Abstract
The global community continues to face the urgent need to develop environmentally friendly methods to increase agricultural productivity. Using plant growth-promoting bacteria (PGPB) as plant growth stimulants could solve this problem, as this practice is more environmentally friendly than using fertilizers. This study characterized the Gordonia aichiensis P6PL2 bacterium associated with Vitis amurensis using whole-genome sequencing and in vitro and in vivo testing. The whole genome size of G. aichiensis P6PL2 was 5,435,824 bp with 5279 open reading frames. G. aichiensis P6PL2 possessed genes for the production of phytohormones (auxins and cytokinins) and an increased bioavailability of nutrients such as nitrogen, phosphorus, potassium, and sulfur. In addition, the presence of genes involved in synthesizing growth stimulants, such as gamma-aminobutyric acid and spermidine, has been demonstrated, as has the presence of genes involved in reducing various abiotic and biotic stress factors. Moreover, the results demonstrated the growth-promoting impact of a single application of G. aichiensis P6PL2 on seedlings and 30-day rice plants. This paper has shown and discussed the potential importance of G. aichiensis P6PL2 for agriculture.
1. Introduction
The utilization of organic and inorganic fertilizers and pesticides in agricultural practices is deleterious to the environment [1,2]. The population is growing rapidly, and experts predict that by 2050, the demand for agricultural products could increase by up to 60% to 110% [3]. Consequently, the use of fertilizers and pesticides will increase. Various approaches have been proposed to boost crop yields, including classical breeding and genetic modification. However, in recent years, research into the use of different microorganisms in agriculture has received particular attention [4,5,6].
Plants interact with a variety of microbial communities from different environments. Bacteria that enter into symbiosis with plants and have a positive effect on their growth and development are known as plant growth-promoting bacteria (PGPB) [7]. These are of particular interest to the agriculture industry. This group of bacteria can directly influence plant growth directly by synthesizing or degrading phytohormones, such as auxins, cytokinins, and ethylene, and increasing the bioavailability of various nutrients, including nitrogen, phosphorus, potassium, sulfur, etc. [7,8,9,10].
On the other hand, PGPBs have multiple indirect mechanisms that influence plant growth. One way to stimulate plant growth is to reduce the effects of stress. Studies show that bacteria can reduce the effects of abiotic stresses, such as salinity [11,12], drought [13,14], heavy metal exposure [15,16], and oxidative stress [17,18]. Moreover, bacteria have the capacity to purify contaminated soil by means of the degradation of hydrocarbons [19]. Also, bacteria can increase a plant’s resistance to biotic stresses by suppressing pathogens through the synthesis of fungicides and antibacterial substances [20,21] and by inducing systemic plant resistance [22,23]. Based on the above, using bacteria to stimulate and protect plants can be an alternative to traditional farming methods. The use of PGPBs allows a combined effect that covers several needs. For example, Bacillus altitudinis KRS010 showed antagonistic activity towards pathogenic fungi such as Botrytis cinerea, Colletotrichum falcatum and C. gloeosporiodes, Fusarium graminarum, F. oxysporum, and Verticilium dahliae. In addition, it has the ability to increase nutrient availability and stimulate cotton plant growth [24].
Currently, the market for microbial-based biofertilizers is growing rapidly in regions such as North America, Europe, Asia Pacific, Latin America, Middle East, and Africa [25]. The variety of commercial biologics is also growing, and they are being developed from different microorganisms and their combinations, and for different crop groups. For example, preparations based on nitrogen-fixing bacteria such as Azoter (Azoter, Győr, Hungary), Bio Gold (Bio Power Lanka, Colombo, Sri Lanka), and TwinN (Mapleton Agri Biotec, Mapleton, Australia), as well as preparations based on bacteria capable of dissolving phosphate and potassium such as CataPult (Bio-Tech Organics, Virginia, Australia), Symbion van Plus (T Stanes and Company LTD, Tamil Nadu, India), and many others are widely represented [25,26,27]. In general, representatives of Azotobacter, Bacillus, and Pseudomonas genera are used as bacteria for the production of such preparations, and bioprospecting for the search of new bacterial genera remains an urgent task.
Wild plants constitute a fascinating subject of investigation in the context of endophyte isolation. These plants inhabit their natural environment, where they encounter a multitude of biotic and abiotic stressors. It is plausible that endophytes confer wild plants with some degree of resistance to these stressors, thereby enhancing their ecological fitness and survival strategies. The wild Amur grapevine Vitis amurensis Rupr is native to Asia and often grows on hillsides or in ravines at high elevations. This species is highly resistant to cold and is able to survive at −40 °C [28]. This species is also highly resistant to various diseases of the vine, such as powdery mildew [29], white rot of grapes, and anthracnose [30]. Based on the above, V. amurensis, as a wild-growing species that is resistant to adverse stress factors, may become valuable representatives of the bacteriome for agriculture.
The genus Gordonia was first proposed by Tsukamura [31] and is classified within the family Gordoniaceae. It is characterized as an aerobic Gram-positive bacteria [32]. This genus is widely distributed and can be found in soil [33,34], water [35], plants [36], sludge [37], and medical specimens [38]. It is well-known that the Gordonia genus is capable of degrading various hydrocarbons [39,40]. It has also been demonstrated that Gordonia sp. S2RP-17 stimulated the growth of Zea mays in soils contaminated with diesel fuel [41]. It was also shown that Gordonia sp. JPA2 stimulated the growth of Cenchrus americanus under salt stress [42]. Also, G. aichiensis is considered to be a pathogen and has been isolated from human clinical specimens, such as sputum [43]. However, according to Ramanan et al. [44], there have been no reported cases of clinical infection and its role as a pathogen remains unclear. It is worth noting that, among the microorganisms capable of degrading hydrocarbons, it is not uncommon to find species which can be pathogenic to humans, animals or plants. Most of these microorganisms are opportunist pathogens, meaning that they can only infect those who are already ill or have a compromised immune system. They can survive in various environments, and human beings are just one host they can infect [45]. For example, strains of Pseudomonas spp. and Bacillus spp. consume petroleum hydrocarbons as a source of carbon, but they are safe for humans [46,47,48]. Also, according to the BacDive database [49], G. aichiensis belongs to biosafety level 1 risk group (French classification), indicating that this species is safe for healthy people.
Despite the fact that many species belonging to the Gordonia genus have been described, little is known about their ability to stimulate plant growth and the mechanisms behind this process. Also, the specific molecular and genetic mechanisms that enable these bacteria to perform these functions remain unexplored. Therefore, in the present study, we characterized the V. amurensis-associated bacterium G. aichiensis P6PL2 using a comprehensive approach that included full genome sequencing, specialized media, analytical tests, and seedling tests. Additionally, its ability to stimulate plant growth was analyzed.
2. Materials and Methods
2.1. Bacterial Strain
The Gordonia aichiensis strain P6PL2 was isolated from the leaf tissue of a visually healthy V. amurensis plant. The leaf surface was sterilized with 70% ethanol for one min and 10% H2O2 for two minutes. The leaf tissues were then washed with sterile distilled water five times [50]. The sterile sheet was homogenized in a sterile mortar and diluted with 200 µL of sterile water. Next, 70 μL of the resulting solution was plated onto Reasoner’s 2A agar (R2A) nutrient medium and incubated at 26 °C for 48 h. After this time, the strain was seeded and purified. G. aichiensis P6PL2 was placed in the laboratory collection (Laboratory of Biotechnology, Federal Scientific Center of the East Asia Terrestrial Biodiversity, Far Eastern Branch of the Russian Academy of Sciences, Vladivostok, Russia).
2.2. Genomic DNA Isolation, Sequencing, Assembly and Annotation
The reparation and culture conditions of the bacterial strain have been described in a previous study [51]. Total DNA was isolated using the hexadecyltrimethylammonium bromide (CTAB) method with modifications [52,53].
Whole-genome sequencing was performed by “SYNTOL” (Moscow, Russia) and “GENOANALYTICA” (Moscow, Russia) using the MiSeq Illumina (Illumina, San Diego, CA, USA) and MinION (Oxford Nanopore Technologies, Oxford Science Park, Oxford, UK) instruments, respectively. The hybrid assembly of the G. aichiensis P6PL2 whole-genome sequence was performed using the program Unicycler v0.5.1 [54]. Genome annotation was performed using the program Prokka 1.14.6. [55] and the RAST 2.0 (http://rast.nmpdr.org/, accessed on 5 April 2025) server [56]. PGPB trait genes were searched using the PGPT-Pred PlaBase predictor [57].
2.3. Genome-Based Taxonomic Analysis
Phylogenomic analysis was performed using the Kbase server (https://www.kbase.us/, accessed on 10 April 2025). The genome set was created using the Batch Create Genome Set v1.2.0 function and included 21 genomes (Table S1). A phylogenetic tree was constructed based on 49 genetic markers using the Species Tree-v2.2.0 [58] function. Orthologous Average Nucleotide Identity was determined using the Orthologous Average Nucleotide Identity Tool (OAT) program [59], and DNA-DNA hybridization (dDDH) was calculated using GGDC 3.0 [60].
2.4. Comparative Genomics
The genomes of the four closest strains, namely Gordonia sputi NBRC 100414 (GCF_000248055.1), Gordonia aichiensis NBRC 108223 (GCF_000332975.1), Gordonia polyisoprenivorans VH2 (GCF_000247715.1), and Gordonia desulfuricans NBRC 100010 (GCF_001485495.1), were selected for a comparison with the G. aichiensis P6PL2 genome. The Venn diagram was constructed using OrthoVenn3 [61]. The tRNA and rRNA genes were searched using tRNAscan-SE v 2.0 [62] and RNAmmer v1.2 [63], respectively.
2.5. Signs of PGPB In Vitro
The isolate was tested for nitrogen (N) fixation ability using Ashby agar plates. Incubation was carried out at 26 °C for 72 h, after which the ability to grow on Ashby agar plates was assessed as an indication of nitrogen fixation. To determine phosphate solubilization activity, the isolates were grown in Pikowski’s medium with tricalcium phosphate as the insoluble phosphate. Incubation was performed under the same conditions, and the formation of a transparent halo was evaluated as a positive result. The ability to solubilize potassium was evaluated using Alexandrovsky agar. Incubation was performed at 26 °C for 96 h. The formation of a clear halo was evaluated as a positive result. The ability to oxidize sulfur was tested using modified Thiosulfate Agar (TSA) with Bromcresol Purple as an indicator. Incubation was carried out at 26 °C for 48 h. The appearance of a yellow halo was evaluated as a positive result.
To search for phytohormones, a 50 mL sample of overnight culture of G. aichiensis P6PL2 grown in R2B medium was placed in an analytical mill (IKA A11 basic; IKA Werke GmbH & Co. KG, Staufen, Germany) and ground to obtain a homogeneous mass. This was then re-treated with a Sonicator Q55 ultrasonic homogenizer (QSonica, Newtown, CT, USA) for 5 min. Next, extraction with ethyl acetate was carried out at a ratio of 1:2 (i.e., 100 mL of ethyl acetate was added to 50 mL of the medium), stirring continuously on a heated magnetic stirrer at 40 °C for 40 min. After stirring, the extract was spun in 50 mL falcon tubes at 3500 rpm for five min. The upper ethyl acetate fractions were transferred to a 250 mL flask using an automatic pipette. The extract volume was then increased to 3–4 mL using a rotary evaporator. The extract was subsequently dispersed into 1.5 mL tubes and dried in a concentrator at 40 °C. The dry extract was redissolved in 1 mL of methanol and filtered through a Discovery® DSC-18 SPE tube bed filter (50 mg, Supelco, Bellefonte, PA, USA). The extract was analyzed by HPLC-DAD using an LC-20 AD XR HPLC analytical system (Shimadzu, Kyoto, Japan).
2.6. Seed Inoculation, Evaluation of Biometric Parameters
The seeds of the Oryza sativa cultivar “Dubrava” were sterilized, as were the grape leaf tissues (see Section 2.1. Bacterial Strain). The overnight culture of G. aichiensis P6PL2 was centrifuged at 3000 rpm for five minutes to allow sedimentation. The resulting supernatant was drained and the resulting suspension was diluted with sterile water to a concentration of 3 × 108 CFU/mL. The sterile seeds were then placed in the bacterial solution and incubated for one hour. The excess moisture was then dried off. The seeds of the control group were subjected to the same manipulations, but sterile water was used instead of the bacterial solution.
Two groups were used for seed inoculation studies of G. aichiensis P6PL2: 7-day-old seedlings grown in Petri dishes on 2 layers of filter paper with 6 mL of sterile water, and 30-day-old plants grown in pots. All plants were cultivated in a climate chamber at 25 °C with a photoperiod of 16 h light/8 h dark, relative humidity of 70%, and light intensity of 250 mmol m−2s−1. After each time interval, the length of the root or stem (for coleoptile seedlings) was measured. Then, the plants were dried for 3 days at 30 °C. For 30-day-old plants, the root and stem were separated from each other before the dry biomass was measured.
Three independent experiments were performed, each comprising ten technical repeats. The data are presented as the mean ± standard error (SE) and were analyzed using a Student’s t-test, with a p-value of less than 0.05 being considered statistically significant.
3. Results and Discussion
3.1. Phenotypic Manifestation of PGPB Properties
To confirm the presence of some plant growth stimulation functions, the genes of which were represented in the studied strain, and Petri dish tests and HPLC-MS analysis were performed. As a result, G. aichiensis P6PL2 was found to grow on Ashby’s medium (Figure 1a), indicating a potential ability to fix nitrogen, and to solubilize phosphate on Pikowski’s medium (Figure 1b). However, the ability to oxidize sulfur and dissolve potassium was not confirmed (Figure 1c,d). Additionally, the HPLC-MS analysis detected the phytohormones indole-3-acetic acid (IAA) and its precursor indole-3-acetoaldehyde (Figure 1e,f) along with trans-zeatin (Figure 1g) in the G. aichiensis P6PL2 bacterial culture extract.
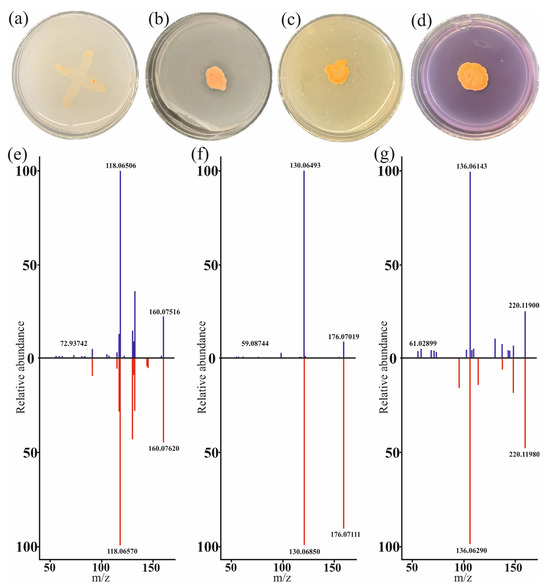
Figure 1.
In vitro test of plant growth-promoting traits of Gordonia aichiensis strain P6PL2: (a) nitrogen fixation on Ashby’s medium; (b) phosphate solubilization on Pikowski’s medium; (c) potassium solubilization on Alexandrovsky’s medium; (d) sulfur oxidation on TSA medium; (e) MS/MS mass spectrum of indole-3-acetoaldehyde production; (f) MS/MS mass spectrum of indole-3-acetic acid production; (g) MS/MS mass spectrum of trans-zeatin production. The mass spectra of the substances found in the G. aichiensis P6PL2 extract are shown in blue for (e–g); the reference mass spectra are indicated in red.
3.2. Effect of Gordonia aichiensis P6PL2 Inoculation on the Growth of Oryza sativa
In this study, we examined the effect of the inoculation of G. aichiensis P6PL2 seeds of O. sativa cultivar Dubrava on the growth of 7-day-old seedlings in Petri dishes and 30-day-old potted plants. G. aichiensis P6PL2 stimulated both types of plant under normal conditions, primarily through promoting root growth (Figure 2 and Figure 3).
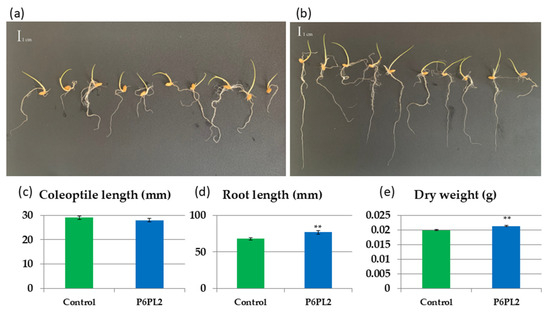
Figure 2.
The effect of Gordonia aichiensis P6PL2 inoculation on 7-day-old Oryza sativa seedlings. (a) control; (b) seedlings inoculated with G. aichiensis P6PL2; (c) coleoptile length; (d) root length; (e) total dry weight of seedlings. Data are presented as mean ± SE. **—significantly different from the control values at p ≤ 0.01 according to Student’s t-test.
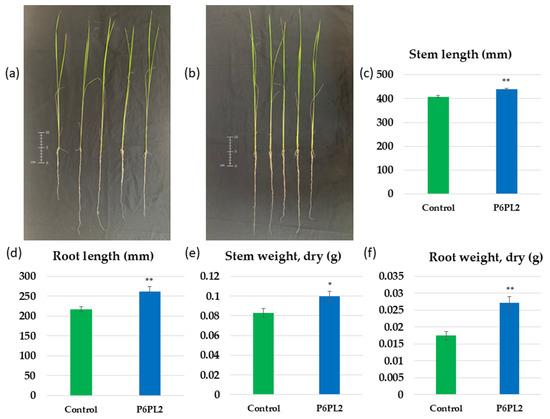
Figure 3.
The effect of Gordonia aichiensis P6PL2 inoculation on 30-day-old Oryza sativa plants. (a) control; (b) plants inoculated with G. aichiensis P6PL2; (c) stem length; (d) root length; (e) stem dry weight; (f) stem dry weight. Data are presented as mean ± SE. *, **—significantly different from the control values at p ≤ 0.05 and 0.01 by Student’s t-test.
The inoculation of rice seeds with G. aichiensis P6PL2 resulted in a significant increase in root length of 12.9% in 7-day-old seedlings compared to the control (67.92 mm vs. 76.69 mm, Figure 2d). In addition, the dry weight of the inoculated seedlings increased by a significant 10.5% (0.019 g vs. 0.021 g, Figure 2e). However, there was no significant change in coleoptile length in the seedlings inoculated with the tested strains (Figure 2c).
The inoculation of rice seeds with G. aichiensis P6PL2 resulted in a significant 7.5% increase in both stem length (407.7 mm vs. 438.35 mm) (Figure 3c) and stem dry weight (0.082 g vs. 0.099 g) (Figure 3e) in 30-day-old plants. The same trend of increasing root length, as observed in seedlings, was also evident in 30-day-old plants: the root length of inoculated plants increased by 20.8% (from 217.87 mm vs. 262.58 mm) (Figure 3d), and the root dry weight increased by 55.37% (from 0.017 g vs. 0.027 g) (Figure 3f). The obtained data indicate the potential importance of G. aichiensis P6PL2 in agriculture. However, no data were found on the effect of the Gordonia genus, in particular the G. aichiensis species, on plant growth under normal conditions.
3.3. Phylogenetic Identification
A phylogenetic analysis based on 49 core, universal genes defined by clusters of orthologous groups (COG) gene families demonstrated that the strain P6PL2 has the highest genetic similarity to the G. aichiensis strain NBRC 108223 (Figure 4a). Average nucleotide identity (ANI) and digital DNA-DNA hybridization (dDDH) analyses using the eight most closely related species showed the highest similarity of strain P6PL2 98.93% (ANI) (Figure 4b) and 94.1% (dDDH) (Table S2) to G. aichiensis NBRC 108223. Based on the result of the phylogenetic analysis, and given that the ANI and dDDH values were both above the gold standard thresholds of >95% and >70%, respectively [64], we hypothesize that this strain belongs to the species G. aichiensis.
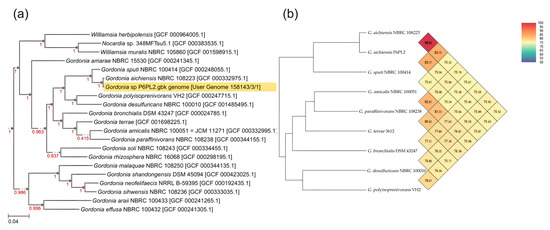
Figure 4.
The phylogenetic analysis of Gordonia aichiensis P6PL2: (a) phylogenetic tree based on 49 genetic markers using the Species Tree-v2.2.0. NCBI RefSeq assembly numbers are listed next to the species name and duplicated in (Table S1); (b) Orthologous Average Nucleotide Identity analysis was determined using the Orthologous Average Nucleotide Identity Tool.
3.4. Genomic Features and Comparison of Genetic Characteristics
Assembly of the G. aichiensis P6PL2 hybrid genome associated with V. amurensis generated a sequence length of 5,435,824 bp consisting of eight contigs, with an average GC content of 65.1% (Table 1). The genome contains 5279 protein-coding sequences (CDS), of which 4233 were functionally annotated and 921 were hypothetical (Table S3). In addition, the G. aichiensis P6PL2 genome contains 79 tRNA genes, 6 rRNA genes, and eight sites encoding prophages (Table 1).

Table 1.
Genomic features of the Gordonia aichiensis P6PL2 and related members of the Gordonia genus.
OrthoVenn analysis revealed that Gordonia sputi NBRC 100414 contains 3.928 gene clusters, Gordonia polyisoprenivorans VH2 contains 3981 clusters, Gordonia desulfuricans NBRC 100010 contains 3724 clusters, Gordonia aichiensis P6PL2 contains 4233 clusters, and Gordonia aichiensis NBRC 108223 contains 4297 gene clusters (Figure 5). A comparative analysis of the genetic clusters of the five selected Gordonia strains revealed that 2825 genetic clusters were present in all strains. A total of 3790 genetic clusters were shared between P6PL2 and G. aichiensis NBRC 108223. G. aichiensis P6PL2 had 3565 common clusters with G. sputi NBRC 100414, as well as 3377 common clusters with G. polyisoprenivorans VH2. Conversely, P6PL2 had 3083 clusters in common with G. desulfuricans NBRC 100010. G. aichiensis P6PL2 had 25 unique genetic clusters relative to the other strains studied.
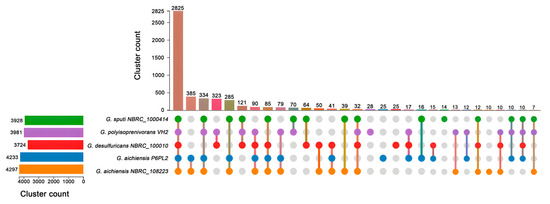
Figure 5.
UpSet diagram of Gordonia aichiensis P6PL2, Gordonia polyisoprenivorans VH2, Gordonia desulfuricans NBRC_100010, Gordonia aichiensis NBRC_108223, and Gordonia sputi NBRC_100414 built using the OrthoVenn3 program. The numbers of gene clusters are between the genome subsets.
3.5. Genetic Elements of Gordonia aichiensis P6PL2 Responsible for Plant–Bacterial Interactions
The PGPT-Pred PlaBase predictor identified 2293 genes (Table S4) in the G. aichiensis P6PL2 genome that are potentially associated with the stimulation of plant growth. Of all the genes detected, 39% had a direct effect on plant growth. This included 13% of genes related to phytohormone synthesis and plant signaling, 13% related to bio-fertilization, and 13% related to bio-remediation. The genes related to indirect effect factors accounted for 60% of the total, with 25% relating to the colonization of plant systems, 19% relating to the control of biotic and abiotic stresses, 15% relating to competitive exclusion, and 1% relating to the stimulation of the plant immune response. One percent of the genes belonged to putative functions (Figure 6). Although many genes potentially involved in plant–bacterial interactions have been identified using the PlaBase database, this article will focus only on the most interesting results.
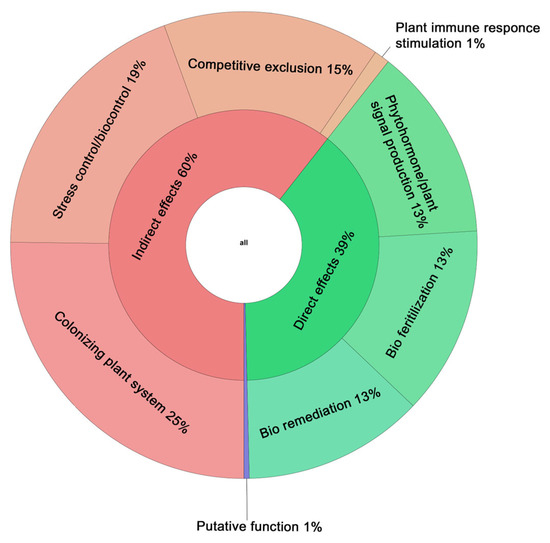
Figure 6.
Genomic characterization based on PGPT-Pred analysis. Through PGPT-Pred PlaBase analysis in the Gordonia aichiensis P6PL2 genome, 2293 genes were identified that are associated with the possible stimulation of plant growth, 39% of which were direct effect factors on plant growth, 60% were related to indirect effect factors, and 1% of the genes belonged to putative functions.
3.5.1. Production of Phytohormones and Other Growth Stimulants
Plant hormones play important roles in plant growth, defense, and productivity [65]. Phytohormones include auxins, cytokinins (CK), jasmonates, ethylene, abscisic acid (ABA), salicylic acid (SA), strigolactones (SL), gibberellins (GA), and brassinosteroids (BR) [66]. Phytohormone production is one of the most important properties of PGPB [7].
The plant hormone auxin plays an important role processes, including the formation of lateral roots, leaves, and flowers [67]. The ability to synthesize auxins is found in many bacterial genera such as Azotobacter [68,69], Azospirillum [70,71], Bacillus [72,73,74], Enterobacter [75,76], Mycobacterium [77,78], Pseudomonas [79,80], Rahnella [81,82], and others.
There are few studies on auxin synthesis in the genus Gordonia and it has been shown that Gordonia sp. JPA2 exhibited the ability to synthesize IAA and stimulated the growth of Cenchrus americanus [42]; on the other hand, Gordonia sp. ST45 did not possess the ability to synthesize IAA [83].
The genes responsible for auxin biosynthesis were found in the genome of G. aichiensis P6PL2, in particular the tryptophan operon trpABCDES, as well as genes responsible for the indole-3-acetoaldehyde (IAAld) pathway of auxin biosynthesis (aldA and aldB) (Table S5). HPLC-MS data confirm the presence of IAA and its possible biosynthesis pathway that was described by McClerklin et al. [84] when indole-3-acetoaldehyde was detected (Figure 1e); the putative IAA biosynthesis pathway is show below (Figure 7a).
Cytokinins are another important phytohormone that regulate the cell cycle, and inhibit the degradation of chlorophyll, nucleic acids, and proteins [85]. The ability to synthesize cytokinins has been found in various bacterial genera such as Azospirillum [86], Bacillus [87,88], and Pseudomonas [89]. Information on cytokinin production in the genus Gordonia was not found.
The genome of G. aichiensis P6PL2 contains genes responsible for cytokinin biosynthesis, namely ipt encoding isopentenyltransferase, which catalyzes the conversion of AMP into isopentenyladenine riboside 5′-monophosphate [90]. In addition, the yvdD gene encoding cytokinin riboside 5′-monophosphate phosphoribohydrolase, a member of the Lonely Guy (LOG) family, was found to catalyze the final step of cytokinin biosynthesis [91]. The ability to produce cytokinins was confirmed by HPLC-MS data, which showed the presence of a zeatin-type cytokinin, namely trans-zeatin (Figure 1g); the putative trans-zeatin biosynthesis pathway is shown below (Figure 7b).
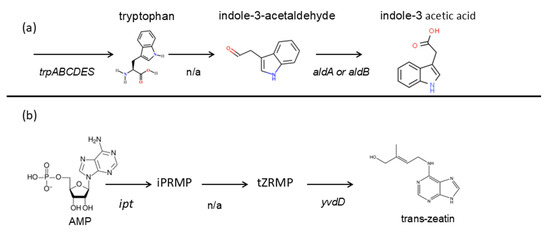
Figure 7.
Putative pathways of phytohormone biosynthesis in Gordonia aichiensis P6PL2. (a) Simplified auxin biosynthesis pathway by [84]; (b) simplified cytokinin biosynthesis pathway by [92]. AMP—adenosine monophosphate; iPRMP—isopentenyladenine riboside 5′-monophosphate; tZRMP—trans-zeatin riboside 5′-monophosphate; n/a—genes are missing.
Other substances that regulate plant growth and development were found to have their biosynthesis genes in G. aichiensis P6PL2. These substances were gamma-aminobutyric acid (GABA) and spermidine (Table S5). The application of exogenous GABA stimulates photosynthesis in Zea mays, thereby improving the development of maize seedlings [93]. It is hypothesized that spermidine plays a role in promoting plant growth and development by participating in the production of substances such as steroids, auxins, statins, and terpenes [91]. It has also been demonstrated that the spermidine synthesizing bacterium Bacillus subtilis OKB105, which synthesizes spermidine, stimulates the growth of Nicotiana tabacum [94].
3.5.2. Biofertilization
Another one of the direct plant growth-promoting properties of PGPB is the increased bioavailability of nutrients that are most often found in low-bioavailability forms, such as nitrogen, phosphorus, sulfur, and potassium [95].
Phosphate and potassium solubilizing bacteria are known to stimulate plant growth [96], and this ability is characteristic of many bacterial genera such as Azotobacter, Bacillus, Enterobacter, Pantoea, Rahnella, etc. [96,97]. The ability to solubilize phosphate was previously shown in the genus Gordonia [98]. The genome of G. aichiensis P6PL2 contains genes responsible for synthesizing various acids that contribute to the bioavailability of potassium and phosphorus. These include lactic, oxalic, butyric, malonic, acetic, pyruvic, and succinic acids (Table S6). In addition, other genes involved in the assimilation and metabolism of potassium and phosphorus have been discovered (Table S6). In addition, 18 genes responsible for sulfur assimilation were found (Table S6), including cysADHJKNTW. However, under in vitro testing conditions, G. aichiensis P6PL2 has been shown to have only have a phosphate solubilization ability (Figure 1b–d). Moreover, 19 genes associated with the process of iron assimilation and the creation of siderophores and hemophores were identified (Table S6). The production of siderophores has been found in many bacterial genera positioned as biocontrol agents, for example, Azospirillum brasilense, Bacillus subtilis, and Pseudomonas fluroscens. Siderophores synthesized by bacteria also positively regulate plant growth [99].
Also, in the genome of P6PL2, 44 genes involved in the increase of nitrogen bioavailability, including the assimilation of atmospheric nitrogen, ammonia metabolism, and urea metabolism, were found (Table S6). Ammonium and urea are used as some of the most popular nitrogen fertilizers [100,101]. Gao et al. demonstrated that the ammonia-assimilating bacterium Enterobacter sp. B12 promoted wheat growth as well as increased plant nitrogen content [102]. The G. aichiensis P6PL2 demonstrated the ability to grow on nitrogen-free medium, which demonstrates the potential for nitrogen fixation (Figure 1a). However, only nifU and nifS, which are responsible for nitrogen fixation [84] (Table S6), were identified from the nif operon. This is insufficient to confirm that this strain is capable of nitrogen fixation. Further study is required to confirm this property.
3.5.3. Bioremediation
As already mentioned, representatives of the genus Gordonia are often characterized by the ability to degrade various xenobiotics, including hydrocarbons. Thus, Gordonia sihwensis MTZ096 isolated from compost demonstrated the ability to degrade n-hexadecanes [103]. Gordonia polyisoprenivorans ZM27 demonstrated the ability to degrade n-hexadecanes [104], and Gordonia sp. SoCg degraded n-alkanes [105]. In addition, it was shown, that Gordonia sp. S2RP-17 stimulated the growth of Zea mays in soils contaminated with diesel fuel [42]. The G. aichiensis P6PL2 genome contains genes encoding di- and monooxygenases (involved in the degradation of various aromatic compounds, such as dioxygenases (benABC, pcaBCDGH)) (Table S7). It was demonstrated that the introducing of Serratia marcescens S2I7, which possesses the above-mentioned dioxygenases genes, into the rhizosphere resulted in the degradation of benzo(a)pyrene in soil [106]. In addition, genes involved in the degradation of hydrocarbons and steroids were identified (Table S7). Based on the obtained data, we hypothesize that G. aichiensis P6PL2 exhibits bioremediation properties, making it an interesting candidate for further research in this area.
3.5.4. Resistance to Biotic and Abiotic Stresses
PGPB can reduce the negative effects of various stressors [107], and the use of bacteria to reduce stress effects is becoming more popular [108]. Streptomyces pactum Act 12 has been shown to reduce soil ph. Also, this strain reduced lipid peroxidation, thereby stimulating wheat growth [109]. The inoculation of soybean with Pseudomonas fluorescens, P. putida, and Bacillus subtilis has been shown to mitigate the deleterious effects of salt stress [110]. It has been repeatedly shown that bacteria can inhibit the growth and development of plant pathogens [111,112].
Genes potentially involved in resistance to abiotic and biotic stress factors were found in G. aichiensis P6PL2. Thus, the genes responsible for salt stress resistance were found. These include genes involved in glutamate and proline synthesis (Table S8), as well as genes encoding oxidoreductases and terpenoid synthesis, which are involved in resistance to oxidative stress. The genes responsible for resistance to biotic stress factors were represented by genes that synthesized volatile organic compounds, namely 3-butanediol, as well as genes involved in the activation of systemic plant resistance (Table S8). It is also worth noting that synthesized 3-butanediol synthesized by bacteria activates systemic plant resistance against bacterial pathogens [113,114].
The whole-genome sequence of G. aichiensis P6PL2 also contained genes for resistance to heavy metals (Table S8). Thus, the GlpF gene encoding an arsenic entry channel [115], as well as genes of the ars operon responsible for resistance to arsenic were found, but the ars operon arsABCR did not include the arsM gene involved in the methylation and subsequent conversion of As III to the gaseous compound As(CH)3 [116]. In addition, 14 genes responsible for cobalt resistance, 9 genes for copper resistance, 8 genes for nickel resistance, and 5 genes for selenium resistance were found (Table S7). However, these were not assigned to complete sets of functional genes. The ability of G. aichiensis P6PL2 remains to be determined and requires further investigation.
In addition, G. aichiensis P6PL2 possesses vitamin biosynthesis genes, namely niacin (vitamin B3), pyridoxine (vitamin B6), and folic acid (vitamin B9) (Table S9). These vitamins are presumably also involved in resistance to various stress factors. The application of exogenous vitamin B3 has been shown to reduce the effects of drought on wheat [117]. Pyridoxine and folic acid can reduce the effects of salt and oxidative stress [118,119].
4. Conclusions
The bacterium G. aichiensis P6PL2, associated with grape V. amurensis was isolated; phylogenetic analysis confirmed that the P6PL2 strain belongs to the species G. aichiensis. This strain was found to be capable of synthesizing phytohormones, particularly IAA and trans-zeatin. It was also found that this strain has the potential to increase the bioavailability of phosphate and nitrogen. The genome of G. aichiensis P6PL2 contains genes that suggest that this strain is able to reduce the impact of various stress factors, as well as participate in the purification of soil from various xenobiotics. However, this fact requires additional research.
Moreover, experiments on O. sativa seedlings and 30-day-old potted plants revealed that a single application of G. aichiensis P6PL2 suspension significantly stimulated rice growth, primarily by increasing the root system. However, the mechanism of this effect on growth remains unclear. This paper therefore details the potential importance of G. aichiensis P6PL2 for agriculture.
The present study comprises the preliminary stage in developing biologics based on G. aichiensis P6PL2 bacteria, aimed at promoting plant growth and improving agronomic traits. In the future, we will focus on studying the effects of this bacterium on grape growth and quality in industrial vineyards.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae11070735/s1: Table S1: List of genomes used in phylogenetic analysis; Table S2: Comparison of G. aichiensis P6PL2 with G. aichiensis NBRC_108223 using digital DNA-DNA hybridization (dDDH); Table S3: Some characteristics of bacterial genomes used for upset construction; Table S4: Results of PGPT-Pred analysis; Table S5: Found genes involved in the biosynthesis of phytohormones and other growth regulators; Table S6: List of genes found to be involved in increasing the bioavailability of nutrients; Table S7: List of found genes involved in bioremediation (aromatic compounds and hydrocarbons); Table S8: List of genes found to be involved in resistance to biotic and abiotic factors; Table S9: List of found genes involved in vitamin biosynthesis (B3, B6, B9).
Author Contributions
O.A.A. and A.A.A. performed the research design, data analysis, paper preparation, and experimental process. O.A.A., A.A.A. and A.R.S. collected the material. A.R.S. performed HPLC analysis. O.A.A. performed the isolation of DNA for NGS. N.N.N., A.A.A. and Z.V.O. performed the bioinformatic analysis and visualization. O.A.A., A.A.A. and K.V.K. were responsible for the writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The NGS data and their bioinformatic analysis were supported by a grant from the Russian Science Foundation (grant number 22–74–10001, https://rscf.ru/project/22-74-10001) (accessed on 23 May 2025). The G. aichiensis P6PL2 strain isolation and maintenance in the endophyte collection was carried out within the state assignment of Ministry of Science and Higher Education of the Russian Federation (theme number 124012200181-4).
Data Availability Statement
The complete genome sequence for G. aichiensis P6PL2 can be found in the following NCBI database “BioProject PRJNA1267753”.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rashmi, I.; Roy, T.; Kartika, K.S.; Pal, R.; Coumar, V.; Kala, S.; Shinoji, K.C. Organic and Inorganic Fertilizer Contaminants in Agriculture: Impact on Soil and Water Resources. In Contaminants in Agriculture: Sources, Impacts and Management; Naeem, M., Ansari, A.A., Gill, S.S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 3–41. ISBN 978-3-030-41552-5. [Google Scholar]
- Gnanaprakasam, P.D.; Vanisree, A.J. Recurring Detrimental Impact of Agrochemicals on the Ecosystem, and a Glimpse of Organic Farming as a Possible Rescue. Environ. Sci. Pollut. Res. 2022, 29, 75103–75112. [Google Scholar] [CrossRef] [PubMed]
- Molotoks, A.; Stehfest, E.; Doelman, J.; Albanito, F.; Fitton, N.; Dawson, T.P.; Smith, P. Global Projections of Future Cropland Expansion to 2050 and Direct Impacts on Biodiversity and Carbon Storage. Glob. Change Biol. 2018, 24, 5895–5908. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, M.F.; Tan, B.C. Genetic Modification Techniques in Plant Breeding: A Comparative Review of CRISPR/Cas and GM Technologies. Hortic. Plant J. 2024; in press. [Google Scholar] [CrossRef]
- Abdul Aziz, M.; Masmoudi, K. Molecular Breakthroughs in Modern Plant Breeding Techniques. Hortic. Plant J. 2025, 11, 15–41. [Google Scholar] [CrossRef]
- Kumari, E.; Kumari, S.; Das, S.S.; Mahapatra, M.; Sahoo, J.P. Plant Growth-Promoting Bacteria (PGPB) for Sustainable Agriculture: Current Prospective and Future Challenges. AgroEnviron. Sustain. 2023, 1, 274–285. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Mosqueda, M.d.C.; Santoyo, G.; Glick, B.R. Recent Advances in the Bacterial Phytohormone Modulation of Plant Growth. Plants 2023, 12, 606. [Google Scholar] [CrossRef]
- Poria, V.; Dębiec-Andrzejewska, K.; Fiodor, A.; Lyzohub, M.; Ajijah, N.; Singh, S.; Pranaw, K. Plant Growth-Promoting Bacteria (PGPB) Integrated Phytotechnology: A Sustainable Approach for Remediation of Marginal Lands. Front. Plant Sci. 2022, 13, 999866. [Google Scholar] [CrossRef]
- Gunjal, A.B.; Glick, B.R. Plant Growth-Promoting Bacteria (PGPB) in Horticulture. Proc. Indian Natl. Sci. Acad. 2024, 90, 1–11. [Google Scholar] [CrossRef]
- Peng, M.; Jiang, Z.; Zhou, F.; Wang, Z. From Salty to Thriving: Plant Growth Promoting Bacteria as Nature’s Allies in Overcoming Salinity Stress in Plants. Front. Microbiol. 2023, 14, 1169809. [Google Scholar] [CrossRef]
- Safdarian, M.; Askari, H.; Nematzadeh, G.; Sofo, A. Halophile Plant Growth-Promoting Rhizobacteria Induce Salt Tolerance Traits in Wheat Seedlings (Triticum aestivum L.). Pedosphere 2020, 30, 684–693. [Google Scholar] [CrossRef]
- Bouremani, N.; Cherif-Silini, H.; Silini, A.; Rabhi, N.E.H.; Bouket, A.C.; Belbahri, L. Osmotolerant Plant Growth Promoting Bacteria Mitigate Adverse Effects of Drought Stress on Wheat Growth. AIMS Microbiol. 2024, 10, 507–541. [Google Scholar] [CrossRef] [PubMed]
- Mahreen, N.; Yasmin, S.; Asif, M.; Yahya, M.; Ejaz, K.; Mehboob-ur-Rahman; Yousaf, S.; Amin, I.; Zulfiqar, S.; Imran, A.; et al. Mitigation of Water Scarcity with Sustained Growth of Rice by Plant Growth Promoting Bacteria. Front. Plant Sci. 2023, 14, 1081537. [Google Scholar] [CrossRef]
- Joshi, S.; Gangola, S.; Bhandari, G.; Bhandari, N.S.; Nainwal, D.; Rani, A.; Malik, S.; Slama, P. Rhizospheric Bacteria: The Key to Sustainable Heavy Metal Detoxification Strategies. Front. Microbiol. 2023, 14, 1229828. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, A.W.; Yasmin, H.; Hassan, M.N.; Khan, N.; Jan, B.L.; Mumtaz, S. Heavy Metal–Resistant Plant Growth–Promoting Citrobacter werkmanii Strain WWN1 and Enterobacter cloacae Strain JWM6 Enhance Wheat (Triticum aestivum L.) Growth by Modulating Physiological Attributes and Some Key Antioxidants Under Multi-Metal Stress. Front. Microbiol. 2022, 13, 815704. [Google Scholar] [CrossRef]
- Rossi, M.; Borromeo, I.; Capo, C.; Glick, B.R.; Del Gallo, M.; Pietrini, F.; Forni, C. PGPB Improve Photosynthetic Activity and Tolerance to Oxidative Stress in Brassica Napus Grown on Salinized Soils. Appl. Sci. 2021, 11, 11442. [Google Scholar] [CrossRef]
- González-Reguero, D.; Robas-Mora, M.; Probanza, A.; Jiménez, P.A. Evaluation of the Oxidative Stress Alleviation in Lupinus albus Var. Orden Dorado by the Inoculation of Four Plant Growth-Promoting Bacteria and Their Mixtures in Mercury-Polluted Soils. Front. Microbiol. 2022, 13, 907557. [Google Scholar] [CrossRef]
- Tara, N.; Afzal, M.; Ansari, T.M.; Tahseen, R.; Iqbal, S.; Khan, Q.M. Combined Use of Alkane-Degrading and Plant Growth-Promoting Bacteria Enhanced Phytoremediation of Diesel Contaminated Soil. Int. J. Phytoremediat. 2014, 16, 1268–1277. [Google Scholar] [CrossRef]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of Plant Growth-Promoting Bacteria for Biocontrol of Plant Diseases: Principles, Mechanisms of Action, and Future Prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef]
- Ranjan, A.; Rajput, V.D.; Prazdnova, E.V.; Gurnani, M.; Bhardwaj, P.; Sharma, S.; Sushkova, S.; Mandzhieva, S.S.; Minkina, T.; Sudan, J.; et al. Nature’s Antimicrobial Arsenal: Non-Ribosomal Peptides from PGPB for Plant Pathogen Biocontrol. Fermentation 2023, 9, 597. [Google Scholar] [CrossRef]
- Jacob, J.; Krishnan, G.V.; Thankappan, D.; Amma, D.K.B.N.S. 4—Endophytic Bacterial Strains Induced Systemic Resistance in Agriculturally Important Crop Plants. In Microbial Endophytes; Kumar, A., Radhakrishnan, E.K., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 75–105. ISBN 978-0-12-819654-0. [Google Scholar]
- Munif, A.; Putri, D.; Mutaqin, K. Induced Resistance and Plant Growth Promotion by Endophytic Bacteria Bacillus Sp. AA2 against Meloidogyne Sp. on Pepper. IOP Conf. Ser. Earth Environ. Sci. 2020, 468, 012040. [Google Scholar] [CrossRef]
- Shan, Y.; Wang, D.; Zhao, F.-H.; Song, J.; Zhu, H.; Li, Y.; Zhang, X.-J.; Dai, X.-F.; Han, D.; Chen, J.-Y. Insights into the Biocontrol and Plant Growth Promotion Functions of Bacillus altitudinis Strain KRS010 against Verticillium dahliae. BMC Biol. 2024, 22, 116. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Reed, L.; Glick, B.R. The Recent Use of Plant-Growth-Promoting Bacteria to Promote the Growth of Agricultural Food Crops. Agriculture 2023, 13, 1089. [Google Scholar] [CrossRef]
- Artyszak, A.; Gozdowski, D. The Effect of Growth Activators and Plant Growth-Promoting Rhizobacteria (PGPR) on the Soil Properties, Root Yield, and Technological Quality of Sugar Beet. Agronomy 2020, 10, 1262. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Liu, S.-Y.; Sun, X.; Fang, Y. Oenological Potential and Health Benefits of Chinese Non-Vitis vinifera Species: An Opportunity to the Revalorization and to Breed New Varieties. Food Res. Int. 2020, 137, 109443. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Y.; Zhang, H.; Huang, H.; Folta, K.M.; Lu, J. Whole Genome Wide Expression Profiles of Vitis amurensis grape Responding to Downy Mildew by Using Solexa Sequencing Technology. BMC Plant Biol. 2010, 10, 234. [Google Scholar] [CrossRef]
- Liu, L.; Li, H. Review: Research Progress in Amur Grape, Vitis amurensis Rupr. Can. J. Plant Sci. 2013, 93, 565–575. [Google Scholar] [CrossRef]
- Tsukamura, M. Proposal of a New Genus, Gordona, for Slightly Acid-Fast Organisms Occurring in Sputa of Patients with Pulmonary Disease and in Soil. Microbiology 1971, 68, 15–26. [Google Scholar] [CrossRef]
- Frantsuzova, E.; Bogun, A.; Shishkina, L.; Vetrova, A.; Solyanikova, I.; Delegan, Y. Insights into the Potential Role of Gordonia alkanivorans Strains in Biotechnologies. Processes 2023, 11, 3184. [Google Scholar] [CrossRef]
- Kämpfer, P.; Young, C.-C.; Chu, J.-N.; Frischmann, A.; Busse, H.-J.; Arun, A.B.; Shen, F.-T.; Rekha, P.D. Gordonia humi Sp. Nov., Isolated from Soil. Int. J. Syst. Evol. Microbiol. 2011, 61, 65–70. [Google Scholar] [CrossRef]
- Kim, Y.S.; Roh, S.G.; Kim, S.B. Gordonia insulae Sp. Nov., Isolated from an Island Soil. Int. J. Syst. Evol. Microbiol. 2020, 70, 2079–2083. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Sun, S.; Chu, F.; Wang, M.; Zhao, Q.; Shi, J.; Jia, R. Identification and Inactivation of Gordonia, a New Chlorine-Resistant Bacterium Isolated from a Drinking Water Distribution System. J. Water Health 2020, 18, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Muangham, S.; Lipun, K.; Thamchaipenet, A.; Matsumoto, A.; Duangmal, K. Gordonia oryzae Sp. Nov., Isolated from Rice Plant Stems (Oryza sativa L.). Int. J. Syst. Evol. Microbiol. 2019, 69, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Riesco, R.; Rose, J.J.A.; Batinovic, S.; Petrovski, S.; Sánchez-Juanes, F.; Seviour, R.J.; Goodfellow, M.; Trujillo, M.E. Gordonia pseudamarae Sp. Nov., a Home for Novel Actinobacteria Isolated from Stable Foams on Activated Sludge Wastewater Treatment Plants. Int. J. Syst. Evol. Microbiol. 2022, 72, 005547. [Google Scholar] [CrossRef]
- Andalibi, F.; Fatahi-Bafghi, M. Gordonia: Isolation and Identification in Clinical Samples and Role in Biotechnology. Folia Microbiol. 2017, 62, 245–252. [Google Scholar] [CrossRef]
- Amin, A.A.; Wahyuni, A.R.T.; Ekawati, A.W.; Kurniawan, A. Analysis of Polycyclic Aromatic Hydrocarbons (PAHs) Bioremediation by Hydrocarbonoclastic Degrading Bacteria (Gordonia terrae). IOP Conf. Ser. Earth Environ. Sci. 2022, 1036, 012028. [Google Scholar] [CrossRef]
- Xue, Y.; Sun, X.; Zhou, P.; Liu, R.; Liang, F.; Ma, Y. Gordonia paraffinivorans Sp. Nov., a Hydrocarbon-Degrading Actinomycete Isolated from an Oil-Producing Well. Int. J. Syst. Evol. Microbiol. 2003, 53, 1643–1646. [Google Scholar] [CrossRef]
- Hong, S.H.; Ryu, H.; Kim, J.; Cho, K.-S. Rhizoremediation of Diesel-Contaminated Soil Using the Plant Growth-Promoting Rhizobacterium Gordonia Sp. S2RP-17. Biodegradation 2011, 22, 593–601. [Google Scholar] [CrossRef]
- Kayasth, M.; Kumar, V.; Gera, R. Gordonia Sp.: A Salt Tolerant Bacterial Inoculant for Growth Promotion of Pearl Millet under Saline Soil Conditions. 3 Biotech 2014, 4, 553–557. [Google Scholar] [CrossRef]
- Aoyama, K.; Kang, Y.; Yazawa, K.; Gonoi, T.; Kamei, K.; Mikami, Y. Characterization of Clinical Isolates of Gordonia Species in Japanese Clinical Samples During 1998–2008. Mycopathologia 2009, 168, 175–183. [Google Scholar] [CrossRef]
- Ramanan, P.; Deziel, P.J.; Wengenack, N.L. Gordonia Bacteremia. J. Clin. Microbiol. 2013, 51, 3443–3447. [Google Scholar] [CrossRef]
- Rojo, F.; Martínez, J.L. Hydrocarbon Degraders as Pathogens. In Health Consequences of Microbial Interactions with Hydrocarbons, Oils, and Lipids; Springer: Cham, Switzerland, 2019; pp. 1–15. ISBN 978-3-319-72473-7. [Google Scholar]
- Banerjee, S.; Bedics, A.; Tóth, E.; Kriszt, B.; Soares, A.R.; Bóka, K.; Táncsics, A. Isolation of Pseudomonas aromaticivorans Sp. Nov from a Hydrocarbon-Contaminated Groundwater Capable of Degrading Benzene-, Toluene-, m- and p-Xylene under Microaerobic Conditions. Front. Microbiol. 2022, 13, 929128. [Google Scholar] [CrossRef]
- Kaida, N.; Habib, S.; Yasid, N.A.; Shukor, M.Y. Biodegradation of Petroleum Hydrocarbons by Bacillus Spp.: A Review. Bioremediat. Sci. Technol. Res. 2018, 6, 14–21. [Google Scholar] [CrossRef]
- Das, A.; Das, N.; Rajkumari, J.; Pandey, P.; Pandey, P. Exploring the Bioremediation Potential of Bacillus Spp. for Sustainable Mitigation of Hydrocarbon Contaminants. Environ. Sustain. 2024, 7, 135–156. [Google Scholar] [CrossRef]
- Schober, I.; Koblitz, J.; Sardà Carbasse, J.; Ebeling, C.; Schmidt, M.L.; Podstawka, A.; Gupta, R.; Ilangovan, V.; Chamanara, J.; Overmann, J.; et al. BacDive in 2025: The Core Database for Prokaryotic Strain Data. Nucleic Acids Res. 2024, 53, D748–D756. [Google Scholar] [CrossRef] [PubMed]
- Nityagovsky, N.N.; Ananev, A.A.; Suprun, A.R.; Ogneva, Z.V.; Dneprovskaya, A.A.; Tyunin, A.P.; Dubrovina, A.S.; Kiselev, K.V.; Sanina, N.M.; Aleynova, O.A. Distribution of Plasmopara viticola Causing Downy Mildew in Russian Far East Grapevines. Horticulturae 2024, 10, 326. [Google Scholar] [CrossRef]
- Ananev, A.A.; Ogneva, Z.V.; Nityagovsky, N.N.; Suprun, A.R.; Kiselev, K.V.; Aleynova, O.A. Whole Genome Sequencing of Bacillus velezensis AMR25, an Effective Antagonist Strain against Plant Pathogens. Microorganisms 2024, 12, 1533. [Google Scholar] [CrossRef]
- Echt, C.S.; Erdahl, L.A.; McCoy, T.J. Genetic Segregation of Random Amplified Polymorphic DNA in Diploid Cultivated Alfalfa. Genome 1992, 35, 84–87. [Google Scholar] [CrossRef]
- Aleynova, O.A.; Nityagovsky, N.N.; Ananev, A.A.; Suprun, A.R.; Ogneva, Z.V.; Dneprovskaya, A.A.; Beresh, A.A.; Tyunin, A.P.; Dubrovina, A.S.; Kiselev, K.V. The Endophytic Microbiome of Wild Grapevines Vitis amurensis Rupr. and Vitis coignetiae Pulliat Growing in the Russian Far East. Plants 2023, 12, 2952. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving Bacterial Genome Assemblies from Short and Long Sequencing Reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid Annotations Using Subsystems Technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Patz, S.; Gautam, A.; Becker, M.; Ruppel, S.; Rodríguez-Palenzuela, P.; Huson, D. PLaBAse: A Comprehensive Web Resource for Analyzing the Plant Growth-Promoting Potential of Plant-Associated Bacteria. BioRxiv 2021. [Google Scholar] [CrossRef]
- Chivian, D.; Jungbluth, S.P.; Dehal, P.S.; Wood-Charlson, E.M.; Canon, R.S.; Allen, B.H.; Clark, M.M.; Gu, T.; Land, M.L.; Price, G.A.; et al. Metagenome-Assembled Genome Extraction and Analysis from Microbiomes Using KBase. Nat. Protoc. 2023, 18, 208–238. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Ouk Kim, Y.; Park, S.-C.; Chun, J. OrthoANI: An Improved Algorithm and Software for Calculating Average Nucleotide Identity. Int. J. Syst. Evol. Microbiol. 2016, 66, 1100–1103. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.-P.; Göker, M. Genome Sequence-Based Species Delimitation with Confidence Intervals and Improved Distance Functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef]
- Sun, J.; Lu, F.; Luo, Y.; Bie, L.; Xu, L.; Wang, Y. OrthoVenn3: An Integrated Platform for Exploring and Visualizing Orthologous Data across Genomes. Nucleic Acids Res. 2023, 51, W397–W403. [Google Scholar] [CrossRef]
- Chan, P.P.; Lin, B.Y.; Mak, A.J.; Lowe, T.M. tRNAscan-SE 2.0: Improved Detection and Functional Classification of Transfer RNA Genes. Nucleic Acids Res. 2021, 49, 9077–9096. [Google Scholar] [CrossRef]
- Lagesen, K.; Hallin, P.; Rødland, E.A.; Stærfeldt, H.-H.; Rognes, T.; Ussery, D.W. RNAmmer: Consistent and Rapid Annotation of Ribosomal RNA Genes. Nucleic Acids Res. 2007, 35, 3100–3108. [Google Scholar] [CrossRef]
- Richter, M.; Rosselló-Móra, R. Shifting the Genomic Gold Standard for the Prokaryotic Species Definition. Proc. Natl. Acad. Sci. USA 2009, 106, 19126–19131. [Google Scholar] [CrossRef]
- Mukherjee, A.; Gaurav, A.K.; Singh, S.; Yadav, S.; Bhowmick, S.; Abeysinghe, S.; Verma, J.P. The Bioactive Potential of Phytohormones: A Review. Biotechnol. Rep. 2022, 35, e00748. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, V.; Skokan, R.; Depaepe, T.; Kurtović, K.; Haluška, S.; Vosolsobě, S.; Vaculíková, R.; Pil, A.; Dobrev, P.I.; Motyka, V.; et al. Phytohormone Profiling in an Evolutionary Framework. Nat. Commun. 2024, 15, 3875. [Google Scholar] [CrossRef] [PubMed]
- Sosnowski, J.; Truba, M.; Vasileva, V. The Impact of Auxin and Cytokinin on the Growth and Development of Selected Crops. Agriculture 2023, 13, 724. [Google Scholar] [CrossRef]
- García-Tabares, F.; Herraiz-Tomico, T.; Amat-Guerri, F.; Garcia Bilbao, J.L. Production of 3-Indoleacetic Acid and 3-Indolelactic Acid in Azotobacter vinelandii Cultures Supplemented with Tryptophan. Appl. Microbiol. Biotechnol. 1987, 25, 502–506. [Google Scholar] [CrossRef]
- Chennappa, G.; Adkar-Purushothama, C.R.; Suraj, U.; Tamilvendan, K.; Sreenivasa, M.Y. Pesticide Tolerant Azotobacter Isolates from Paddy Growing Areas of Northern Karnataka, India. World J. Microbiol. Biotechnol. 2014, 30, 1–7. [Google Scholar] [CrossRef]
- Ganusova, E.E.; Banerjee, I.; Seats, T.; Alexandre, G. Indole-3-Acetic Acid (IAA) Protects Azospirillum brasilense from Indole-Induced Stress. Appl. Environ. Microbiol. 2025, 91, e02384-24. [Google Scholar] [CrossRef]
- Molina, R.; Rivera, D.; Mora, V.; López, G.; Rosas, S.; Spaepen, S.; Vanderleyden, J.; Cassán, F. Regulation of IAA Biosynthesis in Azospirillum brasilense Under Environmental Stress Conditions. Curr. Microbiol. 2018, 75, 1408–1418. [Google Scholar] [CrossRef]
- Wagi, S.; Ahmed, A. Bacillus Spp.: Potent Microfactories of Bacterial IAA. PeerJ 2019, 7, e7258. [Google Scholar] [CrossRef]
- Goud, M.S.; Sharma, S.K.; Kharbikar, L.L.; Prasanna, R.; Sangwan, S.; Dahuja, A.; Dixit, A. Bacillus Species Consortium with Tryptophan-Dependent and -Independent Pathways Mediated Production of IAA and Its Derivatives Modulates Soil Biological Properties, Growth and Yield of Wheat. Plant Soil 2025, 508, 71–97. [Google Scholar] [CrossRef]
- Abo Elsoud, M.M.; Hasan, S.F.; Elhateir, M.M. Optimization of Indole-3-Acetic Acid Production by Bacillus velezensis Isolated from Pyrus Rhizosphere and Its Effect on Plant Growth. Biocatal. Agric. Biotechnol. 2023, 50, 102714. [Google Scholar] [CrossRef]
- Kumar Ghosh, P.; Kumar Sen, S.; Kanti Maiti, T. Production and Metabolism of IAA by Enterobacter Spp. (Gammaproteobacteria) Isolated from Root Nodules of a Legume Abrus precatorius L. Biocatal. Agric. Biotechnol. 2015, 4, 296–303. [Google Scholar] [CrossRef]
- Luziatelli, F.; Melini, F.; Bonini, P.; Melini, V.; Cirino, V.; Ruzzi, M. Production of Indole Auxins by Enterobacter Sp. Strain P-36 under Submerged Conditions. Fermentation 2021, 7, 138. [Google Scholar] [CrossRef]
- Karmakar, J.; Goswami, S.; Pramanik, K.; Maiti, T.K.; Kar, R.K.; Dey, N. Growth Promoting Properties of Mycobacterium and Bacillus on Rice Plants under Induced Drought. Plant Sci. Today 2021, 8, 49–57. [Google Scholar] [CrossRef]
- Golubev, S.N.; Muratova, A.Y.u.; Panchenko, L.V.; Shchyogolev, S.Y.u.; Turkovskaya, O.V. Mycolicibacterium Sp. Strain PAM1, an Alfalfa Rhizosphere Dweller, Catabolizes PAHs and Promotes Partner-Plant Growth. Microbiol. Res. 2021, 253, 126885. [Google Scholar] [CrossRef]
- AL-Habib, A.A.S. IAA Production by Pseudomonas putida Associated with Rhizosphere of Some Medicine Plants. IOP Conf. Ser. Earth Environ. Sci. 2021, 735, 012076. [Google Scholar] [CrossRef]
- Chen, B.; Luo, S.; Wu, Y.; Ye, J.; Wang, Q.; Xu, X.; Pan, F.; Khan, K.Y.; Feng, Y.; Yang, X. The Effects of the Endophytic Bacterium Pseudomonas fluorescens Sasm05 and IAA on the Plant Growth and Cadmium Uptake of Sedum Alfredii Hance. Front. Microbiol. 2017, 8, 2538. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Hou, X.; Yan, Y.; Liu, T.; Dai, X.; Igarashi, Y.; Fan, L.; Yang, C.; Luo, F. Plant Growth-Promoting and Arsenic Accumulation Reduction Effects of Two Endophytic Bacteria Isolated from Brassica Napus. J. Plant Growth Regul. 2024, 43, 76–88. [Google Scholar] [CrossRef]
- Thoa, N.T.K.; Mai, D.T.H.; Hiu, B.L.; Duong, C.A.; Chau, N.N.B.; Nghiep, N.M.; Van Minh, N.; Quoc, N.B. Roles of β-Indole Acetic Acid (IAA) Producing Endophytic Bacteria on the Recovery of Plant Growth and Survival Ability of Sugarcane Infected White Leaf Disease (SWLD). Curr. Microbiol. 2022, 79, 389. [Google Scholar] [CrossRef]
- Alotaibi, F.; St-Arnaud, M.; Hijri, M. In-Depth Characterization of Plant Growth Promotion Potentials of Selected Alkanes-Degrading Plant Growth-Promoting Bacterial Isolates. Front. Microbiol. 2022, 13, 863702. [Google Scholar] [CrossRef]
- McClerklin, S.A.; Lee, S.G.; Harper, C.P.; Nwumeh, R.; Jez, J.M.; Kunkel, B.N. Indole-3-Acetaldehyde Dehydrogenase-Dependent Auxin Synthesis Contributes to Virulence of Pseudomonas syringae Strain DC3000. PLoS Pathog. 2018, 14, e1006811. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.; Meng, Z.; Wang, B.; Chen, M. Research Progress on the Roles of Cytokinin in Plant Response to Stress. Int. J. Mol. Sci. 2020, 21, 6574. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, M.S.; Ali, H.H.; Iqbal, M.A.; Erinle, K.O.; Javed, T.; Iqbal, J.; Hashmi, M.I.U.; Mumtaz, M.Z.; Salama, E.A.A.; Kalaji, H.M.; et al. Cytokinin Production by Azospirillum brasilense Contributes to Increase in Growth, Yield, Antioxidant, and Physiological Systems of Wheat (Triticum aestivum L.). Front. Microbiol. 2022, 13, 886041. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Veselov, S.U.; Melentiev, A.I.; Martynenko, E.V.; Kudoyarova, G.R. Ability of Bacterium Bacillus Subtilis to Produce Cytokinins and to Influence the Growth and Endogenous Hormone Content of Lettuce Plants. Plant Soil. 2005, 272, 201–209. [Google Scholar] [CrossRef]
- Park, Y.-G.; Mun, B.-G.; Kang, S.-M.; Hussain, A.; Shahzad, R.; Seo, C.-W.; Kim, A.-Y.; Lee, S.-U.; Oh, K.Y.; Lee, D.Y.; et al. Bacillus aryabhattai SRB02 Tolerates Oxidative and Nitrosative Stress and Promotes the Growth of Soybean by Modulating the Production of Phytohormones. PLoS ONE 2017, 12, e0173203. [Google Scholar] [CrossRef]
- Großkinsky, D.K.; Tafner, R.; Moreno, M.V.; Stenglein, S.A.; García de Salamone, I.E.; Nelson, L.M.; Novák, O.; Strnad, M.; van der Graaff, E.; Roitsch, T. Cytokinin Production by Pseudomonas fluorescens G20-18 Determines Biocontrol Activity against Pseudomonas syringae in Arabidopsis. Sci. Rep. 2016, 6, 23310. [Google Scholar] [CrossRef] [PubMed]
- Nouioui, I.; Cortés-albayay, C.; Carro, L.; Castro, J.F.; Gtari, M.; Ghodhbane-Gtari, F.; Klenk, H.-P.; Tisa, L.S.; Sangal, V.; Goodfellow, M. Genomic Insights into Plant-Growth-Promoting Potentialities of the Genus Frankia. Front. Microbiol. 2019, 10, 1457. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Hernández, A.G.; Glick, B.R.; Rossi, M.J. Plant Growth-Promoting Activities and Genomic Analysis of the Stress-Resistant Bacillus megaterium STB1, a Bacterium of Agricultural and Biotechnological Interest. Biotechnol. Rep. 2020, 25, e00406. [Google Scholar] [CrossRef] [PubMed]
- Frébortová, J.; Frébort, I. Biochemical and Structural Aspects of Cytokinin Biosynthesis and Degradation in Bacteria. Microorganisms 2021, 9, 1314. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Ashraf, U.; Li, G.; Li, Y.; Lu, W.; Gao, L.; Han, F.; Hu, J. Exogenous γ-Aminobutyric Acid (GABA) Application Improved Early Growth, Net Photosynthesis, and Associated Physio-Biochemical Events in Maize. Front. Plant Sci. 2016, 7, 919. [Google Scholar] [CrossRef]
- Xie, S.-S.; Wu, H.-J.; Zang, H.-Y.; Wu, L.-M.; Zhu, Q.-Q.; Gao, X.-W. Plant Growth Promotion by Spermidine-Producing Bacillus subtilis OKB105. Mol. Plant-Microbe Interact. 2014, 27, 655–663. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.d.C.; Flores, A.; Rojas-Sánchez, B.; Urtis-Flores, C.A.; Morales-Cedeño, L.R.; Valencia-Marin, M.F.; Chávez-Avila, S.; Rojas-Solis, D.; Santoyo, G. Plant Growth-Promoting Bacteria as Bioinoculants: Attributes and Challenges for Sustainable Crop Improvement. Agronomy 2021, 11, 1167. [Google Scholar] [CrossRef]
- Bakhshandeh, E.; Pirdashti, H.; Lendeh, K.S. Phosphate and Potassium-Solubilizing Bacteria Effect on the Growth of Rice. Ecol. Eng. 2017, 103, 164–169. [Google Scholar] [CrossRef]
- Timofeeva, A.; Galyamova, M.; Sedykh, S. Prospects for Using Phosphate-Solubilizing Microorganisms as Natural Fertilizers in Agriculture. Plants 2022, 11, 2119. [Google Scholar] [CrossRef]
- Hoberg, E.; Marschner, P.; Lieberei, R. Organic Acid Exudation and pH Changes by Gordonia Sp. and Pseudomonas fluorescens Grown with P Adsorbed to Goethite. Microbiol. Res. 2005, 160, 177–187. [Google Scholar] [CrossRef]
- Deb, C.; Tatung, M. Siderophore Producing Bacteria as Biocontrol Agent against Phytopathogens for a Better Environment: A Review. S. Afr. J. Bot. 2024, 165, 153–162. [Google Scholar] [CrossRef]
- Swify, S.; Mažeika, R.; Baltrusaitis, J.; Drapanauskaitė, D.; Barčauskaitė, K. Review: Modified Urea Fertilizers and Their Effects on Improving Nitrogen Use Efficiency (NUE). Sustainability 2024, 16, 188. [Google Scholar] [CrossRef]
- Li, C.-K.; Chen, R.-Y. Ammonium Bicarbonate Used as a Nitrogen Fertilizer in China. Fertil. Res. 1980, 1, 125–136. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Q.; Chen, Y.; Yang, Y.; Zhou, C.; Yu, J.; Li, Y.; Qiu, L. Ammonia-Assimilating Bacteria Promote Wheat (Triticum aestivum) Growth and Nitrogen Utilization. Microorganisms 2025, 13, 43. [Google Scholar] [CrossRef]
- Silva, N.M.; Oliveira, A.M.S.A.d.; Pegorin, S.; Giusti, C.E.; Ferrari, V.B.; Barbosa, D.; Martins, L.F.; Morais, C.; Setubal, J.C.; Vasconcellos, S.P.; et al. Characterization of Novel Hydrocarbon-Degrading Gordonia paraffinivorans and Gordonia sihwensis Strains Isolated from Composting. PLoS ONE 2019, 14, e0215396. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Li, H.; Wu, H.; Ren, H.; Kong, X.; Lu, Z. Resting for Viability: Gordonia polyisoprenivorans ZM27, a Robust Generalist for Petroleum Bioremediation under Hypersaline Stress. Environ. Pollut. 2024, 360, 124618. [Google Scholar] [CrossRef] [PubMed]
- Lo Piccolo, L.; De Pasquale, C.; Fodale, R.; Puglia, A.M.; Quatrini, P. Involvement of an Alkane Hydroxylase System of Gordonia Sp. Strain SoCg in Degradation of Solid n-Alkanes. Appl. Environ. Microbiol. 2011, 77, 1204–1213. [Google Scholar] [CrossRef]
- Kotoky, R.; Pandey, P. Rhizosphere Assisted Biodegradation of Benzo(a)Pyrene by Cadmium Resistant Plant-Probiotic Serratia Marcescens S2I7, and Its Genomic Traits. Sci. Rep. 2020, 10, 5279. [Google Scholar] [CrossRef]
- Abdou, A.; Alkhateeb, O.; Eldin, H.; Ghazzawy, H.; Albadrani, M.; Al-harbi, N.; Al-Shammari, W.; Abdelaal, K. Application of Plant Growth-Promoting Bacteria as an Eco-Friendly Strategy for Mitigating the Harmful Effects of Abiotic Stress on Plants. Phyton 2023, 92, 3305–3321. [Google Scholar] [CrossRef]
- Fanai, A.; Bohia, B.; Lalremruati, F.; Lalhriatpuii, N.; Lalrokimi; Lalmuanpuii, R.; Singh, P.K.; Zothanpuia. Plant Growth Promoting Bacteria (PGPB)-Induced Plant Adaptations to Stresses: An Updated Review. PeerJ 2024, 12, e17882. [Google Scholar] [CrossRef]
- Ali, A.; Guo, D.; Li, Y.; Shaheen, S.M.; Wahid, F.; Antoniadis, V.; Abdelrahman, H.; Al-Solaimani, S.G.; Li, R.; Tsang, D.C.W.; et al. Streptomyces pactum Addition to Contaminated Mining Soils Improved Soil Quality and Enhanced Metals Phytoextraction by Wheat in a Green Remediation Trial. Chemosphere 2021, 273, 129692. [Google Scholar] [CrossRef]
- Abulfaraj, A.A.; Jalal, R.S. Use of Plant Growth-Promoting Bacteria to Enhance Salinity Stress in Soybean (Glycine max L.) Plants. Saudi J. Biol. Sci. 2021, 28, 3823–3834. [Google Scholar] [CrossRef]
- Ali, M.A.; Ahmed, T.; Ibrahim, E.; Rizwan, M.; Chong, K.P.; Yong, J.W.H. A Review on Mechanisms and Prospects of Endophytic Bacteria in Biocontrol of Plant Pathogenic Fungi and Their Plant Growth-Promoting Activities. Heliyon 2024, 10, e31573. [Google Scholar] [CrossRef]
- Bonaterra, A.; Badosa, E.; Daranas, N.; Francés, J.; Roselló, G.; Montesinos, E. Bacteria as Biological Control Agents of Plant Diseases. Microorganisms 2022, 10, 1759. [Google Scholar] [CrossRef]
- Ryu, C.-M.; Farag, M.A.; Hu, C.-H.; Reddy, M.S.; Kloepper, J.W.; Paré, P.W. Bacterial Volatiles Induce Systemic Resistance in Arabidopsis. Plant Physiol. 2004, 134, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Lee, S.J.; Moon, J.H.; Park, K.H.; Yang, K.Y.; Cho, B.H.; Kim, K.Y.; Kim, Y.W.; Lee, M.C.; Anderson, A.J.; et al. GacS-Dependent Production of 2R, 3R-Butanediol by Pseudomonas chlororaphis O6 Is a Major Determinant for Eliciting Systemic Resistance Against Erwinia carotovora but Not Against Pseudomonas syringae Pv. Tabaci in Tobacco. Mol. Plant-Microbe Interact. 2006, 19, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Pramanik, K.; Ghosh, S.K.; Pal, P.; Mondal, T.; Soren, T.; Maiti, T.K. Unraveling the Role of Plant Growth-Promoting Rhizobacteria in the Alleviation of Arsenic Phytotoxicity: A Review. Microbiol. Res. 2021, 250, 126809. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Wang, G. Genomic Evidence Reveals the Extreme Diversity and Wide Distribution of the Arsenic-Related Genes in Burkholderiales. PLoS ONE 2014, 9, e92236. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, N.; Bukhari, M.A.; Ahmad, T.; Ahmad, Z.; Jatoi, W.N.; Abbas, S.M.; Latif, A.; Raza, A.; Aurangzaib, M.; Hashem, A.; et al. Exogenously Applied Nicotinic Acid Alleviates Drought Stress by Enhancing Morpho-Physiological Traits and Antioxidant Defense Mechanisms in Wheat. Ecotoxicol. Environ. Saf. 2023, 263, 115350. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Tian, Y.; Hou, X.; Hou, X.; Jia, Z.; Li, M.; Hao, M.; Jiang, Y.; Wang, Q.; Pu, Q.; et al. Multiple Forms of Vitamin B6 Regulate Salt Tolerance by Balancing ROS and Abscisic Acid Levels in Maize Root. Stress Biol. 2022, 2, 39. [Google Scholar] [CrossRef]
- Alsamadany, H.; Mansour, H.; Elkelish, A.; Ibrahim, M.F.M. Folic Acid Confers Tolerance against Salt Stress-Induced Oxidative Damages in Snap Beans through Regulation Growth, Metabolites, Antioxidant Machinery and Gene Expression. Plants 2022, 11, 1459. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).