google Scholar]
- Çelik, H. Rootstocks used in viticulture and their importance for grape growing—A review. Anadolu J. Aegean Agric. Res. Inst. 1996, 6, 127–148. [Google Scholar]
- Ollat, N.; Peccoux, A.; Papura, D.; Esmenjaud, D.; Marguerit, E.; Tandonnet, J.P.; Bordenave, L.; Cookson, S.J.; Barrieu, F.; Rossdeutsch, L.; et al. Rootstocks as a component of adaptation to environment. In Grapevine in a Changing Environment; Gerós, H., Chaves, M.M., Gil, H.M., Delrot, S., Eds.; John Wiley & Sons: Chichester, UK, 2016; pp. 68–108. [Google Scholar] [CrossRef]
- Gökbayrak, Z.; Söylemezoğlu, G.; Akkurt, M.; Çelik, H. Determination of grafting compatibility of grapevine with electrophoretic methods. Sci. Hortic. 2007, 113, 343–357. [Google Scholar] [CrossRef]
- Gramaje, D.; Armengol, J. Fungal trunk pathogens in the grapevine propagation process: Potential inoculum sources, detection, identification, and management strategies. Plant Dis. 2011, 95, 1040–1055. [Google Scholar] [CrossRef] [PubMed]
- Waite, H.; Whitelaw-Weckert, M.; Torley, P. Grapevine propagation: Principles and methods for the production of high-quality grapevine planting material. N. Z. J. Crop Hortic. Sci. 2015, 43, 144–161. [Google Scholar] [CrossRef]
- Santarosa, E.; Dutra de Souza, P.V.; de Araujo Mariath, J.E.; Lourosa, G.V. Physiological interaction between rootstock-scion: Effects on xylem vessels in Cabernet Sauvignon and Merlot grapevines. Am. J. Enol. Vitic. 2016, 67, 65–76. [Google Scholar] [CrossRef]
- Delrot, S.; Grimplet, J.; Carbonell-Bejerano, P.; Schwandner, A.; Bert, P.F.; Bavaresco, L.; Dalla Costa, L.; Gaspero, G.; Duchêne, E.; Hausmann, L.; et al. Genetic and genomic approaches for adaptation of grapevine to climate change. In Genomic Designing of Climate-Smart Fruit Crops; Kole, C., Ed.; Springer: Cham, Switzerland, 2020; pp. 1–39. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.C.; Alcaraz-López, C.; Muries, B.; Mota-Cadenas, C.; Carvajal, M. Physiological aspects of rootstock–scion interactions. Sci. Hortic. 2010, 127, 112–118. [Google Scholar] [CrossRef]
- Pina, A.; Errea, P.; Martens, H.J. Graft union formation and cell-to-cell communication via plasmodesmata in compatible and incompatible stem unions of Prunus spp. Sci. Hortic. 2012, 143, 144–150. [Google Scholar] [CrossRef]
- Warschefsky, E.J.; Klein, L.L.; Frank, M.H.; Chitwood, D.H.; Londo, J.P.; Von Wettberg, E.J.B.; Miller, A.J. Rootstocks: Diversity, domestication, and impacts on shoot phenotypes. Trends Plant Sci. 2016, 21, 418–437. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Wang, T.; Guo, S.; Ling, N.; Shen, Q. Plant grafting shapes complexity and co-occurrence of rhizobacterial assemblages. Microb. Ecol. 2020, 80, 643–655. [Google Scholar] [CrossRef]
- Tedesco, S.; Erban, A.; Gupta, S.; Kopka, J.; Fevereiro, P.; Kragler, F.; Pina, A. The impact of metabolic scion–rootstock interactions in different grapevine tissues and phloem exudates. Metabolites 2021, 11, 349. [Google Scholar] [CrossRef]
- Cookson, S.J.; Clemente Moreno, M.J.; Hevin, C.; Nyamba Mendome, L.Z.; Delrot, S.; Trossat-Magnin, C.; Ollat, N. Graft union formation in grapevine induces transcriptional changes related to cell wall modification, wounding, hormone signalling, and secondary metabolism. J. Exp. Bot. 2013, 64, 2997–3008. [Google Scholar] [CrossRef]
- Rasool, A.; Mansoor, S.; Bhat, K.M.; Hassan, G.I.; Baba, T.R.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A.; Paray, B.A.; Ahmad, P. Mechanisms underlying graft union formation and rootstock scion interaction in horticultural plants. Front. Plant Sci. 2020, 11, 590847. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, S.; Pina, A.; Fevereiro, P.; Kragler, F. A phenotypic search on graft compatibility in grapevine. Agronomy 2020, 10, 706. [Google Scholar] [CrossRef]
- Vršič, S.; Pulko, B.; Kocsis, L. Factors influencing grafting success and compatibility of grape rootstocks. Sci. Hortic. 2015, 181, 168–173. [Google Scholar] [CrossRef]
- Migicovsky, Z.; Cousins, P.; Jordan, L.M.; Myles, S.; Striegler, R.K.; Verdegaal, P.; Chitwood, D.H. Grapevine rootstocks affect growth-related scion phenotypes. Plant Direct 2021, 5, e00324. [Google Scholar] [CrossRef]
- Chen, Y.; Fei, Y.; Howell, K.; Chen, D.; Clingeleffer, P.; Zhang, P. Rootstocks for grapevines now and into the future: Selection of rootstocks based on drought tolerance, soil nutrient availability, and soil pH. Aust. J. Grape Wine Res. 2024, 2024, 6704238. [Google Scholar] [CrossRef]
- Zhang, L.; Marguerit, E.; Rossdeutsch, L.; Ollat, N.; Gambetta, G.A. The influence of grapevine rootstocks on scion growth and drought resistance. Theor. Exp. Plant Physiol. 2016, 28, 143–157. [Google Scholar] [CrossRef]
- Roux Le, D.J. The collection and storage of vineyard grafting material. VORI Leaflet 1988, 209, 1988. [Google Scholar] [CrossRef]
- Sucu, S.; Yağcı, A.; Yıldırım, K. Changes in morphological, physiological traits and enzyme activity of grafted and ungrafted grapevine rootstocks under drought stress. Erwerbs-Obstbau 2018, 60, 127–136. [Google Scholar] [CrossRef]
- Richards, M. Propagation of grapes by grafting. Plant Propagator 1976, 22, 8–10. [Google Scholar]
- Küçükyumuk, C. Effects of Different Irrigation Intervals and Mulch Applications on Nursery Quality and Success Rate in Grafted Vines. Ph.D. Thesis, Süleyman Demirel University, Isparta, Türkiye, 2009. [Google Scholar]
- Aslan, K.A.; Özcan, S.; Kösetürkmen, S.; Yağcı, A.; Sakar, E.; Odabaşıoğlu, M.İ.; Kılıç, D. Comparison of different grape variety–rootstock combinations for production of grape grafted grapevine saplings in the city of Gaziantep. Selcuk J. Agric. Food Sci. 2015, 27, 210–216. [Google Scholar]
- Ollat, N.; Geny, L.; Soyer, J. Les boutures fructifères de vigne: Validation d’un modèle d’étude du développement de la physiologie de la vigne, I. Caractéristiques de l’appareil végétatif. J. Int. Sci. Vigne Vin 1998, 32, 1–9. [Google Scholar]
- Keller, M. Developmental physiology. In The Science of Grapevines, 3rd ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 199–277. [Google Scholar] [CrossRef]
- Eichhorn, K.W.; Lorenz, D.H. Phänologische Entwicklungsstadien der Rebe. Nachr. Dtsch. Pflanzenschutzd. 1977, 29, 119–120. [Google Scholar]
- Damborska, M. Results of investigations on the callus formation on rootstock and scion of vines. Vinohrad 1981, 19, 8–9. [Google Scholar]
- Assunção, M.; Canas, S.; Cruz, S.; Brazão, J.; Zanol, G.C.; Eiras-Dias, J.E. Graft compatibility of Vitis spp.: The role of phenolic acids and flavanols. Sci. Hortic. 2016, 207, 140–145. [Google Scholar] [CrossRef]
- Çelik, M.; Kısmalı, İ. The researches on the effects of some rootstocks on yield, quality and vegetative growth of Round Seedless cultivar. J. Agric. Fac. Ege Univ. 2003, 40, 1–8. [Google Scholar]
- Gargın, S.; İşçi, B.; Altındişli, A. A research on the affinity coefficients of some grape varieties grafting with 41 B Grape rootstock. Soma Voc. Sch. Tech. Sci. J. 2011, 1, 75–86. [Google Scholar]
- Van Leeuwen, C.; Roby, J.P.; De Rességuier, L. Soil-related terroir factors: A review. OENO One 2018, 52, 173–188. [Google Scholar] [CrossRef]
- Satisha, J.; Ramteke, S.D.; Karibasappa, G.S. Physiological and biochemical characterisation of grape rootstocks. S. Afr. J. Enol. Vitic. 2007, 28, 163–168. [Google Scholar] [CrossRef]
- Bouquet, A. Differences observed in the graft compatibility between some cultivars of Muscadine grape (Vitis rotundifolia Michx.) and European grape (Vitis vinifera L. cv. Cabernet Sauvignon). VITIS-J. Grapevine Res. 2016, 19, 99. [Google Scholar]
- Bekişli, M.İ.; Gürsöz, S.; Bilgiç, C. Investigation of the production of grafted grapevine saplings in some variety-rootstock combinations: Germinate room performance. Harran J. Agric. Food Sci. 2015, 19, 24–37. [Google Scholar]
- Cangi, R. A study on the effects of rootstocks on the development of grapevine grafted grapevine saplingss. In Proceedings of the 4th Viticulture Symposium, Yalova, Türkiye, 20–23 October 1998; pp. 412–416. [Google Scholar]
- Çelik, S. Bağcılık (Ampeloloji); Trakya University Faculty of Agriculture: Tekirdağ, Türkiye, 1998; pp. 223–227. [Google Scholar]
- Marasco, R.; Rolli, E.; Fusi, M.; Michoud, G.; Daffonchio, D. Grapevine rootstocks shape underground bacterial microbiome and networking but not potential functionality. Microbiome 2018, 6, 3. [Google Scholar] [CrossRef]
- Vink, S.; Chrysargyris, A.; Tzortzakis, N.; Salles, J.F. Bacterial community dynamics varies with soil management and irrigation practices in grapevines (Vitis vinifera L.). Appl. Soil Ecol. 2021, 158, 103807. [Google Scholar] [CrossRef]
- Blank, M.; Hofmann, M.; Stoll, M. Seasonal differences in Vitis vinifera L. cv. Pinot noir and wine quality in relation to climate. OENO One 2019, 53, 189–203. [Google Scholar] [CrossRef]
- İşçi, B.; Altındişli, A. A research on take ratio of some grape varieties of 41B and 110 R Grape rootstock. J. Agric. Fac. Ege Univ. 2006, 43, 13–25. [Google Scholar]
- Köse, B.; Çelik, H.; Karabulut, B. Determination of callusing performance and vine grafted grapevine saplings characteristics on different rootstocks of ‘Merzifon Karası’ grape variety (Vitis vinifera L.). Anadolu J. Agric. Sci. 2015, 30, 87–94. [Google Scholar] [CrossRef]
- Köse, B.; Ateş, S.; Çelik, H. The effects of grafted vine yield and growth of ‘Foxy Grape’ (Vitis labrusca L.) and Shiraz (Vitis vinifera L.) grape on grafted different rootstocks in the heavy textured soil conditions. Harran J. Agric. Food Sci. 2016, 20, 135–145. [Google Scholar]
- Gündeşli, M.A. The effects of some Grape rootstocks on grafting success and the quality of grafted vine in Kabarcik and Hönüsü grapevine cultivars. Turk. J. Agric. Nat. Sci. 2018, 5, 331–338. [Google Scholar]
- Dardeniz, A.; Şahin, A.O. The effects of the combinations of different varieties and rootstocks on the vegetative growth and nursery plant ratio for the production of grafted vine rootstocks. Bahçe 2005, 34, 1–9. [Google Scholar]
- Baydar, N.G.; Ece, M. Comparation of different scion/rootstock combinations in the production of grafted grapevines in Isparta condition. Süleyman Demirel Univ. J. Nat. Appl. Sci. 2005, 9, 49–53. [Google Scholar]
- Blank, M.; Tittmann, S.; Ben Ghozlen, N.; Stoll, M. Grapevine rootstocks result in differences in leaf composition (Vitis vinifera L. cv. Pinot noir) detected through non-invasive fluorescence sensor technology. Aust. J. Grape Wine Res. 2018, 24, 327–334. [Google Scholar] [CrossRef]
- Çoban, H.; Kara, S. Investigations on the effect of some grape (Vitis vinifera L.) varieties grafted on different rootstocks on the quality of grapevine grafted grapevine saplingss. Anadolu J. Aegean Agric. Res. Inst. 2003, 13, 176–187. [Google Scholar]
- Tandonnet, J.P.; Cookson, S.J.; Vivin, P.; Ollat, N. Scion genotype controls biomass allocation and root development in grafted grapevine. Aust. J. Grape Wine Res. 2009, 16, 290–300. [Google Scholar] [CrossRef]
- Aloni, B.; Cohen, R.; Karni, L.; Aktas, H.; Edelstein, M. Hormonal signaling in rootstock–scion interactions. Sci. Hortic. 2010, 127, 119–126. [Google Scholar] [CrossRef]
- Herms, C.H.; Hennessy, R.C.; Bak, F.; Dresbøll, D.B.; Nicolaisen, M.H. Back to our roots: Exploring the role of root morphology as a mediator of beneficial plant–microbe interactions. Environ. Microbiol. 2022, 24, 3264–3272. [Google Scholar] [CrossRef]
- Çakır, A.; Karaca, N.; Sidfar, M.; Baral, Ç.; Söylemezoğlu, G. Determination of grafting success of Sultani Seedless grape variety on different Grape rootstocks. Yuzuncu Yıl Univ. J. Agric. Sci. 2013, 23, 229–235. [Google Scholar]
- Saleem, M.; Hu, J.; Jousset, A. More than the sum of its parts: Microbiome biodiversity as a driver of plant growth and soil health. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 145–168. [Google Scholar] [CrossRef]
- Gautier, A.T.; Chambaud, C.; Brocard, L.; Ollat, N.; Gambetta, G.A.; Delrot, S.; Cookson, S.J. Merging genotypes: Graft union formation and scion–rootstock interactions. J. Exp. Bot. 2019, 70, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; Wang, X.; Li, H.; Xu, X.; Wu, T.; Zhang, X.; Wang, Y.; Han, Z. Apple scion cultivars regulate the rhizosphere microbiota of scion/rootstock combinations. Appl. Soil Ecol. 2022, 170, 104305. [Google Scholar] [CrossRef]


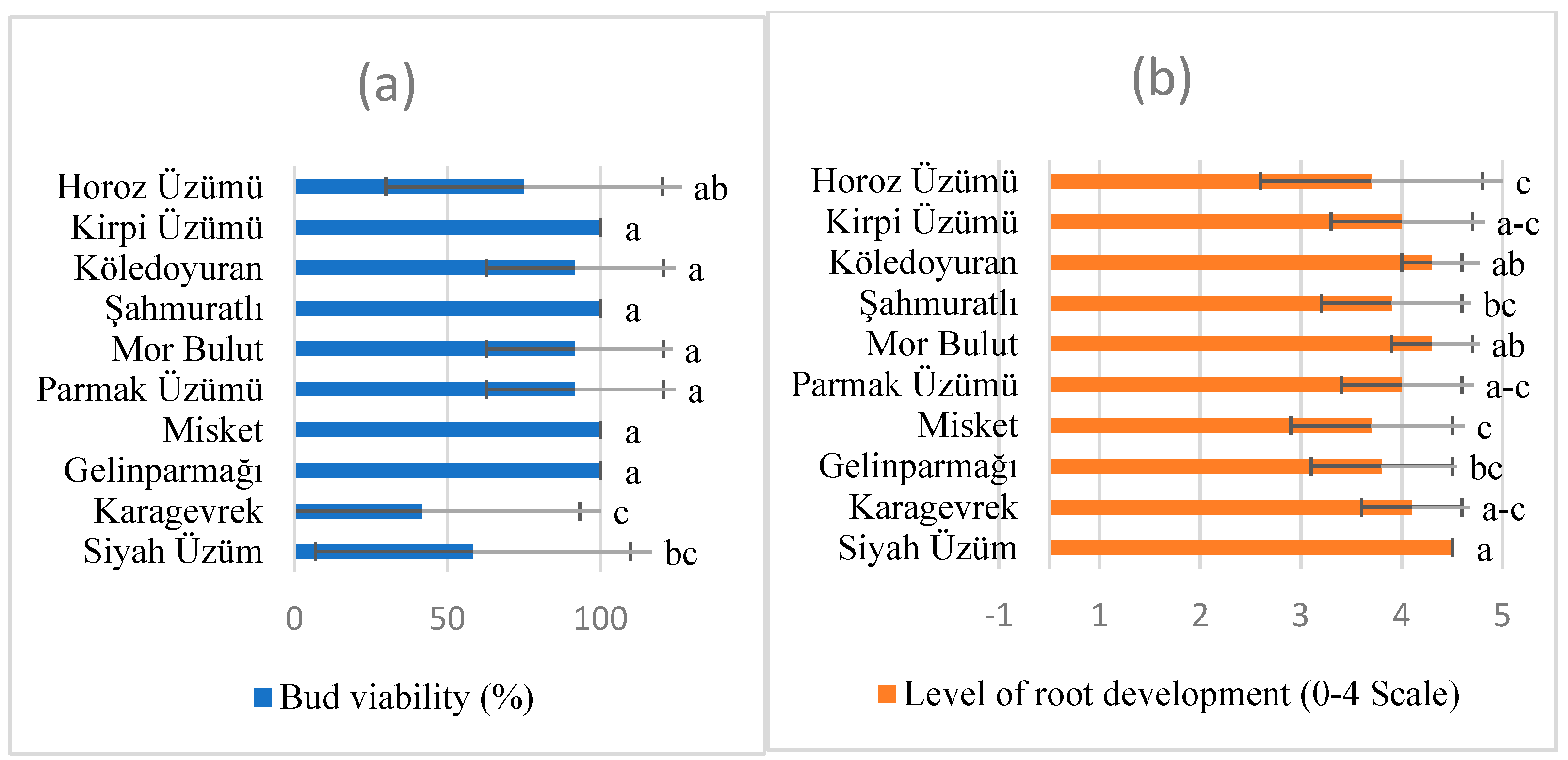
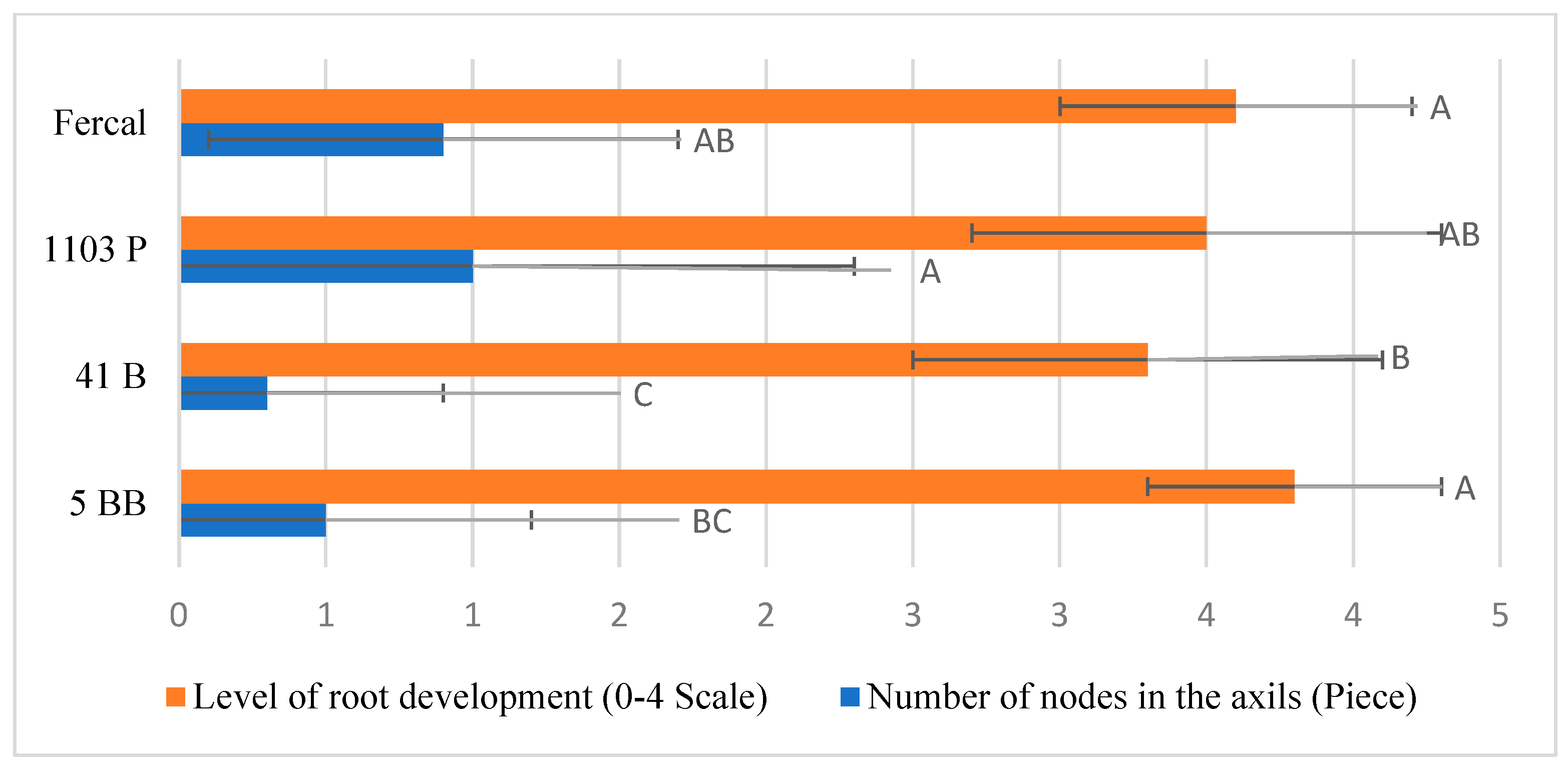



| Characteristics | Variety Df | Variety F | Variety p | Rootstock Df | Rootstok F | Rootstock p | Variety × Rootstock Df | Variety × Rootstock F | Variety × Rootstock P |
|---|---|---|---|---|---|---|---|---|---|
| Grafting success rate | 9 | 94.77 | p < 0.001 | 3 | 91.839 | p < 0.001 | 27 | 39.356 | p < 0.001 |
| Callus development level | 9 | 0.964 | p = 0.476 | 3 | 1.855 | p = 0.144 | 27 | 0.592 | p = 0.937 |
| Bud viability status | 9 | 4.254 | p < 0.001 | 3 | 0.857 | p = 0.467 | 27 | 0.381 | p = 0.997 |
| Bud sprouting status | 9 | 0.585 | p = 0.806 | 3 | 0.65 | p = 0.585 | 27 | 0.141 | p = 1.000 |
| Shoot length from bud | 9 | 1.444 | p = 0.184 | 3 | 1.28 | p = 0.287 | 27 | 2.378 | p = 0.002 |
| Basal root formation on cutting | 9 | 0.206 | p = 0.993 | 3 | 1.913 | p = 0.134 | 27 | 0.326 | p = 0.999 |
| Callus development at cutting base | 9 | 0.585 | p = 0.806 | 3 | 1.005 | p = 0.395 | 27 | 0.755 | p = 0.792 |
| Grafted grapevine saplings efficiency | 9 | 71.472 | p < 0.001 | 3 | 143.592 | p < 0.001 | 27 | 32.123 | p < 0.001 |
| First quality grafted grapevine saplings ratio | 9 | 67.134 | p < 0.001 | 3 | 90.616 | p < 0.001 | 27 | 35.841 | p < 0.001 |
| Second quality grafted grafted grapevine saplings ratio | 9 | 67.144 | p < 0.001 | 3 | 90.592 | p < 0.001 | 27 | 35.853 | p < 0.001 |
| Rootstock thickness | 9 | 4.246 | p < 0.001 | 3 | 64.109 | p < 0.001 | 27 | 3.445 | p < 0.001 |
| Graft union thickness | 9 | 5.801 | p < 0.001 | 3 | 39.515 | p < 0.001 | 27 | 3.082 | p < 0.001 |
| Graft shoot thickness | 9 | 2.381 | p = 0.019 | 3 | 28.477 | p < 0.001 | 27 | 2.495 | p = 0.001 |
| Graft shoot length | 9 | 30.437 | p < 0.001 | 3 | 85.504 | p < 0.001 | 27 | 5.368 | p < 0.001 |
| Internode length on graft shoot | 9 | 19.5 | p < 0.001 | 3 | 19.012 | p < 0.001 | 27 | 2.184 | p = 0.004 |
| Number of nodes on graft shoot | 9 | 18.459 | p < 0.001 | 3 | 37.89 | p < 0.001 | 27 | 3.543 | p < 0.001 |
| Total number of nodes | 9 | 14.268 | p < 0.001 | 3 | 30.705 | p < 0.001 | 27 | 3.253 | p < 0.001 |
| Number of lateral shoots on graft shoot | 9 | 7.539 | p < 0.001 | 3 | 15.2 | p < 0.001 | 27 | 2.502 | p = 0.001 |
| Number of nodes in lateral shoots | 9 | 0.727 | p = 0.683 | 3 | 4.894 | p = 0.004 | 27 | 1.3 | p = 0.185 |
| Number of secondary–tertiary basal shoots | 9 | 1.58 | p = 0.135 | 3 | 0.296 | p = 0.828 | 27 | 2.272 | p = 0.003 |
| Number of primary roots | 9 | 26.46 | p < 0.001 | 3 | 123.636 | p < 0.001 | 27 | 55.492 | p < 0.001 |
| Number of secondary roots | 9 | 27.82 | p < 0.001 | 3 | 41.162 | p < 0.001 | 27 | 18.323 | p < 0.001 |
| Root length | 9 | 11.306 | p < 0.001 | 3 | 12.803 | p < 0.001 | 27 | 10.22 | p < 0.001 |
| Root development level | 9 | 2.497 | p = 0.014 | 3 | 3.314 | p = 0.024 | 27 | 1.343 | p = 0.157 |
| Rootstock/Variety Combination | Grafting Success Rate (%) | Scion Length (cm) | Grafted Grapevine Sapling Efficiency (%) | First Quality Grafted Grapevine Sapling Ratio (%) | Second Quality Grafted Grafted Grapevine Saplings Ratio (%) |
|---|---|---|---|---|---|
| 5 BB/Siyah Üzüm | 89.7 ± 2.5 d–e | 2.7 ± 1.5 a–f | 93.93 ± 3.09 ab | 100.0 ± 0.0 a | 0.0 ± 0.0 k |
| 41 B/Siyah Üzüm | 100.0 ± 0.0 a | 1.8 ± 0.8 b–f | 80.07 ± 1.16 hi | 100.0 ± 0.0 a | 0.0 ± 0.0 k |
| 1103 P/Siyah Üzüm | 100.0 ± 0.0 a | 2.2 ± 1.0 b–f | 86.90 ± 0.90 ef | 100.0 ± 0.0 a | 0.0 ± 0.0 k |
| Fercal/Siyah Üzüm | 100.0 ± 0.0 a | 2.7 ± 1.3 a–f | 91.80 ± 3.95 a–c | 100.0 ± 0.0 a | 0.0 ± 0.0 k |
| 5 BB/Karagevrek | 94.4 ± 3.6–d | 4.3 ± 1.8 a | 88.07 ± 2.50 c–e | 96.3 ± 3.8 ab | 3.7 ± 3.8 jk |
| 41 B/Karagevrek | 100.0 ± 0.0 a | 1.5 ± 0.5 d–f | 86.87 ± 3.55 ef | 83.3 ± 6.7 de | 16.7 ± 6.7gh |
| 1103 P/Karagevrek | 97.2 ± 2.2 ab | 1.8 ± 0.3 b–f | 82.47 ± 5.31 gh | 81.5 ± 6.5 d–f | 18.5 ± 6.5 f–h |
| Fercal/Karagevrek | 90.9 ± 6.1 c–e | 2.8 ± 1.3 a–e | 87.87 ± 1.46 de | 95.5 ± 4.5 ab | 4.5 ± 4.5 jk |
| 5 BB/Gelinparmağı | 92.3 ± 3.7 b–e | 3.3 ± 0.3 a–c | 66.47 ± 1.05 mn | 66.6 ± 3.5 hi | 33.4 ± 3.5 cd |
| 41 B/Gelinparmağı | 100.0 ± 0.0 a | 3.5 ± 0.0 ab | 68.67 ± 2.65 l–n | 75.0 ± 3.1 fg | 25.0 ± 3.1 ef |
| 1103 P/Gelinparmağı | 93.3 ± 0.7 b–e | 2.8 ± 0.6 a–e | 76.53 ± 1.87 ij | 76.5 ± 4.4 e–g | 23.5 ± 4.4 e–g |
| Fercal/Gelinparmağı | 100.0 ± 0.0 a | 1.5 ± 0.5 d–f | 93.43 ± 2.44 ab | 100.0 ± 0.0 a | 0.0 ± 0.0 k |
| 5 BB/Misket Üzümü | 96.7 ± 2.4 ab | 2.5 ± 0.5 b–f | 82.53 ± 1.19 gh | 90.1 ± 2.1 bc | 9.9 ± 2.1 ij |
| 41 B/Misket Üzümü | 100.0 ± 0.0 a | 2.5 ± 1.0 b–f | 73.17 ± 1.18 jk | 83.1 ± 2.0 de | 16.9 ± 2.0 gh |
| 1103 P/Misket Üzümü | 97.0 ± 0.4 ab | 1.5 ± 0.0 d–f | 62.47 ± 0.95 o | 57.0 ± 4.0 j | 43.0 ± 4.0 b |
| Fercal/Misket Üzümü | 100.0 ± 0.0 a | 1.0 ± 0.0 f | 71.57 ± 1.21 kl | 70.0 ± 2.0 gh | 30.0 ± 2.0 de |
| 5 BB/Parmak Üzümü | 83.9 ± 3.0 f–g | 2.2 ± 0.8 b–f | 83.30 ± 0.96 f–h | 100.0 ± 0.0 a | 0.0 ± 0.0 k |
| 41 B/Parmak Üzümü | 100.0 ± 0.0 a | 2.8 ± 0.6 a–e | 81.90 ± 1.31 h | 83.3 ± 3.7 de | 16.7 ± 3.7 gh |
| 1103 P/Parmak Üzümü | 89.5 ± 4.6 d–e | 2.0 ± 0.5 b–f | 82.73 ± 3.61 gh | 70.3 ± 5.5 gh | 29.7 ± 5.5 de |
| Fercal/Parmak Üzümü | 100.0 ± 0.0 a | 2.5 ± 1.8 b–f | 86.30 ± 1.74 e–g | 78.5 ± 4.5 ef | 21.5 ± 4.5 fg |
| 5 BB/Mor Bulut | 73.3±4.4 h | 1.8 ± 0.3 b–f | 88.60 ± 2.26 c–e | 100.0 ± 0.0 a | 0.0 ± 0.0 k |
| 41 B/Mor Bulut | 100.0 ± 0.0 a | 3.0 ± 0.5 a–d | 71.17 ± 0.85 kl | 80.0 ± 5.1 d–f | 20.0 ± 5.1 f–h |
| 1103 P/Mor Bulut | 82.1 ± 2.9 g | 2.5 ± 0.9 b–f | 81.93 ± 2.29 h | 100.0 ± 0.0 a | 0.0 ± 0.0 k |
| Fercal/Mor Bulut | 30.0 ± 4.0 i | 3.5 ± 0.5 ab | 81.47 ± 0.49 h | 91.0 ± 2.0 bc | 9.0 ± 2.0 ij |
| 5 BB/Şahmuratlı | 68.8 ± 6.8 h | 2.5 ± 1.5 b–f | 79.37 ± 2.42 hi | 81.3 ± 5.0 d–f | 18.8 ± 5.0 f–h |
| 41 B/Şahmuratlı | 97.6 ± 2.4 ab | 1.2 ± 0.3 ef | 70.20 ± 1.21 k–m | 70.3 ± 1.9 gh | 29.7 ± 1.9 de |
| 1103 P/Şahmuratlı | 94.3 ± 3.7 a–d | 1.7 ± 0.6 c–f | 80.17 ± 1.97 hi | 100.0 ± 0.0 a | 0.0 ± 0.0 k |
| Fercal/Şahmuratlı | 95.8 ± 4.2 a–c | 3.3 ± 0.8 a–c | 71.83 ± 1.70 kl | 66.6 ± 3.5 hi | 33.4 ± 3.5 cd |
| 5 BB/Köledoyuran | 100.0 ± 0.0 a | 2.3 ± 0.6 b–f | 82.67 ± 1.18 gh | 91.6 ± 3.4 bc | 8.4 ± 3.4 ij |
| 41 B/Köledoyuran | 97.4 ± 2.6 ab | 1.0 ± 0.0 f | 82.47 ± 3.08 gh | 85.9 ± 4.0 cd | 14.1 ± 4.0 hi |
| 1103 P/Köledoyuran | 96.9 ± 3.1 ab | 2.7 ± 0.8 a–f | 86.17 ± 1.30 e–g | 100.0 ± 0.0 a | 0.0 ± 0.0 k |
| Fercal/Köledoyuran | 90.0 ± 4.0 d–e | 2.5 ± 0.0 b–f | 91.37 ± 1.00 b–d | 95.3 ± 4.8 ab | 4.7 ± 4.8 jk |
| 5 BB/Kirpi Üzümü | 83.3 ± 3.7 g | 2.0 ± 0.5 b–f | 80.90 ± 2.07 h | 94.7 ± 3.1 ab | 5.3 ± 3.1 jk |
| 41 B/Kirpi Üzümü | 88.6 ± 4.5 e–f | 3.0 ± 1.3 a–d | 68.17 ± 1.55 l–n | 61.0 ± 9.0 ij | 39.0 ± 9.0 bc |
| 1103 P/Kirpi Üzümü | 96.6 ± 3.5 ab | 3.2 ± 0.8 a–d | 87.27 ± 2.22 e | 92.3 ± 2.3 bc | 7.7 ± 2.3 ij |
| Fercal/Kirpi Üzümü | 81.6 ± 0.4 g | 3.0 ± 0.5 a–d | 81.40 ± 0.26 h | 94.1 ± 4.1 ab | 5.9 ± 4.1 jk |
| 5 BB/Horoz Üzümü | 96.8 ± 3.3 ab | 2.3 ± 0.8 b–f | 88.20 ± 2.61 c–e | 93.3 ± 4.3 ab | 6.7 ± 4.3 jk |
| 41 B/Horoz Üzümü | 100.0 ± 0.0 a | 3.2 ± 2.1 a–d | 65.33 ± 1.01 no | 46.2 ± 3.0 k | 53.8 ± 3.0 a |
| 1103 P/Horoz Üzümü | 100.0 ± 0.0 a | 1.5 ± 0.5 d–f | 68.03 ± 0.67 l–n | 58.3 ± 4.7 j | 41.7 ± 4.7 b |
| Fercal/Horoz Üzümü | 93.8 ± 4.3 b–e | 2.3 ± 1.3 b–f | 95.43 ± 1.50 a | 94.1 ± 0.9 ab | 5.9 ± 0.9 jk |
| Rootstock/Variety Combination | Rootstock Thickness (mm) | Graft Union Thickness (mm) | Scion Thickness (mm) | Scion Length (cm) | Internode Length of Scion (cm) | Internode number of Scion |
|---|---|---|---|---|---|---|
| 5 BB/Siyah Üzüm | 12.5 ± 0.4 c–e | 19.2 ± 1.0 b–g | 3.6 ± 0.3 f–i | 40.3 ± 9.6 c–f | 7.3 ± 1.0 a | 7.7 ± 1.5 e–k |
| 41 B/Siyah Üzüm | 10.0 ± 0.3 h–l | 15.0 ± 0.5 kl | 3.1 ± 0.5 hi | 19.7 ± 0.6 p–s | 4.0 ± 0.5 f–i | 5.0 ± 0.0 k–n |
| 1103 P/Siyah Üzüm | 10.3 ± 0.6 g–l | 17.3 ± 1.1 d–k | 3.7 ± 0.5 c–i | 37.8 ± 5.0 d–h | 5.2 ± 0.8 b–f | 9.7 ± 1.5 b–g |
| Fercal/Siyah Üzüm | 10.3 ± 0.7 g–l | 16.9 ± 2.0 e–k | 4.5 ± 0.7 b–d | 47.8 ± 8.1 bc | 6.5 ± 1.3 a–c | 11.0 ± 1.7 bc |
| 5 BB/Karagevrek | 12.2 ± 1.7 c–g | 19.4 ± 2.7 b–f | 3.9 ± 0.3 c–i | 39.2 ± 2.6 d–g | 7.0 ± 1.0 a | 8.0 ± 0.0 d–j |
| 41 B/Karagevrek | 8.3 ± 0.4 l | 15.4 ± 1.1 j–l | 3.0 ± 0.3 i | 17.8 ± 6.3 q–t | 4.2 ± 0.8 e–i | 6.0 ± 0.0 j–n |
| 1103 P/Karagevrek | 10.0 ± 0.1 h–l | 16.9 ± 0.8 f–k | 3.3 ± 0.3 g–i | 34.0 ± 6.7 e–k | 6.0 ± 0.9 a–d | 7.3 ± 0.6 f–k |
| Fercal/Karagevrek | 14.5 ± 0.8 ab | 25.2 ± 2.6 a | 4.8 ± 0.3 ab | 42.5 ± 2.6 c–e | 6.2 ± 1.0 a–d | 9.3 ± 1.5 b–h |
| 5 BB/Gelinparmağı | 9.9 ± 1.3 h–l | 16.3 ± 1.2 g–l | 3.5 ± 0.7 f–i | 14.3 ± 2.0 r–u | 3.2 ± 0.8 g–k | 4.7 ± 1.2 l–n |
| 41 B/Gelinparmağı | 8.5 ± 0.8 kl | 16.1 ± 1.3 h–l | 3.3 ± 0.4 g–i | 10.3 ± 2.8 tu | 2.2 ± 0.3 jk | 3.7 ± 2.1 n |
| 1103 P/Gelinparmağı | 10.4 ± 0.9 f–k | 15.2 ± 2.1 j–l | 3.6 ± 0.3 f–i | 25.3 ± 4.1 k–q | 4.0 ± 0.5 f–i | 8.0 ± 1.7 d–j |
| Fercal/Gelinparmağı | 13.3 ± 0.2 b–c | 20.5 ± 0.9 bc | 4.5 ± 0.3 b–e | 21.3 ± 3.3 n–s | 2.5 ± 0.5 i–k | 7.0 ± 2.6 g–l |
| 5 BB/Misket Üzümü | 10.3 ± 1.2 g–l | 17.1 ± 0.7 d–k | 3.4 ± 0.7 f–i | 20.5 ± 8.2 o–s | 3.8 ± 1.0 f–j | 8.3 ± 1.5 c–j |
| 41 B/Misket Üzümü | 9.3 ± 0.7 i–l | 14.2 ± 0.7 kl | 3.6 ± 0.2 f–i | 19.0 ± 2.6 p–t | 2.7 ± 1.3 i–k | 7.0 ± 1.0 g–l |
| 1103 P/Misket Üzümü | 8.9 ± 0.5 j–l | 13.7 ± 1.6 l | 3.1 ± 0.6 hi | 14.8 ± 4.5 r–u | 1.8 ± 0.3 k | 5.7 ± 0.6 j–n |
| Fercal/Misket Üzümü | 10.0 ± 0.5 h–l | 16.3 ± 2.5 g–l | 3.5 ± 0.8 f–i | 24.5 ± 4.4 l–q | 3.5 ± 0.5 f–k | 7.7 ± 1.2 e–k |
| 5 BB/Parmak Üzümü | 12.9 ± 1.5 b–d | 20.5 ± 0.9 bc | 4.6 ± 0.4 b–c | 34.5 ± 2.3 e–k | 5.0 ± 0.0 c–g | 12.0 ± 1.0 ab |
| 41 B/Parmak Üzümü | 10.7 ± 1.5 e–j | 17.0 ± 0.3 e–k | 3.2 ± 0.4 g–i | 19.8 ± 2.3 o–s | 3.8 ± 1.3 f–j | 6.7 ± 1.2 h–m |
| 1103 P/Parmak Üzümü | 9.7 ± 0.6 i–l | 16.5 ± 0.8 f–l | 3.4 ± 0.6 f–i | 32.2 ± 5.3 f–l | 3.0 ± 1.3 h–k | 10.0 ± 0.0 b–f |
| Fercal/Parmak Üzümü | 10.7 ± 1.6 e–j | 18.1 ± 1.0 c–j | 4.1 ± 0.4 b–g | 34.8 ± 3.3 e–j | 3.5 ± 0.9 f–k | 10.0 ± 2.0 b–f |
| 5 BB/Mor Bulut | 13.1 ± 1.1 b–d | 20.1 ± 0.9 b–d | 3.8 ± 0.3 c–i | 28.0 ± 1.0 i–p | 5.8 ± 0.3 a–e | 6.3 ± 0.6 i–m |
| 41 B/Mor Bulut | 10.4 ± 0.4 f–k | 18.8 ± 0.2 b–i | 3.1 ± 0.4 hi | 8.8 ± 2.4 u | 2.5 ± 0.5 i–k | 4.3 ± 1.2 mn |
| 1103 P/Mor Bulut | 9.9 ± 1.3 h–l | 15.8 ± 1.5 i–l | 3.8 ± 0.5 c–i | 31.2 ± 3.9 g–m | 4.8 ± 0.8 c–h | 11.0 ± 0.0 bc |
| Fercal/Mor Bulut | 13.5 ± 1.2 a–c | 19.3 ± 2.4 b–f | 4.3 ± 0.6 b–f | 34.2 ± 4.3 e–k | 4.8 ± 1.2 c–h | 10.7 ± 0.6 b–d |
| 5 BB/Şahmuratlı | 12.3 ± 2.4 c–f | 16.7 ± 0.6 f–l | 3.8 ± 0.5 c–i | 23.5 ± 4.8 l–r | 3.0 ± 1.0 h–k | 7.7 ± 0.6 e–k |
| 41 B/Şahmuratlı | 9.3 ± 0.6 i–l | 16.4 ± 1.0 f–l | 3.6 ± 0.6 e–i | 14.0 ± 4.1 s–u | 2.5 ± 0.9 i–k | 7.3 ± 0.6 f–k |
| 1103 P/Şahmuratlı | 9.7 ± 0.3 i–l | 16.1 ± 1.0 h–l | 3.6 ± 0.3 f–i | 27.0 ± 3.5 j–q | 3.5 ± 0.9 f–k | 9.0 ± 1.0 c–i |
| Fercal/Şahmuratlı | 12.5 ± 1.0 c–e | 19.0 ± 1.3 b–h | 3.4 ± 0.7 f–i | 21.2 ± 2.0 n–s | 2.0 ± 0.0 k | 7.7 ± 2.5 e–k |
| 5 BB/Köledoyuran | 11.3 ± 1.4 d–i | 16.8 ± 1.3 f–k | 3.5 ± 0.2 f–i | 30.0 ± 2.6 h–n | 5.0 ± 0.5 c–g | 10.7 ± 3.5 b–d |
| 41 B/Köledoyuran | 9.3 ± 1.4 i–l | 16.2 ± 1.2 g–l | 3.9 ± 0.6 c–h | 31.8 ± 11.8 f–l | 4.5 ± 1.5 d–h | 9.7±0.6 b–g |
| 1103 P/Köledoyuran | 10.0 ± 0.2 h–l | 16.9 ± 0.9 e–k | 4.1±0.1 b–g | 43.8 ± 0.3 cd | 5.0 ± 0.9 c–g | 14.0 ± 1.0 a |
| Fercal/Köledoyuran | 15.1 ± 0.5 a | 21.2 ± 0.9 b | 5.4 ± 0.6 a | 57.7 ± 5.6 a | 4.8 ± 0.8 c–h | 14.0 ± 2.0 a |
| 5 BB/Kirpi Üzümü | 10.4 ± 0.4 f–k | 17.2 ± 1.5 d–k | 3.7 ± 0.5 c–i | 28.0 ± 2.6 i–p | 4.2 ± 1.3 e–i | 10.3 ± 0.6 b–e |
| 41 B/Kirpi Üzümü | 8.6 ± 1.3 kl | 16.1 ± 2.1 h–l | 3.1 ± 0.5 hi | 18.3 ± 3.7 q–t | 3.3 ± 0.3 f–k | 6.7 ± 1.2 h–m |
| 1103 P/Kirpi Üzümü | 9.6 ± 0.5 i–l | 16.4 ± 2.4 f–l | 3.6 ± 0.5 e–i | 25.3 ± 3.0 k–q | 4.5 ± 0.9 d–h | 10.0 ± 1.7 b–f |
| Fercal/Kirpi Üzümü | 12.2 ± 1.1 c–g | 19.9 ± 2.0 b–e | 4.5 ± 0.4 b–d | 36.8 ± 0.8 d–i | 4.2 ± 0.3 e–i | 10.7 ± 0.6 b–d |
| 5 BB/Horoz Üzümü | 11.8 ± 2.1 c–h | 18.7 ± 2.4 b–i | 3.3 ± 0.4 f–i | 29.0 ± 4.1 h–o | 6.3 ± 1.5 a–c | 7.7 ± 0.6 e–k |
| 41 B/Horoz Üzümü | 9.2 ± 0.1 j–l | 15.2 ± 1.4 j–l | 3.6 ± 0.7 d–i | 21.5 ± 7.9 n–s | 3.5 ± 1.0 f–k | 6.7 ± 1.5 h–m |
| 1103 P/Horoz Üzümü | 9.5 ± 0.4 i–l | 15.0 ± 0.8 kl | 3.1 ± 0.2 hi | 22.2 ± 2.3 m–s | 4.8 ± 0.8 c–h | 6.0 ± 0.0 j–n |
| Fercal/Horoz Üzümü | 13.0 ± 0.6 b–d | 18.8 ± 2.5 b–i | 4.9 ± 0.2 ab | 53.5 ± 3.0 ab | 6.8 ± 1.8 ab | 10.7 ± 1.2 b–d |
| Rootstock/Variety Combination | Total Number of Nodes on Scion | Number of Lateral Shoots on Scion | Number of Secondary–Tertiary Basal Shoots | Number of Primary Roots | Number of Secondary Roots | Root Length (cm) |
|---|---|---|---|---|---|---|
| 5 BB/Siyah Üzüm | 8.0 ± 1.7 g–l | 0.3 ± 0.6 ef | 0.3 ± 0.6 cd | 68.0 ± 2.0 b | 23.0 ± 3.0 c–i | 9.0 ± 2.0 mn |
| 41 B/Siyah Üzüm | 5.0 ± 0.0 k–m | 0.0 ± 0.0 f | 0.0 ± 0.0 d | 17.7 ± 0.6 l–n | 37.3 ± 5.4 b | 9.4 ± 0.5 l–n |
| 1103 P/Siyah Üzüm | 10.3 ± 2.5 d–i | 0.3 ± 0.6 ef | 0.3 ± 0.6 cd | 33.0 ± 1.0 g–j | 28.0 ± 4.0 c–e | 10.5 ± 0.5 k–n |
| Fercal/Siyah Üzüm | 11.7 ± 2.3 c–g | 0.7 ± 0.6 ef | 0.0 ± 0.0 d | 28.0 ± 4.0 i–k | 38.0 ± 8.0 b | 17.0 ± 4.0 c–g |
| 5 BB/Karagevrek | 8.0 ± 0.0 g–l | 0.0 ± 0.0 f | 0.3 ± 0.6 cd | 36.0 ± 2.0 f–i | 26.0 ± 6.0 c–g | 13.5 ± 1.5 f–m |
| 41 B/Karagevrek | 6.0 ± 0.0 j–m | 0.0 ± 0.0 f | 0.0 ± 0.0 d | 17.0 ± 2.0 l–o | 70.0 ± 10.0 a | 31.0 ± 6.0 a |
| 1103 P/Karagevrek | 9.3 ± 3.2 e–j | 1.0 ± 1.0 ef | 0.0 ± 0.0 d | 40.0 ± 7.0 e–g | 15.0 ± 3.0 j–m | 20.0 ± 3.0 c |
| Fercal/Karagevrek | 9.7 ± 2.1 d–j | 1.0 ± 1.0 ef | 0.3 ± 0.6 cd | 35.0 ± 5.0 f–j | 14.0 ± 1.0 k–m | 13.5 ± 2.5 f–m |
| 5 BB/Gelinparmağı | 4.7 ± 1.2 k–m | 0.3 ± 0.6 ef | 0.3 ± 0.6 cd | 8.0 ± 1.0 p | 16.0 ± 2.0 i–m | 12.0 ± 3.0 h–n |
| 41 B/Gelinparmağı | 3.7 ± 2.1 m | 0.7 ± 1.2 ef | 0.0 ± 0.0 d | 19.0 ± 6.0 lm | 19.0 ± 4.0 g–l | 8.0 ± 0.0 n |
| 1103 P/Gelinparmağı | 12.0 ± 1.7 c–g | 3.7 ± 0.6 a–c | 0.0 ± 0.0 d | 28.0 ± 4.0 i–k | 16.0 ± 4.0 i–m | 17.5 ± 1.5 c–f |
| Fercal/Gelinparmağı | 10.0 ± 4.4 d–j | 2.0 ± 0.0 c–e | 1.0 ± 0.0 a–c | 97.0 ± 8.0 a | 13.0 ± 2.0 lm | 9.0 ± 1.0 mn |
| 5 BB/Misket Üzümü | 9.7 ± 2.1 d–j | 1.7 ± 0.6 d–f | 0.0 ± 0.0 d | 49.0 ± 9.0 cd | 20.0 ± 3.0 f–l | 11.5 ± 0.5 i–n |
| 41 B/Misket Üzümü | 7.0 ± 1.0 h–m | 0.7 ± 0.6 ef | 0.0 ± 0.0 d | 15.0 ± 3.0 l–p | 14.0 ± 3.0 k–m | 19.5 ± 2.5 c |
| 1103 P/Misket Üzümü | 6.3 ± 0.6 i–m | 0.7 ± 0.6 ef | 0.3 ± 0.6 cd | 6.7 ± 0.6 p | 14.0 ± 0.0 k–m | 9.0 ± 2.0 mn |
| Fercal/Misket Üzümü | 8.7 ± 2.1 f–k | 1.0 ± 1.0 ef | 0.0 ± 0.0 d | 15.0 ± 1.0 l–p | 17.0 ± 3.0 h–m | 12.5 ± 2.5 g–n |
| 5 BB/Parmak Üzümü | 13.7 ± 0.6 b–d | 1.7 ± 0.6 d–f | 0.3 ± 0.6 cd | 14.0 ± 2.0 l–p | 20.0 ± 6.0 f–l | 14.0 ± 1.0 e–l |
| 41 B/Parmak Üzümü | 6.7 ± 1.2 i–m | 0.0 ± 0.0 f | 0.7 ± 0.6 b–d | 42.0 ± 7.0 d–f | 26.0 ± 3.0 c–g | 18.5 ± 2.5 c–e |
| 1103 P/Parmak Üzümü | 11.3 ± 1.5 c–g | 1.3 ± 0.6 d–f | 0.3 ± 0.6 cd | 46.0 ± 5.0 c–e | 28.0 ± 4.0 c–e | 16.5 ± 3.5 c–h |
| Fercal/Parmak Üzümü | 11.3 ± 3.1 c–g | 1.3 ± 0.6 d–f | 0.0 ± 0.0 d | 54.0 ± 6.0 c | 23.0 ± 3.0 c–i | 16.5 ± 1.0 c–h |
| 5 BB/Mor Bulut | 6.7 ± 0.6 i–m | 0.7 ± 0.6 ef | 0.3±0.6 cd | 41.0±6.0 e–g | 30.0±5.0 c | 16.0 ± 1.0 c–i |
| 41 B/Mor Bulut | 4.3 ± 1.2 lm | 0.7 ± 0.6 ef | 0.3 ± 0.6 cd | 9.7 ± 2.2 n–p | 22.7 ± 1.4 d–i | 17.0 ± 3.6 c–g |
| 1103 P/Mor Bulut | 15.0±0.0 a–c | 4.0 ± 0.0 ab | 1.7 ± 0.6 a | 18.0±4.0 l–n | 17.0±1.0 h–m | 19.0±4.0 cd |
| Fercal/Mor Bulut | 11.3±0.6 c–g | 0.7±0.6 ef | 0.3±0.6 cd | 19.0±3.0 lm | 21.0±1.0 e–k | 11.0±4.0 j–n |
| 5 BB/Şahmuratlı | 9.7±3.1 d–j | 1.0±1.0 ef | 0.3±0.6 cd | 40.0±7.0 e–g | 20.0±1.0 f–l | 10.0±3.0 k–n |
| 41 B/Şahmuratlı | 8.0±1.0 g–l | 0.7±0.6 ef | 0.0±0.0 d | 10.0±2.0 n–p | 25.0±3.0 c–g | 14.5±2.5 d–k |
| 1103 P/Şahmuratlı | 11.0 ± 1.0 c–h | 2.0 ± 0.0 c–e | 0.0 ± 0.0 d | 21.0 ± 1.0 kl | 15.0 ± 4.0 j–m | 17.0 ± 0.0 c–g |
| Fercal/Şahmuratlı | 10.0 ± 4.6 d–j | 2.0 ± 2.0 c–e | 0.7 ± 0.6 b–d | 27.0 ± 4.0 j–k | 10.0 ± 1.0 m | 12.0 ± 2.0 h–n |
| 5 BB/Köledoyuran | 12.3 ± 5.0 c–f | 2.0 ± 2.0 c–e | 0.3 ± 0.6 cd | 28.7 ± 1.2 h–k | 17.0 ± 2.0 h–m | 20.0 ± 1.0 c |
| 41 B/Köledoyuran | 13.7 ± 0.6 b–d | 1.7 ± 1.2 d–f | 0.0 ± 0.0 d | 30.0 ± 3.0 h–j | 27.0 ± 4.0 c–f | 15.5 ± 1.0 c–j |
| 1103 P/Köledoyuran | 18.3 ± 1.5 a | 5.0 ± 1.7 a | 0.3 ± 0.6 cd | 30.0 ± 1.0 h–j | 16.0 ± 2.0 i–m | 12.0 ± 2.0 h–n |
| Fercal/Köledoyuran | 17.0 ± 3.6 ab | 3.0 ± 1.7 b–d | 0.0 ± 0.0 d | 37.0 ± 4.0 f–h | 11.0 ± 0.0 m | 16.5 ± 3.0 c–h |
| 5 BB/Kirpi Üzümü | 12.0 ± 0.0 c–g | 1.7 ± 0.6 d–f | 0.0 ± 0.0 d | 31.0 ± 3.0 h–j | 15.0 ± 1.0 j–m | 11.0 ± 0.0 j–n |
| 41 B/Kirpi Üzümü | 6.7 ± 1.2 i–m | 0.0 ± 0.0 f | 1.3 ± 1.2 ab | 12.0 ± 2.0 m–p | 22.0 ± 4.0 d–j | 12.0 ± 2.0 h–n |
| 1103 P/Kirpi Üzümü | 12.0 ± 2.6 c–g | 2.0 ± 1.0 c–e | 0.0 ± 0.0 d | 50.0 ± 8.0 c | 21.0 ± 2.0 e–k | 25.0 ± 4.0 b |
| Fercal/Kirpi Üzümü | 13.0 ± 1.0 c–e | 3.0 ± 1.0 b–d | 0.3 ± 0.6 cd | 9.0 ± 0.0 op | 26.0 ± 4.0 c–g | 11.0 ± 1.0 j–n |
| 5 BB/Horoz Üzümü | 8.3 ± 1.5 f–l | 0.7 ± 1.2 ef | 1.0 ± 1.0 a–c | 52.0 ± 7.0 c | 29.0 ± 1.0 cd | 11.0 ± 2.0 j–n |
| 41 B/Horoz Üzümü | 8.3 ± 1.5 f–l | 0.7 ± 1.2 ef | 0.0 ± 0.0 d | 8.0 ± 1.0 p | 19.0 ± 3.0 g–l | 16.5 ± 1.5 c–h |
| 1103 P/Horoz Üzümü | 6.0 ± 0.0 j–m | 0.0 ± 0.0 f | 0.3 ± 0.6 cd | 18.0 ± 5.0 l–n | 13.0 ± 4.0 lm | 12.5 ± 2.5 g–n |
| Fercal/Horoz Üzümü | 12.3 ± 1.5 c–f | 1.7 ± 2.1 d–f | 0.3 ± 0.6 cd | 53.0 ± 7.0 c | 24.0 ± 4.0 c–h | 19.5 ± 3.5 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daler, S.; Kılıç, T.; Hatterman-Valenti, H.; Kaya, O. Graft Compatibility of Local Grapevine Varieties with Grapevine Rootstocks in Yozgat Province. Horticulturae 2025, 11, 803. https://doi.org/10.3390/horticulturae11070803
Daler S, Kılıç T, Hatterman-Valenti H, Kaya O. Graft Compatibility of Local Grapevine Varieties with Grapevine Rootstocks in Yozgat Province. Horticulturae. 2025; 11(7):803. https://doi.org/10.3390/horticulturae11070803
Chicago/Turabian StyleDaler, Selda, Tuğba Kılıç, Harlene Hatterman-Valenti, and Ozkan Kaya. 2025. "Graft Compatibility of Local Grapevine Varieties with Grapevine Rootstocks in Yozgat Province" Horticulturae 11, no. 7: 803. https://doi.org/10.3390/horticulturae11070803
APA StyleDaler, S., Kılıç, T., Hatterman-Valenti, H., & Kaya, O. (2025). Graft Compatibility of Local Grapevine Varieties with Grapevine Rootstocks in Yozgat Province. Horticulturae, 11(7), 803. https://doi.org/10.3390/horticulturae11070803








