Exploring the Role of Vertical and Horizontal Pathways in the Formation of Lettuce Plant Endospheric Bacterial Communities: A Comparative Study of Hydroponic and Soil Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Preparation
2.2. Vegetation Experiment
2.3. DNA Extraction, Amplicon Library Preparation and Sequencing
2.4. Statistical Analysis
3. Results and Discussion
3.1. Endophytic Bacterial Microbiome of Lettuce Seeds
3.2. Composition of the Endospheric Bacterial Microbiome of Lettuce Plants and Substrates of Its Growth
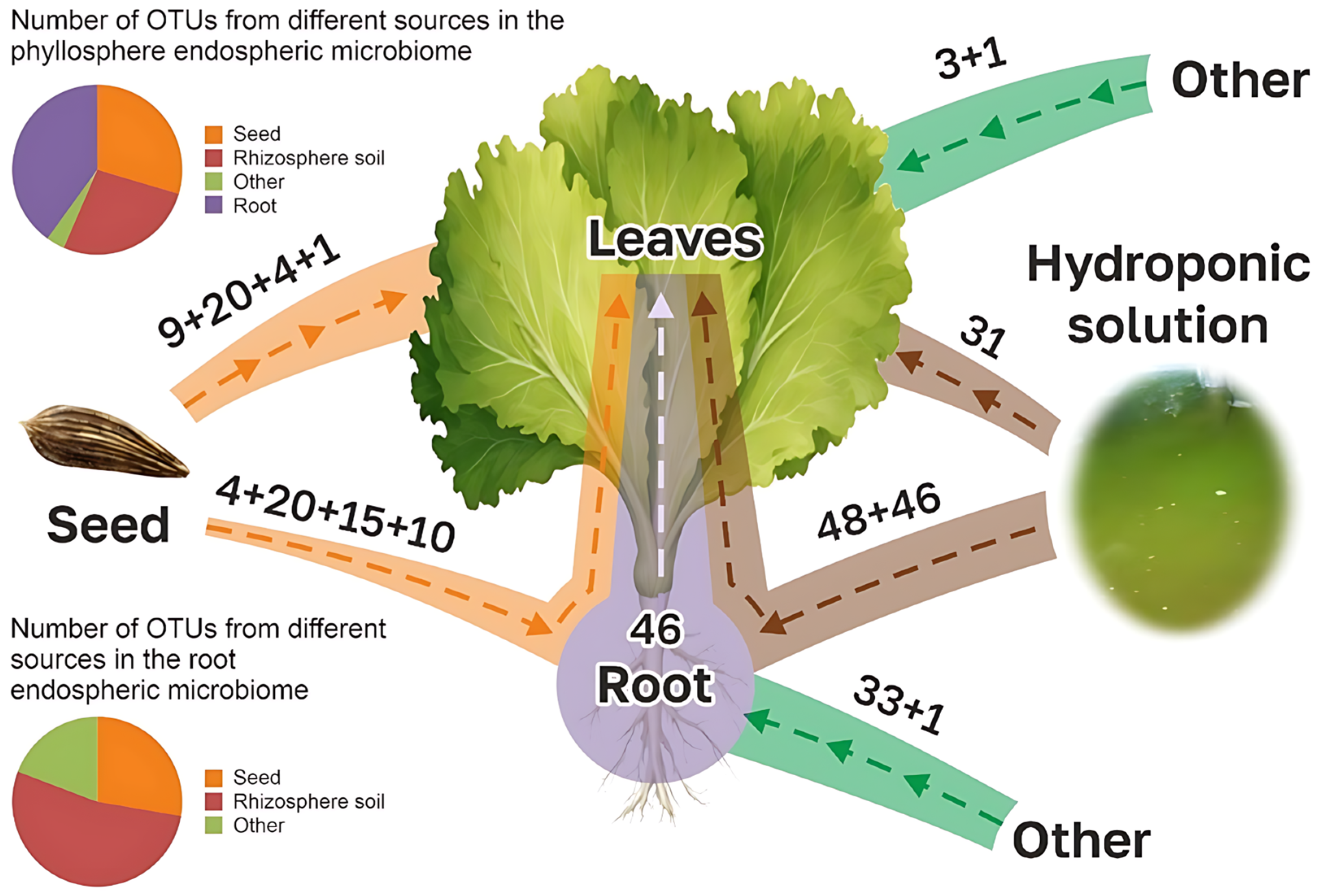
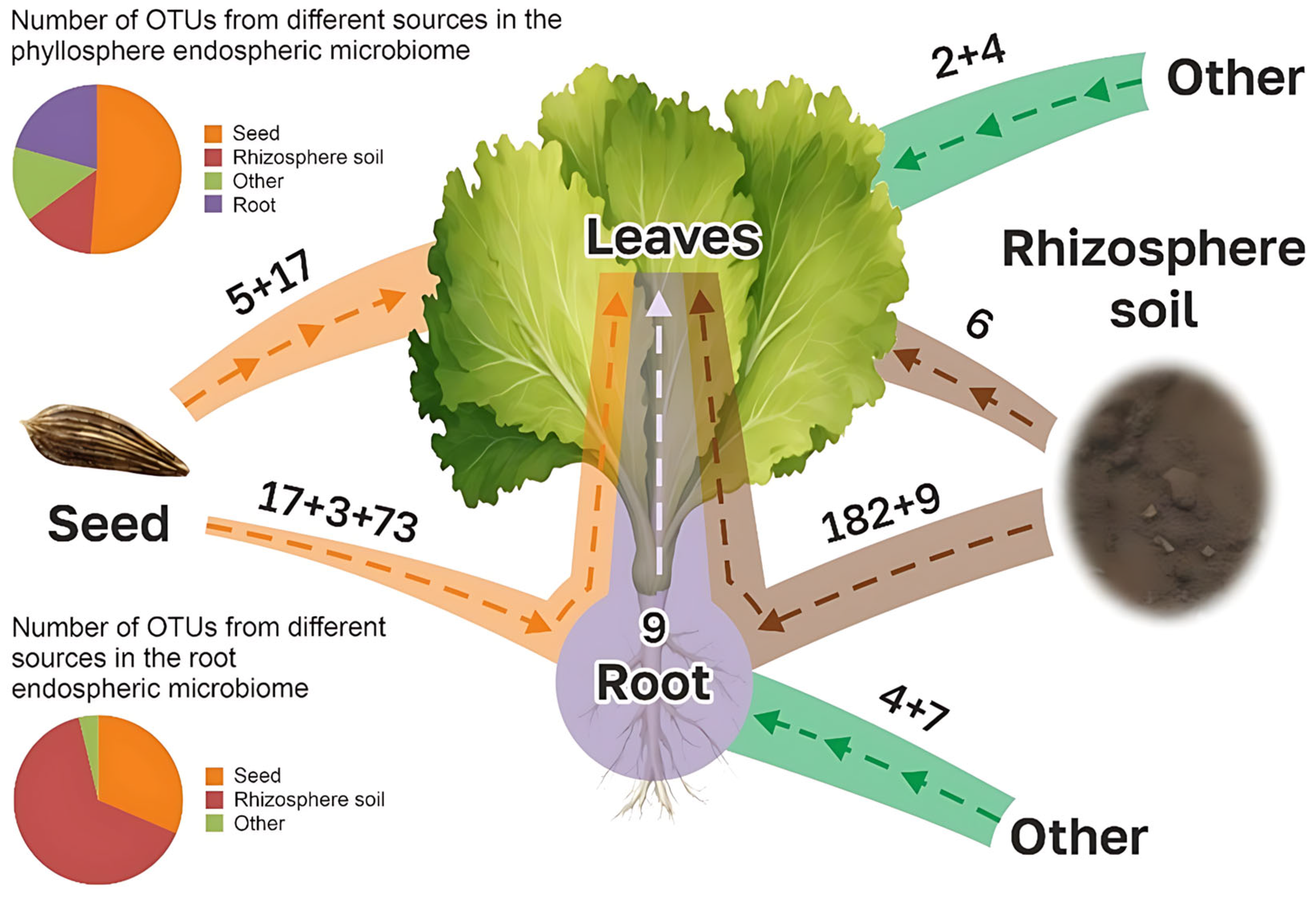
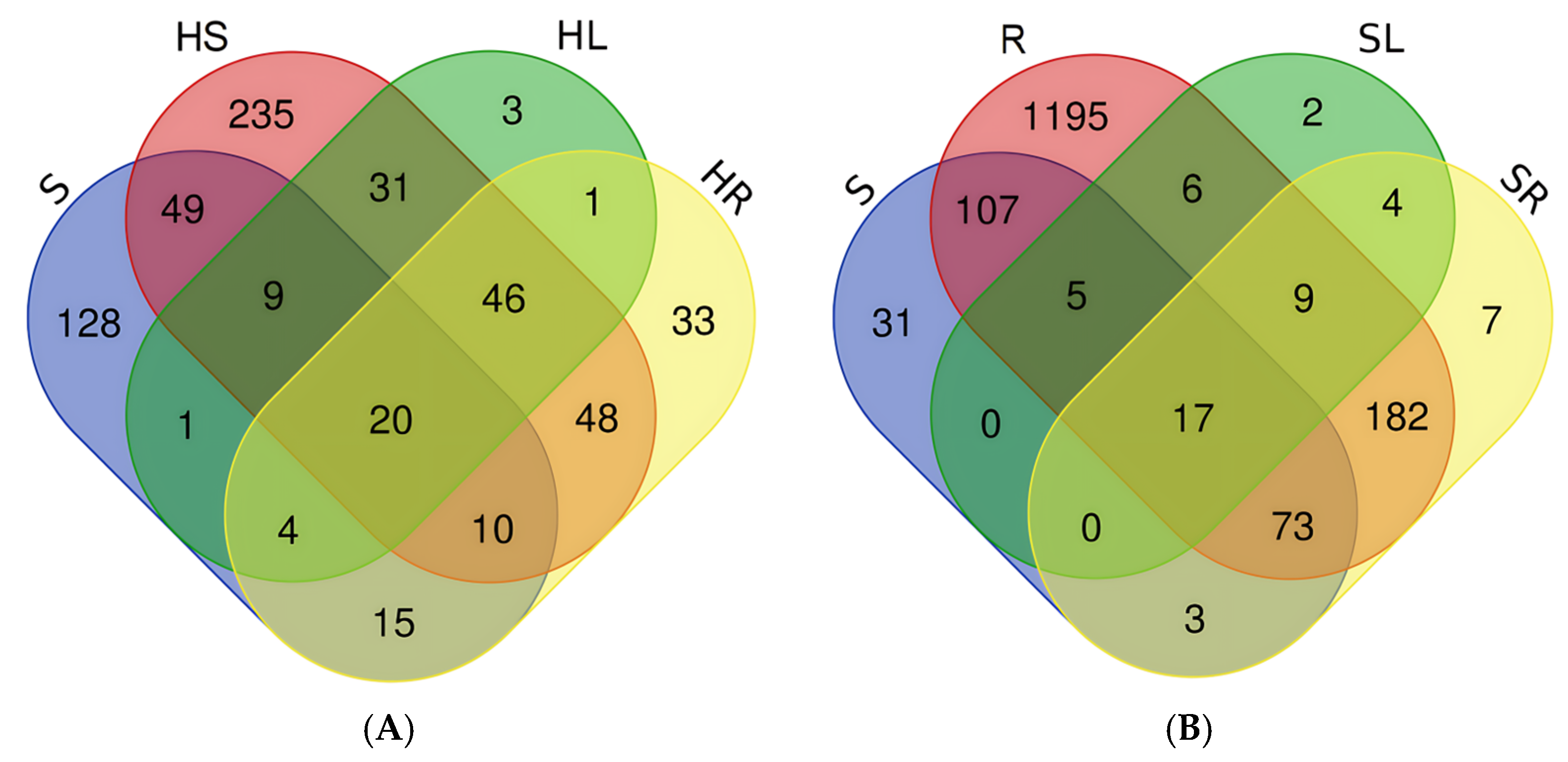
3.3. Comparison of Microbiomes of Lettuce Plants with Microbes of Seeds and Substrates
- (1)
- Vertical colonization (seed -> root)
- (2)
- Vertical colonization (seed -> leaf)
- (3)
- Vertical colonization (seed -> substrate)
- (4)
- Horizontal colonization (substrate -> root)
- (5)
- Horizontal colonization (substrate -> leaf)
- (6)
- Horizontal colonization (leaf -> root)
- (7)
- Horizontal colonization (other sources -> root and leaf)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Adeleke, B.S.; Muller, D.; Babalola, O.O. A Metagenomic Lens into Endosphere Microbial Communities, Promises, and Discoveries. Lett. Appl. Microbiol. 2023, 76, ovac030. [Google Scholar] [CrossRef]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The Importance of the Microbiome of the Plant Holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–Microbiome Interactions: From Community Assembly to Plant Health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant Beneficial Endophytic Bacteria: Mechanisms, Diversity, Host Range and Genetic Determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef]
- Compant, S.; Clément, C.; Sessitsch, A. Plant Growth-Promoting Bacteria in the Rhizo- and Endosphere of Plants: Their Role, Colonization, Mechanisms Involved and Prospects for Utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Amine Hassani, M.; Durán, P.; Hacquard, S. Microbial Interactions within the Plant Holobiont. Microbiome 2018, 6, 58. [Google Scholar]
- Macaya-Sanz, D.; Witzell, J.; Collada, C.; Gil, L.; Martín, J.A. Core Endophytic Mycobiome in Ulmus Minor and Its Relation to Dutch Elm Disease Resistance. Front. Plant Sci. 2023, 14, 1125942. [Google Scholar] [CrossRef]
- Lemanceau, P.; Blouin, M.; Muller, D.; Moënne-Loccoz, Y. Let the Core Microbiota Be Functional. Trends Plant Sci. 2017, 22, 583–595. [Google Scholar] [CrossRef]
- Li, F.; He, X.; Sun, Y.; Zhang, X.; Tang, X.; Li, Y.; Yi, Y. Distinct Endophytes Are Used by Diverse Plants for Adaptation to Karst Regions. Sci. Rep. 2019, 9, 5246. [Google Scholar] [CrossRef]
- Fitzpatrick, C.R.; Salas-González, I.; Conway, J.M.; Finkel, O.M.; Gilbert, S.; Russ, D.; Pereira, P.J.; Teixeira, L.; Dangl, J.L. The Plant Microbiome: From Ecology to Reductionism and Beyond. Annu. Rev. Microbiol. 2020, 74, 81–100. [Google Scholar] [CrossRef]
- Newcombe, G.; Harding, A.; Ridout, M.; Busby, P.E. A Hypothetical Bottleneck in the Plant Microbiome. Front. Microbiol. 2018, 9, 1645. [Google Scholar] [CrossRef]
- Liu, F.; Hewezi, T.; Lebeis, S.L.; Pantalone, V.; Grewal, P.S.; Staton, M.E. Soil Indigenous Microbiome and Plant Genotypes Cooperatively Modify Soybean Rhizosphere Microbiome Assembly. BMC Microbiol. 2019, 19, 201. [Google Scholar] [CrossRef] [PubMed]
- Acuña, J.J.; Hu, J.; Inostroza, N.G.; Valenzuela, T.; Perez, P.; Epstein, S.; Sessitsch, A.; Zhang, Q.; Jorquera, M.A. Endophytic Bacterial Communities in Ungerminated and Germinated Seeds of Commercial Vegetables. Sci. Rep. 2023, 13, 19829. [Google Scholar] [CrossRef]
- Ibáñez, F.; Tonelli, M.L.; Muñoz, V.; Figueredo, M.S.; Fabra, A. Bacterial Endophytes of Plants: Diversity, Invasion Mechanisms and Effects on the Host; Springer: Cham, Switzerland, 2017; Volume 1, pp. 25–40. ISBN 9783319665405. [Google Scholar]
- Johnston-Monje, D.; Gutiérrez, J.P.; Lopez-Lavalle, L.A.B. Seed-Transmitted Bacteria and Fungi Dominate Juvenile Plant Microbiomes. Front. Microbiol. 2021, 12, 737616. [Google Scholar] [CrossRef] [PubMed]
- Poupin, M.J.; González, B. Embracing Complexity in Plant–Microbiome Systems. Environ. Microbiol. Rep. 2024, 16, e70000. [Google Scholar] [CrossRef] [PubMed]
- Pagán, I. Transmission through Seeds: The Unknown Life of Plant Viruses. PLOS Pathog. 2022, 18, e1010707. [Google Scholar] [CrossRef]
- Simonin, M.; Préveaux, A.; Marais, C.; Garin, T.; Arnault, G.; Sarniguet, A.; Barret, M. Transmission of Synthetic Seed Bacterial Communities to Radish Seedlings: Impact on Microbiota Assembly and Plant Phenotype. Peer Community J. 2023, 3, e95. [Google Scholar] [CrossRef]
- Nelson, E.B. The Seed Microbiome: Origins, Interactions, and Impacts. Plant Soil 2018, 422, 7–34. [Google Scholar] [CrossRef]
- Shade, A.; Jacques, M.A.; Barret, M. Ecological Patterns of Seed Microbiome Diversity, Transmission, and Assembly. Curr. Opin. Microbiol. 2017, 37, 15–22. [Google Scholar] [CrossRef]
- Johnston-Monje, D.; Mousa, W.K.; Lazarovits, G.; Raizada, M.N. Impact of Swapping Soils on the Endophytic Bacterial Communities of Pre-Domesticated, Ancient and Modern Maize. BMC Plant Biol. 2014, 14, 233. [Google Scholar] [CrossRef]
- Johnston-Monje, D.; Raizada, M.N. Conservation and Diversity of Seed Associated Endophytes in Zea across Boundaries of Evolution, Ethnography and Ecology. PLoS ONE 2011, 6, e20396. [Google Scholar] [CrossRef] [PubMed]
- Bright, M.; Bulgheresi, S. A Complex Journey: Transmission of Microbial Symbionts. Nat. Rev. Microbiol. 2010, 8, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Barret, M.; Briand, M.; Bonneau, S.; Préveaux, A.; Valière, S.; Bouchez, O.; Hunault, G.; Simoneau, P.; Jacquesa, M.A. Emergence Shapes the Structure of the Seed Microbiota. Appl. Environ. Microbiol. 2015, 81, 1257–1266. [Google Scholar] [CrossRef]
- Leff, J.W.; Lynch, R.C.; Kane, N.C.; Fierer, N. Plant Domestication and the Assembly of Bacterial and Fungal Communities Associated with Strains of the Common Sunflower, Helianthus Annuus. New Phytol. 2017, 214, 412–423. [Google Scholar] [CrossRef]
- Yang, L.; Danzberger, J.; Schöler, A.; Schröder, P.; Schloter, M.; Radl, V. Dominant Groups of Potentially Active Bacteria Shared by Barley Seeds Become Less Abundant in Root Associated Microbiome. Front. Plant Sci. 2017, 8, 1005. [Google Scholar] [CrossRef]
- Rahman, M.M.; Flory, E.; Koyro, H.W.; Abideen, Z.; Schikora, A.; Suarez, C.; Schnell, S.; Cardinale, M. Consistent Associations with Beneficial Bacteria in the Seed Endosphere of Barley (Hordeum vulgare L.). Syst. Appl. Microbiol. 2018, 41, 386–398. [Google Scholar] [CrossRef]
- Eyre, A.W.; Wang, M.; Oh, Y.; Dean, R.A. Identification and Characterization of the Core Rice Seed Microbiome. Phytobiomes J. 2019, 3, 148–157. [Google Scholar] [CrossRef]
- Wassermann, B.; Rybakova, D.; Adam, E.; Zachow, C.; Bernhard, M.; Muller, M.; Mancinelli, R.; Berg, G. Studying Seed Microbiomes. In The Plant Microbiome Methods and Protocols; Humana: New York, NY, USA, 2021; pp. 1–22. [Google Scholar]
- Van Elsas, J.D.; Turner, S.; Bailey, M.J. Horizontal Gene Transfer in the Phytosphere. New Phytol. 2003, 157, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Raaijmakers, J.M. Saving Seed Microbiomes. ISME J. 2018, 12, 1167–1170. [Google Scholar] [CrossRef]
- Simonin, M.; Briand, M.; Chesneau, G.; Rochefort, A.; Marais, C.; Sarniguet, A.; Barret, M. Seed Microbiota Revealed by a Large-scale Meta-analysis Including 50 Plant Species. New Phytol. 2022, 234, 1448–1463. [Google Scholar] [CrossRef]
- Hardoim, P.R.; Hardoim, C.C.P.; van Overbeek, L.S.; van Elsas, J.D. Dynamics of Seed-Borne Rice Endophytes on Early Plant Growth Stages. PLoS ONE 2012, 7, e30438. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.Z.; Schena, L.; Malacrinò, A. Lettuce Seedlings Rapidly Assemble Their Microbiome from the Environment through Deterministic Processes. Rhizosphere 2024, 30, 100896. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Luo, Y.M.; Freitas, H. Inoculation of Endophytic Bacteria on Host and Non-Host Plants-Effects on Plant Growth and Ni Uptake. J. Hazard. Mater. 2011, 195, 230–237. [Google Scholar] [PubMed]
- Compant, S.; Reiter, B.; Sessitsch, A.; Nowak, J.; Clément, C.; Barka, E.A. Endophytic Colonization of Vitis Vinifera L. by Plant Growth-Promoting Bacterium Burkholderia Sp. Strain PsJN. Appl. Environ. Microbiol. 2005, 71, 1685–1693. [Google Scholar] [CrossRef]
- Khan, Z.; Guelich, G.; Phan, H.; Redman, R.; Doty, S. Bacterial and Yeast Endophytes from Poplar and Willow Promote Growth in Crop Plants and Grasses. ISRN Agron. 2012, 2012, 890280. [Google Scholar]
- Carroll, G. Fungal Endophytes in Stems and Leaves: From Latent Pathogen to Mutualistic Symbiont. Soc. Ecol. 1986, 69, 2–9. [Google Scholar]
- Schlaeppi, K.; Bulgarelli, D. The Plant Microbiome at Work. Mol. Plant-Microbe Interact. 2015, 28, 212–217. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Rott, M.; Schlaeppi, K.; Ver Loren van Themaat, E.; Ahmadinejad, N.; Assenza, F.; Rauf, P.; Huettel, B.; Reinhardt, R.; Schmelzer, E.; et al. Revealing Structure and Assembly Cues for Arabidopsis Root-Inhabiting Bacterial Microbiota. Nature 2012, 488, 91–95. [Google Scholar] [CrossRef]
- Lundberg, D.S.; Lebeis, S.L.; Paredes, S.H.; Yourstone, S.; Gehring, J.; Malfatti, S.; Tremblay, J.; Engelbrektson, A.; Kunin, V.; Del Rio, T.G.; et al. Defining the Core Arabidopsis Thaliana Root Microbiome. Nature 2013, 501, 86–90. [Google Scholar] [CrossRef]
- Peiffer, J.A.; Spor, A.; Koren, O.; Jin, Z.; Tringe, S.G.; Dangl, J.L.; Buckler, E.S.; Ley, R.E. Diversity and Heritability of the Maize Rhizosphere Microbiome under Field Conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6548–6553. [Google Scholar] [CrossRef]
- Schlaeppi, K.; Dombrowski, N.; Oter, R.G.; Ver Loren Van Themaat, E.; Schulze-Lefert, P. Quantitative Divergence of the Bacterial Root Microbiota in Arabidopsis Thaliana Relatives. Proc. Natl. Acad. Sci. USA 2014, 111, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.; Johnson, C.; Santos-Medellín, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S.; Eisen, J.A.; Sundaresan, V.; Jeffery, L.D. Structure, Variation, and Assembly of the Root-Associated Microbiomes of Rice. Proc. Natl. Acad. Sci. USA 2015, 112, E911–E920. [Google Scholar] [CrossRef]
- He, J.; Zhang, L.; Van Dingenen, J.; Desmet, S.; Goormachtig, S.; Calonne-Salmon, M.; Declerck, S. Arbuscular Mycorrhizal Hyphae Facilitate Rhizobia Dispersal and Nodulation in Legumes. ISME J. 2024, 18, wrae185. [Google Scholar] [CrossRef]
- Sengupta, S.; Ganguli, S.; Singh, P.K. Metagenome Analysis of the Root Endophytic Microbial Community of Indian Rice (O. sativa L.). Genomics Data 2017, 12, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jeon, J.; Lee, K.K.; Lee, Y.H. Longitudinal Transmission of Bacterial and Fungal Communities from Seed to Seed in Rice. Commun. Biol. 2022, 5, 772. [Google Scholar] [CrossRef] [PubMed]
- Campisano, A.; Antonielli, L.; Pancher, M.; Yousaf, S.; Pindo, M.; Pertot, I. Bacterial Endophytic Communities in the Grapevine Depend on Pest Management. PLoS ONE 2014, 9, e112763. [Google Scholar] [CrossRef]
- Tian, B.Y.; Cao, Y.; Zhang, K.Q. Metagenomic Insights into Communities, Functions of Endophytes, and Their Associates with Infection by Root-Knot Nematode, Meloidogyne Incognita, in Tomato Roots. Sci. Rep. 2015, 5, 17087. [Google Scholar] [CrossRef]
- Akinsanya, M.A.; Goh, J.K.; Lim, S.P.; Ting, A.S.Y. Diversity, Antimicrobial and Antioxidant Activities of Culturable Bacterial Endophyte Communities in Aloe Vera. FEMS Microbiol. Lett. 2015, 362, 184. [Google Scholar] [CrossRef]
- Yang, R.; Liu, P.; Ye, W. Illumina-Based Analysis of Endophytic Bacterial Diversity of Tree Peony (Paeonia Sect. Moutan) Roots and Leaves. Braz. J. Microbiol. 2017, 48, 695–705. [Google Scholar] [CrossRef]
- Hong, C.E.; Kim, J.U.; Lee, J.W.; Bang, K.H.; Jo, I.H. Metagenomic Analysis of Bacterial Endophyte Community Structure and Functions in Panax Ginseng at Different Ages. 3Biotech 2019, 9, 300. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Yang, J.; Zhang, R.; Gao, J.; Zhao, X.; Zhao, J.; Zhao, D.; Zhang, X. Insights into Endophytic Bacterial Community Structures of Seeds Among Various Oryza sativa L. Rice Genotypes. J. Plant Growth Regul. 2019, 38, 93–102. [Google Scholar] [CrossRef]
- Abedinzadeh, M.; Etesami, H.; Alikhani, H.A. Characterization of Rhizosphere and Endophytic Bacteria from Roots of Maize (Zea mays L.) Plant Irrigated with Wastewater with Biotechnological Potential in Agriculture. Biotechnol. Reports 2019, 21, e00305. [Google Scholar] [CrossRef]
- Wicaksono, W.A.; Cernava, T.; Wassermann, B.; Abdelfattah, A.; Soto-Giron, M.J.; Toledo, G.V.; Virtanen, S.M.; Knip, M.; Hyöty, H.; Berg, G. The Edible Plant Microbiome: Evidence for the Occurrence of Fruit and Vegetable Bacteria in the Human Gut. Gut Microbes 2023, 15, 2258565. [Google Scholar] [CrossRef]
- Žiarovská, J.; Urbanová, L.; Moravčíková, D.; Artimová, R.; Omelka, R.; Medo, J. Varieties of Lettuce Forming Distinct Microbial Communities Inhabiting Roots and Rhizospheres with Various Responses to Osmotic Stress. Horticulturae 2022, 8, 1174. [Google Scholar] [CrossRef]
- Damerum, A.; Arnold, E.; Bernard, V.; Smith, H.; Taylor, G. Good and Bad Lettuce Leaf Microbes? Unravelling the Genetic Architecture of the Microbiome to Inform Plant Breeding for Enhanced Food Safety and Reduced Food Waste. bioRxiv, 2021; bioRxiv: 2021.08.06.455490. [Google Scholar]
- Vlasselaer, L.; Crauwels, S.; Lievens, B.; De Coninck, B. Unveiling the Microbiome of Hydroponically Cultivated Lettuce: Impact of Phytophthora Cryptogea Infection on Plant-Associated Microorganisms. FEMS Microbiol. Ecol. 2024, 100, fiae010. [Google Scholar] [CrossRef] [PubMed]
- Galieva, G.; Kuryntseva, P.; Selivanovskaya, S.; Brusko, V.; Garifullin, B.; Dimiev, A.; Galitskaya, P. Glutamic-N,N-Diacetic Acid as an Innovative Chelating Agent in Microfertilizer Development: Biodegradability, Lettuce Growth Promotion, and Impact on Endospheric Bacterial Communities. Soil Syst. 2024, 8, 67. [Google Scholar] [CrossRef]
- Moroenyane, I.; Tremblay, J.; Yergeau, E. Soybean Microbiome Recovery After Disruption Is Modulated by the Seed and Not the Soil Microbiome. Phytobiomes J. 2021, 5, 418–431. [Google Scholar] [CrossRef]
- Bintarti, A.F.; Sulesky-Grieb, A.; Stopnisek, N.; Shade, A. Endophytic Microbiome Variation Among Single Plant Seeds. Phytobiomes J. 2022, 6, 45–55. [Google Scholar] [CrossRef]
- Barra, P.J.; Inostroza, N.G.; Acuña, J.J.; Mora, M.L.; Crowley, D.E.; Jorquera, M.A. Formulation of Bacterial Consortia from Avocado (Persea americana Mill.) and Their Effect on Growth, Biomass and Superoxide Dismutase Activity of Wheat Seedlings under Salt Stress. Appl. Soil Ecol. 2016, 102, 80–91. [Google Scholar] [CrossRef]
- ISO 10390:2021; Soil, Treated Biowaste and Sludge—Determination of PH. International Organization for Standardization: Geneva, Switzerland, 2021; Volume 2021, p. 10.
- ISO 11265:1994; ISO Soil Quality—Determination Specific Electrical Conductivity. International Organization for Standardization: Geneva, Switzerland, 1994.
- ISO 10694:1995; ISO Soil Quality—Determination of Organic and Total Carbon After Dry Combustion (Elementary Analysis). International Organization for Standardization: Geneva, Switzerland, 1995.
- ISO 13878:1998; Soil Quality—Determination of Total Nitrogen Content by Dry Combustion (“Elemental Analysis”). International Organization for Standardization: Geneva, Switzerland, 1998.
- ISO 13320: 2009; Particle Size Analysis—Laser Diffraction Methods. International Organization for Standardization: Geneva, Switzerland, 2009.
- Baudoin, W.; Nono-Womdim, R.; Lutaladio, N.; Hodder, A. Good Agricultural Practices for Greenhouse Vegetable Crops Principles for Mediterranean Climate Areas; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; ISBN 9789251076491. [Google Scholar]
- Danilova, N.; Galieva, G.; Kuryntseva, P.; Selivanovskaya, S.; Galitskaya, P. Influence of the Antibiotic Oxytetracycline on the Morphometric Characteristics and Endophytic Bacterial Community of Lettuce (Lactuca sativa L.). Microorganisms 2023, 11, 2828. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Geofis. Int. 2004, 43, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.B.; Hellmann, J.J.; Ricketts, T.H.; Bohannan, B.J.M. Counting the Uncountable: Statistical Approaches to Estimating Microbial Diversity. Appl. Environ. Microbiol. 2001, 67, 4399–4406. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C. A Mathematical Theory of Communication. J. Franklin Inst. 1948, 27, 379–423. [Google Scholar]
- Beisel, J.N.; Moreteau, J.C. A Simple Formula for Calculating the Lower Limit of Shannon’s Diversity Index. Ecol. Modell. 1997, 99, 289–292. [Google Scholar] [CrossRef]
- Chalita, M.; Kim, Y.O.; Park, S.; Oh, H.S.; Cho, J.H.; Moon, J.; Baek, N.; Moon, C.; Lee, K.; Yang, J.; et al. EzBioCloud: A Genome-Driven Database and Platform for Microbiome Identification and Discovery. Int. J. Syst. Evol. Microbiol. 2024, 74, 006421. [Google Scholar] [CrossRef]
- Wang, W.; Zhai, Y.; Cao, L.; Tan, H.; Zhang, R. Illumina-Based Analysis of Core Actinobacteriome in Roots, Stems, and Grains of Rice. Microbiol. Res. 2016, 190, 12–18. [Google Scholar] [CrossRef]
- Navya, B.; Babu, S. Comparative Metataxonamic Analyses of Seeds and Leaves of Traditional Varieties and Hybrids of Cucumber (Cucumis sativus L.) Reveals Distinct and Core Microbiome. Heliyon 2023, 9, e20216. [Google Scholar] [CrossRef]
- Kumar, K.; Verma, A.; Pal, G.; Anubha; White, J.F.; Verma, S.K. Seed Endophytic Bacteria of Pearl Millet (Pennisetum glaucum L.) Promote Seedling Development and Defend Against a Fungal Phytopathogen. Front. Microbiol. 2021, 12, 774293. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, A.J.; Zong, Q.; Du, Z.; Guo, Q.; Liu, T.; Chen, W.; Gao, L. Microbiome Signature of Endophytes in Wheat Seed Response to Wheat Dwarf Bunt Caused by Tilletia controversa Kühn. Microbiol. Spectr. 2023, 11, e00390-22. [Google Scholar] [CrossRef]
- Chouhan, U.; Gamad, U.; Choudhari, J.K. Metagenomic Analysis of Soybean Endosphere Microbiome to Reveal Signatures of Microbes for Health and Disease. J. Genet. Eng. Biotechnol. 2023, 21, 84. [Google Scholar] [CrossRef]
- Kim, H.; Lee, Y.H. Spatiotemporal Assembly of Bacterial and Fungal Communities of Seed-Seedling-Adult in Rice. Front. Microbiol. 2021, 12, 708475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, Y.N.; Wang, X.; Liao, K.; He, S.; Zhao, X.; Guo, H.; Zhao, D.; Wei, H.L. Dynamics of Rice Microbiomes Reveal Core Vertically Transmitted Seed Endophytes. Microbiome 2022, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, C.E.; Antonielli, L.; Mitter, B.; Sessitsch, A. Assembly of Endophytic Communities of Setaria viridis Plants When Grown in Different Soils and Derived from Different Seeds. Phytobiomes J. 2024, 8, 34–45. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Tack, A.J.M.; Lobato, C.; Wassermann, B.; Berg, G. From Seed to Seed: The Role of Microbial Inheritance in the Assembly of the Plant Microbiome. Trends Microbiol. 2023, 31, 346–355. [Google Scholar] [CrossRef]
- Kumar, A.; Solanki, M.K.; Wang, Z.; Solanki, A.C.; Singh, V.K.; Divvela, P.K. Revealing the Seed Microbiome: Navigating Sequencing Tools, Microbial Assembly, and Functions to Amplify Plant Fitness. Microbiol. Res. 2024, 279, 127549. [Google Scholar] [CrossRef] [PubMed]
- López-López, A.; Rogel, M.A.; Ormeño-Orrillo, E.; Martínez-Romero, J.; Martínez-Romero, E. Phaseolus Vulgaris Seed-Borne Endophytic Community with Novel Bacterial Species Such as Rhizobium endophyticum sp. nov. Syst. Appl. Microbiol. 2010, 33, 322–327. [Google Scholar] [CrossRef]
- Rosenblueth, M.; López-López, A.; Martínez, J.; Rogel, M.A.; Toledo, I.; Martínez-Romero, E. Seed Bacterial Endophytes: Common Genera, Seed-to-Seed Variability and Their Possible Role in Plants. Acta Hortic. 2012, 938, 39–48. [Google Scholar] [CrossRef]
- Sun, Z.; Adeleke, B.S.; Shi, Y.; Li, C. The Seed Microbiomes of Staple Food Crops. Microb. Biotechnol. 2023, 16, 2236–2249. [Google Scholar] [CrossRef]
- Solanki, M.K.; Wang, F.Y.; Wang, Z.; Li, C.N.; Lan, T.J.; Singh, R.K.; Singh, P.; Yang, L.T.; Li, Y.R. Rhizospheric and Endospheric Diazotrophs Mediated Soil Fertility Intensification in Sugarcane-Legume Intercropping Systems. J. Soils Sediments 2019, 19, 1911–1927. [Google Scholar] [CrossRef]
- Díaz Herrera, S.; Grossi, C.; Zawoznik, M.; Groppa, M.D. Wheat Seeds Harbour Bacterial Endophytes with Potential as Plant Growth Promoters and Biocontrol Agents of Fusarium graminearum. Microbiol. Res. 2016, 186–187, 37–43. [Google Scholar] [CrossRef]
- Ouwerkerk, J.P.; Aalvink, S.; Belzer, C.; de Vos, W.M. Akkermansia Glycaniphila Sp. Nov., an Anaerobic Mucin-Degrading Bacterium Isolated from Reticulated Python faeces. Int. J. Syst. Evol. Microbiol. 2016, 66, 4614–4620. [Google Scholar] [CrossRef]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Karsch-Mizrachi, I. NCBI Taxonomy: A Comprehensive Update on Curation, Resources and Tools. Available online: https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Tree&id=512293&lvl=3&lin=f&keep=1&srchmode=1&unlock (accessed on 3 February 2021).
- Lagkouvardos, I.; Lesker, T.R.; Hitch, T.C.A.; Gálvez, E.J.C.; Smit, N.; Neuhaus, K.; Wang, J.; Baines, J.F.; Abt, B.; Stecher, B.; et al. Sequence and Cultivation Study of Muribaculaceae Reveals Novel Species, Host Preference, and Functional Potential of This yet Undescribed Family. Microbiome 2019, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.F.; Pang, Q.; Yao, L.P.; Zhang, Y.; Peng, C.; Huang, W.; Han, B. Gut Microbiota: A Magical Multifunctional Target Regulated by Medicine Food Homology Species. J. Adv. Res. 2023, 52, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Shao, Q.; Ren, Y.; Ge, W.; Yao, T.; Hu, H.; Huang, J.; Liang, Z.; Han, Y. Assembly, Core Microbiota, and Function of the Rhizosphere Soil and Bark Microbiota in Eucommia ulmoides. Front. Microbiol. 2022, 13, 855317. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, L.J.; Lu, W.C.; Yu, D.; Hang, X.N.; Liao, D.X. Colonization Characteristics of Pseudomonas Poae HT1 in Roots and Stems of Faba Bean and Its Effect on Endophytic Bacterial Diversity. Microbiol. China 2021, 48, 4677–4687. [Google Scholar]
- Rosenberg, E.; De Long, E.F.; Lory, S.; Stackebrandt, E.; Thompson, F. The Prokaryotes: Other Major Lineages of Bacteria and the Archaea; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–1028. [Google Scholar]
- Grothjan, J.J.; Young, E.B. Bacterial Recruitment to Carnivorous Pitcher Plant Communities: Identifying Sources Influencing Plant Microbiome Composition and Function. Front. Microbiol. 2022, 13, 791079. [Google Scholar] [CrossRef]
- Bigott, A.F. A Comparison of Soils and Their Associated Microbial Communities as Affected by Sugarcane Cultivation. Master’s Theses, Louisiana State University, Baton Rouge, LA, USA, 2017. [Google Scholar]
- Chewning, S.S.; Grant, D.L.; Banion, B.S.O.; Gates, A.D.; Kennedy, B.J.; Campagna, S.R.; Lebeis, S.L. Root-Associated Streptomyces Isolates Harboring melC Genes Demonstrate Enhanced Plant Colonization. Phytobiomes J. 2019, 3, 165–176. [Google Scholar] [CrossRef]
- De Tender, C. Microbial Community Analysis in Soil (Rhizosphere) and the Marine (Plastisphere) Environment in Function of Plant Health and Biofilm Formation. Ph.D. Thesis, Ghent University, Gent, Belgium, 2017; p. 254. [Google Scholar]
- Debode, J.; De Tender, C.; Soltaninejad, S.; Van Malderghem, C.; Haegeman, A.; Van der Linden, I.; Cottyn, B.; Heyndrickx, M.; Maes, M. Chitin Mixed in Potting Soil Alters Lettuce Growth, the Survival of Zoonotic Bacteria on the Leaves and Associated Rhizosphere Microbiology. Front. Microbiol. 2016, 7, 565. [Google Scholar] [CrossRef]
- Li, X.; Hu, H.; Ren, Q.; Wang, M.; Du, Y.; He, Y.; Wang, Q. Comparative Analysis of Endophyte Diversity of Dendrobium Officinale Lived on Rock and Tree. Plant Biotechnol. 2023, 40, 145–155. [Google Scholar] [CrossRef]
- Mei, X.; Wu, C.; Zhao, J.; Yan, T.; Jiang, P. Community Structure of Bacteria Associated with Drifting Sargassum Horneri, the Causative Species of Golden Tide in the Yellow Sea. Front. Microbiol. 2019, 10, 1192. [Google Scholar] [CrossRef]
- Wassermann, B. The Seed & Fruit Microbiome: Health Implications for Crops and Consumers. Ph.D. Thesis, Graz University of Technology, Graz, Austria, 2020. [Google Scholar]
- Pu, Q.; Wang, H.T.; Pan, T.; Li, H.; Su, J.Q. Enhanced Removal of Ciprofloxacin and Reduction of Antibiotic Resistance Genes by Earthworm Metaphire vulgaris in Soil. Sci. Total Environ. 2020, 742, 140409. [Google Scholar] [CrossRef] [PubMed]
- Alrashedi, S. Effect of Endophyte-Infected Tall Fescue Toxins on Growth Performance and the Microbial Community in the Rumen and Feces in Pregnant Ewes. Ph.D. Thesis, University of Arkansas, Fayetteville, AR, USA, 2017. [Google Scholar]
- Guo, J.; Ling, N.; Li, Y.; Li, K.; Ning, H.; Shen, Q.; Guo, S.; Vandenkoornhuyse, P. Seed-Borne, Endospheric and Rhizospheric Core Microbiota as Predictors of Plant Functional Traits across Rice Cultivars Are Dominated by Deterministic Processes. New Phytol. 2021, 230, 2047–2060. [Google Scholar] [CrossRef]
- Fernández-González, A.J.; Villadas, P.J.; Gómez-Lama Cabanás, C.; Valverde-Corredor, A.; Belaj, A.; Mercado-Blanco, J.; Fernández-López, M. Defining the Root Endosphere and Rhizosphere Microbiomes from the World Olive Germplasm Collection. Sci. Rep. 2019, 9, 20423. [Google Scholar] [CrossRef]
- Hamaoka, K.; Aoki, Y.; Takahashi, S.; Enoki, S.; Yamamoto, K.; Tanaka, K.; Suzuki, S. Diversity of Endophytic Bacterial Microbiota in Grapevine Shoot Xylems Varies Depending on Wine Grape-Growing Region, Cultivar, and Shoot Growth Stage. Sci. Rep. 2022, 12, 15772. [Google Scholar] [CrossRef] [PubMed]
- Sonam, W.; Liu, Y.; Guo, L. Endophytic Bacteria in the Periglacial Plant Potentilla fruticosa var. albicans Are Influenced by Habitat Type. Ecol. Process. 2023, 12, 57. [Google Scholar] [CrossRef]
- Bodhankar, S.; Grover, M.; Hemanth, S.; Reddy, G.; Rasul, S.; Yadav, S.K.; Desai, S.; Mallappa, M.; Mandapaka, M.; Srinivasarao, C. Maize Seed Endophytic Bacteria: Dominance of Antagonistic, Lytic Enzyme-Producing Bacillus Spp. 3Biotech 2017, 7, 232. [Google Scholar] [CrossRef]
- James, E.K.; Gyaneshwar, P.; Mathan, N.; Barraquio, W.L.; Reddy, P.M.; Iannetta, P.P.M.; Olivares, F.L.; Ladha, J.K. Infection and Colonization of Rice Seedlings by the Plant Growth-Promoting Bacterium Herbaspirillum seropedicae Z67. Mol. Plant-Microbe Interact. 2002, 15, 894–906. [Google Scholar] [CrossRef]
- Adam, E.; Bernhart, M.; Müller, H.; Winkler, J.; Berg, G. The Cucurbita Pepo Seed Microbiome: Genotype-Specific Composition and Implications for Breeding. Plant Soil 2018, 422, 35–49. [Google Scholar] [CrossRef]
- Johnston-Monje, D.; Lundberg, D.S.; Lazarovits, G.; Reis, V.M.; Raizada, M.N. Bacterial Populations in Juvenile Maize Rhizospheres Originate from Both Seed and Soil. Plant Soil 2016, 405, 337–355. [Google Scholar] [CrossRef]
- Santoyo, G. How Plants Recruit Their Microbiome? New Insights into Beneficial Interactions. J. Adv. Res. 2022, 40, 45–58. [Google Scholar] [CrossRef]
- Gundel, P.E.; Rudgers, J.A.; Ghersa, C.M. Incorporating the Process of Vertical Transmission into Understanding of Host-Symbiont Dynamics. Oikos 2011, 120, 1121–1128. [Google Scholar]
- Hodgson, S.; de Cates, C.; Hodgson, J.; Morley, N.J.; Sutton, B.C.; Gange, A.C. Vertical Transmission of Fungal Endophytes Is Widespread in Forbs. Ecol. Evol. 2014, 4, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Bergna, A.; Cernava, T.; Grosch, R.; Zachow, C.; Berg, G. Tomato Seeds Preferably Transmit Plant Beneficial Endophytes. Phytobiomes J. 2018, 2, 183–193. [Google Scholar]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Asaf, S.; Lee, I.J. What Is There in Seeds? Vertically Transmitted Endophytic Resources for Sustainable Improvement in Plant Growth. Front. Plant Sci. 2018, 9, 319267. [Google Scholar] [CrossRef]
- Hardoim, P. The Ecology of Seed Microbiota. In Seed Endophytes; Springer: Cham, Switzerland, 2019; pp. 103–125. [Google Scholar]
- Rochefort, A.; Briand, M.; Marais, C.; Wagner, M.H.; Laperche, A.; Vallée, P.; Barret, M.; Sarniguet, A. Influence of Environment and Host Plant Genotype on the Structure and Diversity of the Brassica Napus Seed Microbiota. Phytobiomes J. 2019, 3, 326–336. [Google Scholar]
- Wassermann, B.; Cernava, T.; Müller, H.; Berg, C.; Berg, G. Seeds of Native Alpine Plants Host Unique Microbial Communities Embedded in Cross-Kingdom Networks. Microbiome 2019, 7, 108. [Google Scholar]
- Rodríguez, C.E.; Antonielli, L.; Mitter, B.; Trognitz, F.; Sessitsch, A. Heritability and Functional Importance of the Setaria viridis Bacterial Seed Microbiome. Phytobiomes J. 2020, 4, 40–52. [Google Scholar] [CrossRef]
- Faddetta, T.; Abbate, L.; Alibrandi, P.; Arancio, W.; Siino, D.; Strati, F.; De Filippo, C.; Fatta Del Bosco, S.; Carimi, F.; Puglia, A.M.; et al. The Endophytic Microbiota of Citrus Limon Is Transmitted from Seed to Shoot Highlighting Differences of Bacterial and Fungal Community Structures. Sci. Rep. 2021, 11, 7078. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Wisniewski, M.; Schena, L.; Tack, A.J.M. Experimental Evidence of Microbial Inheritance in Plants and Transmission Routes from Seed to Phyllosphere and Root. Environ. Microbiol. 2021, 23, 2199–2214. [Google Scholar] [CrossRef]
- Kong, H.G.; Song, G.C.; Ryu, C.M. Inheritance of Seed and Rhizosphere Microbial Communities through Plant-Soil Feedback and Soil Memory. Environ. Microbiol. Rep. 2019, 11, 479–486. [Google Scholar]
- Wolfgang, A.; Zachow, C.; Müller, H.; Grand, A.; Temme, N.; Tilcher, R.; Berg, G. Understanding the Impact of Cultivar, Seed Origin, and Substrate on Bacterial Diversity of the Sugar Beet Rhizosphere and Suppression of Soil-Borne Pathogens. Front. Plant Sci. 2020, 11, 560869. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.M.; Becker-Uncapher, I.; Carlson, M.; Fierer, N. Variable Influences of Soil and Seed-Associated Bacterial Communities on the Assembly of Seedling Microbiomes. ISME J. 2021, 15, 2748–2762. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.; Simonin, M.; Marais, C.; Guillerm-Erckelboudt, A.-Y.; Barret, M.; Sarniguet, A. Transmission of Seed and Soil Microbiota to Seedling. mSystems 2021, 6, e0044621. [Google Scholar]
- Hardoim, P.R.; van Overbeek, L.S.; van Elsas, J.D. Properties of Bacterial Endophytes and Their Proposed Role in Plant Growth. Trends Microbiol. 2008, 16, 463–471. [Google Scholar] [CrossRef]
- Dastogeer, K.M.G.; Tumpa, F.H.; Sultana, A.; Akter, M.A.; Chakraborty, A. Plant Microbiome—An Account of the Factors That Shape Community Composition and Diversity. Curr. Plant Biol. 2020, 23, 100161. [Google Scholar] [CrossRef]
- Compant, S.; Kaplan, H.; Sessitsch, A.; Nowak, J.; Ait Barka, E.; Clément, C. Endophytic Colonization of Vitis Vinifera L. by Burkholderia Phytofirmans Strain PsJN: From the Rhizosphere to Inflorescence Tissues. FEMS Microbiol. Ecol. 2008, 63, 84–93. [Google Scholar] [CrossRef]
- Sessitsch, A.; Reiter, B.; Pfeifer, U.; Wilhelm, E. Cultivation-Independent Population Analysis of Bacterial Endophytes in Three Potato Varieties Based on Eubacterial and Actinomycetes-Specific PCR of 16S RRNA Genes. FEMS Microbiol. Ecol. 2002, 39, 23–32. [Google Scholar] [CrossRef]
- Berg, G.; Krechel, A.; Ditz, M.; Sikora, R.A.; Ulrich, A.; Hallmann, J. Endophytic and Ectophytic Potato-Associated Bacterial Communities Differ in Structure and Antagonistic Function against Plant Pathogenic Fungi. FEMS Microbiol. Ecol. 2005, 51, 215–229. [Google Scholar] [CrossRef]
- Okunishi, S.; Sako, K.; Mano, H.; Imamura, A.; Morisaki, H. Bacterial Flora of Endophytes in the Maturing Seed of Cultivated Rice (Oryza sativa). Microbes Environ. 2005, 20, 168–177. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant Growth-Promoting Bacterial Endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed]
- Del Carmen Orozco-Mosqueda, M.; Duan, J.; DiBernardo, M.; Zetter, E.; Campos-García, J.; Glick, B.R.; Santoyo, G. The Production of ACC Deaminase and Trehalose by the Plant Growth Promoting Bacterium pseudomonas sp. UW4 Synergistically Protect Tomato Plants against Salt Stress. Front. Microbiol. 2019, 10, 458514. [Google Scholar]
- Montejano-Ramírez, V.; García-Pineda, E.; Valencia-Cantero, E. Bacterial Compound N,N-Dimethylhexadecylamine Modulates Expression of Iron Deficiency and Defense Response Genes in Medicago Truncatula Independently of the Jasmonic Acid Pathway. Plants 2020, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- González, V.; Santamaría, R.I.; Bustos, P.; Pérez-Carrascal, O.M.; Vinuesa, P.; Juárez, S.; Martínez-Flores, I.; Cevallos, M.Á.; Brom, S.; Martínez-Romero, E.; et al. Phylogenomic Rhizobium Species Are Structured by a Continuum of Diversity and Genomic Clusters. Front. Microbiol. 2019, 10, 441237. [Google Scholar] [CrossRef]
- Poole, P.; Ramachandran, V.; Terpolilli, J. Rhizobia: From Saprophytes to Endosymbionts. Nat. Rev. Microbiol. 2018, 16, 291–303. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C. The Endophytic Continuum. Mycol. Res. 2005, 109, 661–686. [Google Scholar] [CrossRef]






| Phylum | Class | Order | Family | Genus | OTU | R. Abundance, % |
|---|---|---|---|---|---|---|
| Actinobacteria | Hermoleophilia | Gaiellales | N/A | N/A | N/A | 1.20 |
| Bacteroidetes | Bacteroidia | Bacteroidales | F082 | N/A | N/A | 2.82 |
| Bacteroidetes | Bacteroidia | Bacteroidales | Muribaculaceae | N/A | N/A | 6.00 |
| Chlamydiae | Chlamydiae | Chlamydiales | Simkaniaceae | N/A | N/A | 4.62 |
| Firmicutes | Bacilli | Bacillales | Bacillaceae | Bacillus | N/A | 3.90 |
| Firmicutes | Bacilli | Lactobacillales | Lactobacillaceae | Lactobacillus | N/A | 1.92 |
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Butyrivibrio | N/A | 1.20 |
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Lachnospiraceae NK4A136 group | N/A | 2.46 |
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | Ruminococcus | N/A | 2.10 |
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | [Eubacterium] coprostanoligenes group | N/A | 3.24 |
| Firmicutes | Erysipelotrichia | Erysipelotrichales | Erysipelotrichaceae | Turicibacter | N/A | 4.20 |
| Planctomycetes | Phycisphaerae | Tepidisphaerales | WD2101 soil group | N/A | 0.96 | |
| Verrucomicrobia | Verrucomicrobiae | Verrucomicrobiales | Akkermansiaceae | Akkermansia | N/A | 30.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuryntseva, P.; Pronovich, N.; Galieva, G.; Galitskaya, P.; Selivanovskaya, S. Exploring the Role of Vertical and Horizontal Pathways in the Formation of Lettuce Plant Endospheric Bacterial Communities: A Comparative Study of Hydroponic and Soil Systems. Horticulturae 2025, 11, 762. https://doi.org/10.3390/horticulturae11070762
Kuryntseva P, Pronovich N, Galieva G, Galitskaya P, Selivanovskaya S. Exploring the Role of Vertical and Horizontal Pathways in the Formation of Lettuce Plant Endospheric Bacterial Communities: A Comparative Study of Hydroponic and Soil Systems. Horticulturae. 2025; 11(7):762. https://doi.org/10.3390/horticulturae11070762
Chicago/Turabian StyleKuryntseva, Polina, Nataliya Pronovich, Gulnaz Galieva, Polina Galitskaya, and Svetlana Selivanovskaya. 2025. "Exploring the Role of Vertical and Horizontal Pathways in the Formation of Lettuce Plant Endospheric Bacterial Communities: A Comparative Study of Hydroponic and Soil Systems" Horticulturae 11, no. 7: 762. https://doi.org/10.3390/horticulturae11070762
APA StyleKuryntseva, P., Pronovich, N., Galieva, G., Galitskaya, P., & Selivanovskaya, S. (2025). Exploring the Role of Vertical and Horizontal Pathways in the Formation of Lettuce Plant Endospheric Bacterial Communities: A Comparative Study of Hydroponic and Soil Systems. Horticulturae, 11(7), 762. https://doi.org/10.3390/horticulturae11070762







