Whole Genome of Gordonia aichiensis P6PL2 Associated with Vitis amurensis That Stimulates Plant Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain
2.2. Genomic DNA Isolation, Sequencing, Assembly and Annotation
2.3. Genome-Based Taxonomic Analysis
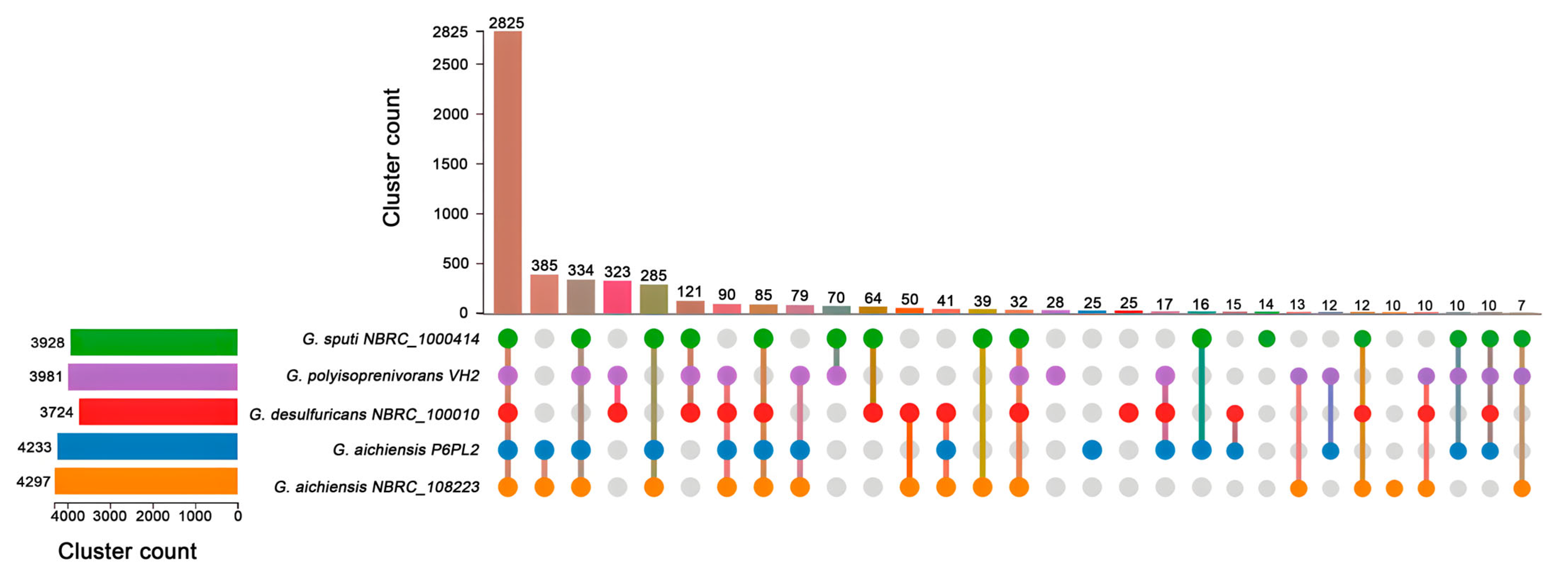
2.4. Comparative Genomics
2.5. Signs of PGPB In Vitro
2.6. Seed Inoculation, Evaluation of Biometric Parameters
3. Results and Discussion
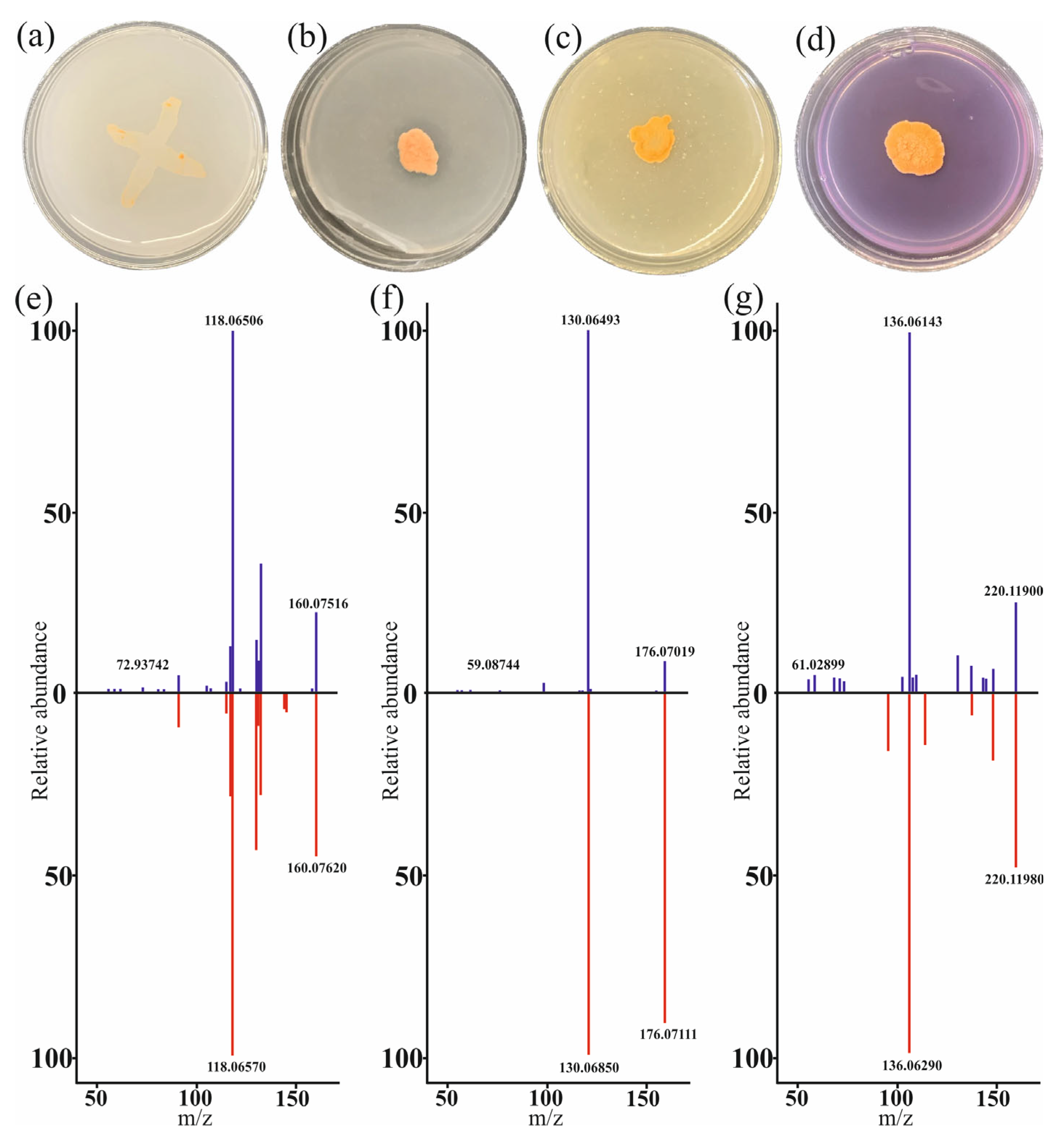
3.1. Phenotypic Manifestation of PGPB Properties
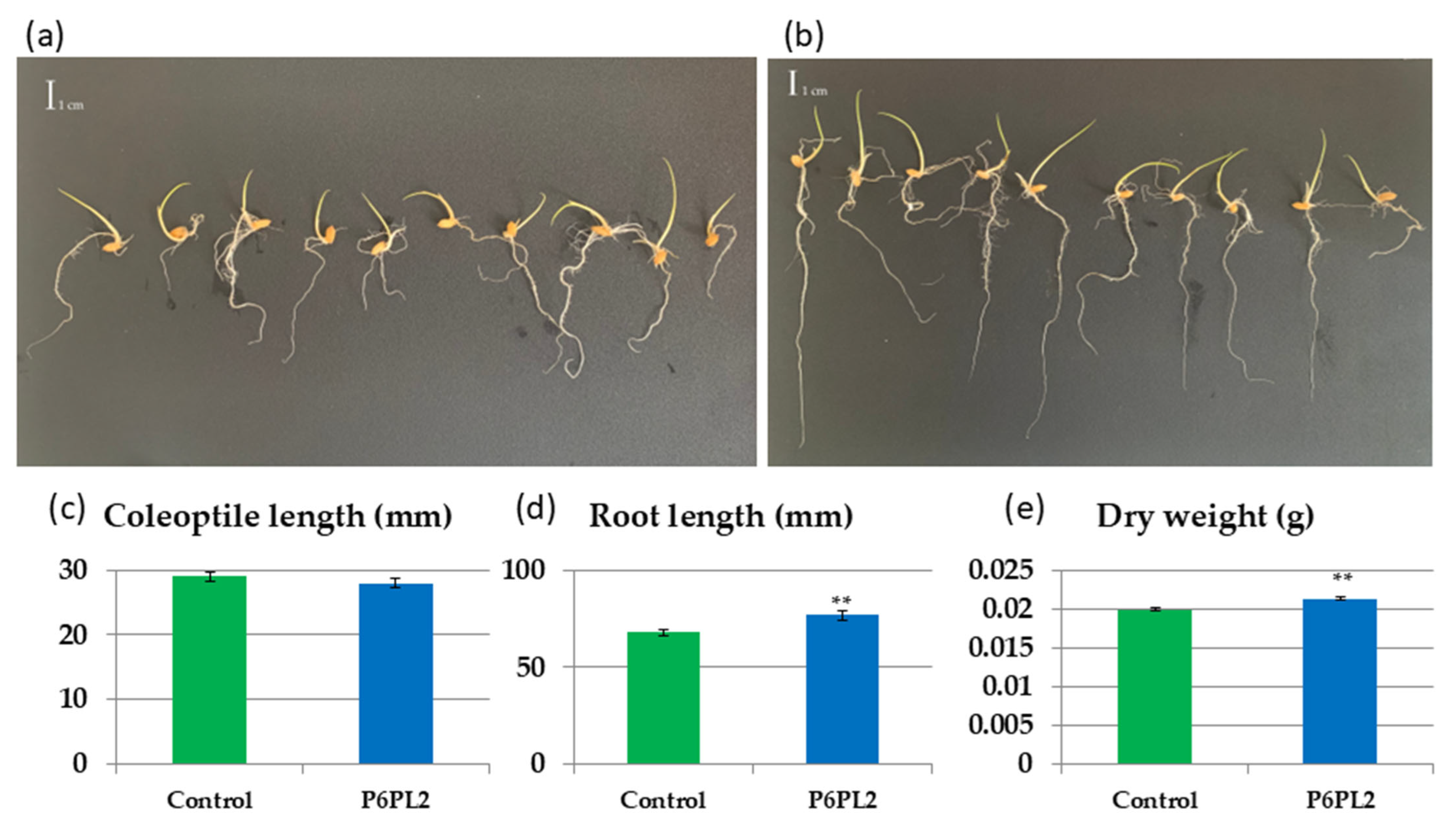
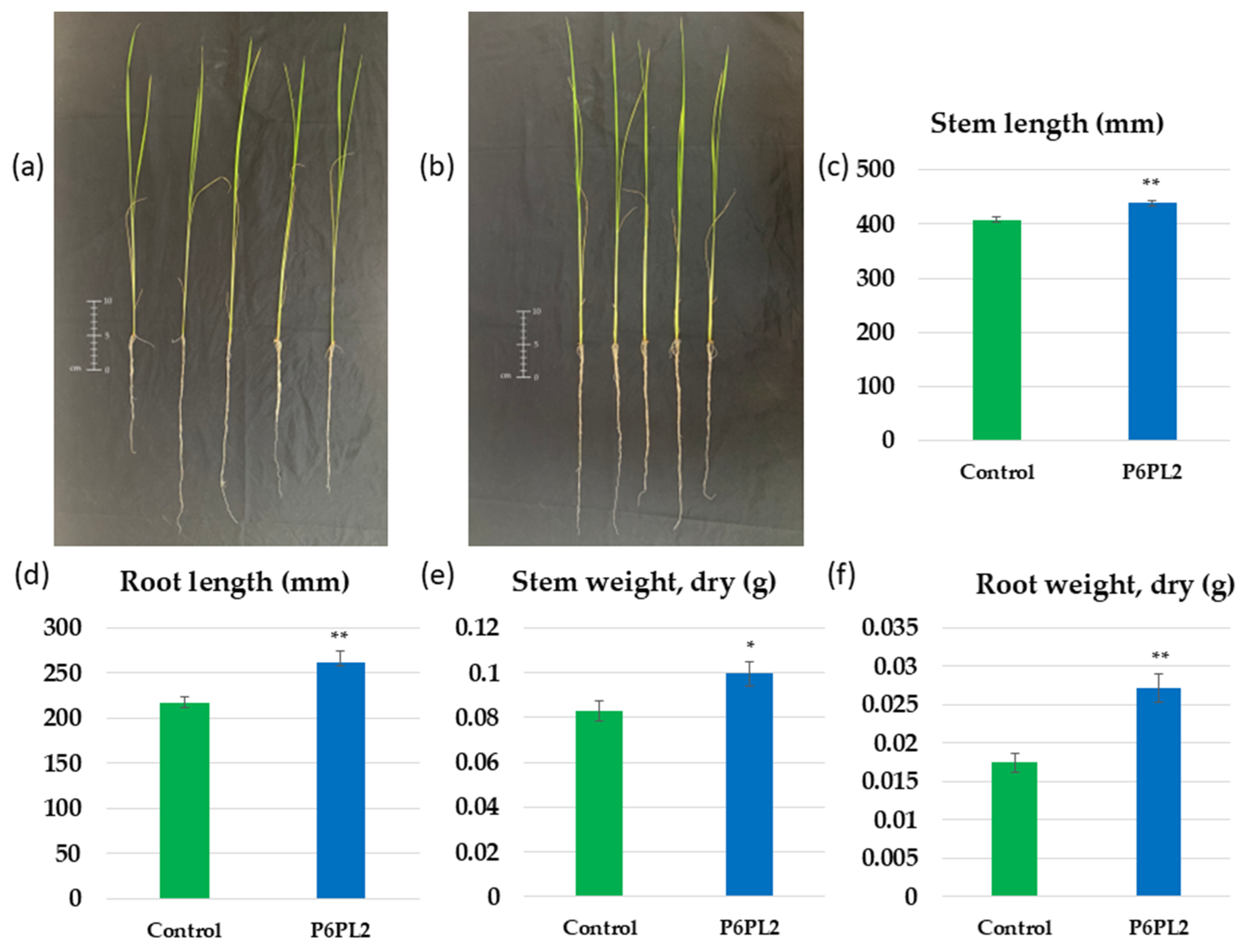
3.2. Effect of Gordonia aichiensis P6PL2 Inoculation on the Growth of Oryza sativa
3.3. Phylogenetic Identification
3.4. Genomic Features and Comparison of Genetic Characteristics
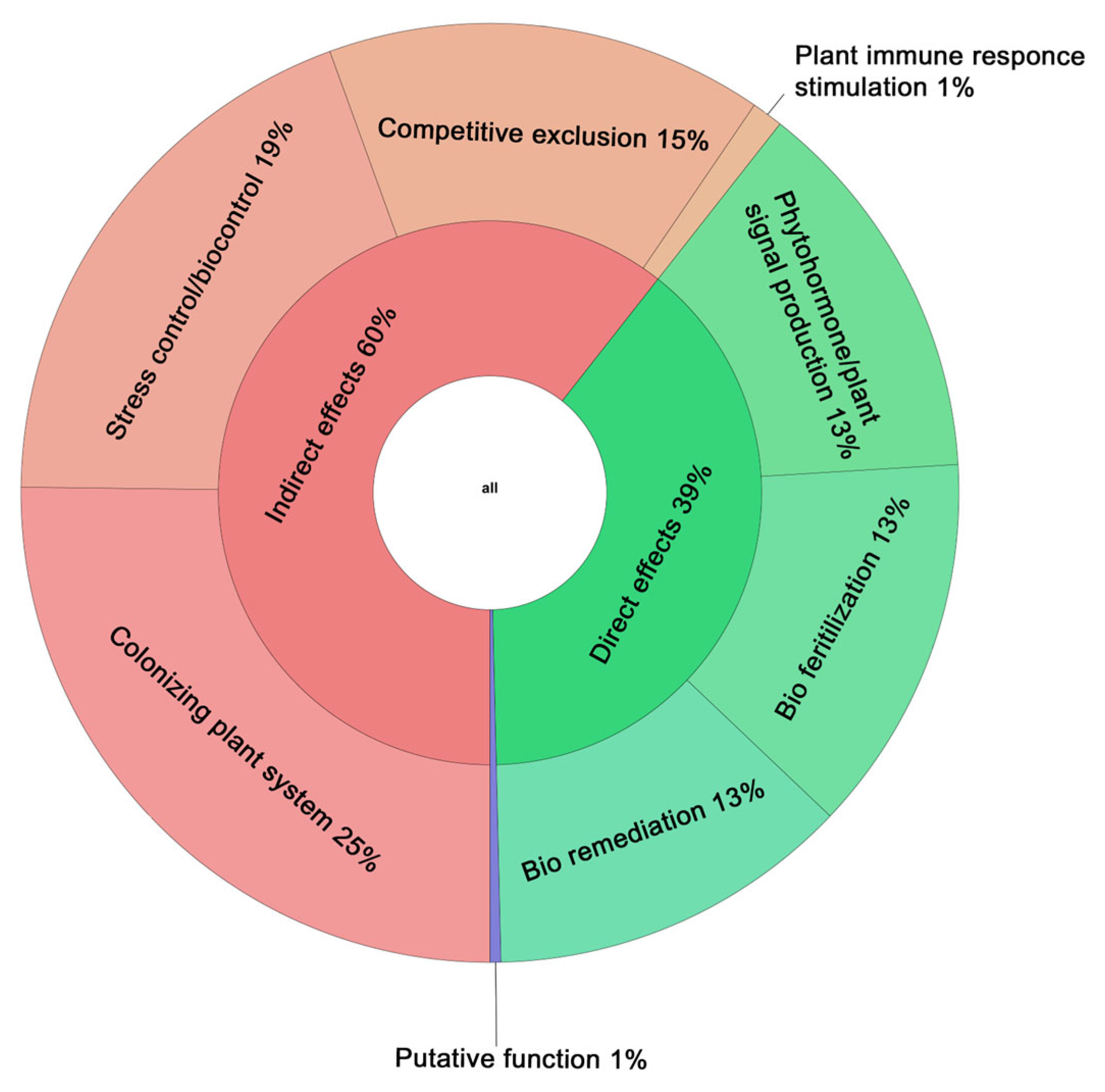
3.5. Genetic Elements of Gordonia aichiensis P6PL2 Responsible for Plant–Bacterial Interactions
3.5.1. Production of Phytohormones and Other Growth Stimulants

3.5.2. Biofertilization
3.5.3. Bioremediation
3.5.4. Resistance to Biotic and Abiotic Stresses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rashmi, I.; Roy, T.; Kartika, K.S.; Pal, R.; Coumar, V.; Kala, S.; Shinoji, K.C. Organic and Inorganic Fertilizer Contaminants in Agriculture: Impact on Soil and Water Resources. In Contaminants in Agriculture: Sources, Impacts and Management; Naeem, M., Ansari, A.A., Gill, S.S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 3–41. ISBN 978-3-030-41552-5. [Google Scholar]
- Gnanaprakasam, P.D.; Vanisree, A.J. Recurring Detrimental Impact of Agrochemicals on the Ecosystem, and a Glimpse of Organic Farming as a Possible Rescue. Environ. Sci. Pollut. Res. 2022, 29, 75103–75112. [Google Scholar] [CrossRef] [PubMed]
- Molotoks, A.; Stehfest, E.; Doelman, J.; Albanito, F.; Fitton, N.; Dawson, T.P.; Smith, P. Global Projections of Future Cropland Expansion to 2050 and Direct Impacts on Biodiversity and Carbon Storage. Glob. Change Biol. 2018, 24, 5895–5908. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, M.F.; Tan, B.C. Genetic Modification Techniques in Plant Breeding: A Comparative Review of CRISPR/Cas and GM Technologies. Hortic. Plant J. 2024; in press. [Google Scholar] [CrossRef]
- Abdul Aziz, M.; Masmoudi, K. Molecular Breakthroughs in Modern Plant Breeding Techniques. Hortic. Plant J. 2025, 11, 15–41. [Google Scholar] [CrossRef]
- Kumari, E.; Kumari, S.; Das, S.S.; Mahapatra, M.; Sahoo, J.P. Plant Growth-Promoting Bacteria (PGPB) for Sustainable Agriculture: Current Prospective and Future Challenges. AgroEnviron. Sustain. 2023, 1, 274–285. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Mosqueda, M.d.C.; Santoyo, G.; Glick, B.R. Recent Advances in the Bacterial Phytohormone Modulation of Plant Growth. Plants 2023, 12, 606. [Google Scholar] [CrossRef]
- Poria, V.; Dębiec-Andrzejewska, K.; Fiodor, A.; Lyzohub, M.; Ajijah, N.; Singh, S.; Pranaw, K. Plant Growth-Promoting Bacteria (PGPB) Integrated Phytotechnology: A Sustainable Approach for Remediation of Marginal Lands. Front. Plant Sci. 2022, 13, 999866. [Google Scholar] [CrossRef]
- Gunjal, A.B.; Glick, B.R. Plant Growth-Promoting Bacteria (PGPB) in Horticulture. Proc. Indian Natl. Sci. Acad. 2024, 90, 1–11. [Google Scholar] [CrossRef]
- Peng, M.; Jiang, Z.; Zhou, F.; Wang, Z. From Salty to Thriving: Plant Growth Promoting Bacteria as Nature’s Allies in Overcoming Salinity Stress in Plants. Front. Microbiol. 2023, 14, 1169809. [Google Scholar] [CrossRef]
- Safdarian, M.; Askari, H.; Nematzadeh, G.; Sofo, A. Halophile Plant Growth-Promoting Rhizobacteria Induce Salt Tolerance Traits in Wheat Seedlings (Triticum aestivum L.). Pedosphere 2020, 30, 684–693. [Google Scholar] [CrossRef]
- Bouremani, N.; Cherif-Silini, H.; Silini, A.; Rabhi, N.E.H.; Bouket, A.C.; Belbahri, L. Osmotolerant Plant Growth Promoting Bacteria Mitigate Adverse Effects of Drought Stress on Wheat Growth. AIMS Microbiol. 2024, 10, 507–541. [Google Scholar] [CrossRef] [PubMed]
- Mahreen, N.; Yasmin, S.; Asif, M.; Yahya, M.; Ejaz, K.; Mehboob-ur-Rahman; Yousaf, S.; Amin, I.; Zulfiqar, S.; Imran, A.; et al. Mitigation of Water Scarcity with Sustained Growth of Rice by Plant Growth Promoting Bacteria. Front. Plant Sci. 2023, 14, 1081537. [Google Scholar] [CrossRef]
- Joshi, S.; Gangola, S.; Bhandari, G.; Bhandari, N.S.; Nainwal, D.; Rani, A.; Malik, S.; Slama, P. Rhizospheric Bacteria: The Key to Sustainable Heavy Metal Detoxification Strategies. Front. Microbiol. 2023, 14, 1229828. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, A.W.; Yasmin, H.; Hassan, M.N.; Khan, N.; Jan, B.L.; Mumtaz, S. Heavy Metal–Resistant Plant Growth–Promoting Citrobacter werkmanii Strain WWN1 and Enterobacter cloacae Strain JWM6 Enhance Wheat (Triticum aestivum L.) Growth by Modulating Physiological Attributes and Some Key Antioxidants Under Multi-Metal Stress. Front. Microbiol. 2022, 13, 815704. [Google Scholar] [CrossRef]
- Rossi, M.; Borromeo, I.; Capo, C.; Glick, B.R.; Del Gallo, M.; Pietrini, F.; Forni, C. PGPB Improve Photosynthetic Activity and Tolerance to Oxidative Stress in Brassica Napus Grown on Salinized Soils. Appl. Sci. 2021, 11, 11442. [Google Scholar] [CrossRef]
- González-Reguero, D.; Robas-Mora, M.; Probanza, A.; Jiménez, P.A. Evaluation of the Oxidative Stress Alleviation in Lupinus albus Var. Orden Dorado by the Inoculation of Four Plant Growth-Promoting Bacteria and Their Mixtures in Mercury-Polluted Soils. Front. Microbiol. 2022, 13, 907557. [Google Scholar] [CrossRef]
- Tara, N.; Afzal, M.; Ansari, T.M.; Tahseen, R.; Iqbal, S.; Khan, Q.M. Combined Use of Alkane-Degrading and Plant Growth-Promoting Bacteria Enhanced Phytoremediation of Diesel Contaminated Soil. Int. J. Phytoremediat. 2014, 16, 1268–1277. [Google Scholar] [CrossRef]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of Plant Growth-Promoting Bacteria for Biocontrol of Plant Diseases: Principles, Mechanisms of Action, and Future Prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef]
- Ranjan, A.; Rajput, V.D.; Prazdnova, E.V.; Gurnani, M.; Bhardwaj, P.; Sharma, S.; Sushkova, S.; Mandzhieva, S.S.; Minkina, T.; Sudan, J.; et al. Nature’s Antimicrobial Arsenal: Non-Ribosomal Peptides from PGPB for Plant Pathogen Biocontrol. Fermentation 2023, 9, 597. [Google Scholar] [CrossRef]
- Jacob, J.; Krishnan, G.V.; Thankappan, D.; Amma, D.K.B.N.S. 4—Endophytic Bacterial Strains Induced Systemic Resistance in Agriculturally Important Crop Plants. In Microbial Endophytes; Kumar, A., Radhakrishnan, E.K., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 75–105. ISBN 978-0-12-819654-0. [Google Scholar]
- Munif, A.; Putri, D.; Mutaqin, K. Induced Resistance and Plant Growth Promotion by Endophytic Bacteria Bacillus Sp. AA2 against Meloidogyne Sp. on Pepper. IOP Conf. Ser. Earth Environ. Sci. 2020, 468, 012040. [Google Scholar] [CrossRef]
- Shan, Y.; Wang, D.; Zhao, F.-H.; Song, J.; Zhu, H.; Li, Y.; Zhang, X.-J.; Dai, X.-F.; Han, D.; Chen, J.-Y. Insights into the Biocontrol and Plant Growth Promotion Functions of Bacillus altitudinis Strain KRS010 against Verticillium dahliae. BMC Biol. 2024, 22, 116. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Reed, L.; Glick, B.R. The Recent Use of Plant-Growth-Promoting Bacteria to Promote the Growth of Agricultural Food Crops. Agriculture 2023, 13, 1089. [Google Scholar] [CrossRef]
- Artyszak, A.; Gozdowski, D. The Effect of Growth Activators and Plant Growth-Promoting Rhizobacteria (PGPR) on the Soil Properties, Root Yield, and Technological Quality of Sugar Beet. Agronomy 2020, 10, 1262. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Liu, S.-Y.; Sun, X.; Fang, Y. Oenological Potential and Health Benefits of Chinese Non-Vitis vinifera Species: An Opportunity to the Revalorization and to Breed New Varieties. Food Res. Int. 2020, 137, 109443. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Y.; Zhang, H.; Huang, H.; Folta, K.M.; Lu, J. Whole Genome Wide Expression Profiles of Vitis amurensis grape Responding to Downy Mildew by Using Solexa Sequencing Technology. BMC Plant Biol. 2010, 10, 234. [Google Scholar] [CrossRef]
- Liu, L.; Li, H. Review: Research Progress in Amur Grape, Vitis amurensis Rupr. Can. J. Plant Sci. 2013, 93, 565–575. [Google Scholar] [CrossRef]
- Tsukamura, M. Proposal of a New Genus, Gordona, for Slightly Acid-Fast Organisms Occurring in Sputa of Patients with Pulmonary Disease and in Soil. Microbiology 1971, 68, 15–26. [Google Scholar] [CrossRef]
- Frantsuzova, E.; Bogun, A.; Shishkina, L.; Vetrova, A.; Solyanikova, I.; Delegan, Y. Insights into the Potential Role of Gordonia alkanivorans Strains in Biotechnologies. Processes 2023, 11, 3184. [Google Scholar] [CrossRef]
- Kämpfer, P.; Young, C.-C.; Chu, J.-N.; Frischmann, A.; Busse, H.-J.; Arun, A.B.; Shen, F.-T.; Rekha, P.D. Gordonia humi Sp. Nov., Isolated from Soil. Int. J. Syst. Evol. Microbiol. 2011, 61, 65–70. [Google Scholar] [CrossRef]
- Kim, Y.S.; Roh, S.G.; Kim, S.B. Gordonia insulae Sp. Nov., Isolated from an Island Soil. Int. J. Syst. Evol. Microbiol. 2020, 70, 2079–2083. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Sun, S.; Chu, F.; Wang, M.; Zhao, Q.; Shi, J.; Jia, R. Identification and Inactivation of Gordonia, a New Chlorine-Resistant Bacterium Isolated from a Drinking Water Distribution System. J. Water Health 2020, 18, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Muangham, S.; Lipun, K.; Thamchaipenet, A.; Matsumoto, A.; Duangmal, K. Gordonia oryzae Sp. Nov., Isolated from Rice Plant Stems (Oryza sativa L.). Int. J. Syst. Evol. Microbiol. 2019, 69, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Riesco, R.; Rose, J.J.A.; Batinovic, S.; Petrovski, S.; Sánchez-Juanes, F.; Seviour, R.J.; Goodfellow, M.; Trujillo, M.E. Gordonia pseudamarae Sp. Nov., a Home for Novel Actinobacteria Isolated from Stable Foams on Activated Sludge Wastewater Treatment Plants. Int. J. Syst. Evol. Microbiol. 2022, 72, 005547. [Google Scholar] [CrossRef]
- Andalibi, F.; Fatahi-Bafghi, M. Gordonia: Isolation and Identification in Clinical Samples and Role in Biotechnology. Folia Microbiol. 2017, 62, 245–252. [Google Scholar] [CrossRef]
- Amin, A.A.; Wahyuni, A.R.T.; Ekawati, A.W.; Kurniawan, A. Analysis of Polycyclic Aromatic Hydrocarbons (PAHs) Bioremediation by Hydrocarbonoclastic Degrading Bacteria (Gordonia terrae). IOP Conf. Ser. Earth Environ. Sci. 2022, 1036, 012028. [Google Scholar] [CrossRef]
- Xue, Y.; Sun, X.; Zhou, P.; Liu, R.; Liang, F.; Ma, Y. Gordonia paraffinivorans Sp. Nov., a Hydrocarbon-Degrading Actinomycete Isolated from an Oil-Producing Well. Int. J. Syst. Evol. Microbiol. 2003, 53, 1643–1646. [Google Scholar] [CrossRef]
- Hong, S.H.; Ryu, H.; Kim, J.; Cho, K.-S. Rhizoremediation of Diesel-Contaminated Soil Using the Plant Growth-Promoting Rhizobacterium Gordonia Sp. S2RP-17. Biodegradation 2011, 22, 593–601. [Google Scholar] [CrossRef]
- Kayasth, M.; Kumar, V.; Gera, R. Gordonia Sp.: A Salt Tolerant Bacterial Inoculant for Growth Promotion of Pearl Millet under Saline Soil Conditions. 3 Biotech 2014, 4, 553–557. [Google Scholar] [CrossRef]
- Aoyama, K.; Kang, Y.; Yazawa, K.; Gonoi, T.; Kamei, K.; Mikami, Y. Characterization of Clinical Isolates of Gordonia Species in Japanese Clinical Samples During 1998–2008. Mycopathologia 2009, 168, 175–183. [Google Scholar] [CrossRef]
- Ramanan, P.; Deziel, P.J.; Wengenack, N.L. Gordonia Bacteremia. J. Clin. Microbiol. 2013, 51, 3443–3447. [Google Scholar] [CrossRef]
- Rojo, F.; Martínez, J.L. Hydrocarbon Degraders as Pathogens. In Health Consequences of Microbial Interactions with Hydrocarbons, Oils, and Lipids; Springer: Cham, Switzerland, 2019; pp. 1–15. ISBN 978-3-319-72473-7. [Google Scholar]
- Banerjee, S.; Bedics, A.; Tóth, E.; Kriszt, B.; Soares, A.R.; Bóka, K.; Táncsics, A. Isolation of Pseudomonas aromaticivorans Sp. Nov from a Hydrocarbon-Contaminated Groundwater Capable of Degrading Benzene-, Toluene-, m- and p-Xylene under Microaerobic Conditions. Front. Microbiol. 2022, 13, 929128. [Google Scholar] [CrossRef]
- Kaida, N.; Habib, S.; Yasid, N.A.; Shukor, M.Y. Biodegradation of Petroleum Hydrocarbons by Bacillus Spp.: A Review. Bioremediat. Sci. Technol. Res. 2018, 6, 14–21. [Google Scholar] [CrossRef]
- Das, A.; Das, N.; Rajkumari, J.; Pandey, P.; Pandey, P. Exploring the Bioremediation Potential of Bacillus Spp. for Sustainable Mitigation of Hydrocarbon Contaminants. Environ. Sustain. 2024, 7, 135–156. [Google Scholar] [CrossRef]
- Schober, I.; Koblitz, J.; Sardà Carbasse, J.; Ebeling, C.; Schmidt, M.L.; Podstawka, A.; Gupta, R.; Ilangovan, V.; Chamanara, J.; Overmann, J.; et al. BacDive in 2025: The Core Database for Prokaryotic Strain Data. Nucleic Acids Res. 2024, 53, D748–D756. [Google Scholar] [CrossRef] [PubMed]
- Nityagovsky, N.N.; Ananev, A.A.; Suprun, A.R.; Ogneva, Z.V.; Dneprovskaya, A.A.; Tyunin, A.P.; Dubrovina, A.S.; Kiselev, K.V.; Sanina, N.M.; Aleynova, O.A. Distribution of Plasmopara viticola Causing Downy Mildew in Russian Far East Grapevines. Horticulturae 2024, 10, 326. [Google Scholar] [CrossRef]
- Ananev, A.A.; Ogneva, Z.V.; Nityagovsky, N.N.; Suprun, A.R.; Kiselev, K.V.; Aleynova, O.A. Whole Genome Sequencing of Bacillus velezensis AMR25, an Effective Antagonist Strain against Plant Pathogens. Microorganisms 2024, 12, 1533. [Google Scholar] [CrossRef]
- Echt, C.S.; Erdahl, L.A.; McCoy, T.J. Genetic Segregation of Random Amplified Polymorphic DNA in Diploid Cultivated Alfalfa. Genome 1992, 35, 84–87. [Google Scholar] [CrossRef]
- Aleynova, O.A.; Nityagovsky, N.N.; Ananev, A.A.; Suprun, A.R.; Ogneva, Z.V.; Dneprovskaya, A.A.; Beresh, A.A.; Tyunin, A.P.; Dubrovina, A.S.; Kiselev, K.V. The Endophytic Microbiome of Wild Grapevines Vitis amurensis Rupr. and Vitis coignetiae Pulliat Growing in the Russian Far East. Plants 2023, 12, 2952. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving Bacterial Genome Assemblies from Short and Long Sequencing Reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid Annotations Using Subsystems Technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Patz, S.; Gautam, A.; Becker, M.; Ruppel, S.; Rodríguez-Palenzuela, P.; Huson, D. PLaBAse: A Comprehensive Web Resource for Analyzing the Plant Growth-Promoting Potential of Plant-Associated Bacteria. BioRxiv 2021. [Google Scholar] [CrossRef]
- Chivian, D.; Jungbluth, S.P.; Dehal, P.S.; Wood-Charlson, E.M.; Canon, R.S.; Allen, B.H.; Clark, M.M.; Gu, T.; Land, M.L.; Price, G.A.; et al. Metagenome-Assembled Genome Extraction and Analysis from Microbiomes Using KBase. Nat. Protoc. 2023, 18, 208–238. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Ouk Kim, Y.; Park, S.-C.; Chun, J. OrthoANI: An Improved Algorithm and Software for Calculating Average Nucleotide Identity. Int. J. Syst. Evol. Microbiol. 2016, 66, 1100–1103. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.-P.; Göker, M. Genome Sequence-Based Species Delimitation with Confidence Intervals and Improved Distance Functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef]
- Sun, J.; Lu, F.; Luo, Y.; Bie, L.; Xu, L.; Wang, Y. OrthoVenn3: An Integrated Platform for Exploring and Visualizing Orthologous Data across Genomes. Nucleic Acids Res. 2023, 51, W397–W403. [Google Scholar] [CrossRef]
- Chan, P.P.; Lin, B.Y.; Mak, A.J.; Lowe, T.M. tRNAscan-SE 2.0: Improved Detection and Functional Classification of Transfer RNA Genes. Nucleic Acids Res. 2021, 49, 9077–9096. [Google Scholar] [CrossRef]
- Lagesen, K.; Hallin, P.; Rødland, E.A.; Stærfeldt, H.-H.; Rognes, T.; Ussery, D.W. RNAmmer: Consistent and Rapid Annotation of Ribosomal RNA Genes. Nucleic Acids Res. 2007, 35, 3100–3108. [Google Scholar] [CrossRef]
- Richter, M.; Rosselló-Móra, R. Shifting the Genomic Gold Standard for the Prokaryotic Species Definition. Proc. Natl. Acad. Sci. USA 2009, 106, 19126–19131. [Google Scholar] [CrossRef]
- Mukherjee, A.; Gaurav, A.K.; Singh, S.; Yadav, S.; Bhowmick, S.; Abeysinghe, S.; Verma, J.P. The Bioactive Potential of Phytohormones: A Review. Biotechnol. Rep. 2022, 35, e00748. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, V.; Skokan, R.; Depaepe, T.; Kurtović, K.; Haluška, S.; Vosolsobě, S.; Vaculíková, R.; Pil, A.; Dobrev, P.I.; Motyka, V.; et al. Phytohormone Profiling in an Evolutionary Framework. Nat. Commun. 2024, 15, 3875. [Google Scholar] [CrossRef] [PubMed]
- Sosnowski, J.; Truba, M.; Vasileva, V. The Impact of Auxin and Cytokinin on the Growth and Development of Selected Crops. Agriculture 2023, 13, 724. [Google Scholar] [CrossRef]
- García-Tabares, F.; Herraiz-Tomico, T.; Amat-Guerri, F.; Garcia Bilbao, J.L. Production of 3-Indoleacetic Acid and 3-Indolelactic Acid in Azotobacter vinelandii Cultures Supplemented with Tryptophan. Appl. Microbiol. Biotechnol. 1987, 25, 502–506. [Google Scholar] [CrossRef]
- Chennappa, G.; Adkar-Purushothama, C.R.; Suraj, U.; Tamilvendan, K.; Sreenivasa, M.Y. Pesticide Tolerant Azotobacter Isolates from Paddy Growing Areas of Northern Karnataka, India. World J. Microbiol. Biotechnol. 2014, 30, 1–7. [Google Scholar] [CrossRef]
- Ganusova, E.E.; Banerjee, I.; Seats, T.; Alexandre, G. Indole-3-Acetic Acid (IAA) Protects Azospirillum brasilense from Indole-Induced Stress. Appl. Environ. Microbiol. 2025, 91, e02384-24. [Google Scholar] [CrossRef]
- Molina, R.; Rivera, D.; Mora, V.; López, G.; Rosas, S.; Spaepen, S.; Vanderleyden, J.; Cassán, F. Regulation of IAA Biosynthesis in Azospirillum brasilense Under Environmental Stress Conditions. Curr. Microbiol. 2018, 75, 1408–1418. [Google Scholar] [CrossRef]
- Wagi, S.; Ahmed, A. Bacillus Spp.: Potent Microfactories of Bacterial IAA. PeerJ 2019, 7, e7258. [Google Scholar] [CrossRef]
- Goud, M.S.; Sharma, S.K.; Kharbikar, L.L.; Prasanna, R.; Sangwan, S.; Dahuja, A.; Dixit, A. Bacillus Species Consortium with Tryptophan-Dependent and -Independent Pathways Mediated Production of IAA and Its Derivatives Modulates Soil Biological Properties, Growth and Yield of Wheat. Plant Soil 2025, 508, 71–97. [Google Scholar] [CrossRef]
- Abo Elsoud, M.M.; Hasan, S.F.; Elhateir, M.M. Optimization of Indole-3-Acetic Acid Production by Bacillus velezensis Isolated from Pyrus Rhizosphere and Its Effect on Plant Growth. Biocatal. Agric. Biotechnol. 2023, 50, 102714. [Google Scholar] [CrossRef]
- Kumar Ghosh, P.; Kumar Sen, S.; Kanti Maiti, T. Production and Metabolism of IAA by Enterobacter Spp. (Gammaproteobacteria) Isolated from Root Nodules of a Legume Abrus precatorius L. Biocatal. Agric. Biotechnol. 2015, 4, 296–303. [Google Scholar] [CrossRef]
- Luziatelli, F.; Melini, F.; Bonini, P.; Melini, V.; Cirino, V.; Ruzzi, M. Production of Indole Auxins by Enterobacter Sp. Strain P-36 under Submerged Conditions. Fermentation 2021, 7, 138. [Google Scholar] [CrossRef]
- Karmakar, J.; Goswami, S.; Pramanik, K.; Maiti, T.K.; Kar, R.K.; Dey, N. Growth Promoting Properties of Mycobacterium and Bacillus on Rice Plants under Induced Drought. Plant Sci. Today 2021, 8, 49–57. [Google Scholar] [CrossRef]
- Golubev, S.N.; Muratova, A.Y.u.; Panchenko, L.V.; Shchyogolev, S.Y.u.; Turkovskaya, O.V. Mycolicibacterium Sp. Strain PAM1, an Alfalfa Rhizosphere Dweller, Catabolizes PAHs and Promotes Partner-Plant Growth. Microbiol. Res. 2021, 253, 126885. [Google Scholar] [CrossRef]
- AL-Habib, A.A.S. IAA Production by Pseudomonas putida Associated with Rhizosphere of Some Medicine Plants. IOP Conf. Ser. Earth Environ. Sci. 2021, 735, 012076. [Google Scholar] [CrossRef]
- Chen, B.; Luo, S.; Wu, Y.; Ye, J.; Wang, Q.; Xu, X.; Pan, F.; Khan, K.Y.; Feng, Y.; Yang, X. The Effects of the Endophytic Bacterium Pseudomonas fluorescens Sasm05 and IAA on the Plant Growth and Cadmium Uptake of Sedum Alfredii Hance. Front. Microbiol. 2017, 8, 2538. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Hou, X.; Yan, Y.; Liu, T.; Dai, X.; Igarashi, Y.; Fan, L.; Yang, C.; Luo, F. Plant Growth-Promoting and Arsenic Accumulation Reduction Effects of Two Endophytic Bacteria Isolated from Brassica Napus. J. Plant Growth Regul. 2024, 43, 76–88. [Google Scholar] [CrossRef]
- Thoa, N.T.K.; Mai, D.T.H.; Hiu, B.L.; Duong, C.A.; Chau, N.N.B.; Nghiep, N.M.; Van Minh, N.; Quoc, N.B. Roles of β-Indole Acetic Acid (IAA) Producing Endophytic Bacteria on the Recovery of Plant Growth and Survival Ability of Sugarcane Infected White Leaf Disease (SWLD). Curr. Microbiol. 2022, 79, 389. [Google Scholar] [CrossRef]
- Alotaibi, F.; St-Arnaud, M.; Hijri, M. In-Depth Characterization of Plant Growth Promotion Potentials of Selected Alkanes-Degrading Plant Growth-Promoting Bacterial Isolates. Front. Microbiol. 2022, 13, 863702. [Google Scholar] [CrossRef]
- McClerklin, S.A.; Lee, S.G.; Harper, C.P.; Nwumeh, R.; Jez, J.M.; Kunkel, B.N. Indole-3-Acetaldehyde Dehydrogenase-Dependent Auxin Synthesis Contributes to Virulence of Pseudomonas syringae Strain DC3000. PLoS Pathog. 2018, 14, e1006811. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.; Meng, Z.; Wang, B.; Chen, M. Research Progress on the Roles of Cytokinin in Plant Response to Stress. Int. J. Mol. Sci. 2020, 21, 6574. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, M.S.; Ali, H.H.; Iqbal, M.A.; Erinle, K.O.; Javed, T.; Iqbal, J.; Hashmi, M.I.U.; Mumtaz, M.Z.; Salama, E.A.A.; Kalaji, H.M.; et al. Cytokinin Production by Azospirillum brasilense Contributes to Increase in Growth, Yield, Antioxidant, and Physiological Systems of Wheat (Triticum aestivum L.). Front. Microbiol. 2022, 13, 886041. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Veselov, S.U.; Melentiev, A.I.; Martynenko, E.V.; Kudoyarova, G.R. Ability of Bacterium Bacillus Subtilis to Produce Cytokinins and to Influence the Growth and Endogenous Hormone Content of Lettuce Plants. Plant Soil. 2005, 272, 201–209. [Google Scholar] [CrossRef]
- Park, Y.-G.; Mun, B.-G.; Kang, S.-M.; Hussain, A.; Shahzad, R.; Seo, C.-W.; Kim, A.-Y.; Lee, S.-U.; Oh, K.Y.; Lee, D.Y.; et al. Bacillus aryabhattai SRB02 Tolerates Oxidative and Nitrosative Stress and Promotes the Growth of Soybean by Modulating the Production of Phytohormones. PLoS ONE 2017, 12, e0173203. [Google Scholar] [CrossRef]
- Großkinsky, D.K.; Tafner, R.; Moreno, M.V.; Stenglein, S.A.; García de Salamone, I.E.; Nelson, L.M.; Novák, O.; Strnad, M.; van der Graaff, E.; Roitsch, T. Cytokinin Production by Pseudomonas fluorescens G20-18 Determines Biocontrol Activity against Pseudomonas syringae in Arabidopsis. Sci. Rep. 2016, 6, 23310. [Google Scholar] [CrossRef] [PubMed]
- Nouioui, I.; Cortés-albayay, C.; Carro, L.; Castro, J.F.; Gtari, M.; Ghodhbane-Gtari, F.; Klenk, H.-P.; Tisa, L.S.; Sangal, V.; Goodfellow, M. Genomic Insights into Plant-Growth-Promoting Potentialities of the Genus Frankia. Front. Microbiol. 2019, 10, 1457. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Hernández, A.G.; Glick, B.R.; Rossi, M.J. Plant Growth-Promoting Activities and Genomic Analysis of the Stress-Resistant Bacillus megaterium STB1, a Bacterium of Agricultural and Biotechnological Interest. Biotechnol. Rep. 2020, 25, e00406. [Google Scholar] [CrossRef] [PubMed]
- Frébortová, J.; Frébort, I. Biochemical and Structural Aspects of Cytokinin Biosynthesis and Degradation in Bacteria. Microorganisms 2021, 9, 1314. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Ashraf, U.; Li, G.; Li, Y.; Lu, W.; Gao, L.; Han, F.; Hu, J. Exogenous γ-Aminobutyric Acid (GABA) Application Improved Early Growth, Net Photosynthesis, and Associated Physio-Biochemical Events in Maize. Front. Plant Sci. 2016, 7, 919. [Google Scholar] [CrossRef]
- Xie, S.-S.; Wu, H.-J.; Zang, H.-Y.; Wu, L.-M.; Zhu, Q.-Q.; Gao, X.-W. Plant Growth Promotion by Spermidine-Producing Bacillus subtilis OKB105. Mol. Plant-Microbe Interact. 2014, 27, 655–663. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.d.C.; Flores, A.; Rojas-Sánchez, B.; Urtis-Flores, C.A.; Morales-Cedeño, L.R.; Valencia-Marin, M.F.; Chávez-Avila, S.; Rojas-Solis, D.; Santoyo, G. Plant Growth-Promoting Bacteria as Bioinoculants: Attributes and Challenges for Sustainable Crop Improvement. Agronomy 2021, 11, 1167. [Google Scholar] [CrossRef]
- Bakhshandeh, E.; Pirdashti, H.; Lendeh, K.S. Phosphate and Potassium-Solubilizing Bacteria Effect on the Growth of Rice. Ecol. Eng. 2017, 103, 164–169. [Google Scholar] [CrossRef]
- Timofeeva, A.; Galyamova, M.; Sedykh, S. Prospects for Using Phosphate-Solubilizing Microorganisms as Natural Fertilizers in Agriculture. Plants 2022, 11, 2119. [Google Scholar] [CrossRef]
- Hoberg, E.; Marschner, P.; Lieberei, R. Organic Acid Exudation and pH Changes by Gordonia Sp. and Pseudomonas fluorescens Grown with P Adsorbed to Goethite. Microbiol. Res. 2005, 160, 177–187. [Google Scholar] [CrossRef]
- Deb, C.; Tatung, M. Siderophore Producing Bacteria as Biocontrol Agent against Phytopathogens for a Better Environment: A Review. S. Afr. J. Bot. 2024, 165, 153–162. [Google Scholar] [CrossRef]
- Swify, S.; Mažeika, R.; Baltrusaitis, J.; Drapanauskaitė, D.; Barčauskaitė, K. Review: Modified Urea Fertilizers and Their Effects on Improving Nitrogen Use Efficiency (NUE). Sustainability 2024, 16, 188. [Google Scholar] [CrossRef]
- Li, C.-K.; Chen, R.-Y. Ammonium Bicarbonate Used as a Nitrogen Fertilizer in China. Fertil. Res. 1980, 1, 125–136. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Q.; Chen, Y.; Yang, Y.; Zhou, C.; Yu, J.; Li, Y.; Qiu, L. Ammonia-Assimilating Bacteria Promote Wheat (Triticum aestivum) Growth and Nitrogen Utilization. Microorganisms 2025, 13, 43. [Google Scholar] [CrossRef]
- Silva, N.M.; Oliveira, A.M.S.A.d.; Pegorin, S.; Giusti, C.E.; Ferrari, V.B.; Barbosa, D.; Martins, L.F.; Morais, C.; Setubal, J.C.; Vasconcellos, S.P.; et al. Characterization of Novel Hydrocarbon-Degrading Gordonia paraffinivorans and Gordonia sihwensis Strains Isolated from Composting. PLoS ONE 2019, 14, e0215396. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Li, H.; Wu, H.; Ren, H.; Kong, X.; Lu, Z. Resting for Viability: Gordonia polyisoprenivorans ZM27, a Robust Generalist for Petroleum Bioremediation under Hypersaline Stress. Environ. Pollut. 2024, 360, 124618. [Google Scholar] [CrossRef] [PubMed]
- Lo Piccolo, L.; De Pasquale, C.; Fodale, R.; Puglia, A.M.; Quatrini, P. Involvement of an Alkane Hydroxylase System of Gordonia Sp. Strain SoCg in Degradation of Solid n-Alkanes. Appl. Environ. Microbiol. 2011, 77, 1204–1213. [Google Scholar] [CrossRef]
- Kotoky, R.; Pandey, P. Rhizosphere Assisted Biodegradation of Benzo(a)Pyrene by Cadmium Resistant Plant-Probiotic Serratia Marcescens S2I7, and Its Genomic Traits. Sci. Rep. 2020, 10, 5279. [Google Scholar] [CrossRef]
- Abdou, A.; Alkhateeb, O.; Eldin, H.; Ghazzawy, H.; Albadrani, M.; Al-harbi, N.; Al-Shammari, W.; Abdelaal, K. Application of Plant Growth-Promoting Bacteria as an Eco-Friendly Strategy for Mitigating the Harmful Effects of Abiotic Stress on Plants. Phyton 2023, 92, 3305–3321. [Google Scholar] [CrossRef]
- Fanai, A.; Bohia, B.; Lalremruati, F.; Lalhriatpuii, N.; Lalrokimi; Lalmuanpuii, R.; Singh, P.K.; Zothanpuia. Plant Growth Promoting Bacteria (PGPB)-Induced Plant Adaptations to Stresses: An Updated Review. PeerJ 2024, 12, e17882. [Google Scholar] [CrossRef]
- Ali, A.; Guo, D.; Li, Y.; Shaheen, S.M.; Wahid, F.; Antoniadis, V.; Abdelrahman, H.; Al-Solaimani, S.G.; Li, R.; Tsang, D.C.W.; et al. Streptomyces pactum Addition to Contaminated Mining Soils Improved Soil Quality and Enhanced Metals Phytoextraction by Wheat in a Green Remediation Trial. Chemosphere 2021, 273, 129692. [Google Scholar] [CrossRef]
- Abulfaraj, A.A.; Jalal, R.S. Use of Plant Growth-Promoting Bacteria to Enhance Salinity Stress in Soybean (Glycine max L.) Plants. Saudi J. Biol. Sci. 2021, 28, 3823–3834. [Google Scholar] [CrossRef]
- Ali, M.A.; Ahmed, T.; Ibrahim, E.; Rizwan, M.; Chong, K.P.; Yong, J.W.H. A Review on Mechanisms and Prospects of Endophytic Bacteria in Biocontrol of Plant Pathogenic Fungi and Their Plant Growth-Promoting Activities. Heliyon 2024, 10, e31573. [Google Scholar] [CrossRef]
- Bonaterra, A.; Badosa, E.; Daranas, N.; Francés, J.; Roselló, G.; Montesinos, E. Bacteria as Biological Control Agents of Plant Diseases. Microorganisms 2022, 10, 1759. [Google Scholar] [CrossRef]
- Ryu, C.-M.; Farag, M.A.; Hu, C.-H.; Reddy, M.S.; Kloepper, J.W.; Paré, P.W. Bacterial Volatiles Induce Systemic Resistance in Arabidopsis. Plant Physiol. 2004, 134, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Lee, S.J.; Moon, J.H.; Park, K.H.; Yang, K.Y.; Cho, B.H.; Kim, K.Y.; Kim, Y.W.; Lee, M.C.; Anderson, A.J.; et al. GacS-Dependent Production of 2R, 3R-Butanediol by Pseudomonas chlororaphis O6 Is a Major Determinant for Eliciting Systemic Resistance Against Erwinia carotovora but Not Against Pseudomonas syringae Pv. Tabaci in Tobacco. Mol. Plant-Microbe Interact. 2006, 19, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Pramanik, K.; Ghosh, S.K.; Pal, P.; Mondal, T.; Soren, T.; Maiti, T.K. Unraveling the Role of Plant Growth-Promoting Rhizobacteria in the Alleviation of Arsenic Phytotoxicity: A Review. Microbiol. Res. 2021, 250, 126809. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Wang, G. Genomic Evidence Reveals the Extreme Diversity and Wide Distribution of the Arsenic-Related Genes in Burkholderiales. PLoS ONE 2014, 9, e92236. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, N.; Bukhari, M.A.; Ahmad, T.; Ahmad, Z.; Jatoi, W.N.; Abbas, S.M.; Latif, A.; Raza, A.; Aurangzaib, M.; Hashem, A.; et al. Exogenously Applied Nicotinic Acid Alleviates Drought Stress by Enhancing Morpho-Physiological Traits and Antioxidant Defense Mechanisms in Wheat. Ecotoxicol. Environ. Saf. 2023, 263, 115350. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Tian, Y.; Hou, X.; Hou, X.; Jia, Z.; Li, M.; Hao, M.; Jiang, Y.; Wang, Q.; Pu, Q.; et al. Multiple Forms of Vitamin B6 Regulate Salt Tolerance by Balancing ROS and Abscisic Acid Levels in Maize Root. Stress Biol. 2022, 2, 39. [Google Scholar] [CrossRef]
- Alsamadany, H.; Mansour, H.; Elkelish, A.; Ibrahim, M.F.M. Folic Acid Confers Tolerance against Salt Stress-Induced Oxidative Damages in Snap Beans through Regulation Growth, Metabolites, Antioxidant Machinery and Gene Expression. Plants 2022, 11, 1459. [Google Scholar] [CrossRef]

| P6PL2 | NBRC 108223 | NBRC 100414 | VH2 | NBRC 100010 | |
|---|---|---|---|---|---|
| GeneBank acc. number | PRJNA1267753 | GCF_000332975.1 | GCF_000248055.1 | GCF_000247715.1 | GCF_001485495.1 |
| Genome size (bp) | 5,435,824 | 5,092,029 | 4,952,979 | 5,844,299 | 5,428,634 |
| G+C content (mol%) | 65.1 | 65.5 | 65.5 | 67 | 68 |
| Number of Contigs | 8 | 78 | 158 | 2 | 246 |
| Protein-coding genes (CDS) | 5279 | 4613 | 4592 | 5110 | 4705 |
| tRNA | 79 | 48 | 54 | 57 | 58 |
| rRNA | 6 | 3 | 3 | 9 | 3 |
| Prophage | 6 | 2 | 2 | 4 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ananev, A.A.; Aleynova, O.A.; Nityagovsky, N.N.; Suprun, A.R.; Ogneva, Z.V.; Kiselev, K.V. Whole Genome of Gordonia aichiensis P6PL2 Associated with Vitis amurensis That Stimulates Plant Growth. Horticulturae 2025, 11, 735. https://doi.org/10.3390/horticulturae11070735
Ananev AA, Aleynova OA, Nityagovsky NN, Suprun AR, Ogneva ZV, Kiselev KV. Whole Genome of Gordonia aichiensis P6PL2 Associated with Vitis amurensis That Stimulates Plant Growth. Horticulturae. 2025; 11(7):735. https://doi.org/10.3390/horticulturae11070735
Chicago/Turabian StyleAnanev, Alexey A., Olga A. Aleynova, Nikolay N. Nityagovsky, Andrey R. Suprun, Zlata V. Ogneva, and Konstantin V. Kiselev. 2025. "Whole Genome of Gordonia aichiensis P6PL2 Associated with Vitis amurensis That Stimulates Plant Growth" Horticulturae 11, no. 7: 735. https://doi.org/10.3390/horticulturae11070735
APA StyleAnanev, A. A., Aleynova, O. A., Nityagovsky, N. N., Suprun, A. R., Ogneva, Z. V., & Kiselev, K. V. (2025). Whole Genome of Gordonia aichiensis P6PL2 Associated with Vitis amurensis That Stimulates Plant Growth. Horticulturae, 11(7), 735. https://doi.org/10.3390/horticulturae11070735








