Abstract
The commercial production of grapevines (Vitis vinifera L.) relies heavily on rootstocks that are hybrids of non-vinifera parentage. The relatively newly released GRN rootstocks (GRN-1, GRN-2, GRN-3, GRN-4, and GRN-5) were bred from especially under-studied genetic backgrounds. This study aimed to evaluate ungrafted GRN-series grape rootstocks under moderate water-stress conditions and to characterize and compare their physiological performances. Each of the GRN rootstocks had specific physiological characteristics that would make them suitable for a wide range of growing conditions and vineyard management goals. GRN-1 had growth habits which were more vigorous and the highest carbohydrate storage levels, while GRN-2 had the highest level of nitrogen and the largest leaf area, but the lowest levels of carbohydrate storage. GRN-3 was less tolerant to high-salinity soils, and had the longest internodes, while GRN-4 had high boron levels, which supports flowering and fruit set, and short internodes. GRN-5 was consistently moderate across all measured areas, except internode thickness, for which it was the highest. These findings show the variations in physiological growth habits among the ungrafted GRN-series rootstocks and suggest that growth habits, carbohydrate storage, leaf canopy, fruit production, and nutrition vary based on rootstock parentage. Further investigation is needed to determine whether these characteristics persist when grafted onto Vitis vinifera L. scions.
1. Introduction
Winegrapes (Vitis vinifera L.) are commonly grown as a grafted vine using North American species or interspecific hybrids as rootstocks due to their to phylloxera and various nematode species [1,2,3,4,5]. The first use of rootstocks in viticulture was during the phylloxera epidemic in European vineyards during the late 19th century. After botanists observed that North American grape species had tolerance to phylloxera, vineyards were replanted using grafted vines with V. vinifera as the scion and North American grape species as the rootstock to reduce the damage caused by phylloxera [2,3,6,7]. Currently, grape rootstocks are selected for their resistance to phylloxera and for a variety of other desired characteristics, such as ease of rooting, grafting compatibility, resistance to nematodes, tolerance to high lime and salinity, and tolerance to drought [1,6,7,8,9,10,11]. Since grapevines are commonly cultivated in areas with frequent drought events, such as California, selecting rootstocks that allow grapevines to remain productive under water-stress conditions is highly desired. Nematodes are a significant challenge for grapevine cultivation in North America, especially the root-knot (Meloidogyne spp.) and dagger (Xiphinema spp.) nematodes, as their feeding on grapevine roots can lower vine productivity, prevent the transport of nutrients and water within the vine [12], vector viruses [13,14,15,16,17], and can sometimes lead to vine death. Nematode-resistant rootstocks have now become one of the most effective strategies for mitigating the effects of nematode damage [6,9,13,18]. The University of California, Davis developed nematode-resistant rootstocks (UCD GRN series) that were released in 2008 and were bred from diverse genetic combinations. However, important physiological characteristics such as growth parameters, optimal soil conditions, and canopy management strategies remain under-studied.
Five ungrafted grape rootstocks of varying parentage were evaluated in this study. All are resistant to root-knot nematode (Meloidogyne incognita Race 3, M. incognita pathotype Harmony C, Meloidogyne arenaria pathotype Harmony A) and dagger nematode (Xiphinema index), and moderately resistant to root lesion nematode (Pratylenchus vulnus), pin nematode (Paratylenchus hamatus), ring nematode (Mesocriconema xenoplax), and citrus nematode (Tylenchus semipenetrans) [5]. While these rootstocks have been evaluated as to nematode resistance in California [5,6] and limited physiological evaluations have been performed in Texas [19,20] and Missouri [13], a comprehensive evaluation of their fitness and performance under moderate drought conditions has not been completed. This paper evaluates five GRN rootstocks as to their physiological metrics, including canopy architecture, leaf area index, photosynthetic active radiation, carbohydrates, macro- and micronutrient analysis, pruning weights, and internode parameters, in order to evaluate the suitability of GRN rootstocks for a wide range of viticultural environments.
2. Materials and Methods
2.1. Vineyard Site and Experimental Design
The ungrafted GRN rootstocks were planted in 2016 and were studied in 2021 and 2022 in a research vineyard in San Luis Obispo, CA, USA (35°19′02.3″ N 120°41′03.0″ W) located within the San Luis Obispo Coast viticultural area. The vineyard block was organized as a completely randomized design with three replications per rootstock (n = 3). Each replication consisted of three rows of three vines, for a total of nine vines per rootstock block replicate. The GRN rootstock series evaluated included UCD GRN-1 (Vitis rupestris × Muscadinia rotundifolia), UCD GRN-2 ((Vitis rufotomentosa × (‘Dog Ridge’ × ‘Riparia Gloire’)) × ‘Riparia Gloire’), UCD GRN-3 ((V. rufotomentosa × (‘Dog Ridge’ × ‘Riparia Gloire’)) × Vitis champinii c9038), UCD GRN-4 ((V. rufotomentosa × (‘Dog Ridge’ × ‘Riparia Gloire’)) × V. champinii c9038), and UCD GRN-5 ((‘Ramsey’ × ‘Riparia Gloire’) × V. champinii c9021). The dominant soil type at this site is Los Osos loam (USDA NRCS web soil survey, accessed 2023) and is described as a moderately deep, well-drained soil that is formed from weathered sandstone and shale. The vines were planted in rows with a north–south orientation, on a 15% slope, and with a 1.2 m in-row × 2.4 m between-row spacing. The vines were spur-pruned and bilateral cordon trained on a modified vertical shoot-positioned (VSP) trellis system, with the cordon developed 0.889 m from the ground. The modified trellis system had three cross-arms, with the first cross-arm located 0.254 m above the cordon wire. The cross arm had two wires located on both ends separated 0.3048 m apart and two interior rake wires. The next set of cross arms were located 0.254 m above the first cross arm and had two wires on either end and were spaced 0.4064 m apart. The final cross-arm was located 0.254 m above the second cross-arm, with the wires on the ends spaced 0.4572 m apart. Drip irrigation was used at this site, employing two 2.01-L/h pressure-compensating emitters per vine, spaced approximately 0.6 m on either side of the trunk. Grapevines were irrigated at 100% evapotranspiration (ET) demand from budbreak to full canopy size. When the vines were at full canopy size, the vines were irrigated at 75% ET to implement moderate water stress. Vine water status was measured using a pressure chamber (PMS Instruments, Albany, OR, USA) to maintain a target leaf-water potential between −1.1 and −1.3 MPa at mid-day. Multiple leaves from each rootstock were measured. Each leaf was placed inside a plastic bag and cut at the base of the petiole with a sharp razor blade immediately after being bagged. Once cut, the leaf was immediately put into the pressure chamber, and the pressure readings (MPa) were recorded when the sap from the petiole began to emerge from the cut surface.
2.2. Climate Data
Weather data were obtained from California Irrigation Information Management System (CIMIS) station 52 (35°18′20″ N 120°39′42″ W), located 2.42 km from the experimental site. Seasonal (1 April to 31 October) and annual (1 January to 31 December) cumulative growing-degree days (GDD) were calculated from daily maximum and minimum air temperatures, using a baseline temperature of 10 °C (Table 1 and Table 2).

Table 1.
Growing-degree days (GDD); Winkler region classification; and precipitation for San Luis Obispo, California (USA) CIMIS station 52.

Table 2.
Monthly average air temperature, minimum air temperature, and maximum air temperature for San Luis Obispo, CA (USA) CIMIS station 52 during the 2021 and 2022 growing seasons.
2.3. Phenology and Senescence Tracking
Phenology tracking occurred every two weeks throughout the growing season on marked data vines to identify key phenological events. Four random shoots per vine were selected (2 shoots/cordon), and their stages of development were assessed using the Modified Eichhorn–Lorenz (E-L) system [20]. Three vine replicates of each rootstock were evaluated. The phenology of male rootstocks placed an emphasis on degree of cane maturation, from berry set to complete cane maturation, due to the lack of developed fruit. Once the vines reached full cane maturation, the Dodson–Walker Senescence scale [21] was used to track leaf abscission on the same designated data collection vines every two weeks, alongside phenology tracking.
2.4. Canopy Architecture, Leaf Area, and Photosynthetically Active Radiation
Shoot internode length and diameter measurements were collected when grapevines achieved full canopy size, using Neiko 0–200 mm digital calipers (Zhejiang Kangle Group, Wenzhou, China). Measurements were collected from four randomly selected shoots, two from each cordon, at the fourth internode above the base of the shoot. Measurements were collected from three vine replicates per treatment. Internode diameters were measured at the thinnest part of the internode (middle of the internode). Leaf area index and photosynthetically active radiation within the canopy (light penetration) were collected at full canopy using an AccuPAR LP-80 PAR/LAI Ceptometer (METER Group, Inc. USA, Pullman, WA, USA) during the 2021 and 2022 growing seasons. The ceptometer was inserted into the vine between the third and fifth internode above the base, where the shoots originate. The instrument was allowed to stabilize for 5 s before three measurements were recorded, each with a 5 s delay interval. Data was collected between 1200 and 1400 h on full-sun days on which the photosynthetically active radiation (PAR) exceeded 1800 µmol m−2s−1. After the completion of cane maturation, all leaves were removed, collected, and scanned from the three replicate grapevines. Leaves were scanned using an LI-3100C leaf area scanner (LI-COR, Lincoln, NE, USA) to determine the leaf area.
2.5. Nutritional Analysis
Petiole and lamina samples were collected from mature leaves opposite the basal clusters at full bloom during the 2022 growing season. In total, 25–30 lamina and petiole samples were collected per replicate (n = 3). Once tissue samples were collected, petioles and lamina were immediately separated, placed into paper bags, and then sent to Fruit Grower’s Laboratory, Inc. for nutritional analysis. Leaf and petiole samples were subsequently dried, ground, and analyzed using the University of California tissue analysis procedure [22]. The total amount of nitrogen was quantitatively analyzed using sample combustion coupled with thermal conductivity/infrared detection. This analysis is based on sample oxidation by “flash combustion”, which converts organic and inorganic materials into combustion gases. All other nutrients were analyzed using the nitric acid digestion method, which quantitatively determines the concentration using a nitric acid and hydrogen peroxide microwave digestion. The concentrations were then determined using Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES) (Table 3, Table 4 and Table 5).
2.6. Pruning Weights
Pruning weights were collected in February of 2022 and 2023 (Table 6). All vines were pruned to one-bud spurs, and all plant material removed during pruning was tied together using bungee cords and weighed using a hanging scale (Dr. Meter, FS01, San Jose, CA, USA). Data were collected from each vine and pooled within each experimental unit (n = 3).
2.7. Carbohydrate Analysis
Trunk samples were collected from each rootstock and were used for carbohydrate analysis during dormancy at the end of the 2021 growing season (Table 7). The trunk samples were collected by drilling to approximately mid-depth using a 12.6 mm spade drill bit. Trunk samples were immediately dried after collection for 12 h at 55 °C in a forced-air oven. After drying, the samples were ground with a Wiley Mill Model 4 mill (Thomas Scientific, Swedesboro, NJ, USA) until samples were fine enough to pass through a 40-mesh screen. Soluble carbohydrates (free sugars fructose, glucose, and sucrose) were determined through an established procedure [23]. Total non-structural carbohydrates (TNC) and starch were quantitatively derived from the total glucose, free fructose, and free sucrose, where total glucose was enzymatically hydrolyzed at 55 °C with amyloglucosidase for 12 h and analyzed by high-performance liquid chromatography (HPLC) with mass selective detection. The analysis used a Phenomenex Luna NH2 (250 mm × 4.6 mm) HPLC column at a flow rate of 2.75 mL min−1 acetonitrile/water (78:22).
2.8. Statistical Analysis
Vine growth and productivity statistical analyses were performed using JMP 16 statistical software (SAS Institute, Cary, NC, USA). Normality was assessed using a normal quantile plot and a Shapiro–Wilk test. Data that did not meet normality assumptions were transformed using a Box Cox transformation. Homogeneity of variance was evaluated using a Bartlett test. Growth and productivity data were analyzed using one-way and two-way ANOVA, and statistically significant variables (p < 0.05) were subjected to a multiple comparison test and were evaluated using a Fisher’s LSD test. A principal component analysis was performed in R (RStudioversion 2024.04.2 + 764), using internode length, diameter, and pruning weights to identify growth patterns among rootstocks. All data were standardized prior to analysis.
3. Results
3.1. Climate
The 2022 growing season was substantially warmer than the 2021 growing season (Table 1). The 2022 growing season was classified within Winkler Region (III), while 2021 was classified within Winkler Region II. The difference in growing-degree days between the two seasons was 191.1 growing-degree days, while the difference in annual precipitation was 166 mm. Rainfall was higher in 2021, compared to 2022.
3.2. Phenology and Senescence
Progression through the phenological and senescence-based scales generally varied among rootstocks during the 2021 and 2022 growing seasons. Phenology and senescence tracking data were similar in both seasons, with the notable exceptions described below. Therefore, only the 2022 data are presented (Figure 1 and Figure 2). The trend in progression through the numerical scale was similar in both years. However, GRN-5 showed different trends in 2021 and 2022. During 2021, GRN-5 had a later progression, when compared to the other rootstocks, but in 2022, GRN-5 consistently displayed earlier progression throughout the growing season. All other rootstocks had a consistent trend in both years, especially GRN-1. GRN-1 had a significantly slower progression through the numerical scale than the other rootstocks. Senescence tracking displayed a trend similar to the phenology tracking, and varied among rootstocks. GRN-5 had an earlier progression through the senescence scale during both growing seasons. Similarly, GRN-3 and GRN-4 were among the slower-senescing rootstocks in both years. GRN-1 had different trends in these two years. In 2021, GRN-1 was among the earlier senescing rootstocks, but in 2022 GRN-1 lagged significantly behind, and was the last to go into dormancy.
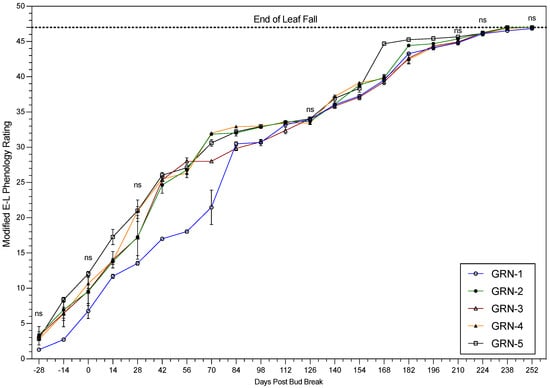
Figure 1.
Phenology ratings of ungrafted GRN rootstocks in the 2022 growing season. One-way analysis of variance (ANOVA) displaying rootstock mean values followed by the standard error of the mean (n = 3). The letters “ns” signify the lack of significant differences based on a Fisher’s LSD test (p < 0.05).
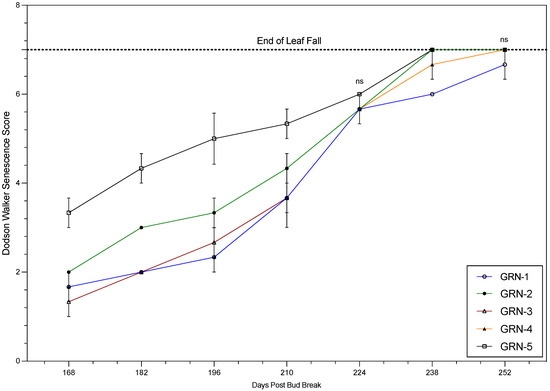
Figure 2.
Senescence scores of ungrafted rootstocks during the 2022 growing season. One-way analysis of variance (ANOVA) displaying rootstock mean values, with standard error bars (n = 3). The letters “ns” signify the lack of significant differences from each other based on a Fisher’s LSD test (p < 0.05).
3.3. Canopy Architecture, Photosynthetically Active Radiation, Leaf Area Index, and Total Leaf Area
Photosynthetically active radiation in the canopy as a percentage of full sun is displayed in Table 3. Light penetration into the canopy varied between growing seasons (p = 0.0009) and among rootstocks (p = 0.0143). There was no significant year-by-rootstock interaction (p = 0.3271). From 2021 to 2022, light penetration into the canopy decreased from 0.2% of full sun to 0.03% of full sun. GRN-4 had the highest percentage of outside PAR, at 0.24%, while GRN-2 had the lowest percentage, at 0.01%. GRN-4 was not significantly different from GRN-3 or GRN-5. The leaf area index and total leaf area (Table 4) had a significant year-by-rootstock interaction (p = 0.0088 and p = 0.0473, respectively). Total leaf area varied significantly among rootstocks, with GRN-1 and GRN-2 having the larger areas in both years. GRN-5 was among the rootstocks with the largest leaf area in 2021 but was among the smallest in 2022. GRN-4 was consistent between both years and had a relatively small total leaf area.

Table 3.
Two-way analysis of variance (ANOVA) with interaction, displaying average % of outside-PAR values for ungrafted GRN rootstocks at full canopy size during the 2021 and 2022 growing seasons. Table shows year and rootstock means followed by the standard error of the mean. Different letters within a column indicate significant differences based on a Fisher’s LSD test (n = 6) (p < 0.05). Significant p-values (<0.05) are displayed in bold.
Table 3.
Two-way analysis of variance (ANOVA) with interaction, displaying average % of outside-PAR values for ungrafted GRN rootstocks at full canopy size during the 2021 and 2022 growing seasons. Table shows year and rootstock means followed by the standard error of the mean. Different letters within a column indicate significant differences based on a Fisher’s LSD test (n = 6) (p < 0.05). Significant p-values (<0.05) are displayed in bold.
| % Outside PAR | |
|---|---|
| Year | |
| 2021 | 0.20 ± 0.06 a |
| 2022 | 0.03 ± 0.01 b |
| Rootstock | |
| GRN-1 | 0.04 ± 0.03 bc |
| GRN-2 | 0.01 ± 0.01 c |
| GRN-3 | 0.18 ± 0.11 ab |
| GRN-4 | 0.24 ± 0.11 a |
| GRN-5 | 0.11 ± 0.04 ab |
| p-value | 0.0044 |
| Year | 0.0009 |
| Rootstock | 0.0143 |
| Y × R | 0.3271 |

Table 4.
Two-way analysis of variance (ANOVA) with interaction, displaying average leaf area index at full canopy size and average total leaf area for ungrafted GRN rootstocks during the 2021 and 2022 growing seasons. The table displays the year-by-rootstock interaction effect followed by the standard error of the mean. Different letters within a column indicate significant differences based on a Fisher’s LSD test (p < 0.05). Significant p-values (<0.05) are displayed in bold.
Table 4.
Two-way analysis of variance (ANOVA) with interaction, displaying average leaf area index at full canopy size and average total leaf area for ungrafted GRN rootstocks during the 2021 and 2022 growing seasons. The table displays the year-by-rootstock interaction effect followed by the standard error of the mean. Different letters within a column indicate significant differences based on a Fisher’s LSD test (p < 0.05). Significant p-values (<0.05) are displayed in bold.
| Year | Rootstock | LAI | Leaf Area (m2) |
|---|---|---|---|
| 2021 | GRN-1 | 7.64 ± 1.89 de | 10.6 ± 1.64 abc |
| GRN-2 | 9.93 ± 1.23 cd | 13.1 ± 2.86 a | |
| GRN-3 | 4.47 ± 2.22 e | 7.72 ± 1.86 bcd | |
| GRN-4 | 3.03 ± 1.02 e | 2.47 ± 0.07 e | |
| GRN-5 | 4.35 ± 0.43 e | 12.1 ± 1.17 ab | |
| 2022 | GRN-1 | 13.6 ± 2.59 bc | 11.0 ± 2.60 ab |
| GRN-2 | 12.3 ± 0.54 bcd | 14.0 ± 0.82 a | |
| GRN-3 | 15.0 ± 2.49 ab | 11.0 ± 1.17 ab | |
| GRN-4 | 18.6 ± 0.49 a | 5.80 ± 0.49 cde | |
| GRN-5 | 14.4 ± 1.60 abc | 5.05 ± 1.09 de | |
| p-value | <0.0001 | 0.001 | |
| Year | <0.0001 | 0.8748 | |
| Rootstock | 0.7985 | 0.0003 | |
| Y × R | 0.0088 | 0.0473 |
Internode length and diameter measurements (Table 5) were consistent between growing seasons (p = 0.05 and p = 0.274, respectively) but varied by rootstock (p < 0.0001). Internode length was lower in 2021 compared to 2022 and was near the threshold for statistical significance (p = 0.05). Internode length measurements were observed to deviate into four different groups. GRN-3 had the longest average internode length, at 102.7 mm, followed by GRN-5, with a size of 82.4 mm. GRN-2 had the third-longest internode length, at 71.1 mm, while GRN-1 and GRN-4 had the shortest lengths, at 57.8 mm and 47.9 mm, respectively. All rootstocks were significantly different from each other, except for GRN-1 and GRN-4. Internode diameters followed a trend similar to that of the internode length measurements. Instead of diverging into four distinct groupings, diameter measurements were split into three groups. GRN-5 and GRN-3 had reversed results when compared to the internode length measurements. For diameter measurements, GRN-5 had the thickest diameter at 8.18 mm, and GRN-3 had a thickness of 7.17 mm. Like the internode length measurements, GRN-1 and GRN-4 also had the thinnest diameter measurements, compared to the other rootstocks. The internode diameter of GRN-2 was not significantly different from GRN-5 or GRN-3, and it had an average diameter of 7.37 mm.

Table 5.
Two-way analysis of variance (ANOVA) with interaction, displaying average internode length and diameter values for ungrafted GRN rootstocks at full canopy size during the 2021 and 2022 growing seasons. The table shows the year and rootstock means followed by the standard error of the mean. Different letters within a column indicate significant differences based on a Fisher’s LSD test (n = 3) (p < 0.05). Significant p-values (<0.05) are displayed in bold.
Table 5.
Two-way analysis of variance (ANOVA) with interaction, displaying average internode length and diameter values for ungrafted GRN rootstocks at full canopy size during the 2021 and 2022 growing seasons. The table shows the year and rootstock means followed by the standard error of the mean. Different letters within a column indicate significant differences based on a Fisher’s LSD test (n = 3) (p < 0.05). Significant p-values (<0.05) are displayed in bold.
| Internode Length (mm) | Internode Diameter (mm) | |
|---|---|---|
| Year | ||
| 2021 | 69.0 ± 3.22 a | 6.85 ± 0.17 a |
| 2022 | 75.8 ± 3.65 a | 7.15 ± 0.25 a |
| Rootstock | ||
| GRN-1 | 57.8 ± 3.06 d | 6.22 ± 0.17 c |
| GRN-2 | 71.1 ± 2.85 c | 7.37 ± 0.22 ab |
| GRN-3 | 102.7 ± 6.25 a | 7.17 ± 0.47 b |
| GRN-4 | 47.9 ± 2.83 d | 6.05 ± 0.22 c |
| GRN-5 | 82.4 ± 3.16 b | 8.18 ± 0.31 a |
| p-value | <0.0001 | 0.0002 |
| Year | 0.05 | 0.274 |
| Rootstock | <0.0001 | <0.0001 |
| Y × R | 0.4742 | 0.9236 |
3.4. Nutrition
A one-way analysis of variance revealed that several nutrient concentrations, including total N (p < 0.0001), nitrate N (p = 0.0002), P (p = 0.0001), Zn (p < 0.0001), Cu (p = 0.0001), B (p = 0.0007), and Na (p = 0.0002), varied significantly among rootstocks (Figure 3, Figure 4 and Figure 5). The total nitrogen concentration was observed to be highest in GRN-2 (4.93%) and lowest in GRN-3 (3.07%). Rootstocks GRN-1 and GRN-4 were not significantly different from each other and had concentrations higher than those of GRN-5 and GRN-3. GRN-2 also had the highest nitrate N concentration (2840.0 ppm), but this was not significantly higher than that of GRN-4 (2073.3 ppm). No significant variation was observed in nitrate N for the other rootstocks. The phosphorus concentration was considerably higher in GRN-1, with a concentration of 1.22%. No significant variations in phosphorus concentrations were observed among the remaining rootstocks. Zinc concentrations varied among rootstocks and were highest in GRN-5 (121.3 ppm) and GRN-1 (110 ppm). Although GRN-2 had the lowest concentration, it was not significantly different from GRN-3 or GRN-4. Copper concentrations also varied among rootstocks, with the highest concentrations in GRN-2 (40.1 ppm) and GRN-5 (34.8 ppm). The lowest copper concentration was observed in GRN-1 (21.9 ppm), but this was not significantly smaller than the concentration found in GRN-3 (24.2 ppm). GRN-4 had the highest boron concentration (60.6 ppm) but was not significantly different from GRN-2 in this respect. In addition to not being different from GRN-4, GRN-2 was also not significantly different from GRN-3 in this respect. GRN-3 was also not different in this respect from GRN-1, which was in turn found not to be different from GRN-5, which had the lowest boron concentration of all the rootstocks. Sodium concentrations also varied among rootstocks, with GRN-3 having the highest, at 0.38% and GRN-1 having the lowest, at 0.12%. No differences between GRN-2, GRN-4, and GRN-5 were observed, and no differences between GRN-1 and GRN-2 were observed.
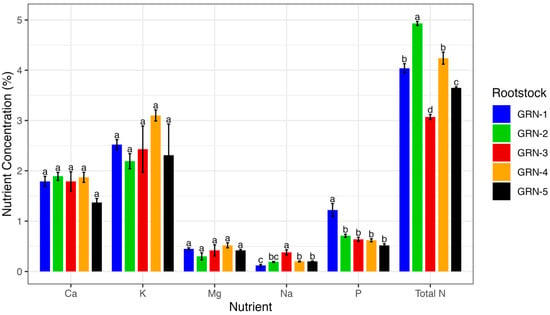
Figure 3.
One-way analysis of variance (ANOVA), displaying average macronutrient concentrations (% dry weight) of ungrafted GRN rootstocks at bloom during the 2022 growing season. Bars represent rootstock means, with error bars indicating the standard error of the mean (n = 3). Different letters above bars indicate significant differences among rootstocks within each macronutrient based on a Fisher’s LSD test (p < 0.05).
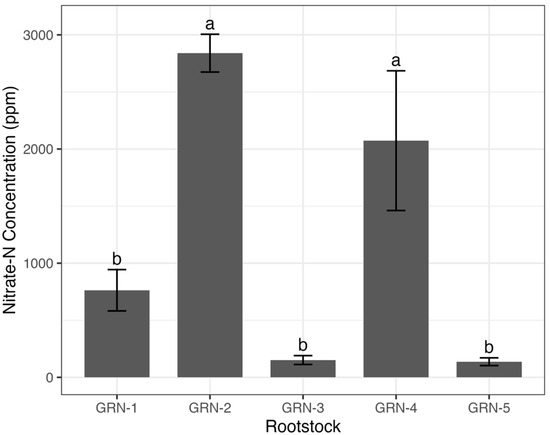
Figure 4.
One-way analysis of variance (ANOVA) displaying average nitrate-nitrogen concentrations (ppm) of ungrafted GRN rootstocks at bloom during the 2022 growing season. Bars represent rootstock means, with error bars indicating the standard error of the mean (n = 3). Different letters above bars indicate significant differences among rootstocks based on a Fisher’s LSD test (p < 0.05).
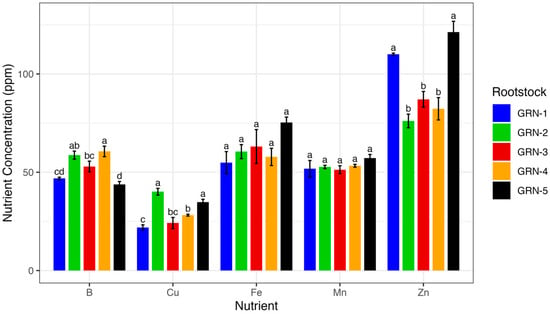
Figure 5.
One-way analysis of variance (ANOVA) displaying average micronutrient concentrations (ppm) of ungrafted GRN rootstocks at bloom during the 2022 growing season. Bars represent rootstock means, with error bars indicating the standard error of the mean (n = 3). Different letters above bars indicate significant differences among rootstocks within each micronutrient based on a Fisher’s LSD test (p < 0.05).
3.5. Pruning Weights
A two-way analysis of variance revealed a year (p = 0.0162) and rootstock effect (p = 0.0006) on pruning weights (Table 6). Average pruning weights increased from 1.68 kg in 2022 to 2.47 kg in 2023. GRN-5 and GRN-2 had the highest pruning weights (3.73 kg and 2.84 kg, respectively), compared to GRN-1 and GRN-3, which had intermediate pruning weights (1.50 kg and 1.53 kg, respectively) and GRN-4, which had the lowest pruning weight (0.78 kg).

Table 6.
Two-way analysis of variance (ANOVA) with interaction, displaying average pruning weight (kg) at dormancy for ungrafted GRN rootstocks for 2022 and 2023. Table shows year and rootstock means, followed by the standard error of the mean. Different letters within a column indicate significant differences based on a Fisher’s LSD test (n = 3) (p < 0.05). Significant p-values (<0.05) are displayed in bold.
Table 6.
Two-way analysis of variance (ANOVA) with interaction, displaying average pruning weight (kg) at dormancy for ungrafted GRN rootstocks for 2022 and 2023. Table shows year and rootstock means, followed by the standard error of the mean. Different letters within a column indicate significant differences based on a Fisher’s LSD test (n = 3) (p < 0.05). Significant p-values (<0.05) are displayed in bold.
| Pruning Weight (kg) | |
|---|---|
| Year | |
| 2022 | 1.68 ± 0.35 b |
| 2023 | 2.47 ± 0.53 a |
| Rootstock | |
| GRN-1 | 1.50 ± 0.21 b |
| GRN-2 | 2.84 ± 0.25 a |
| GRN-3 | 1.53 ± 0.29 b |
| GRN-4 | 0.78 ± 0.14 c |
| GRN-5 | 3.73 ± 1.27 a |
| p-value | 0.0025 |
| Year | 0.0162 |
| Rootstock | 0.0006 |
| Y × R | 0.7137 |
3.6. Total Non-Structural Carbohydrates
Trunk samples were collected during dormancy, and the samples were analyzed for their carbohydrate reserve concentrations (Table 7). Rootstock averages for glucose, fructose, total glucose (total glucose for total non-structural carbohydrates and starch), total non-structural carbohydrates (TNC), and starch are displayed in Table 7. Due to the detection limit of 0.2% for the high-performance liquid chromatography analysis method, sucrose values are not listed, since all rootstocks had concentrations below the 0.2% detection limit. A one-way analysis of variance displayed a rootstock effect for glucose (p = 0.0057), fructose (p = 0.0008), and TNC (p = 0.0227). GRN-1 had the highest concentrations of glucose (1.53%), fructose (1.93%), and total non-structural carbohydrates (13.3%). However, its glucose concentration was not significantly larger than that of GRN-5, which was 1.17%. The glucose concentrations of GRN-3 (0.83%) and GRN-4 (0.90%) were not significantly different from those of GRN-5 or GRN-2, which had the lowest glucose concentration, 0.67%. Aside from GRN-1, which had significantly higher fructose and TNC concentrations, no significant variations in fructose and TNC concentrations were observed among rootstocks GRN-2 through GRN-5.

Table 7.
One-way analysis of variance (ANOVA), displaying average carbohydrate measurements at dormancy of ungrafted GRN rootstocks for 2021. The table shows rootstock means followed by the standard error of the mean. Different letters within a column indicate significant differences based on a Fisher’s LSD test (n = 3) (p < 0.05). Significant p-values (<0.05) are displayed in bold.
Table 7.
One-way analysis of variance (ANOVA), displaying average carbohydrate measurements at dormancy of ungrafted GRN rootstocks for 2021. The table shows rootstock means followed by the standard error of the mean. Different letters within a column indicate significant differences based on a Fisher’s LSD test (n = 3) (p < 0.05). Significant p-values (<0.05) are displayed in bold.
| Rootstock | Glucose (%) | Fructose (%) | Gluc-Tot (%) | TNC (%) | Starch (%) |
|---|---|---|---|---|---|
| GRN-1 | 1.53 ± 0.12 a | 1.93 ± 0.19 a | 11.2 ± 0.15 a | 13.3 ± 0.31 a | 8.73 ± 0.17 a |
| GRN-2 | 0.67 ± 0.23 c | 0.77 ± 0.17 b | 6.53 ± 1.33 a | 7.37 ± 1.27 b | 5.27 ± 1.24 a |
| GRN-3 | 0.83 ± 0.09 bc | 0.93 ± 0.15 b | 7.33 ± 0.63 a | 8.37 ± 0.66 b | 5.87 ± 0.54 a |
| GRN-4 | 0.90 ± 0.06 bc | 1.00 ± 0.06 b | 6.33 ± 1.21 a | 7.50 ± 1.14 b | 4.87 ± 1.08 a |
| GRN-5 | 1.17 ± 0.03 ab | 1.07 ± 0.03 b | 7.40 ± 1.59 a | 8.83 ± 1.73 b | 5.60 ± 1.42 a |
| p-value | 0.0057 | 0.0008 | 0.064 | 0.0227 | 0.126 |
3.7. Principal Component Analysis
Principal component analysis (PCA) revealed that the first principal component (PC1) accounted for 64.9% of the total variation, while the second principal component (PC2) explained an additional 23.8% (Figure 6). PC1 was influenced by all three traits—internode length, internode diameter, and pruning weights—with internode diameter contributing the most (loading = 0.63). PC2 was primarily driven by pruning weights (loading = −0.79), with a moderate contribution from internode length. The PCA plot (Figure 6) displays the clustering of rootstocks along the first two principal components. GRN-1 and GRN-2 displayed tighter clustering compared to the other rootstocks. The other rootstocks, GRN-3, GRN-4, and GRN-5, showed partial clustering with data points that were more dispersed. Within PC1, GRN-1 and GRN-4 were positioned toward the negative axis, corresponding to smaller internode diameters, whereas GRN-2, GRN-3, and GRN-5 were located toward the positive end, indicating larger diameters.
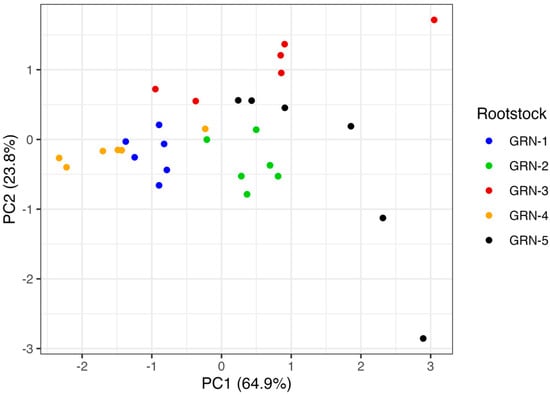
Figure 6.
Principal component analysis of growth traits, including internode length, diameter, and pruning weights, from the 2021 and 2022 growing seasons of ungrafted GRN rootstocks. Each data point represents a rootstock replicate. The first principal component (PC1) explains 64.9% of the variation, while the second principal component (PC2) explains 23.8% of the variation.
4. Discussion
All growth performance parameters measured in this study significantly differed among the GRN rootstocks, except for a few macro- and micronutrients and some carbohydrate reserve parameters. The progressions of phenology and the initiations of senescence varied in both years, and with similar trends. The variation in phenology of Vitis vinifera L. when grafted to various rootstocks has been reported by several studies [21,24,25], but little is known about the phenology of GRN rootstocks. Generally, rootstocks with V. riparia parentage experience earlier phenological development and senescence, and rootstocks with V. rupestris parentage experience delayed phenology and senescence [10]. Most of the GRN rootstocks had some trace of V. riparia in their genetic background, except for GRN-1, which has some V. rupestris in its genetic background. GRN-1 had delayed phenology and senescence compared to the other rootstocks, and this delay could possibly be explained by its V. rupestris parentage [10]. Further research is needed to see if similar trends are observed when the rootstocks are grafted to varying winegrape cultivars.
The GRN rootstocks displayed different growth characteristics, as shown by the variations in canopy sizes, internode measurements, pruning weights, nutrient accumulation, and carbohydrate reserves. These results were expected, considering that GRN rootstocks are hybridizations of multiple Vitis species with varying growth characteristics. These differences in growth characteristics among GRN rootstocks are likely to influence the growth of V. vinifera if they are grafted together. For example, many studies have demonstrated that the pruning weights of V. vinifera are affected by rootstock selection [26,27,28,29]. Although there is a lack of studies that report the pruning weights of ungrafted rootstocks, one can assume that the pruning weights of ungrafted rootstocks will vary among rootstocks, based on the numerous studies that report rootstock/scion growth interactions. The results of this study demonstrate the variation in pruning weights among the ungrafted GRN rootstocks, and these differences are likely due to the variation in parentage among the rootstocks.
The variation in growth characteristics among the GRN rootstocks suggests their potential suitability in diverse vineyard environments. For instance, GRN-1 demonstrated a relatively large leaf area and moderate LAI compared with other rootstocks, along with moderate pruning weights, leading it to be most suitable for environments requiring balanced growth. The internodes were shorter, with moderate diameters, potentially making it suitable for moderate- to higher-density plantings. The GRN-1 vines were higher in phosphorus and nitrogen, which support early root development and growth, and exhibited high zinc levels and low copper and salt levels. They contained the highest glucose, fructose, and TNC (13.3%) of all the rootstocks, factors which promote early-season growth. Due to these factors, it was determined that they are best-suited for vineyards where moderate growth is desired, but may require canopy management to control excessive vine development.
GRN-2 consistently had the largest leaf area each year and a moderate LAI, indicating higher vigor. Partnered with moderate internode length and diameter, it also showed the highest nitrogen and nitrate levels, supporting significant vegetative growth. Phosphorus and potassium levels were both lower than those of other GRN rootstocks. GRN-2 exhibited the lowest levels of glucose, fructose, and TNC (7.37%), indicating lower energy reserves. As a result, it was determined that GRN-2 would be best-suited for low-vigor environments. However, early-season vegetative growth may be reduced due to lower energy reserves, potentially leading to a lower risk of early-season fungal diseases.
GRN-3 showed moderate leaf area and LAI and a moderate response to environmental conditions. Although it had the longest internodes, it exhibited moderate nitrogen, phosphorus, and potassium levels. Its high sodium content (0.38%) makes it less well-suited for high-salinity soils. Moderate levels of glucose, fructose, and TNC (~8.37%) support balanced growth.
GRN-4 exhibited the least vigorous growth pattern, with smaller leaf area, yet a high LAI response to seasonal changes. It has short internodes with moderate internode diameter, showing suitable growth patterns for more vigorous growing conditions. It has moderate nitrogen and high potassium (3.10%), supporting fruit quality and sugar accumulation. The high boron (60.6 ppm) levels benefit flowering and fruit set. Moderate glucose and fructose levels and a TNC of 7.50% indicate balanced energy storage. This rootstock is suitable for high-vigor environments or for varieties that typically struggle with fruit set and flowering.
Finally, GRN-5 had a large total leaf area in 2021 but a small leaf area in 2022. GRN-5 consistently dropped leaves from the lower parts of the shoots throughout the growing season, regardless of water status. Still, in 2022, more leaves were observed to fall, possibly due to increased seasonal temperatures combined with water stress. It had the thickest internode diameters, indicating better structural support, and it exhibited moderate nitrogen levels and low phosphorus and potassium levels. However, it had the highest zinc levels, a factor which supports better metabolic functions. Moderate glucose, fructose, and TNC (8.83%) levels promote moderate growth and energy storage.
As previously mentioned, total non-structural carbohydrates varied by rootstock selection, with GRN-1 having the largest concentration of any rootstock used in this study. GRN-1 was shown to have an isohydric-like stomatal behavior (unpublished data) that is characterized by rapid stomatal closure when the plant is exposed to water stress. Isohydric cultivars are thought to be more carbon-limited than anisohydric cultivars, due to rapid stomatal closure, and have a higher risk of carbohydrate starvation [30,31], but this is not always the case [32,33]. Although there is a need for studies correlating hydraulic strategies and carbohydrate reserves in grapevines, one study by Kannenberg and Phillips [33] investigated the use of hydraulic strategy to predict non-structural carbohydrate dynamics in various tree sapling species. In that study, it was found that there was no link between hydraulic strategy and stored carbohydrates [33]. Moreover, tree saplings classified as highly isohydric maintained carbohydrate reserves during drought conditions even though carbon assimilation rates had decreased [33].
The results of the PCA support the observed variation in growth traits among the GRN rootstocks. PC1, which explained most of the variance (64.9%), was influenced by internode length, diameter, and pruning weights, which have been demonstrated to differ significantly among rootstocks. Internode diameter had the strongest loading (0.63) on PC1, suggesting that it plays an important role in differentiating overall growth. Rootstocks such as GRN-2, GRN-3, and GRN-5, which were positioned along the positive axis of PC1, demonstrated larger internode diameters and, in some cases, higher canopy sizes or pruning weights, reflecting a more vigorous growth characteristic. In contrast, GRN-1 and GRN-4 fell toward the negative end of PC1, which is consistent with their smaller internode diameters and more moderate growth patterns. The clustering patterns observed in the PCA also support growth consistency within some rootstocks. GRN-1 and GRN-2 exhibited tighter clustering, suggesting more uniform growth traits under moderate water-stress conditions. Meanwhile, the dispersion of data points in GRN-3, GRN-4, and GRN-5 may indicate greater within-rootstock variability or differing sensitivity to environmental factors such as reduced water availability.
5. Conclusions
This study demonstrates the variations in physiological growth habits of the GRN rootstocks under moderate water-stress conditions, variations likely influenced by their diverse genetic backgrounds. Significant variation was observed in phenological timing, internode measurements, pruning weights, nutrient accumulation, and carbohydrate reserves. These differences suggest that some GRN rootstocks might be more appropriate for certain vineyard environments or management goals. For instance, GRN-1 is ideal for vigorous growth, while GRN-2 is suited for environments requiring lower vigor. GRN-3 has the highest sodium content, making it less suited for high-salinity environments, and GRN-4’s high boron levels may improve reproductive success. GRN-1 has the highest level of total non-structural carbohydrates, which support vigorous early-season growth, and GRN-2 is characterized as having high nitrogen levels. Overall, the GRN series of rootstocks present a range of physiological characteristics that make them suitable for a variety of growing conditions. Further studies that include grafted combinations with Vitis vinifera scions are necessary to validate these findings and improve rootstock selection in specific viticultural contexts.
Author Contributions
Conceptualization, J.C.D.P.; methodology, J.C.D.P.; software, J.R.M.J.; validation, J.R.M.J. and J.C.D.P.; formal analysis, J.R.M.J.; investigation, J.R.M.J. and J.C.D.P.; resources, J.C.D.P.; data curation, J.R.M.J., J.A.A., M.A.A., A.B., J.H., J.W., M.A., and J.C.D.P.; writing—original draft preparation, J.R.M.J. and J.C.D.P.; writing—review and editing, J.R.M.J. and J.C.D.P.; visualization, J.R.M.J.; supervision, J.C.D.P.; project administration, J.C.D.P.; funding acquisition, J.C.D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Wonderful Nurseries and Duarte Nursery through their plant material contributions. The authors express sincere gratitude to the California State University Agricultural Research Institute (ARI Grant 21-03-100) and the California Grape Rootstock Improvement Commission (Grant 21-451) for generously funding this research.
Data Availability Statement
Data pertaining to this project is being held in computers owned by Washington State University, under control of the research team.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CIMIS | California Irrigation Information Management System |
| GDD | Growing-degree days |
| GRN | A grape rootstock |
| HPLC | High-performance liquid chromatography |
| ICP-AES | Inductively Coupled Plasma Atomic Emission Spectrometry |
| LAI | Leaf area index |
| PAR | Photosynthetically active radiation |
| TNC | Total non-structural carbohydrates |
| UCD | University of California, Davis |
References
- Pongrácz, D.P. Rootstocks for Grapevines, 1st ed.; David Philips Publishers: Cape Town, South Africa, 1983. [Google Scholar]
- Keller, M. The Science of Grapevines, 1st ed.; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Mullins, M.G.; Bouquet, A.; Williams, L.E. Biology of the Grapevine; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Riaz, S.; Lund, K.T.; Granett, J.; Walker, M.A. Population diversity of grape phylloxera in California and evidence for sexual reproduction. Am. J. Enol. Vitic. 2017, 68, 218–227. [Google Scholar] [CrossRef]
- Ferris, H.; Zheng, L.; Walker, M.A. Resistance of grape rootstocks to plant-parasitic nematodes. J. Nematol. 2012, 44, 377–386. [Google Scholar] [PubMed]
- Walker, M.A.; Lund, K.; Agüero, C.; Riaz, S.; Fort, K.; Heinitz, C.; Romero, N. Breeding grape rootstocks for resistance to Phylloxera and nematodes–it’s not always easy. Acta Hortic. 2014, 1045, 89–97. [Google Scholar] [CrossRef]
- Walker, M.A.; Granett, J.; Bettiga, L.J.; Peacock, W.L.; Weber, E.A. Grape Phylloxera. In Grape Pest Management; Bettiga, L.J., Ed.; University of California Agriculture and Natural Resources Publisher: Davis, CA, USA, 2013; Volume 3343, pp. 191–201. [Google Scholar]
- Fort, K.; Fraga, J.; Grossi, D.; Walker, M.A. Early measures of drought tolerance in four grape rootstocks. J. Am. Soc. Hortic. Sci. 2017, 142, 36–46. [Google Scholar] [CrossRef]
- Galet, P. Grape Varieties and Rootstock Varieties; Smith, J., Translator; Oenoplurimédia: Chaintré, France, 1998. [Google Scholar]
- Heinitz, C.C.; Fort, K.; Walker, M.A. Developing drought and salt resistant grape rootstocks. In Proceedings of the XI International Conference on Grapevine Breeding and Genetics, Beijing, China, 28 July–2 August 2014; pp. 305–312. [Google Scholar]
- Heinitz, C.C.; Uretsky, J.; Dodson Peterson, J.C.; Huerta-Acosta, K.G.; Walker, M.A. Crop wild relatives of grape (Vitis vinifera L.) throughout North America. In North American Crop Wild Relatives, Volume 2: Important Species; Greene, S.L., Williams, K.A., Khoury, C.K., Kantar, M.B., Marek, L.F., Eds.; Springer: Cham, Switzerland, 2019; pp. 329–351. [Google Scholar]
- Bridge, J.; Starr, J. Plant Nematodes of Agricultural Importance: A Colour Handbook, 1st ed.; CRC Press: London, UK, 2007. [Google Scholar] [CrossRef]
- Thomas, A.L.; Harris, J.L.; Bergmeier, E.A.; Striegler, R.K. Performance of ‘Chambourcin’ winegrape on nematode-resistant rootstocks in Missouri. Hortte 2020, 30, 597–602. [Google Scholar] [CrossRef]
- McKenry, M.V.; Bettiga, L.J. Nematodes. In Grape Pest Management; Bettiga, L.J., Ed.; University of California Agriculture and Natural Resources Publisher: Davis, CA, USA, 2013; Volume 3343, pp. 449–470. [Google Scholar]
- Powell, C.A.; Forer, L.B.; Longnecker, J.L. Incidence of tomato ringspot virus and tobacco ringspot virus in grapevines in Pennsylvania. Plant Dis. 1990, 74, 702–704. [Google Scholar] [CrossRef]
- van Zyl, S.; Vivier, M.A.; Walker, M.A. Xiphinema index and its relationship to grapevines: A review. S. Afr. J. Enol. Vitic. 2012, 33, 21–32. [Google Scholar] [CrossRef]
- Villate, L.; Plantard, O.; Esmenjaud, D.; Van Helden, M.; Fievet, V.; Hanse, B.; Delemarre, F. Spatial distribution of the dagger nematode Xiphinema index and its associated Grapevine fanleaf virus in French vineyard. Phytopathology 2008, 98, 942–948. [Google Scholar] [CrossRef]
- Esmenjaud, D.; Bouquet, A.; Demangeat, G.; Van Helden, M.; Ollat, N. Nematode-resistant rootstocks as a major component of the management alternative for Grapevine fanleaf virus control in grape. In Proceedings of the V International Phylloxera Symposium, Wien, Austria, 20–21 September 2010; pp. 111–115. [Google Scholar]
- Kamas, J.; Labay, A.; Scheiner, J.J. Evaluation of grapevine rootstocks on slightly acidic and strongly alkaline Texas Hill Country soils. Am. J. Enol. Vitic. 2020, 4 (Suppl. 2), 39–52. [Google Scholar] [CrossRef]
- Scheiner, J.J.; Labay, A.; Kamas, J. Rootstocks improve Blanc du Bois vine performance and fruit quality on alkaline soil. Am. J. Enol. Vitic. 2020, 4 (Suppl. 2), 63–73. [Google Scholar] [CrossRef]
- Peterson, J.C.D.; Walker, M.A. Influence of grapevine rootstock on scion development and initiation of senescence. Am. J. Enol. Vitic. 2017, 1 (Suppl. 2), 48–54. [Google Scholar] [CrossRef]
- Reisenauer, H.M. (Ed.) Soil and Plant-Tissue Testing in California; University of California: Berkeley, CA, USA, 1879. [Google Scholar]
- Johansen, H.N.; Glitsø, V.; Bach Knudsen, K.E. Influence of extraction solvent and temperature on the quantitative determination of oligosaccharides from plant materials by high-performance liquid chromatography. J. Agric. Food Chem. 1996, 44, 1470–1474. [Google Scholar] [CrossRef]
- Miele, A. Rootstock-scion interaction: 6. Phenology, chilling and heat requirements of Cabernet Sauvignon grapevine. Rev. Bras. Frutic. 2019, 41, e-446. [Google Scholar] [CrossRef]
- Munoz, J.R., Jr.; Stauch, S.J.; Wootten, J.; Kitchen, M.; Abreu, M.; Rodriguez, C.J.; Casassa, L.F.; Wolpert, J.A.; Dodson Peterson, J.C. Effect of rootstock on vineyard establishment using green-growing benchgrafts. Agronomy 2023, 13, 1586. [Google Scholar] [CrossRef]
- Ezzahouani, A.; Williams, L.E. The influence of rootstock on leaf water potential, yield, and berry composition of Ruby Seedless grapevines. Am. J. Enol. Vitic 1995, 46, 559–563. [Google Scholar] [CrossRef]
- Satisha, S.J.; Somkuwar, R.G.; Sharma, J.; Upadhyay, A.K.; Adsule, P.G. Influence of rootstocks on growth yield and fruit composition of Thompson seedless grapes grown in the Pune region of India. S. Afr. J. Enol. Vitic. 2010, 31, 1–8. [Google Scholar] [CrossRef][Green Version]
- Li, M.; Guo, Z.; Jia, N.; Yuan, J. Evaluation of eight rootstocks on the growth and berry quality of ‘Marselan’ grapevines. Sci. Hort. 2019, 248, 58–61. [Google Scholar] [CrossRef]
- East, K.E.; Zasada, I.A.; Tarara, J.; Moyer, M.M. Field performance of winegrape rootstocks and fumigation during establishment of a Chardonnay vineyard in Washington. Am. J. Enol. Vitic. 2021, 72, 113–125. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Gerzon, E.; Biton, I.; Yaniv, Y.; Zemach, H.; Netzer, Y.; Schwartz, A.; Fair, A.; Ben-Ari, G. Grapevine anatomy as a possible determinant of isohydric or anisohydric behavior. Am. J. Enol. Vitic. 2015, 66, 340–347. [Google Scholar] [CrossRef]
- Garcia-Forner, N.; Biel, C.; Savé, R.; Martínez-Vilalta, J. Isohydric species are not necessarily more carbon limited than anisohydric species during drought. Tree Physiol. 2017, 37, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Kannenberg, S.A.; Phillips, R.P. Non-structural carbohydrate pools not linked to hydraulic strategies or carbon supply in tree saplings during severe drought and subsequent recovery. Tree Physiol. 2020, 40, 259–271. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).