Genotype × Environment Interaction and Correlations Between Agronomic Traits, Flowering, and Fruit Set in Cassava
Abstract
1. Introduction
2. Materials and Methods
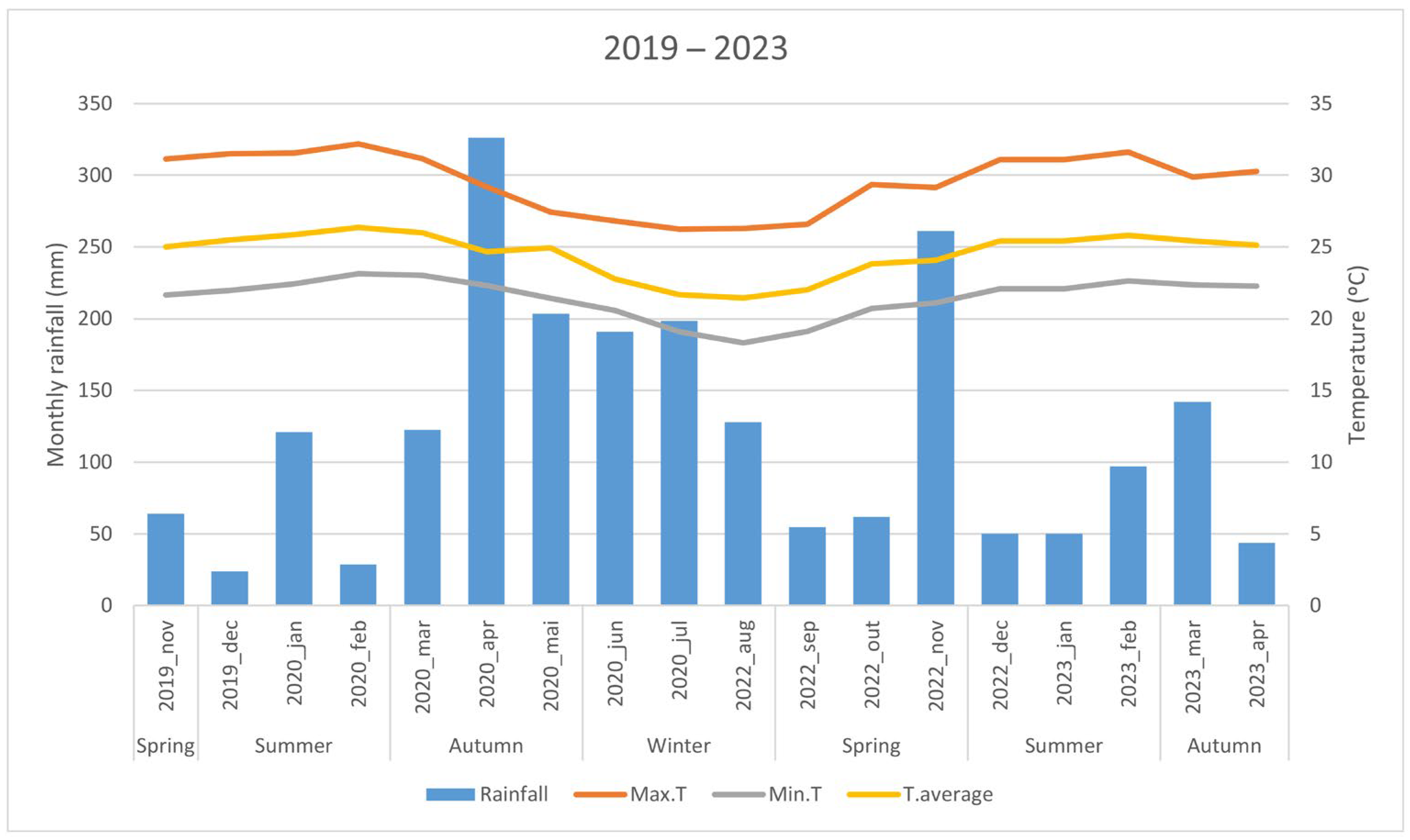
2.1. Plant Material and Environmental Characterization of the Experiments
2.2. Flowering Evaluation
2.3. Agronomic Trait Evaluation
2.4. Data Analyses
3. Results
3.1. General Flowering Traits
3.2. Deviance Analysis of Flowering and Fruiting Traits in Cassava
3.3. Average Performance of Flower and Fruit Set During the Experimental Period
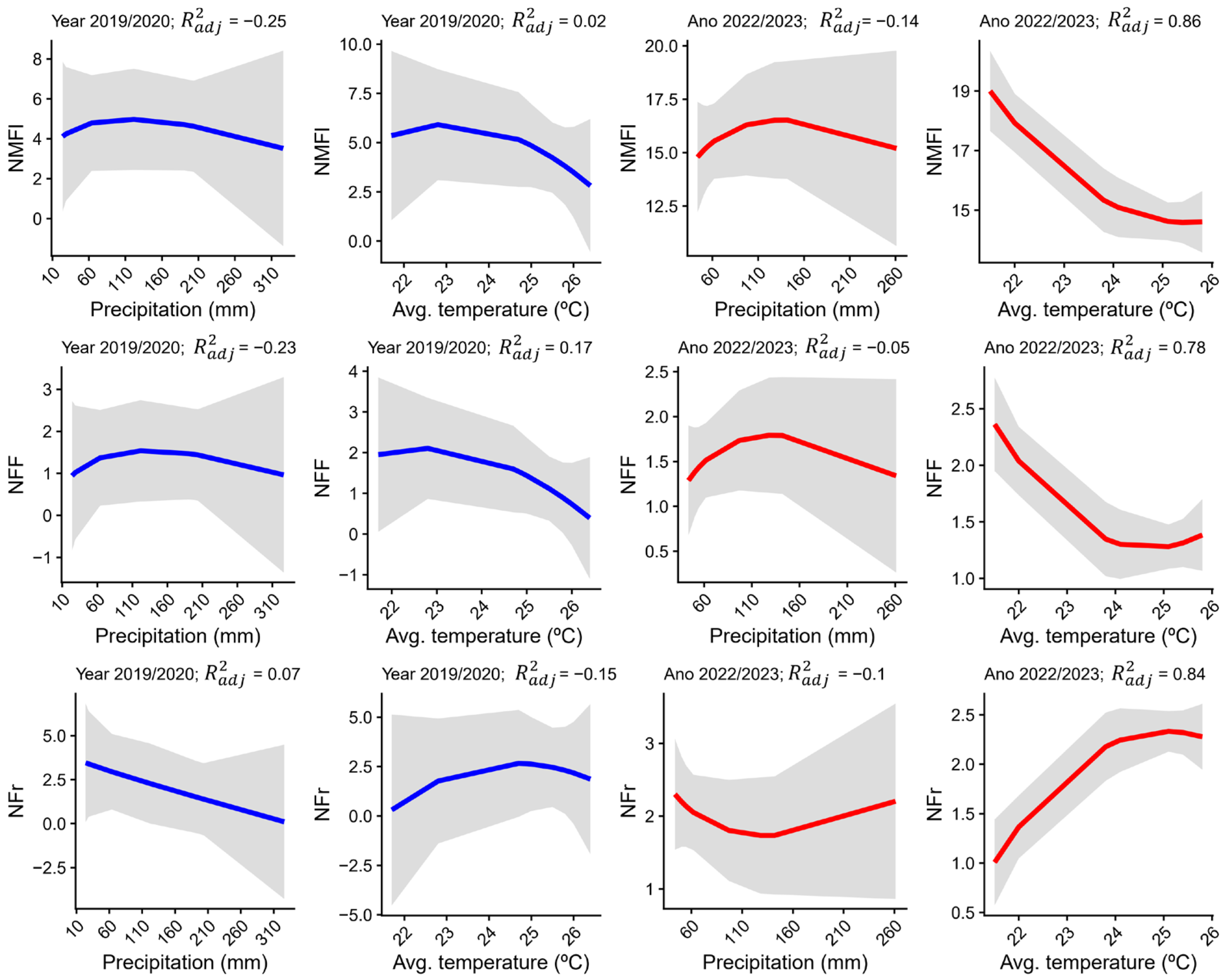
3.4. Relationship Between Flowering and Fruiting with Climatic Variables
3.5. Phenotypic Correlations Between Flowering/Fruiting Traits and Agronomic Attributes
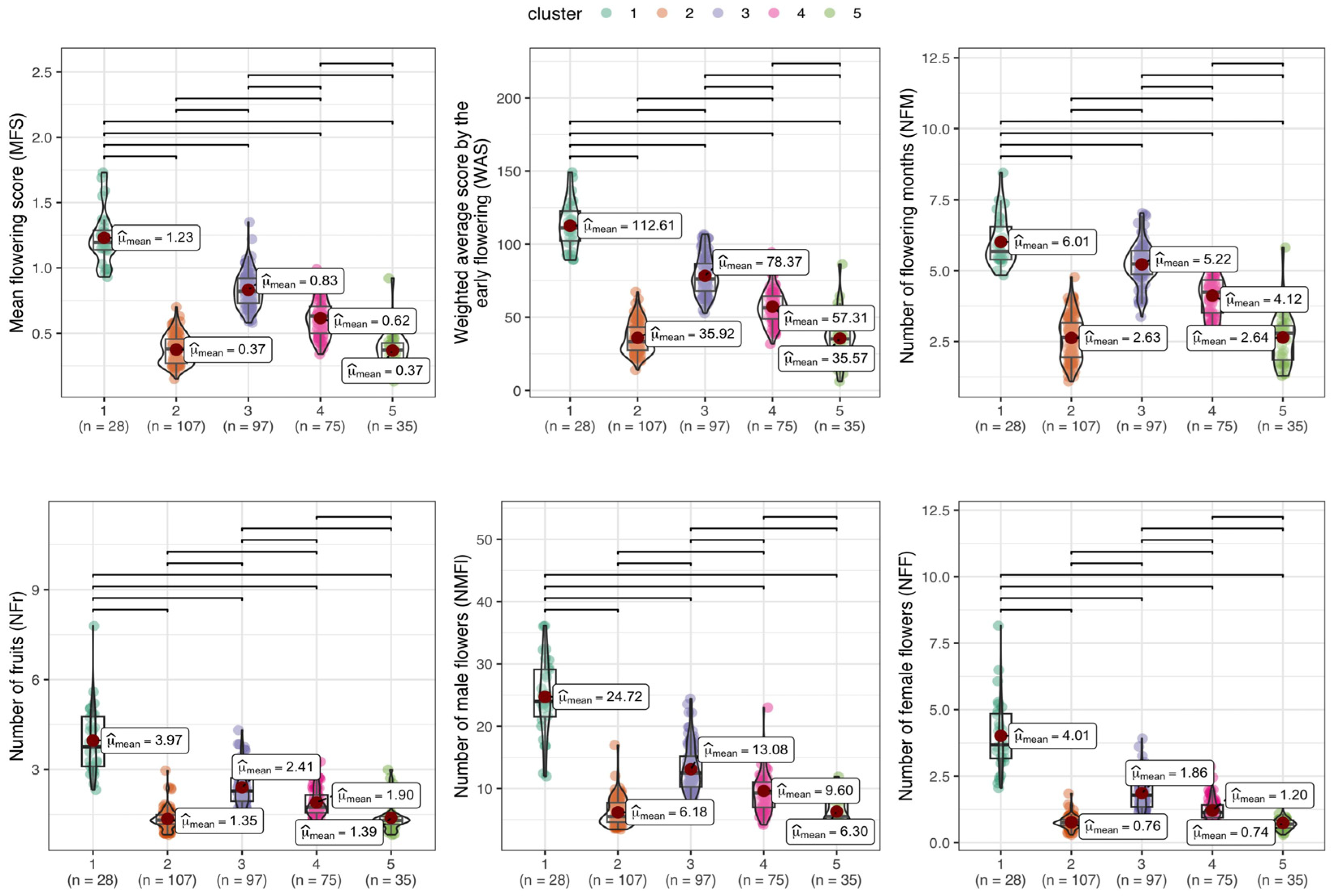
3.6. Discriminant Analysis of Principal Components (DAPC) for Flowering/Fruiting Traits and Agronomic Attributes
4. Discussion
4.1. Genetic Variability and Stability for Flowering and Fruiting Traits in Cassava Germplasm
4.2. Influence of Climatic and Environmental Factors on Flower and Fruit Production
4.3. Correlation Between Flowering/Fruiting Traits and Agronomic Yield Attributes
4.4. Phenotypic Similarity in Flowering, Fruiting, and Agronomic Traits
4.5. Future Perspectives for Cassava Breeding Based on Flowering and Fruiting Traits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allem, A.C. The Origins and Taxonomy of Cassava. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Eds.; CABI Books; CABI Publishing: Wallingford, UK, 2001; pp. 1–16. [Google Scholar] [CrossRef]
- Halsey, M.E.; Olsen, K.M.; Taylor, N.J.; Chavarriaga-Aguirre, P. Reproductive Biology of Cassava (Manihot Esculenta Crantz) and Isolation of Experimental Field Trials. Crop Sci. 2008, 48, 49–58. [Google Scholar] [CrossRef]
- Ceballos, H.; Okogbenin, E.; Pérez, J.C.; López-Valle, L.A.B.; Debouck, D. Cassava. In Root and Tuber Crops; Bradshaw, J.E., Ed.; Springer: New York, NY, USA, 2010; pp. 53–96. [Google Scholar] [CrossRef]
- Tonukari, N.J. Cassava and the Future of Starch. Electron. J. Biotechnol. 2004, 7, 5–8. [Google Scholar] [CrossRef]
- Dankwa, K.O.; Peprah, B.B. Industrialization of Cassava Sector in Ghana: Progress and the Role of Developing High Starch Cassava Varieties. Ghana J. Agric. Sci. 2019, 54, 79–85. [Google Scholar] [CrossRef]
- Egesi, C.N.; Ilona, P.; Ogbe, F.O.; Akoroda, M.; Dixon, A. Genetic Variation and Genotype × Environment Interaction for Yield and Other Agronomic Traits in Cassava in Nigeria. Agron. J. 2007, 99, 1137–1142. [Google Scholar] [CrossRef]
- Bayata, A. Review on Nutritional Value of Cassava for Use as a Staple Food. Sci. J. Anal. Chem. 2019, 7, 83–91. [Google Scholar] [CrossRef]
- Sousa, M.B.E.; de Andrade, L.R.B.; de Souza, E.H.; Alves, A.A.C.; de Oliveira, E.J. Reproductive Barriers in Cassava: Factors and Implications for Genetic Improvement. PLoS ONE 2021, 16, e0260576. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations. Participation of Continents in Cassava Production in 2021. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 28 August 2022).
- IBGE. Levantamento Sistemático da Produção Agrícola. Instituto Brasileiro de Geografia e Estatística. Available online: https://www.ibge.gov.br/estatisticas/economicas/agricultura-e-pecuaria/9201-levantamento-sistematico-da-producao-agricola.html (accessed on 14 February 2025).
- Ramos Abril, L.N.; Pineda, L.M.; Wasek, I.; Wedzony, M.; Ceballos, H. Reproductive Biology in Cassava: Stigma Receptivity and Pollen Tube Growth. Commun. Integr. Biol. 2019, 12, 96–111. [Google Scholar] [CrossRef]
- Ibrahim, Y.; Baguma, Y.; Abincha, W.; Gibson, P.; Edema, R.; Bisikwa, J. Flowering Problems and Their Possible Solution in Cassava Breeding. J. Sci. Agric. 2020, 4, 83–89. [Google Scholar] [CrossRef]
- Ceballos, H.; Davrieux, F.; Talsma, E.F.; Belalcazar, J.; Chavarriaga, P.; Andersson, M.S.; Ceballos, H.; Davrieux, F.; Talsma, E.F.; Belalcazar, J.; et al. Carotenoids in Cassava Roots. In Carotenoids; Cvetkovic, D.J., Nikolic, G.S., Eds.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Alves, A.A.C. Cassava Botany and Physiology. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Eds.; CABI Books; CABI Books: London, UK, 2001; pp. 67–89. [Google Scholar] [CrossRef]
- Souza, L.S.; Diniz, R.P.; de Jesus Neves, R.; Alves, A.A.C.; de Oliveira, E.J. Grafting as a Strategy to Increase Flowering of Cassava. Sci. Hortic. 2018, 240, 544–551. [Google Scholar] [CrossRef]
- Ceballos, H.; Luna, J.; Escobar, A.F.; Ortiz, D.; Pérez, J.C.; Sánchez, T.; Pachón, H.; Dufour, D. Spatial Distribution of Dry Matter in Yellow Fleshed Cassava Roots and Its Influence on Carotenoid Retention upon Boiling. Food Res. Int. 2012, 45, 52–59. [Google Scholar] [CrossRef]
- Adeyemo, O.S.; Hyde, P.T.; Setter, T.L. Identification of FT Family Genes That Respond to Photoperiod, Temperature and Genotype in Relation to Flowering in Cassava (Manihot Esculenta, Crantz). Plant Reprod. 2019, 32, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.S.; Alves, A.A.C.; de Oliveira, E.J. Phenological Diversity of Flowering and Fruiting in Cassava Germplasm. Sci. Hortic. 2020, 265, 109253. [Google Scholar] [CrossRef]
- Didonet, A.D. Importância do período de pré-floração na produtividade do feijoeiro. Pesqui. Agropecuária Trop. 2010, 40, 505–512. [Google Scholar] [CrossRef]
- Mallikarjuna, B.P.; Viswanatha, K.P.; Samineni, S.; Gaur, P.M. Association of Flowering Time with Phenological and Productivity Traits in Chickpea. Euphytica 2019, 215, 77. [Google Scholar] [CrossRef]
- de Souza, M.J.L.; Viana, A.E.S.; Matsumoto, S.N.; de Vasconcelos, R.C.; Sediyama, T.; Morais, O.M. Características agronômicas da mandioca relacionadas à interação entre irrigação, épocas de colheita e cloreto de mepiquat. Acta Sci. Agron. 2010, 32, 45–53. [Google Scholar] [CrossRef][Green Version]
- Rosario-Arellano, J.L.D.; Meneses-Márquez, I.; Leyva-Ovalle, O.R.; Aguilar-Rivera, N.; Bolio-López, G.I.; Andrés-Meza, P. Morphoagronomic and Industrial Performance of Cassava (Manihot Esculenta Crantz) Germplasm for the Production of Starch and Solid Byproducts. AIMS Agric. Food 2020, 5, 617–634. [Google Scholar] [CrossRef]
- Amarullah. Morphological, Physiological and Agronomic Characteristics of Cassava Superior Variety of Coastal Land. IOP Conf. Ser. Earth Environ. Sci. 2021, 748, 012030. [Google Scholar] [CrossRef]
- Carvalho, R.R.B.; Bandeira ESousa, M.; de Oliveira, L.A.; de Oliveira, E.J. Phenotypic Diversity and Selection in Biofortified Cassava Germplasm for Yield and Quality Root Traits. Euphytica Neth. J. Plant Breed. 2022, 218, 173. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.d.S.; Farias, A.R.N.; Mattos, P.L.P.d.; Fukuda, W.M.G. Livro Aspectos Socioeconomicos e Agronomicos da Mandioca; Embrapa Mandioca e Fruticultura: Cruz das Almas, Brazil, 2006; Volume 1. [Google Scholar]
- Kawano, K.; Fukuda, W.; Cenpukdee, U. Genetic and Environmental Effects on Dry Matter Content of Cassava Root. Crop Sci. 1987, 27, 69–74. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 28 August 2023).
- Jombart, T.; Devillard, S.; Balloux, F. Discriminant Analysis of Principal Components: A New Method for the Analysis of Genetically Structured Populations. BMC Genet. 2010, 11, 94. [Google Scholar] [CrossRef]
- Baguma, J.K.; Mukasa, S.B.; Nuwamanya, E.; Alicai, T.; Omongo, C.; Hyde, P.T.; Setter, T.L.; Ochwo-Ssemakula, M.; Esuma, W.; Kanaabi, M.; et al. Flowering and Fruit-Set in Cassava under Extended Red-Light Photoperiod Supplemented with Plant-Growth Regulators and Pruning. BMC Plant Biol. 2023, 23, 335. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zhang, H.; Xin, W.; Ma, K.; Du, D.; Yu, C.; Liu, Y. Dissecting the Genetic Basis of Flowering Time and Height Related-Traits Using Two Doubled Haploid Populations in Maize. Plants 2021, 10, 1585. [Google Scholar] [CrossRef]
- Navas-Lopez, J.F.; León, L.; Rapoport, H.F.; Moreno-Alías, I.; Lorite, I.J.; de la Rosa, R. Genotype, Environment and Their Interaction Effects on Olive Tree Flowering Phenology and Flower Quality. Euphytica 2019, 215, 184. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhao, Z.-H.; Zhao, S.-Q. Signal pathways of flowering time regulation in plant. Hereditas 2006, 28, 1031–1036. [Google Scholar] [PubMed]
- Santos, A.D.; Bandeira e Sousa, M.; Alves, A.A.C.; Oliveira, E.J.D. Environmental Factors Influence the Production of Flowers and Fruits of Cassava. Sci. Hortic. 2024, 323, 112498. [Google Scholar] [CrossRef]
- Lee, Z.; Kim, S.; Choi, S.J.; Joung, E.; Kwon, M.; Park, H.J.; Shim, J.S. Regulation of Flowering Time by Environmental Factors in Plants. Plants 2023, 12, 3680. [Google Scholar] [CrossRef]
- Tun, W.; Yoon, J.; Jeon, J.-S.; An, G. Influence of Climate Change on Flowering Time. J. Plant Biol. 2021, 64, 193–203. [Google Scholar] [CrossRef]
- Xu, Y.; Kong, X.; Guo, Y.; Wang, R.; Yao, X.; Chen, X.; Yan, T.; Wu, D.; Lu, Y.; Dong, J.; et al. Structural Variations and Environmental Specificities of Flowering Time-Related Genes in Brassica Napus. Theor. Appl. Genet. 2023, 136, 42. [Google Scholar] [CrossRef]
- Araldi, R.; Silva, F.M.L.; Ono, E.O.; Rodrigues, J.D. Florescimento em cana-de-açúcar. Ciênc. Rural 2010, 40, 694–702. [Google Scholar] [CrossRef]
- Lessa, L.S.; da Silva Ledo, C.A.; da Silva Santos, V. Effect of Harvesting Times on Agronomic Characteristics of Industrial Cassava Genotypes. Rev. Bras. Ciênc. Agrár. 2019, 14, e5647. [Google Scholar] [CrossRef]
- Rós, A.B.; Hirata, A.C.S.; Araújo, H.S.d.; Narita, N. Crescimento, fenologia e produtividade de cultivares de mandioca. Pesqui. Agropecuária Trop. 2011, 41, 552–558. [Google Scholar] [CrossRef]
- Vieira, E.A.; Fialho, J.d.F.; Carvalho, L.J.C.B. Correlação fenotípica entre caracteres agronômicos em população segregante de mandioca de mesa. Rev. Ceres 2014, 61, 523–529. [Google Scholar] [CrossRef]
- Cach, N.T.; Lenis, J.I.; Perez, J.C.; Morante, N.; Calle, F.; Ceballos, H. Inheritance of Useful Traits in Cassava Grown in Subhumid Conditions. Plant Breed. 2006, 125, 177–182. [Google Scholar] [CrossRef]
- Gomes, C.N.; Carvalho, S.P.d.; Jesus, A.M.S.; Custódio, T.N. Caracterização morfoagronômica e coeficientes de trilha de caracteres componentes da produção em mandioca. Pesqui. Agropecuária Bras. 2007, 42, 1121–1130. [Google Scholar] [CrossRef]
- Esuma, W.; Herselman, L.; Labuschagne, M.; Ramu, P.; Lu, F.; Baguma, Y.; Buckler, E.; Kawuki, R. Genome-Wide Association Mapping of Provitamin A Carotenoid Content in Cassava. Euphytica 2016, 212, 97–110. [Google Scholar] [CrossRef]
- Santos, C.S.D.; Sousa, M.B.; Brito, A.C.; de Oliveira, L.A.; Carvalho, C.W.P.; de Oliveira, E.J. Genome-Wide Association Study of Cassava Starch Paste Properties. PLoS ONE 2022, 17, e0262888. [Google Scholar] [CrossRef]
- Ogbonna, A.C.; Braatz de Andrade, L.R.; Mueller, L.A.; de Oliveira, E.J.; Bauchet, G.J. Comprehensive Genotyping of a Brazilian Cassava (Manihot Esculenta Crantz) Germplasm Bank: Insights into Diversification and Domestication. Theor. Appl. Genet. 2021, 134, 1343–1362. [Google Scholar] [CrossRef]
- Araus, J.L.; Kefauver, S.C.; Zaman-Allah, M.; Olsen, M.S.; Cairns, J.E. Translating High-Throughput Phenotyping into Genetic Gain. Trends Plant Sci. 2018, 23, 451–466. [Google Scholar] [CrossRef]







| Components of Variance | DF | NFr | NMFl | NFF | |
|---|---|---|---|---|---|
| Experiment 1 (2019/2020) | Genotypes (G) (r) | 1 | 8.01 * | 62.43 * | 4.88 * |
| MAP (f) | 8 | 1820.88 * | 2419.20 * | 409.62 * | |
| Block (f) | 13 | 23.95 | 243.41 | 28.75 | |
| Error | 1 | 16.95 | 71.19 | 11.09 | |
| H2 | 0.32 | 0.47 | 0.31 | ||
| Average | 1.89 | 4.16 | 1.33 | ||
| Experiment 2 (2022/2023) | Genotypes (G) (r) | 1 | 5.22 * | 251.69 * | 3.02 * |
| MAP (f) | 8 | 350.52 * | 4405.47 * | 155.02 * | |
| Block (f) | 14 | 27.61 | 2259.93 | 25.03 | |
| Error | 1 | 8.0 | 412.11 | 6.99 | |
| H2 | 0.32 | 0.26 | 0.17 | ||
| Average | 2.07 | 15.18 | 1.51 | ||
| Combined analysis | Genotypes (G) (r) | 1 | 2.70 * | 83.22 * | 2.41 * |
| Environment (E) (f) | 1 | 2.34 ns | 34,823.76 * | 22.46 ns | |
| Interaction G × E (r) | 1 | 12.95 * | 7317.42 * | 2.39 * | |
| MAP (f) | 8 | 352.96 * | 3836.49 * | 376.23 * | |
| Block (f) | 14 | 34.16 | 1225.59 | 16.79 | |
| Error | 1 | 13.56 | 274.79 | 8.92 | |
| H2 | 0.27 | 0.02 | 0.59 | ||
| Average | 2.0 | 10.57 | 1.43 |
| Components of Variance | DF | MFS | WAS | NFM | |
|---|---|---|---|---|---|
| Experiment 1 (2019/2020) | Genotypes (G) (r) | 1 | 0.15 * | 1267.68 * | 4.09 * |
| Block (f) | 13 | 0.24 | 1840.73 | 3.02 | |
| Error | 0.20 | 1280.15 | 3.14 | ||
| H2 | 0.43 | 0.50 | 0.57 | ||
| Average | 0.38 | 35.39 | 2.00 | ||
| Experiment 2 (2022/2023) | Genotypes (G) (r) | 1 | 0.16 * | 1706.15 * | 4.06 * |
| Block (f) | 14 | 0.47 | 5797.63 | 15.33 | |
| Error | 0.15 | 1455.74 | 5.45 | ||
| H2 | 0.52 | 0.54 | 0.43 | ||
| Average | 0.80 | 76.1 | 5.32 | ||
| Combined analysis | Genotypes (G) (r) | 1 | 0.17 * | 1400.82 * | 4.01 * |
| Environment (E) (f) | 1 | 15.23 * | 155,612.5 * | 978.48 * | |
| Interaction G × E (r) | 1 | 0.02 ns | 2455.6 ns | 0.01 ns | |
| Block (f) | 14 | 0.38 | 4756.54 | 18.39 | |
| Error | 0.17 | 1460.39 | 4.55 | ||
| H2 | 0.97 | 0.52 | 0.96 | ||
| Average | 0.62 | 59.07 | 3.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guedes, L.d.S.; e Sousa, M.B.; Oliveira, E.J.d. Genotype × Environment Interaction and Correlations Between Agronomic Traits, Flowering, and Fruit Set in Cassava. Horticulturae 2025, 11, 648. https://doi.org/10.3390/horticulturae11060648
Guedes LdS, e Sousa MB, Oliveira EJd. Genotype × Environment Interaction and Correlations Between Agronomic Traits, Flowering, and Fruit Set in Cassava. Horticulturae. 2025; 11(6):648. https://doi.org/10.3390/horticulturae11060648
Chicago/Turabian StyleGuedes, Luana da Silva, Massaine Bandeira e Sousa, and Eder Jorge de Oliveira. 2025. "Genotype × Environment Interaction and Correlations Between Agronomic Traits, Flowering, and Fruit Set in Cassava" Horticulturae 11, no. 6: 648. https://doi.org/10.3390/horticulturae11060648
APA StyleGuedes, L. d. S., e Sousa, M. B., & Oliveira, E. J. d. (2025). Genotype × Environment Interaction and Correlations Between Agronomic Traits, Flowering, and Fruit Set in Cassava. Horticulturae, 11(6), 648. https://doi.org/10.3390/horticulturae11060648






