Nitrogen Metabolism of Stizolobium aterrimum Grown in Soil Under Toxic Concentrations of Copper (Cu)
Abstract
1. Introduction
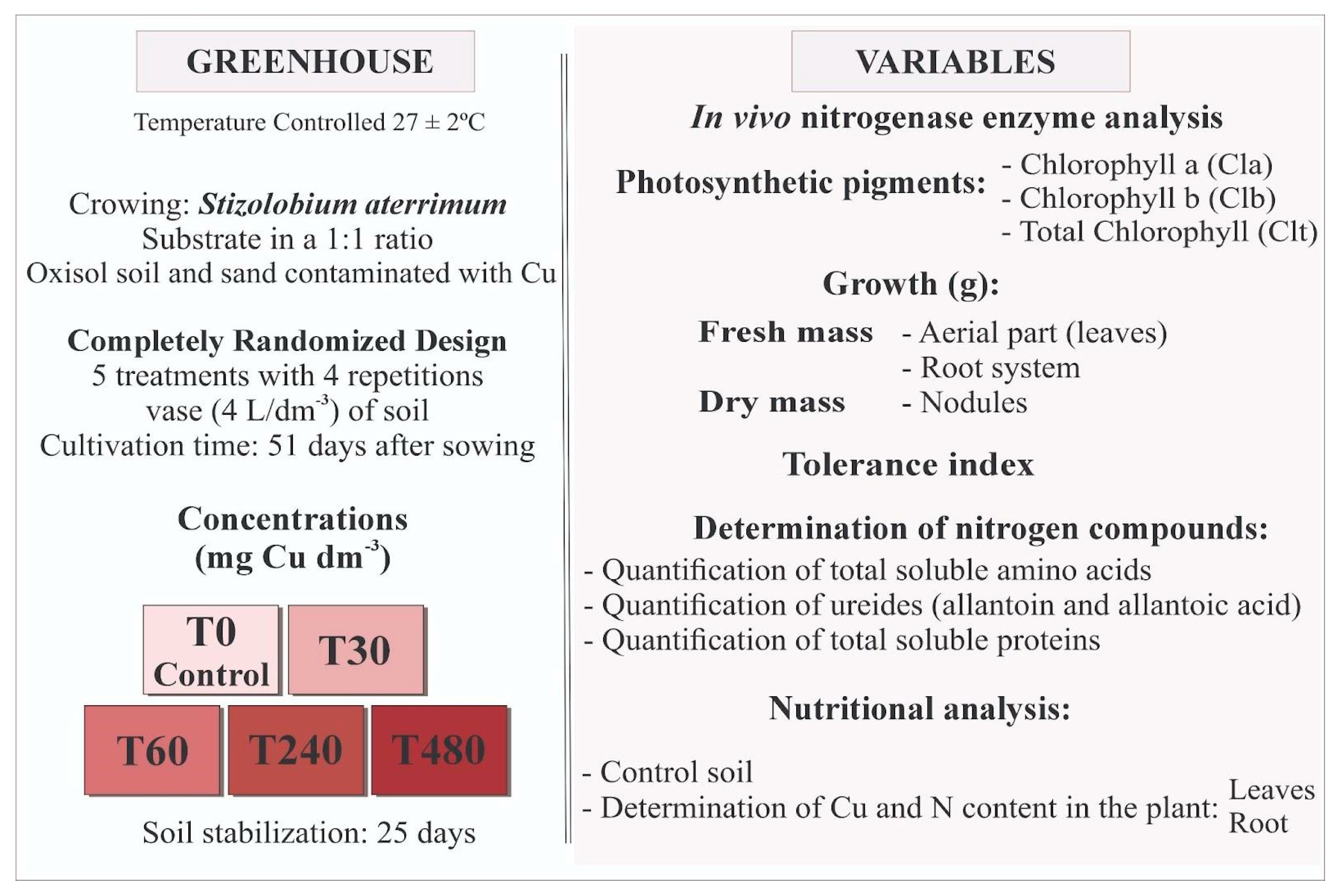
2. Materials and Methods
2.1. Experimental Site and Experimental Design
2.2. Cultivation Conditions
2.3. Experimental Process
2.4. Growth and Partitioned Biomass Production
2.5. Chemical Analysis of Plant Tissue
2.6. Analysis of Chlorophylls
2.7. Analysis of Nitrogen Metabolism and Amino Acids
2.8. Data Analysis
3. Results
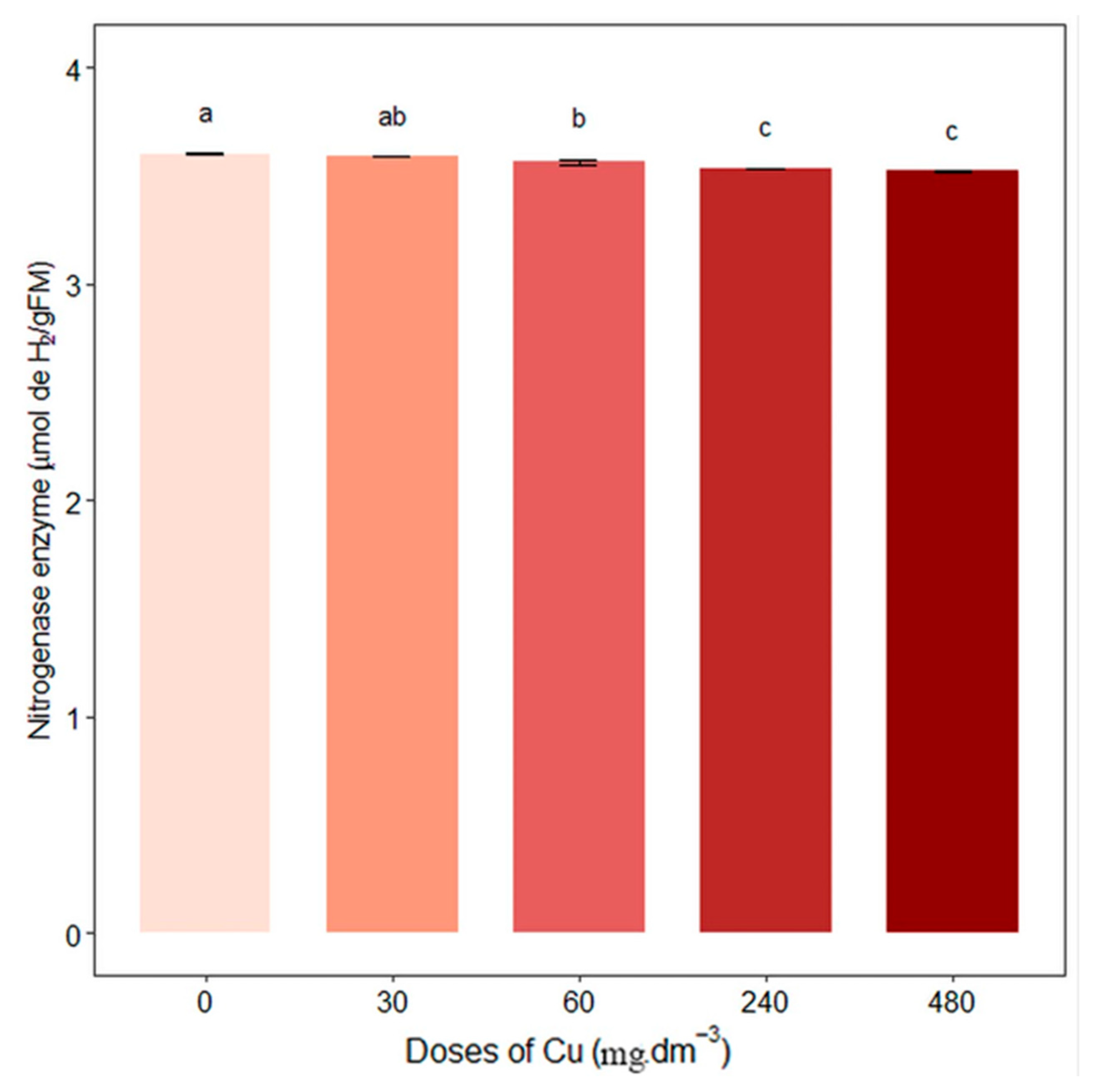
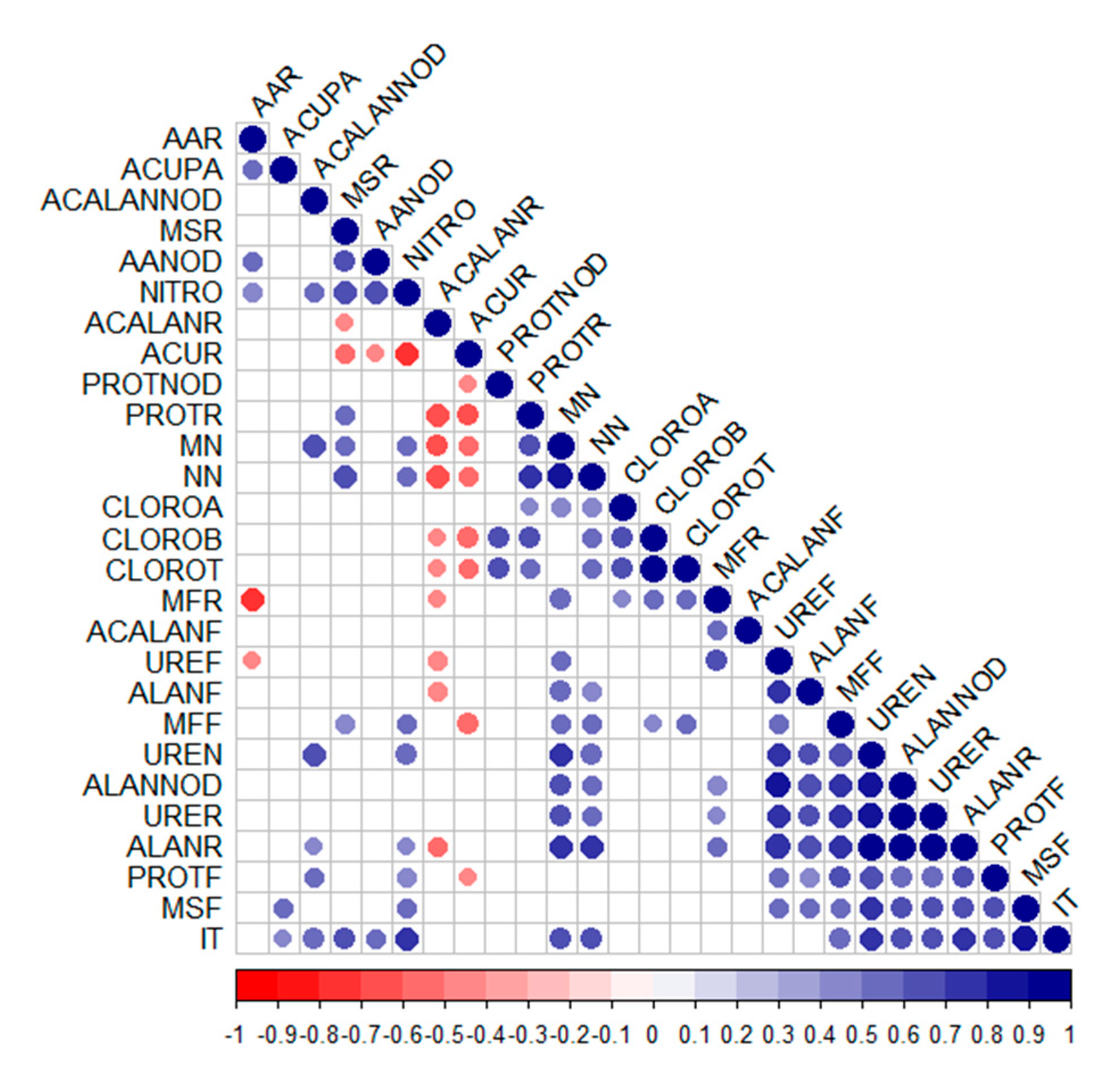
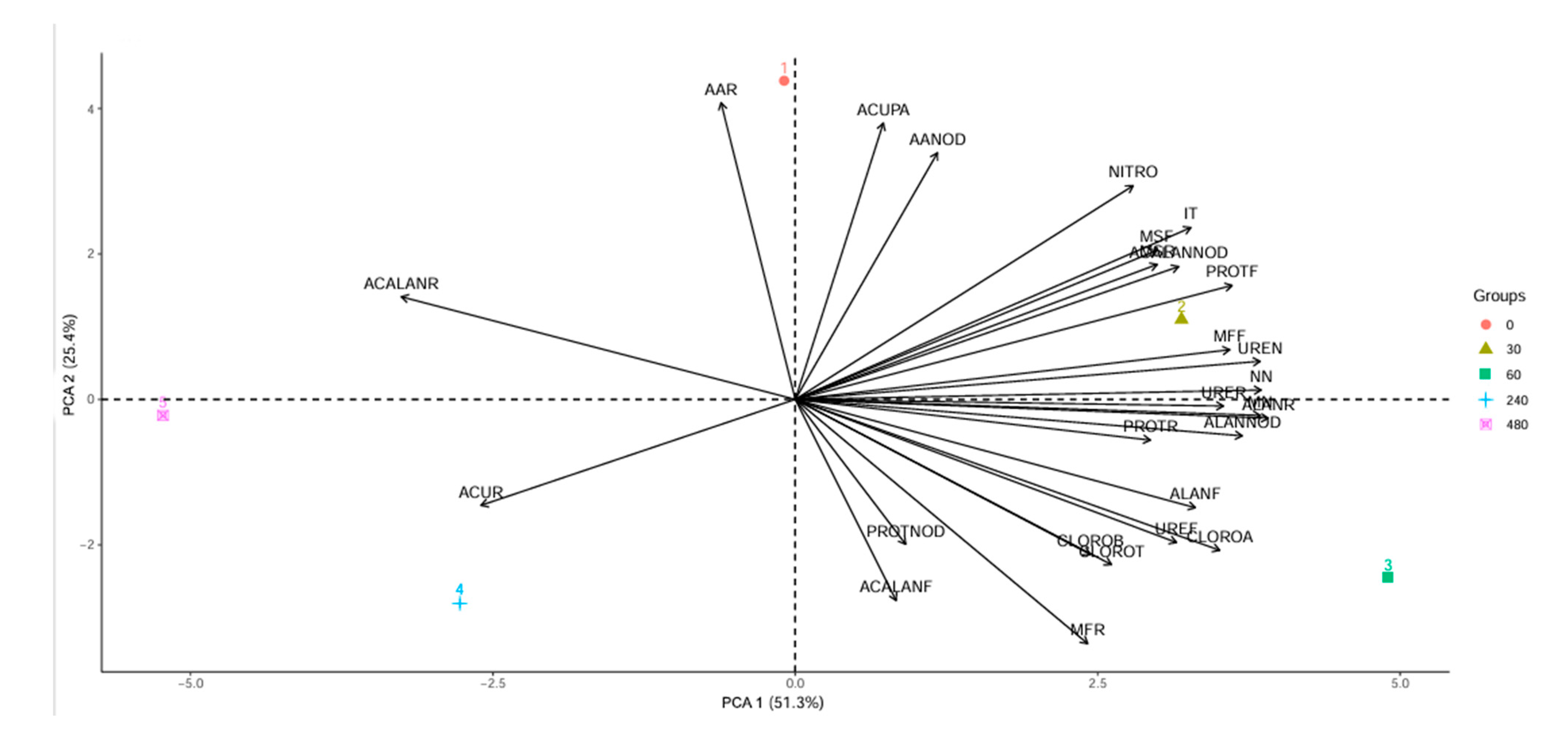
3.1. Nitrogen Metabolism and Amino Acids
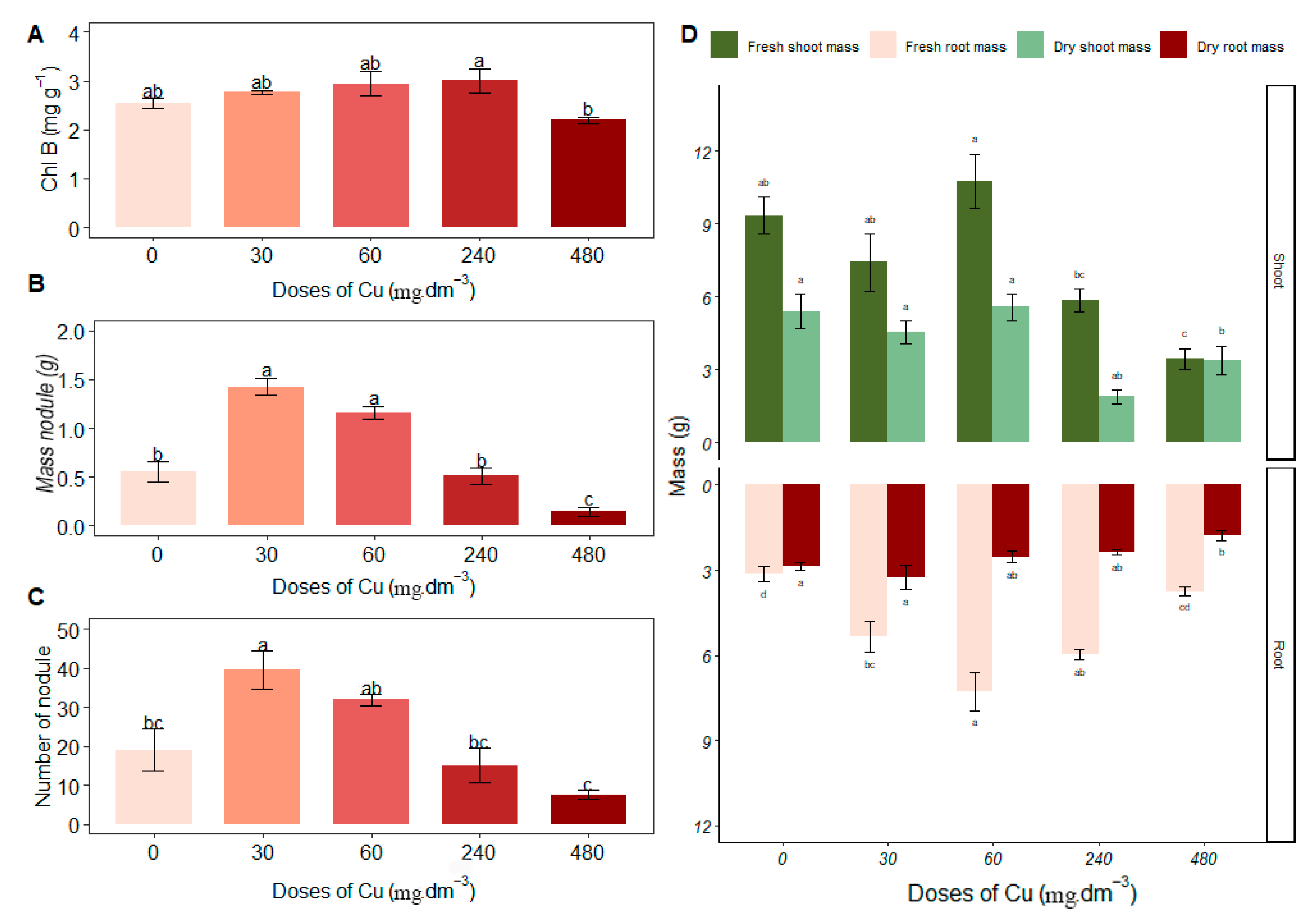
3.2. Chlorophyll, Biomass and Nodules in Plants
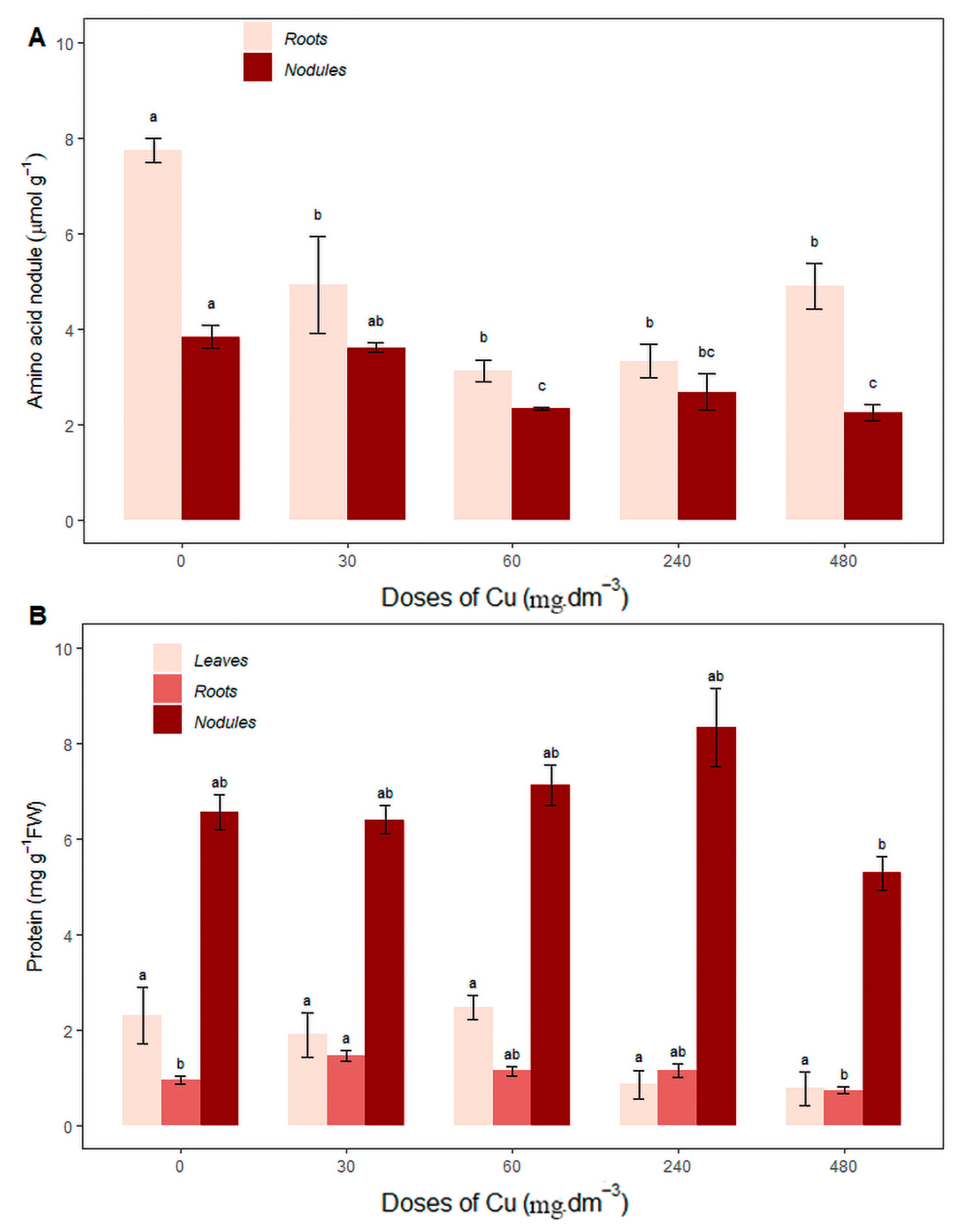
3.3. Nitrogen Compounds: Amino Acids and Proteins
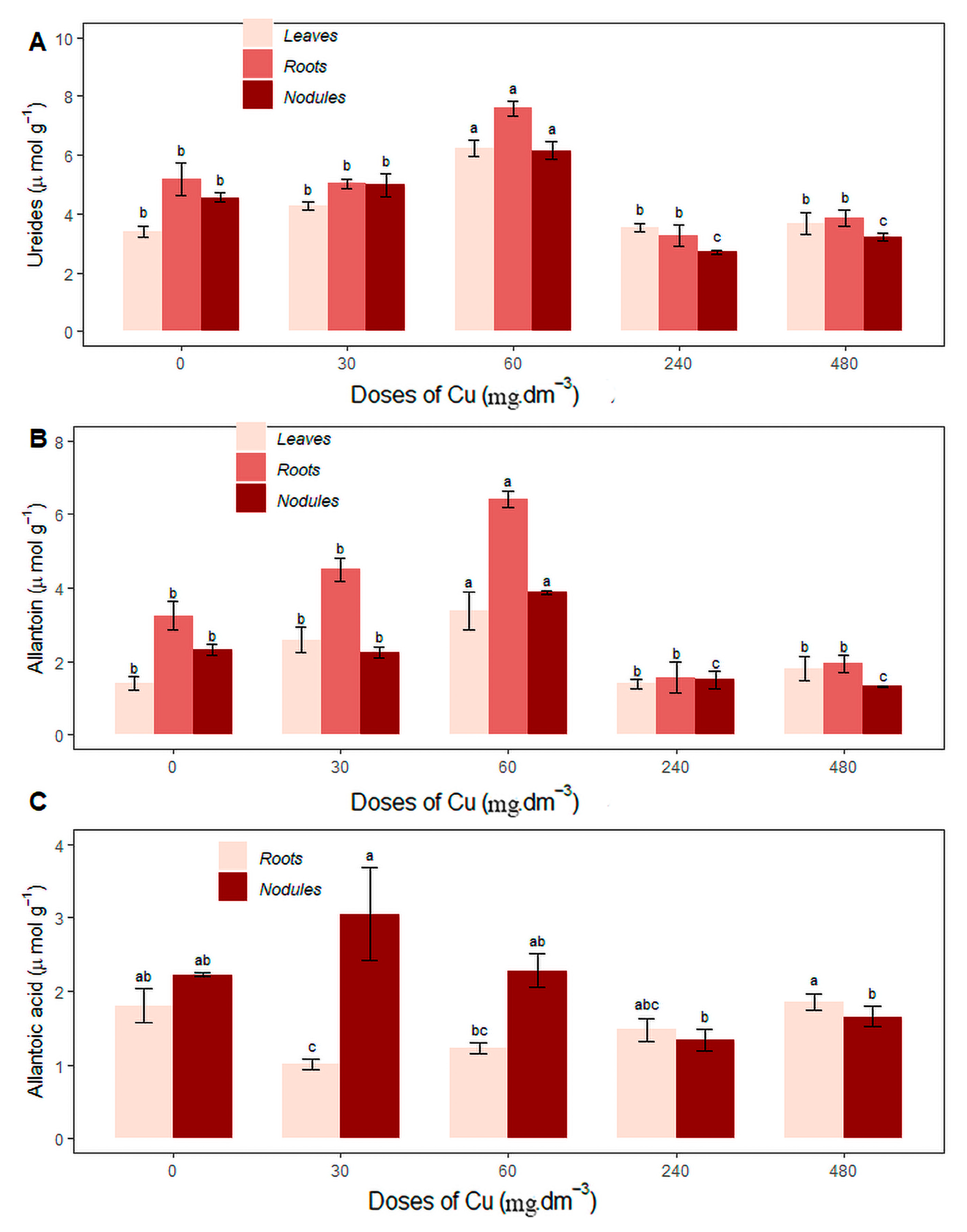
3.4. Nitrogenous Transport Compounds: Ureides, Allantoin and Allantoic Acid
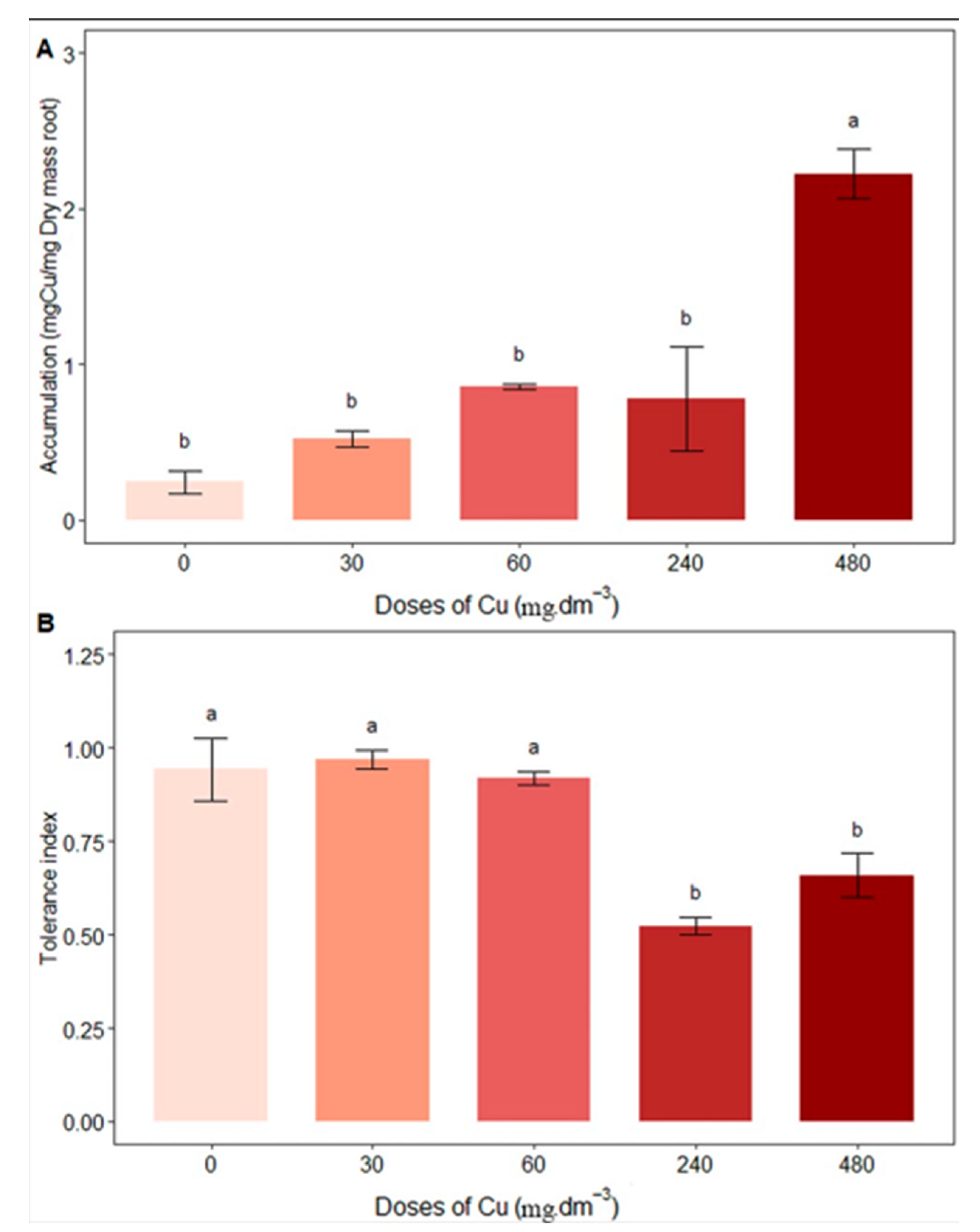
3.5. Copper Accumulation and Tolerance Index of Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goswami, S.; Das, S. Copper Phytoremediation Potential of Calandula officinalis L. and the Role of Antioxidant Enzymes in Metal Tolerance. Ecotoxicol. Environ. Saf. 2016, 126, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Forli, F.; Otto, R.; Vitti, G.C.; do Vale, D.W.; Miyake, R.T.M. Micronutrients Application on Cultivation of Sugarcane Billets. Afr. J. Agric. Res. 2017, 12, 790–794. [Google Scholar] [CrossRef]
- Chia, J.-C.; Yan, J.; Rahmati Ishka, M.; Faulkner, M.M.; Simons, E.; Huang, R.; Smieska, L.; Woll, A.; Tappero, R.; Kiss, A.; et al. Loss of OPT3 Function Decreases Phloem Copper Levels and Impairs Crosstalk between Copper and Iron Homeostasis and Shoot-to-Root Signaling in Arabidopsis Thaliana. Plant Cell 2023, 35, 2157–2185. [Google Scholar] [CrossRef]
- Rehman, M.; Maqbool, Z.; Peng, D.; Liu, L. Morpho-Physiological Traits, Antioxidant Capacity and Phytoextraction of Copper by Ramie (Boehmeria nivea L.) Grown as Fodder in Copper-Contaminated Soil. Environ. Sci. Pollut. Res. Int. 2019, 26, 5851–5861. [Google Scholar] [CrossRef] [PubMed]
- Napoli, M.; Cecchi, S.; Grassi, C.; Baldi, A.; Zanchi, C.A.; Orlandini, S. Phytoextraction of Copper from a Contaminated Soil Using Arable and Vegetable Crops. Chemosphere 2019, 219, 122–129. [Google Scholar] [CrossRef]
- Seliga, H. Nitrogen Fixation in Several Grain Legume Species with Contrasting Sensitivities to Copper Nutrition. Acta Physiol. Plant. 1998, 20, 263–267. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Szteke, B. Trace Elements in Abiotic and Biotic Environments; CRC Press: Boca Raton, FL, USA, 2015; ISBN 978-0-429-16151-3. [Google Scholar]
- Lam, E.J.; Cánovas, M.; Gálvez, M.E.; Montofré, Í.L.; Keith, B.F.; Faz, Á. Evaluation of the Phytoremediation Potential of Native Plants Growing on a Copper Mine Tailing in Northern Chile. J. Geochem. Explor. 2017, 182, 210–217. [Google Scholar] [CrossRef]
- Ghazaryan, K.; Movsesyan, H.; Ghazaryan, N.; Watts, B.A. Copper Phytoremediation Potential of Wild Plant Species Growing in the Mine Polluted Areas of Armenia. Environ. Pollut. 2019, 249, 491–501. [Google Scholar] [CrossRef]
- Saleem, M.H.; Fahad, S.; Khan, S.U.; Ahmar, S.; Ullah Khan, M.H.; Rehman, M.; Maqbool, Z.; Liu, L. Morpho-Physiological Traits, Gaseous Exchange Attributes, and Phytoremediation Potential of Jute (Corchorus capsularis L.) Grown in Different Concentrations of Copper-Contaminated Soil. Ecotoxicol. Environ. Saf. 2020, 189, 109915. [Google Scholar] [CrossRef]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia, A.F.; Seymour, D.; Yuan, Z.-C. Nitrogen Journey in Plants: From Uptake to Metabolism, Stress Response, and Microbe Interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef]
- Starker, C.G.; Parra-Colmenares, A.L.; Smith, L.; Mitra, R.M.; Long, S.R. Nitrogen Fixation Mutants of Medicago Truncatula Fail to Support Plant and Bacterial Symbiotic Gene Expression. Plant Physiol. 2006, 140, 671–680. [Google Scholar] [CrossRef]
- Yanlin, M.; Chengbin, X.; Jianquan, L.; Guangpeng, R. Nutrient-dependent regulation of symbiotic nitrogen fixation in legumes. Hortic. Res. 2024, 12, uhae321. [Google Scholar] [CrossRef]
- Mir, A.R.; Pichtel, J.; Hayat, S. Copper: Uptake, Toxicity and Tolerance in Plants and Management of Cu-Contaminated Soil. BioMetals 2021, 34, 737–759. [Google Scholar] [CrossRef]
- Mergaert, P.; Kereszt, A.; Kondorosi, E. Gene Expression in Nitrogen-Fixing Symbiotic Nodule Cells in Medicago truncatula and Other Nodulating Plants. Plant Cell 2020, 32, 42–68. [Google Scholar] [CrossRef] [PubMed]
- Yruela, I. Cobre em plantas. Braz. J. Plant Physiol. 2005, 17, 145–156. [Google Scholar] [CrossRef]
- de Souza, L.A.; de Andrade, S.A.L.; de Souza, S.C.R.; Schiavinato, M.A. Tolerância e potencial fitorremediador de Stizolobium aterrimum associada ao fungo micorrízico arbuscular Glomus etunicatum em solo contaminado por chumbo. Rev. Bras. Ciênc. Solo 2011, 35, 1441–1451. [Google Scholar] [CrossRef]
- Costa, B.G.P.; Justino, G.C.; Aguiar, L.F.; Souza, L.A.; Camargos, L.S. Boron Phytoremediation: Stizolobium aterrimum Is Tolerant and Can Be Used for Phytomanagement of Boron Excess in Soils. Int. J. Environ. Stud. 2019, 76, 329–337. [Google Scholar] [CrossRef]
- da Silva, M.B.; Bomfim, N.C.P.; da Silva, V.N.; de Lima Frachia, C.; de Souza, L.A.; Justino, G.C.; de Camargos, L.S. Response of Cajanus Cajan to Excess Copper in the Soil: Tolerance and Biomass Production. Physiol. Mol. Biol. Plants 2022, 28, 1335–1345. [Google Scholar] [CrossRef]
- Bomfim, N.C.P.; Aguilar, J.V.; Ferreira, T.C.; dos Santos, B.S.; de Paiva, W.d.S.; de Souza, L.A.; Camargos, L.S. Root Development in Leucaena leucocephala (Lam.) de Wit Enhances Copper Accumulation. Environ. Sci. Pollut. Res. 2023, 30, 80245–80260. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, B.S.; Mendonça, G.W.; Ferreira, T.C.; Bomfim, N.C.P.; de Carvalho, I.F.; Aguilar, J.V.; Camargos, L.S. Exploring the Potential of Crotalaria juncea L. for Phytoremediation: Insights from Gas Exchange, Pigment Quantification, and Growth Measurements under Copper Stress. Horticulturae 2024, 10, 746. [Google Scholar] [CrossRef]
- Sousa, R.R.; Ferraz, T.M.; de Oliveira, J.D.; Nascimento, I.d.O.; Reis, F.d.O.; Costa, N.B. Phytoremediation potential of Canavalia ensiformis in copper- and zinc-contaminated soil. Rev. Em Agronegócio E Meio Ambiente 2024, 17, e11022. [Google Scholar]
- Cambrollé, J.; García, J.L.; Figueroa, M.E.; Cantos, M. Evaluating wild grapevine tolerance to copper toxicity. Chemosphere 2015, 120, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Santoyo-Martínez, M.; Mussali-Galante, P.; Hernández-Plata, I.; Valencia-Cuevas, L.; Flores-Morales, A.; Ortiz-Hernández, L.; Flores-Trujillo, K.; Ramos-Quintana, F.; Tovar-Sánchez, E. Heavy Metal Bioaccumulation and Morphological Changes in Vachellia Campechiana (Fabaceae) Reveal Its Potential for Phytoextraction of Cr, Cu, and Pb in Mine Tailings. Environ. Sci. Pollut. Res. 2020, 27, 11260–11276. [Google Scholar] [CrossRef]
- Santoyo-Martínez, M.; Mussali-Galante, P.; Hernández-Plata, I.; Valencia-Cuevas, L.; Rodríguez, A.; Castrejón-Godínez, M.L.; Tovar-Sánchez, E. Phytoremediation Potential of Crotalaria Pumila (Fabaceae) in Soils Polluted with Heavy Metals: Evidence from Field and Controlled Experiments. Plants 2024, 13, 1947. [Google Scholar] [CrossRef]
- Karczewska, A.; Mocek, A.; Goliński, P.; Mleczek, M. Phytoremediation of Copper-Contaminated Soil. In Phytoremediation: Management of Environmental Contaminants, Volume 2; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 143–170. ISBN 978-3-319-10969-5. [Google Scholar]
- Malavolta, E.; Vitti, G.C.; Olive Tree, S.A. Assessment of the Nutritional Status of Plants: Principles and Applications, 2nd ed.; Brazilian Association for Potashion and Phosphate Research: Piracicaba, Brazil, 1997. [Google Scholar]
- Hiscox, J.D.; Israelstam, G.F. A Method for the Extraction of Chlorophyll from Leaf Tissue without Maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Bieleski, R.L.; Turner, N.A. Separation and Estimation of Amino Acids in Crude Plant Extracts by Thin-Layer Electrophoresis and Chromatography. Anal. Biochem. 1966, 17, 278–293. [Google Scholar] [CrossRef]
- Yemm, E.W.; Cocking, E.C.; Ricketts, R.E. The Determination of Amino-Acids with Ninhydrin. Analyst 1955, 80, 209–214. [Google Scholar] [CrossRef]
- Vogels, G.D.; Van Der Drift, C. Differential Analyses of Glyoxylate Derivatives. Anal. Biochem. 1970, 33, 143–157. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Trannin, I.C.B.; Moreira, F.M.S.; Siqueira, J.O. Crescimento e nodulação de Acacia mangium, Enterolobium contortisiliquum e Sesbania virgata em solo contaminado com metais pesados. Rev. Bras. Ciênc. Solo 2001, 25, 743–753. [Google Scholar] [CrossRef]
- Arguello, J.M.; Raimunda, D.; Padilla-Benavides, T. Mechanisms of Copper Homeostasis in Bacteria. Front. Cell. Infect. Microbiol. 2013, 3, 73. [Google Scholar] [CrossRef]
- Elizalde-Díaz, J.P.; Hernández-Lucas, I.; Medina-Aparicio, L.; Dávalos, A.; Leija, A.; Alvarado-Affantranger, X.; García-García, J.D.; Hernández, G.; Garcia-de los Santos, A. Rhizobium Tropici CIAT 899 copA Gene Plays a Fundamental Role in Copper Tolerance in Both Free Life and Symbiosis with Phaseolus Vulgaris. Microbiology 2019, 165, 651–661. [Google Scholar] [CrossRef]
- Matsuda, A.; Moreira, F.M.d.S.; Siqueira, J.O. Tolerância de rizóbios de diferentes procedências ao zinco, cobre e cádmio. Pesqui. Agropecuária Bras. 2002, 37, 343–355. [Google Scholar] [CrossRef]
- Marques, D.M.; Veroneze Júnior, V.; da Silva, A.B.; Mantovani, J.R.; Magalhães, P.C.; de Souza, T.C. Copper Toxicity on Photosynthetic Responses and Root Morphology of Hymenaea courbaril L. (Caesalpinioideae). Water Air Soil Pollut. 2018, 229, 138. [Google Scholar] [CrossRef]
- Girotto, E.; Ceretta, C.A.; Rossato, L.V.; Farias, J.G.; Brunetto, G.; Miotto, A.; Tiecher, T.L.; de Conti, L.; Lourenzi, C.R.; Schmatz, R.; et al. Biochemical Changes in Black Oat (Avena strigosa Schreb) Cultivated in Vineyard Soils Contaminated with Copper. Plant Physiol. Biochem. 2016, 103, 199–207. [Google Scholar] [CrossRef]
- Lu, M.-Z.; Carter, A.M.; Tegeder, M. Altering Ureide Transport in Nodulated Soybean Results in Whole-Plant Adjustments of Metabolism, Assimilate Partitioning, and Sink Strength. J. Plant Physiol. 2022, 269, 153613. [Google Scholar] [CrossRef]
- Thu, S.W.; Lu, M.-Z.; Carter, A.M.; Collier, R.; Gandin, A.; Sitton, C.C.; Tegeder, M. Role of Ureides in Source-to-Sink Transport of Photoassimilates in Non-Fixing Soybean. J. Exp. Bot. 2020, 71, 4495–4511. [Google Scholar] [CrossRef]
- Gratão, P.L.; Polle, A.; Lea, P.J.; Azevedo, R.A. Making the Life of Heavy Metal-Stressed Plants a Little Easier. Funct. Plant Biol. 2005, 32, 481–494. [Google Scholar] [CrossRef]
- Bertrand, M.; Poirier, I. Photosynthetic Organisms and Excess of Metals. Photosynthetica 2005, 43, 345–353. [Google Scholar] [CrossRef]
- Yruela, I. Copper in Plants: Acquisition, Transport and Interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef]
- Raven, J.; Evans, M.C.W.; Korb, R.E. The Role of Trace Metals in Photosynthetic Electron Transport in O2-Evolving Organisms. Photosynth. Res. 1999, 60, 111–150. [Google Scholar] [CrossRef]
- Nedjimi, B. Phytoremediation: A Sustainable Environmental Technology for Heavy Metals Decontamination. SN Appl. Sci. 2021, 3, 286. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Singh, J.; Taneja, P.K.; Mandal, A. Remediation Techniques for Removal of Heavy Metals from the Soil Contaminated through Different Sources: A Review. Environ. Sci. Pollut. Res. 2020, 27, 1319–1333. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, B.G.P.; Aguilar, J.V.; dos Santos, B.S.; Olivio, M.L.G.; de Souza, R.P.; Ferreira, T.C.; Lapaz, A.d.M.; de Souza, L.A.; de Camargos, L.S. Nitrogen Metabolism of Stizolobium aterrimum Grown in Soil Under Toxic Concentrations of Copper (Cu). Horticulturae 2025, 11, 782. https://doi.org/10.3390/horticulturae11070782
Costa BGP, Aguilar JV, dos Santos BS, Olivio MLG, de Souza RP, Ferreira TC, Lapaz AdM, de Souza LA, de Camargos LS. Nitrogen Metabolism of Stizolobium aterrimum Grown in Soil Under Toxic Concentrations of Copper (Cu). Horticulturae. 2025; 11(7):782. https://doi.org/10.3390/horticulturae11070782
Chicago/Turabian StyleCosta, Beatriz Gonçalves Pereira, Jailson Vieira Aguilar, Beatriz Silvério dos Santos, Maiara Luzia Grigoli Olivio, Roberta Possas de Souza, Tassia Caroline Ferreira, Allan de Marcos Lapaz, Lucas Anjos de Souza, and Liliane Santos de Camargos. 2025. "Nitrogen Metabolism of Stizolobium aterrimum Grown in Soil Under Toxic Concentrations of Copper (Cu)" Horticulturae 11, no. 7: 782. https://doi.org/10.3390/horticulturae11070782
APA StyleCosta, B. G. P., Aguilar, J. V., dos Santos, B. S., Olivio, M. L. G., de Souza, R. P., Ferreira, T. C., Lapaz, A. d. M., de Souza, L. A., & de Camargos, L. S. (2025). Nitrogen Metabolism of Stizolobium aterrimum Grown in Soil Under Toxic Concentrations of Copper (Cu). Horticulturae, 11(7), 782. https://doi.org/10.3390/horticulturae11070782





