Alginate Oligosaccharide Coordinately Modulates Endogenous Phytohormone Profiles to Enhance Tomato Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
- Control: 0 mg·L−1 AOS
- A15: 15 mg·L−1 AOS
- A30: 30 mg·L−1 AOS
- A45: 45 mg·L−1 AOS
2.3. Measurement of Growth Parameters
2.4. Root Morphological Analysis
2.5. Chloroplast Pigments and Pn Measurements
2.6. Phytohormone Profiling
2.7. Data Analysis
3. Results
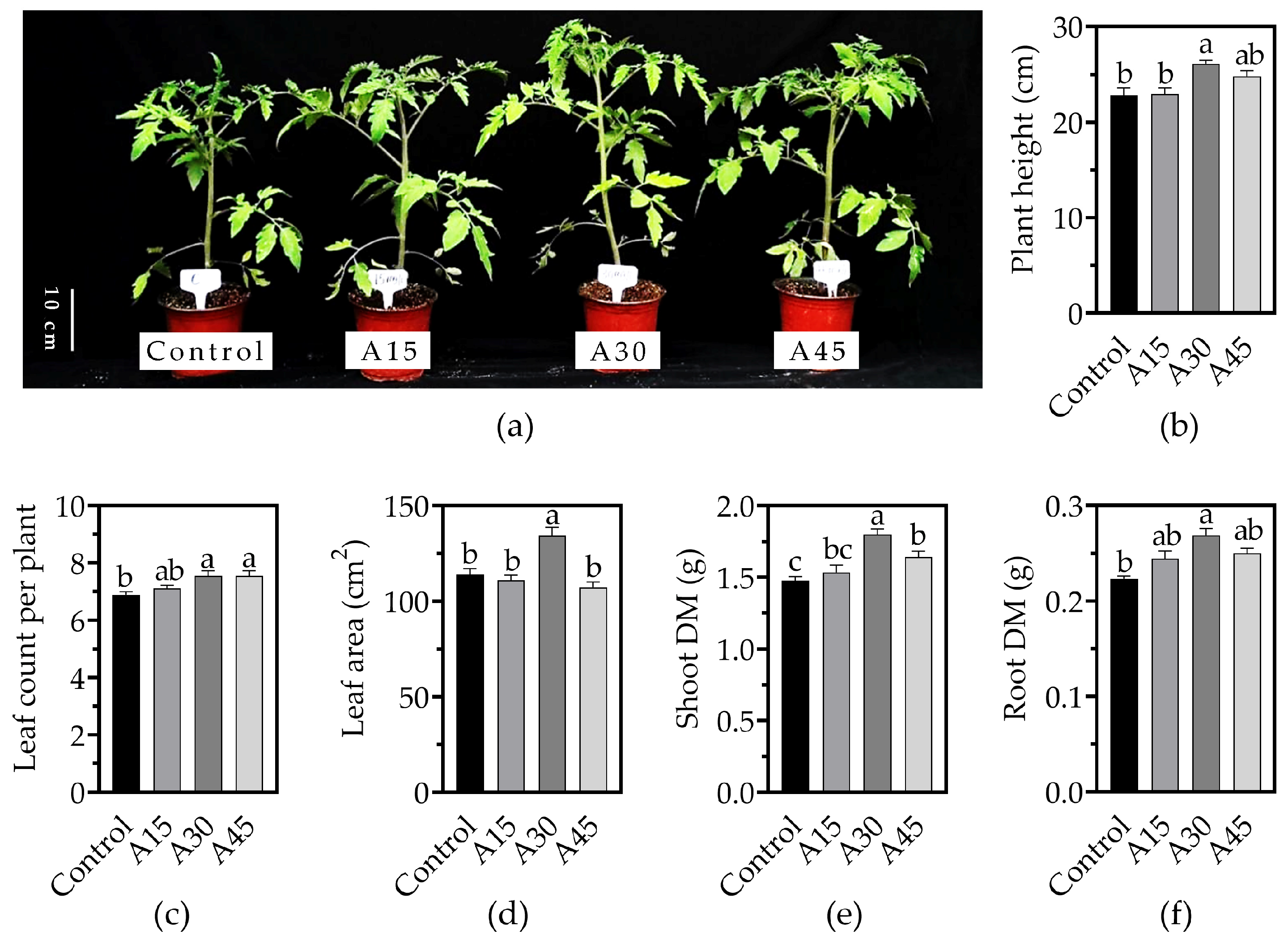
3.1. Effects of AOS Application on the Growth of Tomato Seedlings
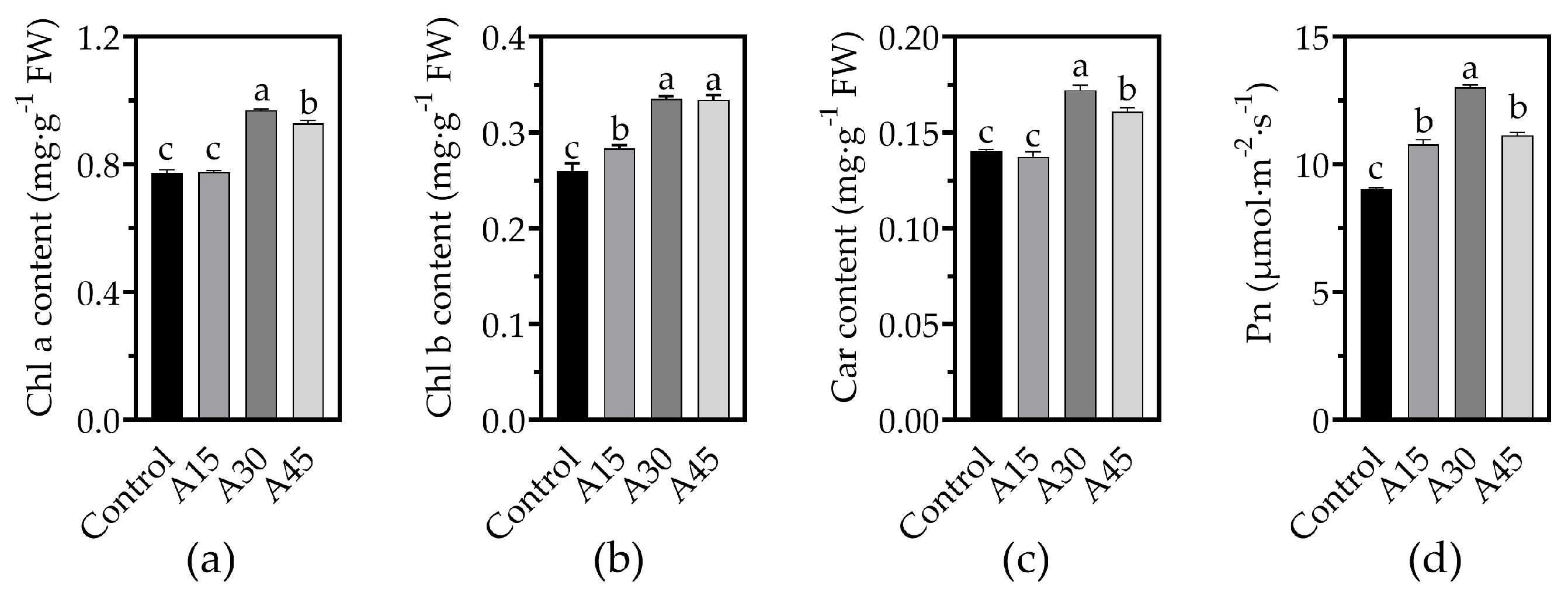
3.2. Effects of AOS Application on Chloroplast Pigment Contents and Photosynthetic Rate of Tomato Leaves
3.3. Effects of AOS Application on the Root Architecture Parameters of Tomato Seedlings
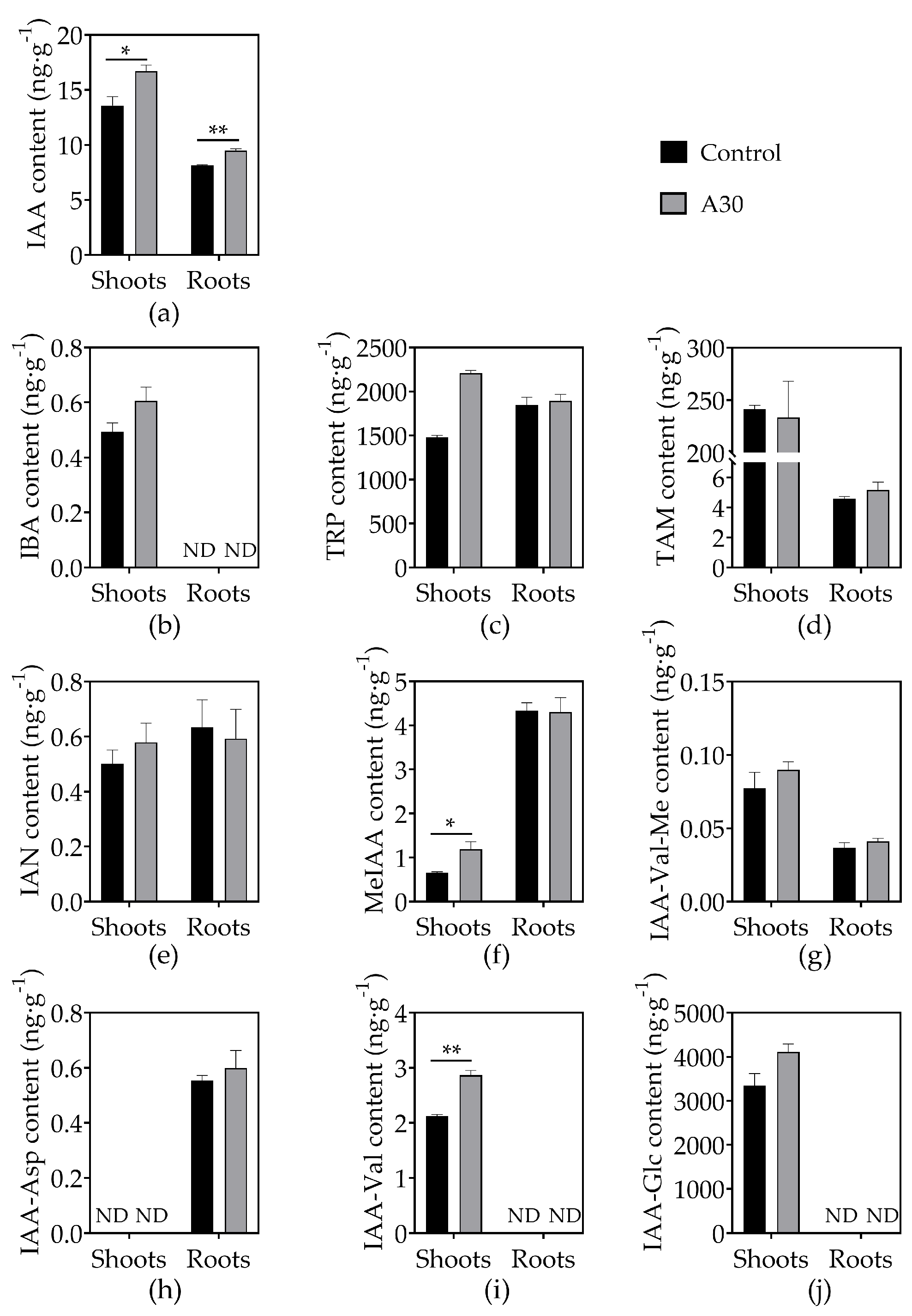
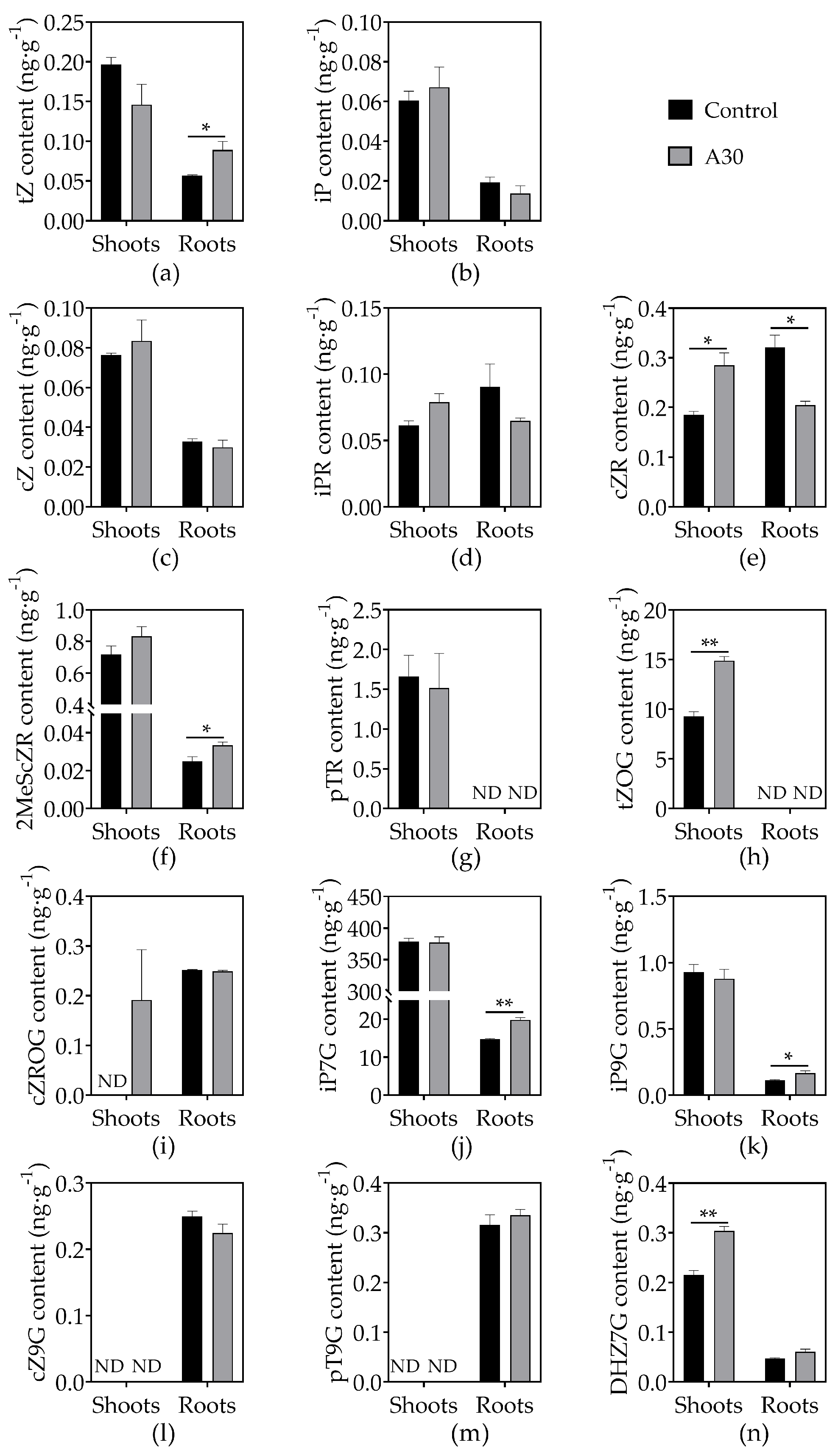
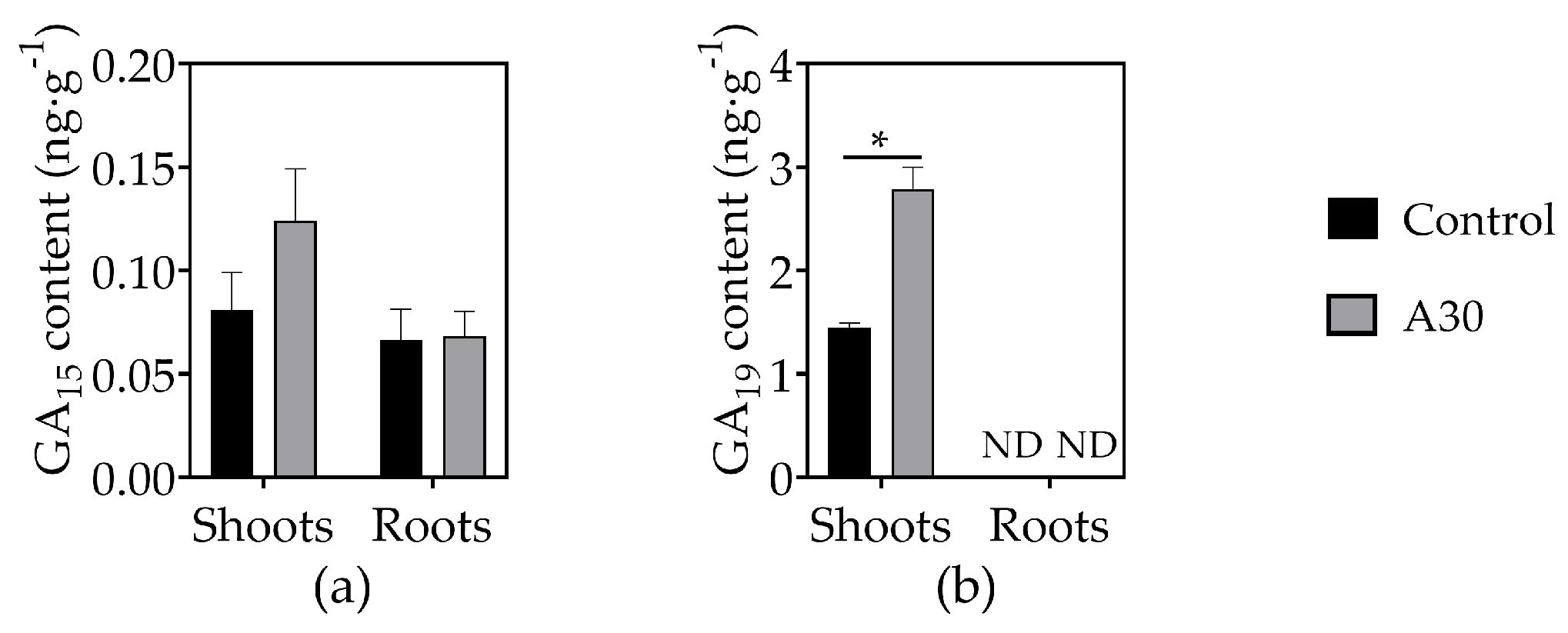
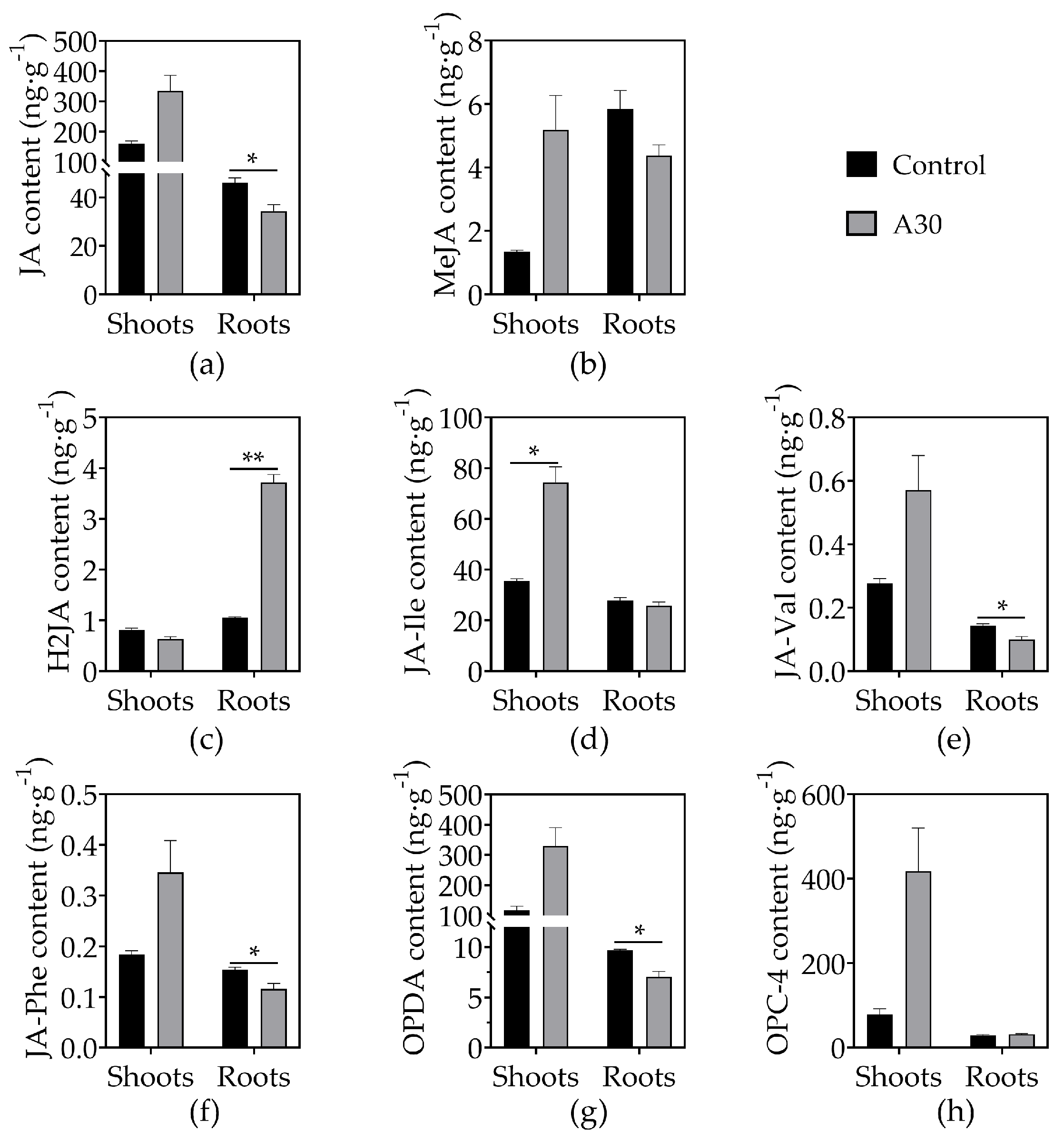
3.4. Effects of AOS on Phytohormone Concentrations in Tomato Seedlings
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2MeScZR | 2-methylthio-cis-zeatin riboside |
| 5DS | 5-deoxystrigol |
| ABA | Abscisic acid |
| ACC | 1-aminocyclopropane-1-carboxylic acid |
| AOS | Alginate oligosaccharide |
| Car | Carotenoids |
| Chl a | Chlorophyll a |
| Chl b | Chlorophyll b |
| CK | Cytokinin |
| cZ | cis-Zeatin |
| cZ9G | cZ N9-glucoside |
| cZR | cZ 9-riboside |
| cZROG | cZR O-glucoside |
| DHZ7G | DHZ N7-glucoside |
| DM | Dry mass |
| GA | Gibberellic acid |
| H2JA | Dihydrojasmonic acid |
| IAA | Indole-3-acetic acid |
| IAA-Asp | Indole-3-acetyl-L-aspartic acid |
| IAA-Glc | 1-O-indol-3-ylacetylglucose |
| IAA-Val | Indole-3-acetyl-L-valine |
| IAA-Val-Me | Indole-3-acetyl-L-valine |
| IAN | 3-indoleacetonitrile |
| IBA | Indole-3-butyric acid |
| iP | Isopentenyladenine |
| iP7G | iP N7-glucoside |
| iP9G | iP N9-glucoside |
| iPR | Isopentenyladenosine |
| JA | Jasmonic acid |
| JA-Ile | Jasmonoyl-L-isoleucine |
| JA-Phe | Jasmonoyl-L-phenylalanine |
| JA-Val | Jasmonoyl-L-valine |
| MeIAA | Methyl indole-3-acetate |
| MeJA | Methyl jasmonate |
| OPC-4 | 3-Oxo-2-(2Z-pentenyl) cyclopentane-1-butyric acid |
| OPDA | 12-oxo-phytodienoic acid |
| Pn | Net photosynthetic rate |
| pT9G | para-topolin-9-glucoside |
| pTR | para-topolin riboside |
| SL | Strigolactone |
| TAM | Tryptamine |
| TRP | L-tryptophan |
| tZ | trans-Zeatin |
| tZOG | tZ O-glucoside |
References
- Mostafalou, S.; Abdollahi, M. Pesticides and human chronic diseases: Evidences, mechanisms, and perspectives. Toxicol. Appl. Pharmacol. 2013, 268, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Velthof, G.L.; Lesschen, J.P.; Webb, J.; Pietrzak, S.; Miatkowski, Z.; Pinto, M.; Kros, J.; Oenema, O. The impact of the nitrates directive on nitrogen emissions from agriculture in the EU-27 during 2000–2008. Sci. Total Environ. 2014, 468–469, 1225–1233. [Google Scholar] [CrossRef]
- Ma, Y. Does “Zero Growth Policy” affect environmental productivity of wheat production in China? Agriculture 2025, 15, 378. [Google Scholar] [CrossRef]
- El Sheikha, A.F.; Allam, A.Y.; Taha, M.; Varzakas, T. How does the addition of biostimulants affect the growth, yield, and quality parameters of the snap bean (Phaseolus vulgaris L.)? How is this reflected in its nutritional value? Appl. Sci. 2022, 12, 776. [Google Scholar] [CrossRef]
- Xing, M.; Cao, Q.; Wang, Y.; Xiao, H.; Zhao, J.; Zhang, Q.; Ji, A.; Song, S. Advances in research on the bioactivity of alginate oligosaccharides. Mar. Drugs 2020, 18, 144. [Google Scholar] [CrossRef]
- Li, L.; Jiang, X.; Guan, H.; Wang, P. Preparation, purification and characterization of alginate oligosaccharides degraded by alginate lyase from Pseudomonas sp. HZJ 216. Carbohydr. Res. 2011, 346, 794–800. [Google Scholar] [CrossRef]
- Yang, J.; Shen, Z.; Sun, Z.; Wang, P.; Jiang, X. Growth stimulation activity of alginate-derived oligosaccharides with different molecular weights and mannuronate/guluronate ratio on Hordeum vulgare L. J. Plant Growth Regul. 2021, 40, 91–100. [Google Scholar] [CrossRef]
- Sarfaraz, A.; Naeem, M.; Nasir, S.; Idrees, M.; Aftab, T.; Hashmi, N.; Khan, M.M.; Moinuddin; Varshney, L. An evaluation of the effects of irradiated sodium alginate on the growth, physiological activities and essential oil production of fennel (Foeniculum vulgare Mill.). J. Med. Plant Res. 2011, 5, 15–21. [Google Scholar]
- Aftab, T.; Khan, M.M.A.; Idrees, M.; Naeem, M.; Moinuddin; Hashmi, N.; Varshney, L. Enhancing the growth, photosynthetic capacity and artemisinin content in Artemisia annua L. by irradiated sodium alginate. Radiat. Phys. Chem. 2011, 80, 833–836. [Google Scholar] [CrossRef]
- Naeem, M.; Idrees, M.; Aftab, T.; Khan, M.M.A.; Varshney, L. Irradiated sodium alginate improves plant growth, physiological activities and active constituents in Mentha arvensis L. J. Appl. Pharm. Sci. 2012, 2, 28–35. [Google Scholar] [CrossRef]
- Ali, A.; Khan, M.M.A.; Uddin, M.; Naeem, M.; Idrees, M.; Hashmi, N.; Dar, T.A.; Varshney, L. Radiolytically depolymerized sodium alginate improves physiological activities, yield attributes and composition of essential oil of Eucalyptus citriodora Hook. Carbohydr. Polym. 2014, 112, 134–144. [Google Scholar] [CrossRef]
- Moussa, H.R.; Taha, M.A.; Dessoky, E.S.; Selem, E. Exploring the perspectives of irradiated sodium alginate on molecular and physiological parameters of heavy metal stressed Vigna radiata L. plants. Physiol. Mol. Biol. Plants 2023, 29, 447–458. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, H.; Wang, W.; Zhao, X.; Du, Y.; Wu, L. Enhancement in photosynthesis characteristics and phytohormones of flowering Chinese cabbage (Brassica campestris L. var. utilis Tsen et Lee) by exogenous alginate oligosaccharides. J. Food Agric. Environ. 2013, 11, 669–675. [Google Scholar]
- Zhang, Y.; Liu, H.; Yin, H.; Wang, W.; Zhao, X.; Du, Y. Nitric oxide mediates alginate oligosaccharides-induced root development in wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2013, 71, 49–56. [Google Scholar] [CrossRef]
- Aitouguinane, M.; El Alaoui-Talibi, Z.; Rchid, H.; Fendri, I.; Abdelkafi, S.; El-Hadj, M.D.O.; Boual, Z.; Le Cerf, D.; Rihouey, C.; Gardarin, C.; et al. Elicitor Activity of low-molecular-weight alginates obtained by oxidative degradation of alginates extracted from Sargassum muticum and Cystoseira myriophylloides. Mar. Drugs 2023, 21, 301. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, D.; Gruda, N.S.; Duan, Z.; Li, X. Fertilizer application rate and nutrient use efficiency in Chinese greenhouse vegetable production. Resour. Conserv. Recycl. 2024, 203, 107431. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, H.; Zhao, X.; Wang, W.; Du, Y.; He, A.; Sun, K. The promoting effects of alginate oligosaccharides on root development in Oryza sativa L. mediated by auxin signaling. Carbohydr. Polym. 2014, 113, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Y.; Yin, H.; Wang, W.; Zhao, X.; Du, Y. Alginate oligosaccharides enhanced Triticum aestivum L. tolerance to drought stress. Plant Physiol. Biochem. 2013, 62, 33–40. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Lin, X.; Yan, G.; Liu, L.; Zheng, H.; Zhao, B.; Tang, J.; Guo, Y.-D. Alginate-derived oligosaccharides promote water stress tolerance in cucumber (Cucumis sativus L.). Plant Physiol. Biochem. 2018, 130, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Howlader, P.; Liu, T.; Sun, X.; Jia, X.; Zhao, X.; Shen, P.; Qin, Y.; Wang, W.; Yin, H. Alginate oligosaccharide (AOS) induced resistance to Pst DC3000 via salicylic acid-mediated signaling pathway in Arabidopsis thaliana. Carbohydr. Polym. 2019, 225, 115221. [Google Scholar] [CrossRef]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014, 4, 63–69. [Google Scholar]
- Liu, J.; Yang, J.; Jiang, C.; Zhou, J.; Zhao, Y.; Huang, L. Volatile organic compound and endogenous phytohormone characteristics during callus browning in Aquilaria sinensis. Ind. Crops Prod. 2021, 168, 113605. [Google Scholar] [CrossRef]
- Li, S.; Ran, S.; Yuan, S.; Chang, K.; Han, M.; Zhong, F. Gibberellin-mediated far-red light-induced leaf expansion in cucumber seedlings. Protoplasma 2024, 261, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzoglou, P.; van Ieperen, W.; Harbinson, J.; van der Meer, M.; Martinakos, S.; Weerheim, K.; Nicole, C.C.S.; Marcelis, L.F.M. Effects of continuous or end-of-day far-red light on tomato plant growth, morphology, light absorption, and fruit production. Front. Plant Sci. 2019, 10, 322. [Google Scholar] [CrossRef]
- Rowland, S.D.; Zumstein, K.; Nakayama, H.; Cheng, Z.; Flores, A.M.; Chitwood, D.H.; Maloof, J.N.; Sinha, N.R. Leaf shape is a predictor of fruit quality and cultivar performance in tomato. New Phytol. 2020, 226, 851–865. [Google Scholar] [CrossRef]
- Horiguchi, G.; Ferjani, A.; Fujikura, U.; Tsukaya, H. Coordination of cell proliferation and cell expansion in the control of leaf size in Arabidopsis thaliana. J. Plant Res. 2006, 119, 37–42. [Google Scholar] [CrossRef]
- Alabadí, D.; Blázquez, M.A. Molecular interactions between light and hormone signaling to control plant growth. Plant Mol. Biol. 2009, 69, 409–417. [Google Scholar] [CrossRef]
- Saini, K.; Markakis, M.N.; Zdanio, M.; Balcerowicz, D.M.; Beeckman, T.; De Veylder, L.; Prinsen, E.; Beemster, G.T.S.; Vissenberg, K. Alteration in auxin homeostasis and signaling by overexpression of PINOID kinase causes leaf growth defects in Arabidopsis thaliana. Front. Plant Sci. 2017, 8, 1009. [Google Scholar] [CrossRef]
- Rai, M.I.; Wang, X.; Thibault, D.M.; Kim, H.J.; Bombyk, M.M.; Binder, B.M.; Shakeel, S.N.; Schaller, G.E. The ARGOS gene family functions in a negative feedback loop to desensitize plants to ethylene. BMC Plant Biol. 2015, 15, 157. [Google Scholar] [CrossRef]
- Spartz, A.K.; Lee, S.H.; Wenger, J.P.; Gonzalez, N.; Itoh, H.; Inzé, D.; Peer, W.A.; Murphy, A.S.; Overvoorde, P.J.; Gray, W.M. The SAUR19 subfamily of SMALL AUXIN UP RNA genes promote cell expansion. Plant J. 2012, 70, 978–990. [Google Scholar] [CrossRef]
- Shohat, H.; Ceriker, H.; Vasuki, H.; Illouz-Eliaz, N.; Blum, S.; Amsellem, Z.; Tarkowska, D.; Aharoni, A.; Eshed, Y.; Weiss, D. Inhibition of gibberellin accumulation by water deficiency promotes fast and long-term ‘drought avoidance’ responses in tomato. New Phytol. 2021, 232, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Ueguchi-Tanaka, M.; Nakajima, M.; Katoh, E.; Ohmiya, H.; Asano, K.; Saji, S.; Hongyu, X.; Ashikari, M.; Kitano, H.; Yamaguchi, I.; et al. Molecular interactions of a soluble gibberellin receptor, GID1, with a rice DELLA protein, SLR1, and gibberellin. Plant Cell 2007, 19, 2140–2155. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, H.; Xu, Y.; Wang, Y.; Zhu, L.; Yu, X.; Kong, F.; Zhou, C.; Han, L. Systematic analysis of gibberellin pathway components in Medicago truncatula reveals the potential application of gibberellin in biomass improvement. Int. J. Mol. Sci. 2020, 21, 7180. [Google Scholar] [CrossRef]
- Xu, Q.; Burgess, P.; Xu, J.; Meyer, W.; Huang, B. Osmotic stress- and salt stress-inhibition and gibberellin-mitigation of leaf elongation associated with up-regulation of genes controlling cell expansion. Environ. Exp. Bot. 2016, 131, 101–109. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Q.; Wu, X.; Chen, F.; Yuan, L.; Mi, G. Gibberellins synthesis is involved in the reduction of cell flux and elemental growth rate in maize leaf under low nitrogen supply. Environ. Exp. Bot. 2018, 150, 198–208. [Google Scholar] [CrossRef]
- Carabelli, M.; Possenti, M.; Sessa, G.; Ciolfi, A.; Sassi, M.; Morelli, G.; Ruberti, I. Canopy shade causes a rapid and transient arrest in leaf development through auxin-induced cytokinin oxidase activity. Genes. Dev. 2007, 21, 1863–1868. [Google Scholar] [CrossRef]
- Raspor, M.; Motyka, V.; Žižková, E.; Dobrev, P.I.; Trávníčková, A.; Zdravković-Korać, S.; Simonović, A.; Ninković, S.; Dragićević, I.C. Cytokinin profiles of AtCKX2-overexpressing potato plants and the impact of altered cytokinin homeostasis on tuberization in vitro. J. Plant Growth Regul. 2012, 31, 460–470. [Google Scholar] [CrossRef]
- Skalák, J.; Vercruyssen, L.; Claeys, H.; Hradilová, J.; Černý, M.; Novák, O.; Plačková, L.; Saiz-Fernández, I.; Skaláková, P.; Coppens, F.; et al. Multifaceted activity of cytokinin in leaf development shapes its size and structure in Arabidopsis. Plant J. 2019, 97, 805–824. [Google Scholar] [CrossRef]
- Shwartz, I.; Levy, M.; Ori, N.; Bar, M. Hormones in tomato leaf development. Dev. Biol. 2016, 419, 132–142. [Google Scholar] [CrossRef]
- Nakayama, H.; Ichihashi, Y.; Kimura, S. Diversity of tomato leaf form provides novel insights into breeding. Breed. Sci. 2023, 73, 76–85. [Google Scholar] [CrossRef]
- Negi, S.; Sukumar, P.; Liu, X.; Cohen, J.D.; Muday, G.K. Genetic dissection of the role of ethylene in regulating auxin-dependent lateral and adventitious root formation in tomato. Plant J. 2010, 61, 3–15. [Google Scholar] [CrossRef]
- Laskowski, M.; Grieneisen, V.A.; Hofhuis, H.; Hove, C.A.T.; Hogeweg, P.; Marée, A.F.M.; Scheres, B. Root system architecture from coupling cell shape to auxin transport. PLoS Biol. 2008, 6, e307. [Google Scholar] [CrossRef] [PubMed]
- Ötvös, K.; Marconi, M.; Vega, A.; O’Brien, J.; Johnson, A.; Abualia, R.; Antonielli, L.; Montesinos, J.C.; Zhang, Y.; Tan, S.; et al. Modulation of plant root growth by nitrogen source-defined regulation of polar auxin transport. EMBO J. 2021, 40, e106862. [Google Scholar] [CrossRef]
- Asim, M.; Ullah, Z.; Oluwaseun, A.; Wang, Q.; Liu, H. Signalling overlaps between nitrate and auxin in regulation of the root system architecture: Insights from the Arabidopsis thaliana. Int. J. Mol. Sci. 2020, 21, 2880. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Jiang, Z.; Zhang, L.; Hussain, I.; Yang, X. Insights on phytohormonal crosstalk in plant response to nitrogen stress: A focus on plant root growth and development. Int. J. Mol. Sci. 2023, 24, 3631. [Google Scholar] [CrossRef]
- Zaman, M.; Kurepin, L.V.; Catto, W.; Pharis, R.P. Enhancing crop yield with the use of N-based fertilizers co-applied with plant hormones or growth regulators. J. Sci. Food Agric. 2015, 95, 1777–1785. [Google Scholar] [CrossRef]
- Kiba, T.; Kudo, T.; Kojima, M.; Sakakibara, H. Hormonal control of nitrogen acquisition: Roles of auxin, abscisic acid, and cytokinin. J. Exp. Bot. 2011, 62, 1399–1409. [Google Scholar] [CrossRef]
- Dello Ioio, R.; Linhares, F.S.; Scacchi, E.; Casamitjana-Martinez, E.; Heidstra, R.; Costantino, P.; Sabatini, S. Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Curr. Biol. 2007, 17, 678–682. [Google Scholar] [CrossRef]
- Jones, B.; Gunnerås, S.A.; Petersson, S.V.; Tarkowski, P.; Graham, N.; May, S.; Dolezal, K.; Sandberg, G.; Ljung, K. Cytokinin regulation of auxin synthesis in Arabidopsis involves a homeostatic feedback loop regulated via auxin and cytokinin signal transduction. Plant Cell 2010, 22, 2956–2969. [Google Scholar] [CrossRef]
- Vellosillo, T.; Martínez, M.; López, M.A.; Vicente, J.; Cascón, T.; Dolan, L.; Hamberg, M.; Castresana, C. Oxylipins produced by the 9-lipoxygenase pathway in Arabidopsis regulate lateral root development and defense responses through a specific signaling cascade. Plant Cell 2007, 19, 831–846. [Google Scholar] [CrossRef]
- Kapulnik, Y.; Delaux, P.-M.; Resnick, N.; Mayzlish-Gati, E.; Wininger, S.; Bhattacharya, C.; Séjalon-Delmas, N.; Combier, J.-P.; Bécard, G.; Belausov, E.; et al. Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis. Planta 2011, 233, 209–216. [Google Scholar] [CrossRef]
- Ruyter-Spira, C.; Kohlen, W.; Charnikhova, T.; van Zeijl, A.; van Bezouwen, L.; de Ruijter, N.; Cardoso, C.; Lopez-Raez, J.A.; Matusova, R.; Bours, R.; et al. Physiological effects of the synthetic strigolactone analog gr24 on root system architecture in Arabidopsis: Another belowground role for strigolactones? Plant Physiol. 2011, 155, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Negi, S.; Ivanchenko, M.G.; Muday, G.K. Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. Plant J. 2008, 55, 175–187. [Google Scholar] [CrossRef]
- Mou, W.; Khare, R.; Polko, J.K.; Taylor, I.; Xu, J.; Xue, D.; Benfey, P.; Van de Poel, B.; Chang, C.; Kieber, J.J. Ethylene-independent modulation of root development by ACC via downregulation of WOX5 and group I CLE peptide expression. Proc. Natl. Acad. Sci. USA 2025, 122, e2417735122. [Google Scholar] [CrossRef]
- Mou, W.; Kao, Y.-T.; Michard, E.; Simon, A.A.; Li, D.; Wudick, M.M.; Lizzio, M.A.; Feijó, J.A.; Chang, C. Ethylene-independent signaling by the ethylene precursor ACC in Arabidopsis ovular pollen tube attraction. Nat. Commun. 2020, 11, 4082. [Google Scholar] [CrossRef]
- Saini, S.; Sharma, I.; Kaur, N.; Pati, P.K. Auxin: A master regulator in plant root development. Plant Cell Rep. 2013, 32, 741–757. [Google Scholar] [CrossRef] [PubMed]
- Larskaya, I.A.; Gorshkova, T.A. Plant oligosaccharides—Outsiders among elicitors? Biochemistry 2015, 80, 881–900. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, J.; Fan, K.; Guo, L.; Hou, L.; Miao, Y.; Sun, M.; Li, Y.; Bai, L. Alginate Oligosaccharide Coordinately Modulates Endogenous Phytohormone Profiles to Enhance Tomato Growth. Horticulturae 2025, 11, 580. https://doi.org/10.3390/horticulturae11060580
Li Y, Wang J, Fan K, Guo L, Hou L, Miao Y, Sun M, Li Y, Bai L. Alginate Oligosaccharide Coordinately Modulates Endogenous Phytohormone Profiles to Enhance Tomato Growth. Horticulturae. 2025; 11(6):580. https://doi.org/10.3390/horticulturae11060580
Chicago/Turabian StyleLi, Yun, Jianxia Wang, Kai Fan, Lingru Guo, Leiping Hou, Yanxiu Miao, Meihua Sun, Yaling Li, and Longqiang Bai. 2025. "Alginate Oligosaccharide Coordinately Modulates Endogenous Phytohormone Profiles to Enhance Tomato Growth" Horticulturae 11, no. 6: 580. https://doi.org/10.3390/horticulturae11060580
APA StyleLi, Y., Wang, J., Fan, K., Guo, L., Hou, L., Miao, Y., Sun, M., Li, Y., & Bai, L. (2025). Alginate Oligosaccharide Coordinately Modulates Endogenous Phytohormone Profiles to Enhance Tomato Growth. Horticulturae, 11(6), 580. https://doi.org/10.3390/horticulturae11060580





