1. Introduction
Gardenia is suitable for growing in warm and humid environments, and is mainly distributed in tropical and subtropical regions. However, it is also found in temperate regions [
1].
Gardenia is a type of four-season evergreen plant. The flowers have ornamental, medicinal, tea, dye extraction, oil extraction, spices and other uses. The fruits have gallbladder, liver protection, blood pressure reduction, swelling and sedation, antipyretic, anti-inflammatory and other functions [
2].
As an abiotic stress, the low-temperatures often encountered in the process of crop growth and development cause physiological damage to crops, resulting in yield decline and economic losses. Short-term low-temperature stress slows the growth and development of crops and reduces the protoplasmic capacity. With the intensification of low-temperature stress, various tissues and organs in crops will be damaged, growth and metabolic activities will be inhibited, and cell death might even be caused [
3]. The root system is an important organ in plants, as it absorbs water and nutrients [
4,
5]. After different degrees of low-temperature stress, the root system will show different changes. Under mild low-temperature stress, the root surface area and root volume increase, and the root tip cell structure remains intact. Under heavy and low-temperature conditions, root growth stops, the cell wall begins to disintegrate, and root tip cells become loose and cause death [
6]. When plants are subjected to low-temperature stress, a large number of reactive oxygen species accumulate, destroying the dynamic balance between the production and removal of original free radicals in the cell [
7]. Studies have confirmed that low-temperature stress significantly increases the production rate of reactive oxygen species in maize seedlings [
8]. Superoxide dismutase (SOD), catalase (POD) and peroxidase (CAT) are a class of enzymatic systems that can effectively remove reactive oxygen species from plants [
8]. Previous studies have shown that low-temperature stress significantly decreased the activities of three antioxidant enzymes (SOD, POD, CAT) in
Phalaenopsis leaves, and that high-intensity low-temperature stress inhibited or destroyed the intracellular antioxidant enzyme system [
9]. Under normal growth conditions, free radicals in rice undergo continuous generation and elimination, but under low-temperature conditions, the free radical generation rate in rice is far greater than the removal rate [
10]. Osmotic adjustment is an important reflection of plants’ adaptation to low temperatures. With a change in the external environment, the contents of the substances (proline, soluble protein and soluble sugar) in plants will change significantly, and their contents will be related to the tolerance of plants to low temperatures [
11]. Research results have confirmed that the soluble protein content in plants is positively correlated with their ability to resist low temperatures [
12]. As a protective substance in plant tissues, an increase in the soluble protein content can improve the water retention capacity of cells, reduce the freezing point of cells, and alleviate the damage caused by low-temperature stress to plants [
12]. Proline can prevent water loss and protect membrane proteins, and its content changes greatly under the influence of temperature; it is of great significance for maintaining the integrity of cell membranes [
13]. The soluble sugar content is the most sensitive index used to reflect plant metabolism under low-temperature stress [
14].
Photosynthesis can promote the cold resistance of plants. The sugars (such as glucose) and amino acids produced by photosynthesis can directly increase the concentration of cell fluids, reduce the freezing point, and prevent the formation of intracellular ice crystals [
15]. For example, cactus produces ‘cactin’ through photosynthesis, which can both resist drought and stabilize the cell membrane structure to resist low-temperature damage [
16]. A high content of cis-unsaturated fatty acids can maintain the fluidity of the chloroplast membrane and ensure normal photosynthesis at low temperatures, thus enhancing cold tolerance [
17]. Spraying potassium dihydrogen phosphate and other fertilizers can improve the photosynthetic efficiency of leaves and increase the cell fluid concentration [
18,
19]. Exogenous substances supplement, such as the addition of Tre, can jointly improve the photosynthetic efficiency and cold resistance of plants, forming a virtuous cycle [
20,
21].
Tre can form a unique protective film on the cell surface under harsh environmental conditions such as high temperatures, low temperatures, high osmotic pressure and dry water loss, effectively protecting the structure of biomolecules from being destroyed, so as to maintain the life process and biological characteristics of living organisms [
22]. Tre is a safe, stable and very reliable natural sugar that has a non-specific protective effect on biological macromolecules and organisms, especially under adverse conditions such as low temperatures, drought, salt damage and high heat, and has an efficient protective effect on a series of biological macromolecules that maintain normal plant life activities [
23].
Exogenous Tre spray can protect plant proteins from damage under abiotic stress conditions such as low temperatures, drought and salt damage [
24]. Studies have shown that exogenous Tre spray can reduce the malondialdehyde (MDA) content and relative permeability of the plasma membrane under salt stress, and increase SOD and POD activities [
25]. Under high-temperature stress, exogenous Tre spray increased the ascorbic acid content, enhanced CAT and ascorbate peroxidase activities, and decreased the MDA and hydrogen peroxide content in wheat seedlings [
26]. At the same time, the exogenous spray of Tre can improve POD and CAT activities and the ascorbic acid content of maize under drought stress, and alleviate oxidative damage under drought stress [
27]. Under the condition of low-temperature treatment, exogenous Tre spray increased the relative water content, proline content and soluble sugar content of wheat, decreased the MDA content, and alleviated the damage caused by low-temperature stress [
28]. Other studies have shown that after applying different concentrations of Tre, various indexes of muskmelon seedlings are improved and the root activity and soluble sugar content are increased, indicating that Tre has an alleviating effect on the physiological and biochemical characteristics of plants [
29].
As a landscape plant, gardenia is suitable for growing in a warm and humid environment, but harsh climates such as a cold spring occur frequently, seriously affecting the growth of gardenia. We hypothesize that Tre can enhance the cold resistance of gardenia through various pathways such as photosynthesis, the antioxidant system, endogenous hormone metabolism and respiration. The main significance of this study is its provision of new technology and theoretical support for the cultivation of gardenia under low-temperature conditions.
2. Materials and Methods
2.1. Plant Material and Experimental Design
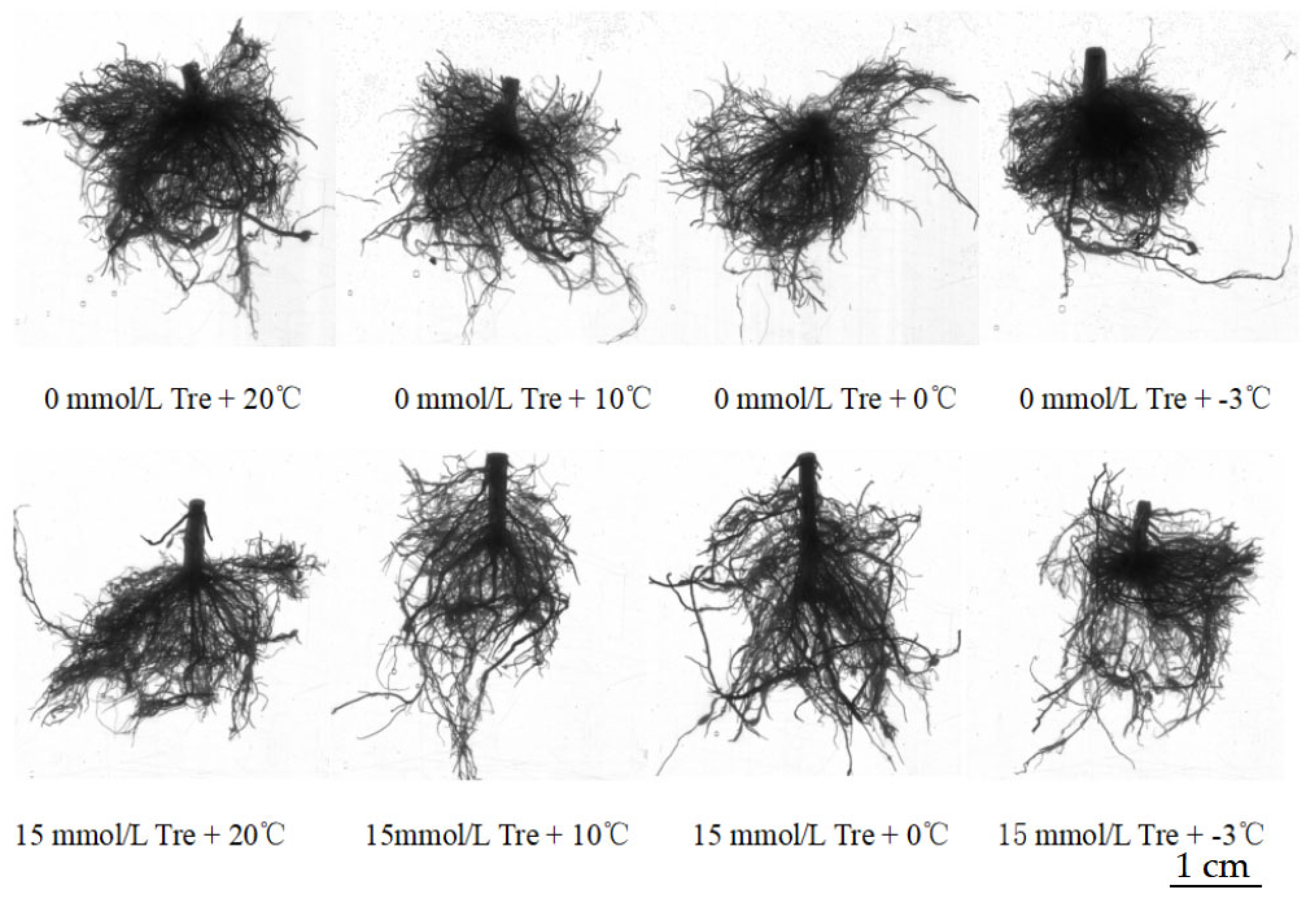
The experiment adopted a two-factor design: (1) Tre treatment (including 0 mmol/L Tre and 15 mmol/L Tre); (2) Low-temperature treatment (20 °C, 10 °C, 0 °C and −3 °C). The experiment consisted of 8 treatments with 5 replicates per treatment group (1 basin per replicate) and a total of 40 pots. In this experiment, gardenia seedlings (given by Jiangxi Academy of Forestry, Nanchang, China) were transplanted into plastic pots (with sterilized river sand) on 12 April 2023, and then the plastic pots were placed in an illumination incubator (18/6 h day/night) at four temperatures (20 °C, 10 °C, 0 °C and −3 °C). Hoaglang nutrient solution was irrigated from the day of planting, and the nutrient solution with a corresponding concentration of Tre was irrigated once every three days. Plants were harvested on 11 June 2023 after 2 months of Tre treatment at 4 temperatures and corresponding concentrations.
2.2. Plant Growth Index and Root System Configuration
Before harvest, the plant height was measured with a ruler (cm) and the number of fully unfolded leaves was measured by the counting method. After harvest, the total weight of the plants and the fresh weight of the above-ground parts and underground parts were measured by electronic balance. After the plants were harvested, root images were obtained using an Epson V700 color image scanner, and root configuration parameters (root length, number of lateral roots, total root surface area, root volume, etc.) were obtained using the root analyzer system (WinRHIZO, Regent Instruments Company, Québec City, QC, Canada).
2.3. Chlorophyll in Leaves, Chlorophyll Fluorescence Parameters and Photosynthetic Intensity Parameters
Before harvesting the plants, healthy and fully unfolded leaves were selected, and the content of chlorophyll a, chlorophyll b and total chlorophyll was measured after wiping the leaves with a clean wet cloth. The chlorophyll fluorescence parameters were determined by IMAGING-PAM (M-series modulated chlorophyll fluorescence meter, Heinz Walz GmbH, Nuremberg, Germany). Before harvesting, the gardenia treated with different treatments was completely unfolded and measured from 09:00 to 11:00. After dark adaptation treatment for 20 min, the blades were fixed on the loading platform. The actual photochemical efficiency (φPSII), maximum photochemical efficiency (Fv′/Fm′), non-photochemical quenching coefficient (NPQ) and photochemical quenching coefficient (qP) of the blades were measured to evaluate the light energy utilization efficiency. Before harvesting the plants, the photosynthetic parameters of the leaves were determined by a Li-6400 photosynthesator. Functional leaves at the 4th to 5th positions with a good physiological status were selected as the measurement objects, and parameters such as the transpiration rate (Tr), net photosynthetic rate (Pn), intercellular CO2 concentration (Ci) and stomatal conductance (Gs) were obtained.
2.4. Activities of Reactive Oxygen Species and Antioxidant Enzymes and Contents of Osmotic Substances in Roots
By using the hydroxylamine oxidation method, employing hydroxylamine (NH2OH) and O2-specific reoxidation reaction, determining the absorbance at a 540 nm wavelength, and calculating the concentration of NO2− using the standard curve, the content of superoxide anion could be determined. Hydrogen peroxide (H2O2) is analyzed by titanium sulfate colorimetry. Titanium sulfate reacts with hydrogen peroxide to produce yellow precipitate. The precipitate can be used to detect the concentration of hydrogen peroxide by measuring the Optical Density (OD value) at 415 nm.
The activities of superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) were determined by the nitrogen blue tetrazole photoreduction method, guaiacol color development method and ammonium molybdate color development method.
The proline (Pro) content was determined by sulfosalicylic acid extraction and the acid indanhydrin color development method. The product was obtained by this method and placed at a 520 nm wavelengths; then, the concentration of proline was converted by detecting the OD value of the wavelength. The soluble sugar was detected by the anthrone color development method. The product obtained by this method was placed at a 620 nm wavelength, and then the content of soluble sugar could be converted by detecting the OD value of the wavelength. The content of malondialdehyde was determined by the thiobarbituric acid (TBA) color development method. The concentration of malondialdehyde was obtained by determining the OD600, OD532 and OD450 of the reaction products on the spectrophotometer. The soluble protein content was determined by the Coomasil bright blue (G-250) staining method. Then, 20 μL of extract solution (enzyme solution) was added to 80 μL, 0.05 mol·L−1 and pH 7.8 phosphate buffer. Then, 2.9 mL of Coomasil bright blue solution was added, and OD595 was measured after the reaction for 2 min. The soluble protein concentration was obtained by calculating the value.
2.5. Endogenous Hormones, Malic Acid and Succinic Acid in Roots
The endogenous hormones in roots were determined using various kits (Nanjing Jiancheng Bioengineering Research Institute Co., Ltd., Nanjing, China), such as the IAA ELISA kit, trans-zeaxin nucleoside (tZR) ELISA kit, abscisic acid (ABA) ELISA kit and gibberellin (GA3) ELISA kit. The above kits are based on the double antibody sandwich method, which detects the hormone content in plant samples. The malic acid and succinic acid contents were determined by high-performance liquid chromatography (HPLC): 1.00 g of gardenia root was accurately weighed and mixed with a blender, ground with 4 mL of extraction solution and centrifuged for 10 min at 10,000 r/min; the residue was added to 2 mL of extraction solution and then extracted, combined with the supernatant, dried in a water bath at 90 °C at a fixed volume of 10 mL, and then extracted with a disposable syringe after whirlpool mixing. It was filtered using a 0.45 μm filter membrane and analyzed using a machine.
2.6. Statistical Analysis
We performed a variance analysis (ANOVA, the GLM procedure) (SAS software 8.1v, SAS Institute, Gaston County, NC, USA) to statistically analyze the data. Microsoft Excel (Version 2013, Microsoft Institute, Redmond, WA, USA) was used for data processing and graphing, and Duncan’s multirange experiment compared significant differences between treatments with p < 0.05.
4. Discussion
The growth of
gardenia is regulated not only by environmental conditions, but also by exogenous substances, which could help plants to resist the stress of adversity. Few studies have reported the effect of exogenous Tre on the growth of
gardenia seedlings under low-temperature stress, but the regulation of other crops and adversity has been reported. Based on low-temperature stress experiments on
Catharanthus roseus, which was used as the experimental material, Wei et al. [
30] discovered that Tre effectively increased plant growth, improved the chlorophyll content and antioxidant enzyme activity of leaves, and improved their cold resistance. Aldesuquy et al. [
31] found that the exogenous application of Tre appeared to mitigate the damaging effect of drought with different magnitudes by counteracting the negative effects of water stress on all growth criteria for wheat root and improving the turgidity of wheat leaf by decreasing the rate of transpiration, increasing the relative water content, decreasing the saturation water deficit, and increasing the water use efficiency for wheat economic yield. In this study, the exogenous application of Tre solution under low-temperature stress significantly improved the growth potential of
gardenia seedlings, such as the plant height, leaf number, total plant weight, above-ground fresh weight, underground fresh weight, root total length, lateral root number, total root surface area and root volume, especially under −3 °C low-temperature stress. Exogenous Tre had the best effect on restoring the growth potential of
gardenia. This is similar to the results of a study by Raza et al. [
32] on exogenous Tre’s effect on the cold tolerance of Rapeseed (
Brassica napus L.) seedlings under low-temperature stress.
When plants encounter low-temperature stress, the chlorophyll will be destroyed, and the chlorophyll content will be forced to decrease, which will eventually weaken the photosynthetic capacity of plants and inhibit the carbon assimilation pathway, resulting in slow plant growth [
33,
34]. Tang et al. [
35] proved that exogenous Tre (10 mmol·L
−1) could significantly increase the contents of chlorophyll a, chlorophyll b and total chlorophyll in the leaves of wheat seedlings under low-temperature stress. This is consistent with the results of this study: low-temperature stress inhibited the synthesis of chlorophyll in the leaves of
gardenia to varying degrees, and exogenous Tre at 15 mmol/L could effectively alleviate the inhibition of low-temperature stress on chlorophyll synthesis in the leaves of
gardenia and moderately restore the contents of chlorophyll a, chlorophyll b and total chlorophyll.
When plant growth is subjected to abiotic stress, the inner membrane of chloroplasts is destroyed, thus affecting plant photosynthesis and growth and development [
36]. The photosynthetic mechanism PSII on the chloroplast thylakoid membrane is particularly sensitive to environmental changes [
36]. The chlorophyll fluorescence parameters φPSII, Fv′/Fm′, qP and NPQ can represent the initial photochemical capacity of PSII and are important indicators used to reflect the effects of environmental stress on photosynthesis [
37]. In the photosynthetic apparatus PSII, Fv′/Fm′ is decreased when plants are subjected to photoinhibition, which indicates that photosystem II is destroyed [
37]. Pilon-Smits [
38] showed that Tre treatment significantly improved the Fv′/Fm′ value in leaves under abiotic stress and restored it to the control level, effectively alleviating the damage caused by abiotic stress to the PSII reaction center, indicating that Tre can alleviate or even restore the damage caused by abiotic stress to PSII. These results also showed that Tre increased the φPSII in leaves under abiotic stress, indicating that the actual photochemical utilization efficiency of leaves increased [
38]. Tre also slows down the decrease in qP and ETR in leaves caused by abiotic stress, and decreases NPQ, indicating that Tre can alleviate problems such as the decrease in the photochemical efficiency and fluorescence yield in leaves caused by abiotic stress [
38]. The above results were similar to the present study: the φPSII, Fv′/Fm′ and qP values of
gardenia leaves decreased to varying degrees under low-temperature stress, and exogenous Tre treatment effectively restored these parameters to the control level, so as to improve the adverse effect of low-temperature stress on the PSII reaction center.
Photosynthesis is very sensitive to abiotic stresses, including strong light, water stress, high temperatures, salt damage, heavy metal toxicity, etc., that reduce the photosynthetic efficiency of plants and thus affect the normal growth of plants [
39]. The photosynthetic intensity parameters can accurately reflect the photosynthetic intensity of plants, which mainly include the net photosynthetic rate (Pn), stomatal conductance (Gs), intercellular carbon dioxide concentration (Ci) and transpiration rate (Tr) [
40]. Plants mainly rely on stomata to exchange external gases (CO
2 and H
2O
2), and CO
2 is the substrate of plant photosynthesis, so the level of Ci and Tr are affected by Gs, further affecting the strength of photosynthesis [
40]. Tr represents the amount of water evaporated per unit leaf area of plant leaves within a certain period of time, and the transpiration pull generated by Tr can absorb and transport water, providing material sources for the plant photosynthesis process [
40]. It has been reported that stress can affect the normal level of various photosynthetic intensity parameters [
41]. With the aggravation of stress, the Pn, Tr and Gs of the leaves of
Leymus chinensis showed a gradual decline, while Ci showed an upward trend [
41]. In order to reduce the damage caused by stress to plant growth and development, researchers have added exogenous substances (such as Tre) to alleviate the adverse effects of stress on plant photosynthesis. Studies have shown that the exogenous addition of Tre can affect the parameters of photosynthetic intensity in wheat leaves under drought stress, and that the appropriate concentration of Tre can significantly increase the chlorophyll content and enhance the values of Pn, Tr and Gs in wheat leaves [
42]. Razzaq et al. [
43] showed that chromium (Cr) stress (100 uM) significantly reduced the Pn, Gs and Tr of
Zea mays leaves, and that the increase in Cr concentration (500 uM) further exacerbated this adverse effect. However, exogenous Tre treatment can effectively reduce these adverse effects caused by Cr stress on
Zea mays leaves, and the effect of 50 mM of Tre is better than that of 25 mM of Tre. These results are consistent with the results of this study: low-temperature stress inhibited the photosynthetic intensity parameters and photosynthetic efficiency of
gardenia; in particular, a low-temperature stress of −3 °C significantly decreased Pn, Gs, Ci and Tr, while 15 mmol/L Tre effectively mitigated the inhibition effects of low-temperature stress on the photosynthetic intensity and photosynthetic efficiency of
gardenia leaves.
Plants will produce abundant ROS when they are subjected to environmental stress. This ROS could destroy macromolecular substances such as DNA, proteins and membrane structures in plant tissues [
44,
45,
46]. In order to avoid the damage caused by excessive ROS accumulation to cells, plants relieve the damage via antioxidant enzymes such as superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), glutathione reductase (GR) and ascorbate peroxidase (APX) [
47,
48,
49,
50]. Luo and Li [
51] showed that heat stress can significantly increase the ROS content (hydrogen peroxide, superoxide anion radical, etc.) in wheat, while exogenous Tre can scavenge the ROS content (hydrogen peroxide, superoxide anion, etc.) by increasing the activities of antioxidant enzymes such as APX, SOD, and CAT, thus alleviating the damage caused by abiotic stress in wheat. Tre treatment significantly enhanced the activities of antioxidant-related enzymes such as APX, CAT, SOD, and GR, as well as the transcription levels of the
AsA-GSH cycle-related gene, which led to a reduction in the ROS (such as hydrogen peroxide) content in peach during cold storage [
52]. According to a study by Akram et al. [
53] in radish (
Raphanus sativus L.), spraying Tre (25 mM) can alleviate the damage caused by water stress to seedlings by enhancing the activities of SOD and POD. Zheng et al. [
54] also confirmed that exogenous Tre (5.0 mM) could induce an increase in the antioxidant enzyme activity (such as SOD and POD) in tea plant under heat stress, indicating that Tre could further stimulate the enzymatic defense system of tea seedlings under heat stress, enhance the antioxidant capacity of plants, and alleviate the damage to cell membranes caused by high-temperature stress. These results are consistent with the results of this study: low-temperature stress promotes the production of excessive ROS in
gardenia, and exogenous Tre can effectively enhance the activity of antioxidant enzymes in
gardenia under low-temperature stress, improve its antioxidant defense ability to reduce the ROS content in vivo, maintain the reoxygen–reduction balance in cells, and protect the structure and function of cell membranes.
Plants under abiotic stress will produce a large number of osmoregulatory substances. Osmoregulatory substances can not only maintain cell turgor and prevent excessive water loss in the protoplasm, but also stabilize the organelle structure, regulate some physiological functions, and alleviate the damage caused to plants under stress. Proline (Pro), malondialdehyde (MDA), soluble protein and soluble sugar are osmoregulatory substances in plants. Hasanuzzaman et al. [
55] confirmed that drought increased the Pro and MDA contents and altered the antioxidant and glyoxalase systems in three Brassica species (
B. napus,
B. campestris and
B. juncea), while Tre reduced the MDA and Pro contents and activity of lipoxygenase enzymes (LOX), further enhancing their drought tolerance. This is consistent with the conclusions of this study: low-temperature stress increased the contents of osmoregulatory substances (Pro and MDA) in the roots of
gardenia, and exogenous Tre effectively alleviated the abnormal accumulation of osmoregulatory substances induced by low temperatures, reduced the damage caused by membrane lipid peroxidation, and improved the cold resistance of
gardenia. In addition, this study also found that soluble protein and soluble sugar were not affected by low-temperature stress and exogenous Tre. However, our results are not quite the same as Zheng et al. [
54]: the contents of PRO and soluble sugar exhibited a significant increase, while the MDA content decreased following treatment with 5.0 mmol·L
−1 Tre under 24 h high-temperature stress (38 °C/29 °C, 12 h/12 h). This may be due to the different responses of Tre to different plants under different abiotic stress conditions; the specific reasons for this need to be further explored.
Plant hormones play an important role in the plant stress response, and can cause adaptive regulatory responses in plants [
56,
57,
58]. When plants encounter abiotic stress such as low temperatures, they can cope with environmental stress by regulating related hormones in the plants [
59,
60]. Cui et al. [
61] treated
Cabernet Sauvignon seedlings with 15 mmol·L
−1 Tre and determined and analyzed the contents of four endogenous hormones (tZR, GA3, IAA and ABA) under low-temperature stress. Compared with the control group, the contents of tZR, GA3 and ABA in the Tre treatment group at −3 °C were increased by 80.03%, 27.66% and 39.14%, respectively, while the contents of IAA were decreased to 0.94 ng·g
−1 [
61]. This is consistent with the findings of this study: low-temperature stress decreased the IAA content but increased the tZR, GA3 and ABA contents in the roots of
gardenia; the 15 mmol/L Tre treatment alleviated the decreasing effect of low-temperature stress on the IAA content in the roots of
gardenia and weakened the increasing effect of low-temperature stress on the tZR and GA3 contents, but had no significant effect on ABA. It can be concluded from the above that Tre treatment has a certain protective effect on the plant hormone synthesis system under low-temperature stress, and can restore the auxin content to a certain extent to restore plant growth.
The normal development of respiratory metabolism plays a vital role in the process of plant growth and development [
62]. When plants face abiotic stress, the appropriate amount of intermediate metabolites is the basis for their adaptation to low temperatures [
62]. As intermediate products of plant respiratory metabolism, succinic acid and malic acid are closely related to plant metabolism [
63]. The succinic acid produced during the tricarboxylic acid cycle, under the action of SDH, produces fumaric acid, which is converted into malic acid by hydration [
63]. A previous study showed that the concentration of root respiratory metabolites is significantly correlated with root activity in rhizosphere soil, and that malic acid and succinic acid as root respiratory metabolites can enhance root activity and promote plant growth [
64]. However, Tre treatment in this study increased the content of malic acid in the roots of
gardenia but decreased the content of succinic acid. This may be different from the response of different plants to Tre stimulation.
Gardenia may respond to the stimulation of exogenous Tre in root respiration through malic acid. Therefore, the application of an appropriate concentration of Tre can promote the production of root respiratory metabolites, enhance root vitality, promote plant growth, and improve plant resistance to low temperatures.