Non-Destructive Detection of Current Internal Disorders and Prediction of Future Appearance in Mango Fruit Using Portable Vis-NIR Spectroscopy
Abstract
1. Introduction
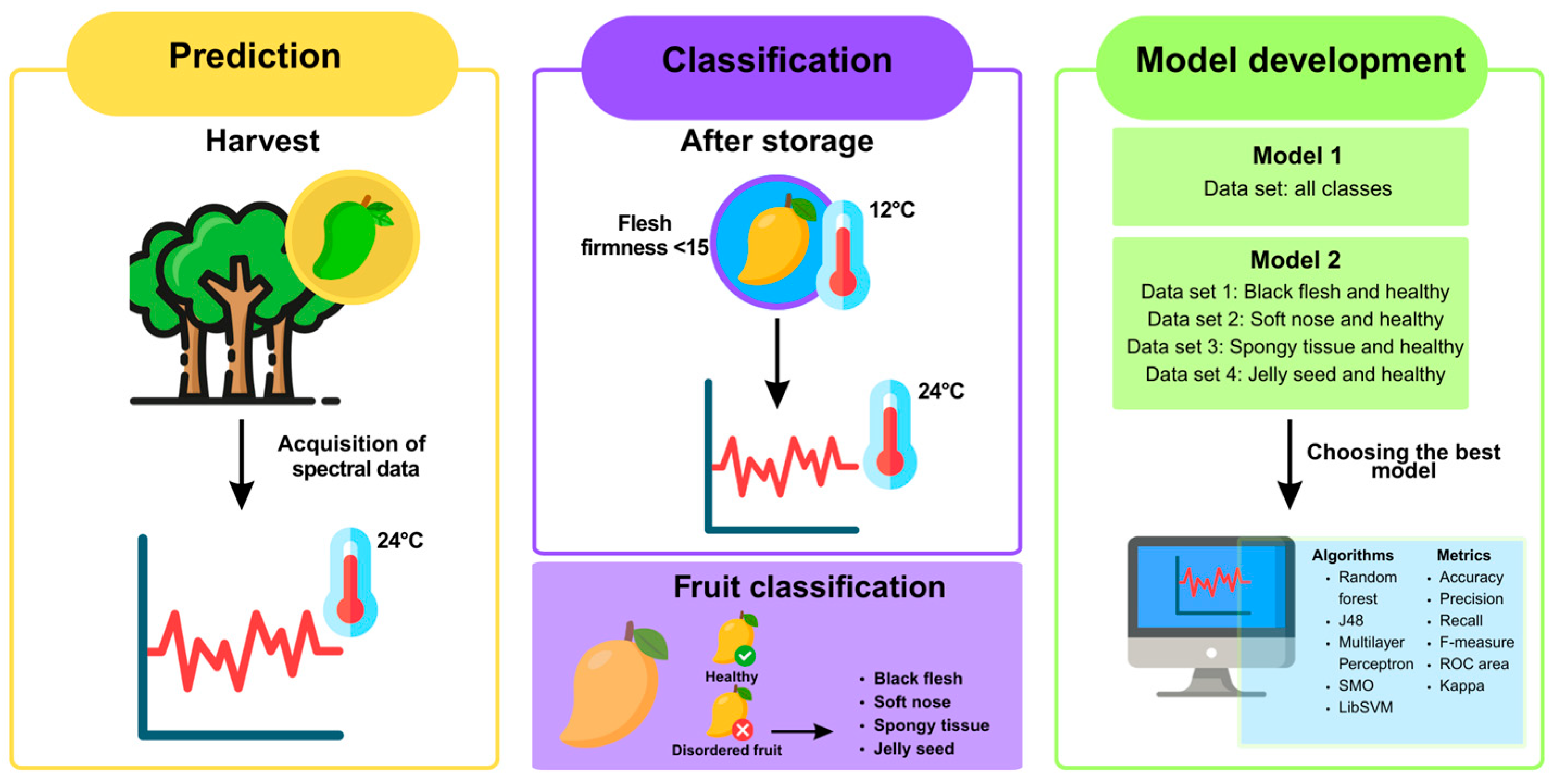
2. Materials and Methods
2.1. Fruit and Disorder Assessment
2.2. Vis-NIR Spectra Acquisition
2.3. Incidence of Disorders
2.4. Model Development
3. Results and Discussion
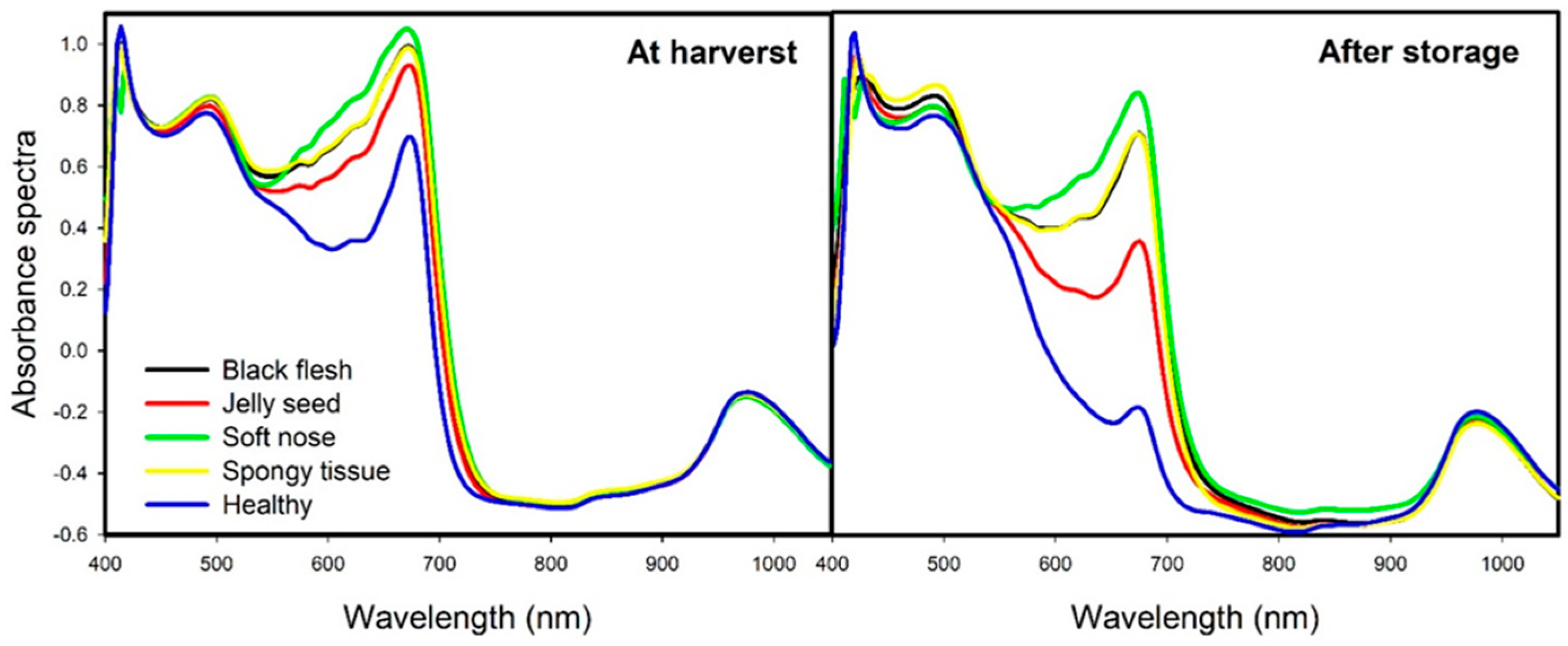
3.1. Interpretation of Spectra
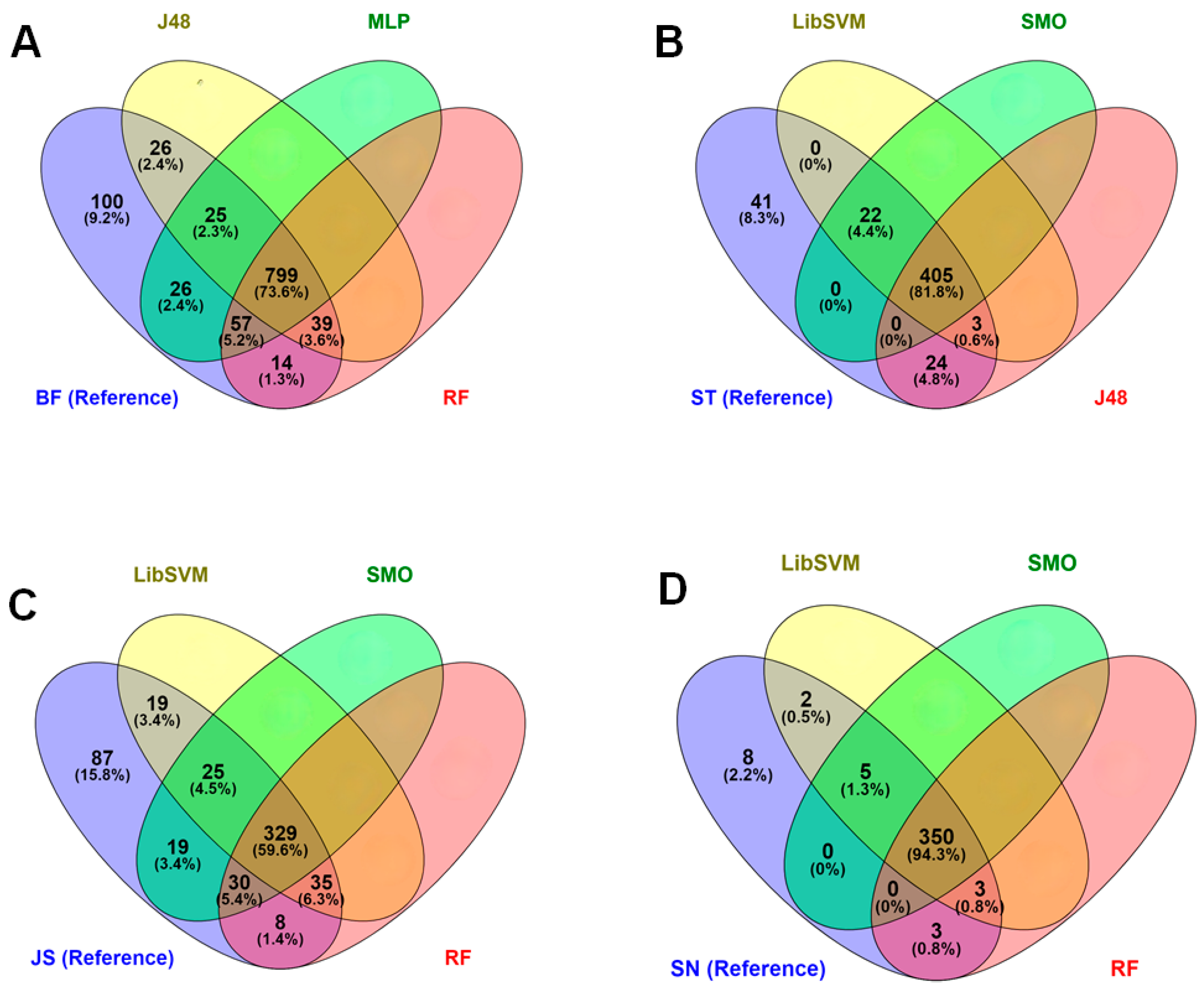
3.2. Discrimination Using At-Harvest Spectra
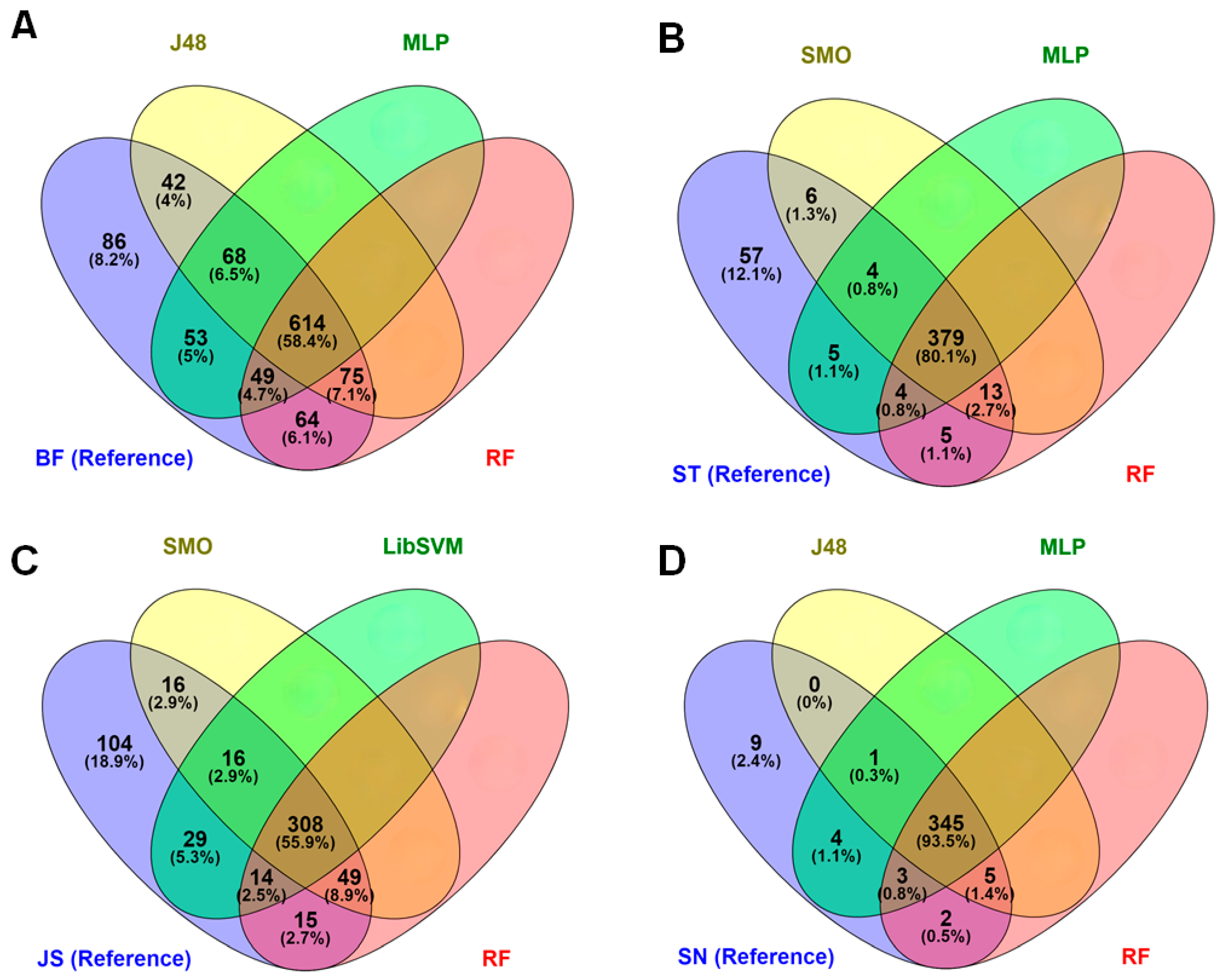
3.3. Discrimination Using After-Storage Spectra
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kiloes, A.M.; Azizan, F.A.; Checco, J.; Joyce, D.; Abdul Aziz, A. What do consumers want in fresh mangoes? A systematic literature review. Int. J. Food Sci. Technol. 2022, 57, 1473–1492. [Google Scholar] [CrossRef]
- Kist, B.B.; Beling, R.R. Brazilian Horti & Fruit Yearbook. Editora Gazeta Santa Cruz, 2023. Available online: https://editoragazeta.com.br/wp-content/uploads/2024/04/HF_2024_DUPLAS.pdf (accessed on 2 January 2025).
- Shivashankar, S. Physiological disorders of mango fruit. In Horticultural Reviews; Janick, J., Ed.; Wiley Online Library: Hoboken, NJ, USA, 2014; pp. 313–348. [Google Scholar] [CrossRef]
- Brecht, J.K. Mango. In Posharvest Physiological Disorders in Fruit and Vegetables; Freitas, S.T., Pareek, S., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 443–466. [Google Scholar] [CrossRef]
- Ullah, M.A.; Khanal, A.; Joyce, P.; White, N.; Macnish, A.; Joyce, D. Internal Disorders of Mango Fruit and Their Management—Physiology, Biochemistry, and Role of Mineral Nutrients. Plants 2024, 13, 2596. [Google Scholar] [CrossRef]
- USDA. Shipping Point and Market Inspection Instructions; United States Department of Agriculture: Washington, DC, USA, 2006. Available online: https://www.ams.usda.gov/sites/default/files/media/Mango_Inspection_Instructions%5B1%5D.pdf (accessed on 19 June 2025).
- Walsh, K.B.; McGlone, V.A.; Han, D.H. The uses of near infra-red spectroscopy in postharvest decision support: A review. Postharvest Biol. Technol. 2020, 163, 111139. [Google Scholar] [CrossRef]
- Clark, C.J.; McGlone, V.A.; Silva, H.N.; Manning, M.A.; Burdon, J.; Mowat, A.D. Prediction of storage disorders of kiwifruit (Actinidia chinensis) based on visible-NIR spectral characteristics at harvest. Postharvest Biol. Technol. 2004, 32, 147–158. [Google Scholar] [CrossRef]
- Kasampalis, D.S.; Tsouvaltzis, P.; Siomos, A.S. Assessment of Melon Fruit Nutritional Composition Using VIS/NIR/SWIR Spectroscopy Coupled with Chemometrics. Preprints 2025, 11, 658. [Google Scholar] [CrossRef]
- Tan, H.; Dong, Y.; Jiang, L.; Fan, W.; Du, G.; Li, P. Simultaneous and non-destructive prediction of multiple internal quality characteristics in mandarin citrus with near-infrared spectroscopy and ensemble learning strategy. J. Food Compos. Anal. 2025, 137, 106961. [Google Scholar] [CrossRef]
- Walsh, J.; Neupane, A.; Koirala, A.; Li, M.; Anderson, N. The evolution of chemometrics coupled with near infrared spectroscopy for fruit quality evaluation. II. The rise of convolutional neural networks. J. Near Infrared Spectrosc. 2023, 31, 109–125. [Google Scholar] [CrossRef]
- Pokhrel, D.R.; Sirisomboon, P.; Khurnpoon, L.; Posom, J.; Saechua, W. Comparing Machine Learning and PLSDA Algorithms for Durian Pulp Classification Using Inline NIR Spectra. Sensors 2023, 23, 5327. [Google Scholar] [CrossRef]
- Tuerxun, A.; Shariff, A.R.M.; Janius, R.; Abbas, Z.; Mahdiraji, G.A. Oil palm fresh fruit bunches maturity prediction by using an optical spectrometer. IOP Conf. Ser. Earth Environ. Sci. 2020, 540, 012085. [Google Scholar] [CrossRef]
- Rivera, N.V.; Gómez-Sanchis, J.; Chanona-Pérez, J.; Carrasco, J.J.; Millán-Giraldo, M.; Lorente, D.; Cubero, S.; Blasco, J. Early detection of mechanical damage in mango using NIR hyperspectral images and machine learning. Biosyst. Eng. 2014, 122, 91–98. [Google Scholar] [CrossRef]
- Kiran, P.R.; Jadhav, P.; Avinash, G.; Aradwad, P.; TV, A.; Bhardwaj, R.; Parray, R.A. Detection and classification of spongy tissue disorder in mango fruit during ripening by using visible-near infrared spectroscopy and multivariate analysis. J. Near Infrared Spectrosc. 2024, 32, 140–151. [Google Scholar] [CrossRef]
- Khanal, A.; Ullah, M.A.; Joyce, P.; White, N.; Macnish, A.; Hoffman, E.; Irving, D.; Webb, R.; Joyce, D. Fruit Sorting Based on Maturity Reduces Internal Disorders in Vapor Heat-Treated ‘B74’Mango. Horticulturae 2024, 10, 1257. [Google Scholar] [CrossRef]
- Seehanam, P.; Chaiya, P.; Theanjumpol, P.; Tiyayon, C.; Ruangwong, O.; Pankasemsuk, T.; Maniwara, P. Internal disorder evaluation of ‘Namdokmai Sithong’ mango by near infrared spectroscopy. Hortic. Environ. Biotechnol. 2022, 63, 665–675. [Google Scholar] [CrossRef]
- Mogollón, R.; Contreras, C.; Silva-Neta, M.L.; Marques, E.J.N.; Zoffoli, J.P.; Freitas, S.T. Non-destructive prediction and detection of internal physiological disorders in ‘Keitt’ mango using a hand-held Vis-NIR spectrometer. Postharvest Biol. Technol. 2020, 167, 111251. [Google Scholar] [CrossRef]
- National Mango Board. Mango Maturity and Ripeness Guide, 2024. Available online: http://mango.org/ (accessed on 5 March 2024).
- Bouckaert, R.R.; Frank, E.; Hall, M.; Kirkby, R.; Reutemann, P.; Seewald, A.; Scuse, D. WEKA Manual (Version 3-9-1); University of Waikato: Hamilton, New Zealand, 2016. [Google Scholar]
- Lanjewar, M.G.; Asolkar, S.; Parab, J.S.; Morajkar, P.P. Detecting starch-adulterated turmeric using Vis-NIR spectroscopy and multispectral imaging with machine learning. J. Food Compos. Anal. 2024, 136, 106700. [Google Scholar] [CrossRef]
- Shiddiq, M.; Candra, F.; Anand, B.; Rabin, M.F. Neural network with k-fold cross validation for oil palm fruit ripeness prediction. TELKOMNIKA (Telecommun. Comput. Electron. Control) 2024, 22, 164–174. [Google Scholar] [CrossRef]
- Sohn, S.I.; Oh, Y.J.; Pandian, S.; Lee, Y.H.; Zaukuu, J.L.Z.; Kang, H.J.; Shin, E.K. Identification of Amaranthus species using visible-near-infrared (vis-NIR) spectroscopy and machine learning methods. Remote Sens. 2021, 13, 4149. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Quinlan, J.R. C4. 5: Programs for Machine Learning; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Chang, C.; Lin, C. LIBSVM: A library for support vector machines. ACM Trans. Intell. Syst. Technol. (TIST) 2011, 2, 1–27. [Google Scholar] [CrossRef]
- Platt, J.C. Sequential Minimal Optimization: A Fast Algorithm for Training Support Vector Machines; Microsoft Research Technical Report; Microsoft: Redmond, WA, USA, 1998. [Google Scholar]
- Rumelhart, D.E.; Hinton, G.E.; Williams, R.J. Learning representations by back-propagating errors. Nature 1986, 323, 533–536. [Google Scholar] [CrossRef]
- Oliveros, J.C.; Venny. An Interactive Tool for Comparing Lists with Venn’s Diagrams. 2007–2005. Available online: https://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 19 June 2025).
- Hossin, M.; Sulaiman, M.N. A review on evaluation metrics for data classification evaluations. Int. J. Data Min. Knowl. Manag. Process 2015, 5, 1. [Google Scholar] [CrossRef]
- Vujović, Ž.D. Classification model evaluation metrics. Int. J. Adv. Comput. Sci. Appl. 2021, 12, 599–606. [Google Scholar] [CrossRef]
- Han, J.; Li, T.; He, Y.; Yang, Z. Predicting the effect of chemicals on fruit using graph neural networks. Sci. Rep. 2024, 14, 8203. [Google Scholar] [CrossRef]
- Li, M.; Li, Z.; Yu, T. Statistical issues. Comment on “proximal sensor data fusion for tropical soil property prediction: Soil fertility properties”. J. South Am. Earth Sci. 2023, 124, 104282. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Q.; Zhang, G. Rapid determination of leaf water content using VIS/NIR spectroscopy analysis with wavelength selection. J. Spectrosc. 2012, 27, 93–105. [Google Scholar] [CrossRef]
- Zur, Y.; Gitelson, A.A.; Chivkunova, O.B.; Merzlyak, M.N. The spectral contribution of carotenoids to light absorption and reflectance in green leaves. In Proceedings of the 2nd International Conference Geospatial Information in Agriculture and Forestry, Lake Buena Vista, FL, USA, 10–12 January 2000; Volume 2, pp. 10–12. [Google Scholar]
- Vanoli, M.; Rizzolo, A.; Lovati, F.; Spinelli, L.; Levoni, P.; Torricelli, A.; Cortellino, G. Non-Destructive Prediction of Carotenoids, Ascorbic Acid, and Total Phenols Contents in ‘Tommy Atkins’ Mangoes Using Absorption and Scattering Properties Measured by Time-Resolved Reflectance Spectroscopy. Agriculture 2024, 14, 1902. [Google Scholar] [CrossRef]
- Lum, G.B.; Shelp, B.J.; Deell, J.R.; Bozzo, G.G. Oxidative metabolism is associated with physiological disorders in fruits stored under multiple environmental stresses. Plant Sci. 2016, 245, 143–152. [Google Scholar] [CrossRef]
- Tridge. Brazil Mango Export Prices. Available online: https://www.tridge.com/intelligences/mango/BR/price (accessed on 10 June 2025).
- Raghavendra, A.; Guru, D.S.; Rao, M.K. Mango internal defect detection based on optimal wavelength selection method using NIR spectroscopy. Artif. Intell. Agric. 2021, 5, 43–51. [Google Scholar] [CrossRef]
- Guru, D.S.; Nandini, D. Mango Internal Defect Detection using NIR Spectroscopy Data. Procedia Comput. Sci. 2025, 258, 947–960. [Google Scholar] [CrossRef]

| Class | Number of Spectra | |||||||
|---|---|---|---|---|---|---|---|---|
| At Harvest | After Storage | |||||||
| Palmer | Keitt | Tommy Atkins | Total | Palmer | Keitt | Tommy Atkins | Total | |
| Healthy | 106 | 5 | 239 | 350 | 106 | 5 | 239 | 349 |
| Black flesh | 25 | 483 | 228 | 736 | 25 | 450 | 227 | 702 |
| Spongy tissue | 43 | 85 | 17 | 145 | 43 | 64 | 17 | 124 |
| Jelly seed | 25 | 62 | 115 | 202 | 25 | 62 | 115 | 202 |
| Soft nose | 4 | 15 | 2 | 21 | 4 | 14 | 2 | 20 |
| Total | 203 | 650 | 601 | 1454 | 203 | 595 | 600 | 1397 |
| Data Set | Algorithms | Accuracy (%) | Precision | Recall | F-Measure | ROC Area (%) | Kappa Statistic |
|---|---|---|---|---|---|---|---|
| All class (n = 1454) | M. P. | 51.2 | 0.49 | 0.51 | 0.50 | 0.68 | 0.24 |
| R. Forest | 62.5 | * | 0.62 | * | 0.76 | 0.55 | |
| LibSVM | 62.2 | 0.51 | 0.62 | 0.55 | 0.66 | 0.36 | |
| SMO | 62.0 | 0.50 | 0.62 | 0.54 | 0.71 | 0.35 | |
| J48 | 50.3 | 0.50 | 0.50 | 0.50 | 0.64 | 0.24 | |
| Black flesh and healthy (n = 1086) | M. P. | 83.6 | 0.84 | 0.84 | 0.84 | 0.88 | 0.63 |
| R. Forest | 83.6 | 0.83 | 0.84 | 0.84 | 0.91 | 0.62 | |
| LibSVM | 82.0 | 0.82 | 0.82 | 0.82 | 0.80 | 0.59 | |
| SMO | 82.0 | 0.82 | 0.82 | 0.82 | 0.80 | 0.59 | |
| J48 | 82.0 | 0.82 | 0.82 | 0.82 | 0.87 | 0.59 | |
| Spongy tissue and healthy (n = 495) | M. P. | 81.6 | 0.81 | 0.82 | 0.81 | 0.78 | 0.53 |
| R. Forest | 85.3 | 0.85 | 0.85 | 0.85 | 0.88 | 0.62 | |
| LibSVM | 86.9 | 0.88 | 0.87 | 0.86 | 0.79 | 0.64 | |
| SMO | 86.3 | 0.87 | 0.86 | 0.85 | 0.78 | 0.63 | |
| J48 | 87.3 | 0.87 | 0.87 | 0.87 | 0.85 | 0.69 | |
| Jelly seed and healthy (n = 552) | M. P. | 72.5 | 0.72 | 0.73 | 0.72 | 0.74 | 0.40 |
| R. Forest | 73.2 | 0.73 | 0.73 | 0.72 | 0.75 | 0.38 | |
| LibSVM | 73.7 | 0.79 | 0.74 | 0.69 | 0.65 | 0.34 | |
| SMO | 72.3 | 0.72 | 0.72 | 0.72 | 0.69 | 0.39 | |
| J48 | 72.5 | 0.72 | 0.73 | 0.72 | 0.72 | 0.40 | |
| Soft nose and healthy (n = 371) | M. P. | 94.6 | 0.95 | 0.95 | 0.95 | 0.85 | 0.54 |
| R. Forest | 96.0 | 0.95 | 0.96 | 0.95 | 0.89 | 0.45 | |
| LibSVM | 97.0 | 0.97 | 0.97 | 0.97 | 0.85 | 0.72 | |
| SMO | 96.0 | 0.96 | 0.96 | 0.96 | 0.80 | 0.61 | |
| J48 | 96.0 | 0.95 | 0.96 | 0.95 | 0.66 | 0.53 |
| Classified as | At harvest (LibSVM) | Class | ||||
|---|---|---|---|---|---|---|
| a | b | c | d | e | ||
| a | 636 | 1 | 0 | 15 | 84 | a = black flesh |
| b | 91 | 0 | 0 | 1 | 53 | b = spongy tissue |
| c | 16 | 0 | 0 | 0 | 5 | c = soft nose |
| d | 132 | 1 | 1 | 9 | 59 | d = jelly seed |
| e | 81 | 0 | 1 | 8 | 260 | e = healthy |
| Data Set | Classified as | At Harvest | Class | |
|---|---|---|---|---|
| a | b | |||
| Black flesh | a | 656 | 80 | a = black flesh |
| b | 98 | 252 | b = healthy | |
| Spongy tissue | a | 109 | 36 | a = spongy tissue |
| b | 27 | 323 | b = healthy | |
| Jelly seed | a | 62 | 140 | a = jelly seed |
| b | 5 | 345 | b = healthy | |
| Soft nose | a | 7 | 14 | a = soft nose |
| b | 2 | 348 | b = healthy | |
| Data set | Algorithms | Accuracy (%) | Precision | Recall | F-measure | ROC Area (%) | Kappa statistic |
|---|---|---|---|---|---|---|---|
| All class (n = 1397) | M. P. | 44.6 | 0.49 | 0.45 | 0.46 | 0.66 | 0.19 |
| R. Forest | 57.8 | * | 0.58 | * | 0.76 | 0.28 | |
| LibSVM | 54.1 | 0.42 | 0.54 | 0.47 | 0.62 | 0.23 | |
| SMO | 54.2 | 0.42 | 0.54 | 0.47 | 0.66 | 0.23 | |
| J48 | 55.9 | 0.44 | 0.44 | 0.44 | 0.60 | 0.14 | |
| Black flesh and healthy (n = 1051) | M. P. | 74.6 | 0.81 | 0.75 | 0.75 | 0.82 | 0.49 |
| R. Forest | 77.0 | 0.77 | 0.77 | 0.77 | 0.86 | 0.47 | |
| LibSVM | 75.0 | 0.76 | 0.75 | 0.70 | 0.63 | 0.32 | |
| SMO | 73.2 | 0.74 | 0.73 | 0.73 | 0.71 | 0.41 | |
| J48 | 76.0 | 0.76 | 0.76 | 0.76 | 0.77 | 0.46 | |
| Spongy tissue and healthy (n = 473) | M. P. | 82.9 | 0.82 | 0.83 | 0.82 | 0.78 | 0.52 |
| R. Forest | 84.8 | 0.85 | 0.85 | 0.83 | 0.83 | 0.55 | |
| LibSVM | 77.2 | 0.75 | 0.77 | 0.75 | 0.63 | 0.31 | |
| SMO | 85.0 | 0.86 | 0.85 | 0.84 | 0.74 | 0.55 | |
| J48 | 81.0 | 0.80 | 0.81 | 0.80 | 0.79 | 0.48 | |
| Jelly seed and healthy (n = 551) | M. P. | 65.9 | 0.65 | 0.66 | 0.65 | 0.65 | 0.23 |
| R. Forest | 70.1 | 0.70 | 0.70 | 0.66 | 0.65 | 0.27 | |
| LibSVM | 66.6 | 0.66 | 0.65 | 0.66 | 0.61 | 0.24 | |
| SMO | 70.6 | 0.70 | 0.71 | 0.70 | 0.68 | 0.30 | |
| J48 | 63.7 | 0.62 | 0.64 | 0.63 | 0.61 | 0.18 | |
| Soft nose and healthy (n = 369) | M. P. | 95.4 | 0.96 | 0.95 | 0.95 | 0.87 | 0.56 |
| R. Forest | 96.2 | 0.96 | 0.96 | 0.95 | 0.85 | 0.45 | |
| LibSVM | 94.0 | 0.92 | 0.94 | 0.93 | 0.57 | 0.18 | |
| SMO | 94.6 | 0.94 | 0.95 | 0.94 | 0.69 | 0.42 | |
| J48 | 95.2 | 0.94 | 0.95 | 0.96 | 0.70 | 0.41 |
| Classified as | After storage (SMO) | Class | ||||
|---|---|---|---|---|---|---|
| a | b | c | d | e | ||
| a | 545 | 1 | 6 | 1 | 149 | a = black flesh |
| b | 67 | 0 | 0 | 1 | 56 | b = spongy tissue |
| c | 112 | 0 | 0 | 0 | 90 | c = soft nose |
| d | 16 | 0 | 0 | 0 | 4 | d = jelly seed |
| e | 136 | 0 | 1 | 0 | 212 | e = healthy |
| Data Set | Classified as | After Storage | Class | |
|---|---|---|---|---|
| a | b | |||
| Black flesh | a | 588 | 114 | a = black flesh |
| b | 135 | 214 | b = healthy | |
| Spongy tissue | a | 62 | 62 | a = spongy tissue |
| b | 9 | 340 | b = healthy | |
| Jelly seed | a | 73 | 129 | a = jelly seed |
| b | 33 | 316 | b = healthy | |
| Soft nose | a | 7 | 13 | a = soft nose |
| b | 1 | 348 | b = healthy | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva Alves, J.; Parente de Carvalho Pires, B.; Ferreira dos Santos, L.; da Silva Ribeiro, T.; Walsh, K.B.; Akio Kido, E.; Tonetto de Freitas, S. Non-Destructive Detection of Current Internal Disorders and Prediction of Future Appearance in Mango Fruit Using Portable Vis-NIR Spectroscopy. Horticulturae 2025, 11, 759. https://doi.org/10.3390/horticulturae11070759
da Silva Alves J, Parente de Carvalho Pires B, Ferreira dos Santos L, da Silva Ribeiro T, Walsh KB, Akio Kido E, Tonetto de Freitas S. Non-Destructive Detection of Current Internal Disorders and Prediction of Future Appearance in Mango Fruit Using Portable Vis-NIR Spectroscopy. Horticulturae. 2025; 11(7):759. https://doi.org/10.3390/horticulturae11070759
Chicago/Turabian Styleda Silva Alves, Jasciane, Bruna Parente de Carvalho Pires, Luana Ferreira dos Santos, Tiffany da Silva Ribeiro, Kerry Brian Walsh, Ederson Akio Kido, and Sergio Tonetto de Freitas. 2025. "Non-Destructive Detection of Current Internal Disorders and Prediction of Future Appearance in Mango Fruit Using Portable Vis-NIR Spectroscopy" Horticulturae 11, no. 7: 759. https://doi.org/10.3390/horticulturae11070759
APA Styleda Silva Alves, J., Parente de Carvalho Pires, B., Ferreira dos Santos, L., da Silva Ribeiro, T., Walsh, K. B., Akio Kido, E., & Tonetto de Freitas, S. (2025). Non-Destructive Detection of Current Internal Disorders and Prediction of Future Appearance in Mango Fruit Using Portable Vis-NIR Spectroscopy. Horticulturae, 11(7), 759. https://doi.org/10.3390/horticulturae11070759







