Native Grasses Enhance Topsoil Organic Carbon and Nitrogen by Improving Soil Aggregates and Microbial Communities in Navel Orange Orchards in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design and Sampling
2.3. Physicochemical Analysis of the Native Grasses
2.4. Soil Physicochemical Analysis, Nutrient Content, and Enzyme Activity
2.5. Fractionation and Physicochemical Analysis of the Soil Aggregates
2.6. Improved Fractionation of the Soil Aggregates and Their Functional Genes and an Analysis of the Microbial Community Diversity
2.6.1. Improved Fractionation of the Soil Aggregates
2.6.2. Real-Time Quantitative Polymerase Chain Reaction (qPCR)
2.6.3. High-Throughput Sequencing and Sequence Processing
2.6.4. Prediction of the Microbial Functional Group
2.7. Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties and Enzyme Activity
3.2. Physicochemical Properties of the Soil Aggregates
3.2.1. Composition and Stability of the Soil Aggregates
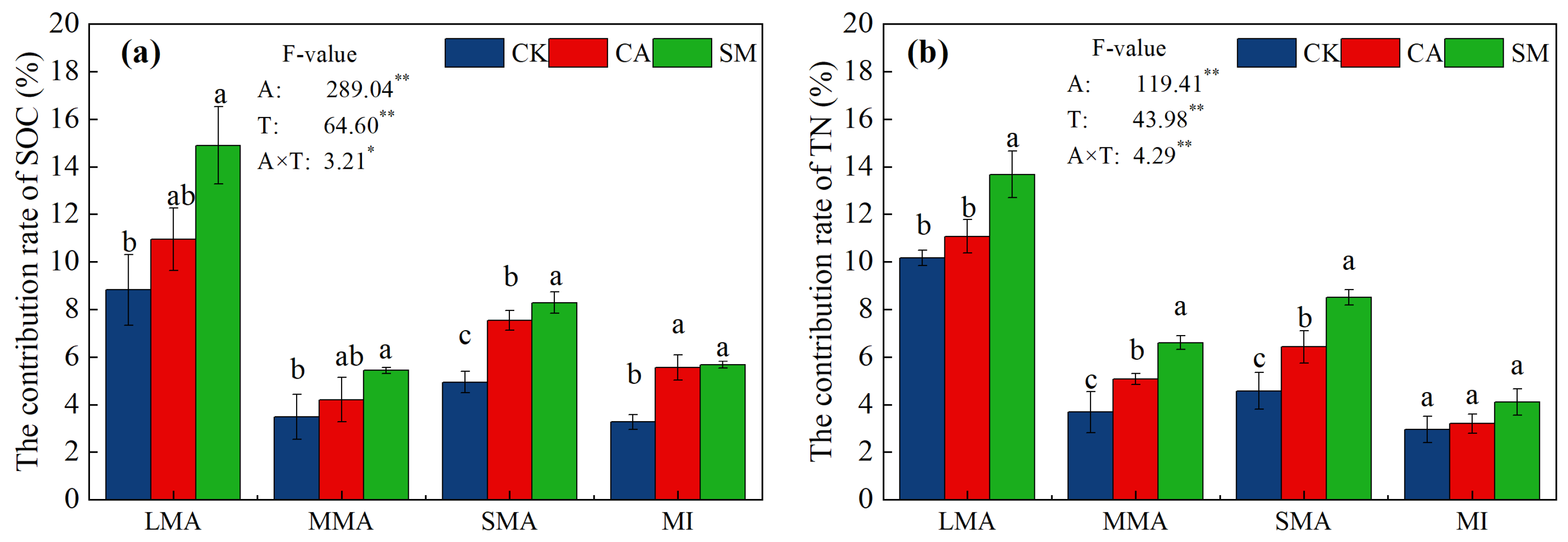
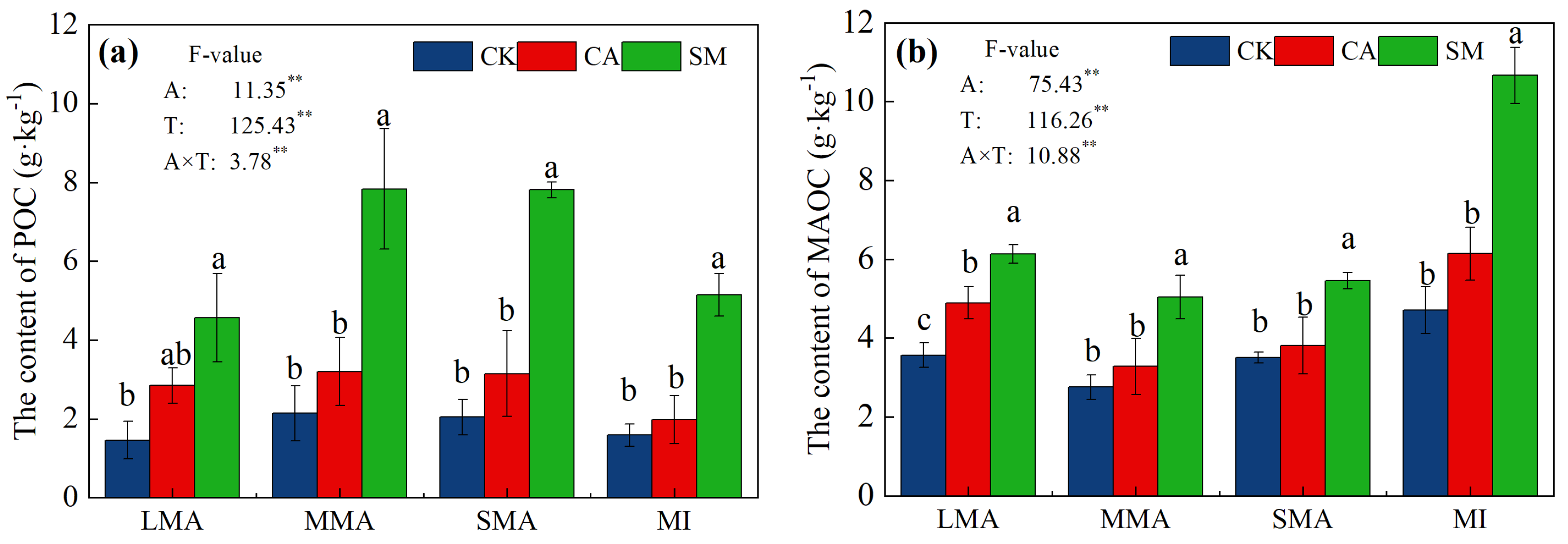
3.2.2. Distribution of Organic Carbon and Nitrogen in the Soil Aggregates
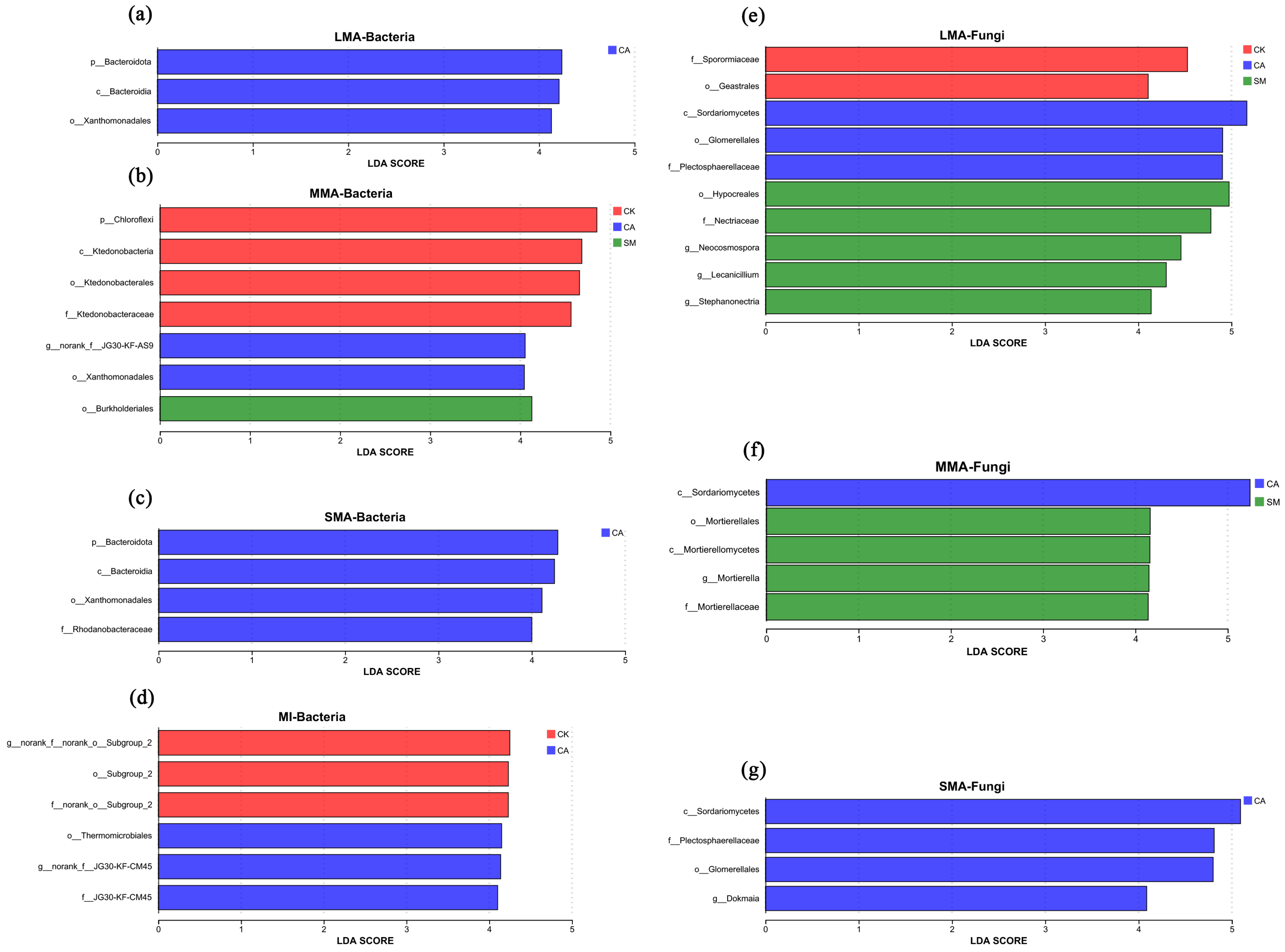
3.3. Microbial Communities in the Soil Aggregates
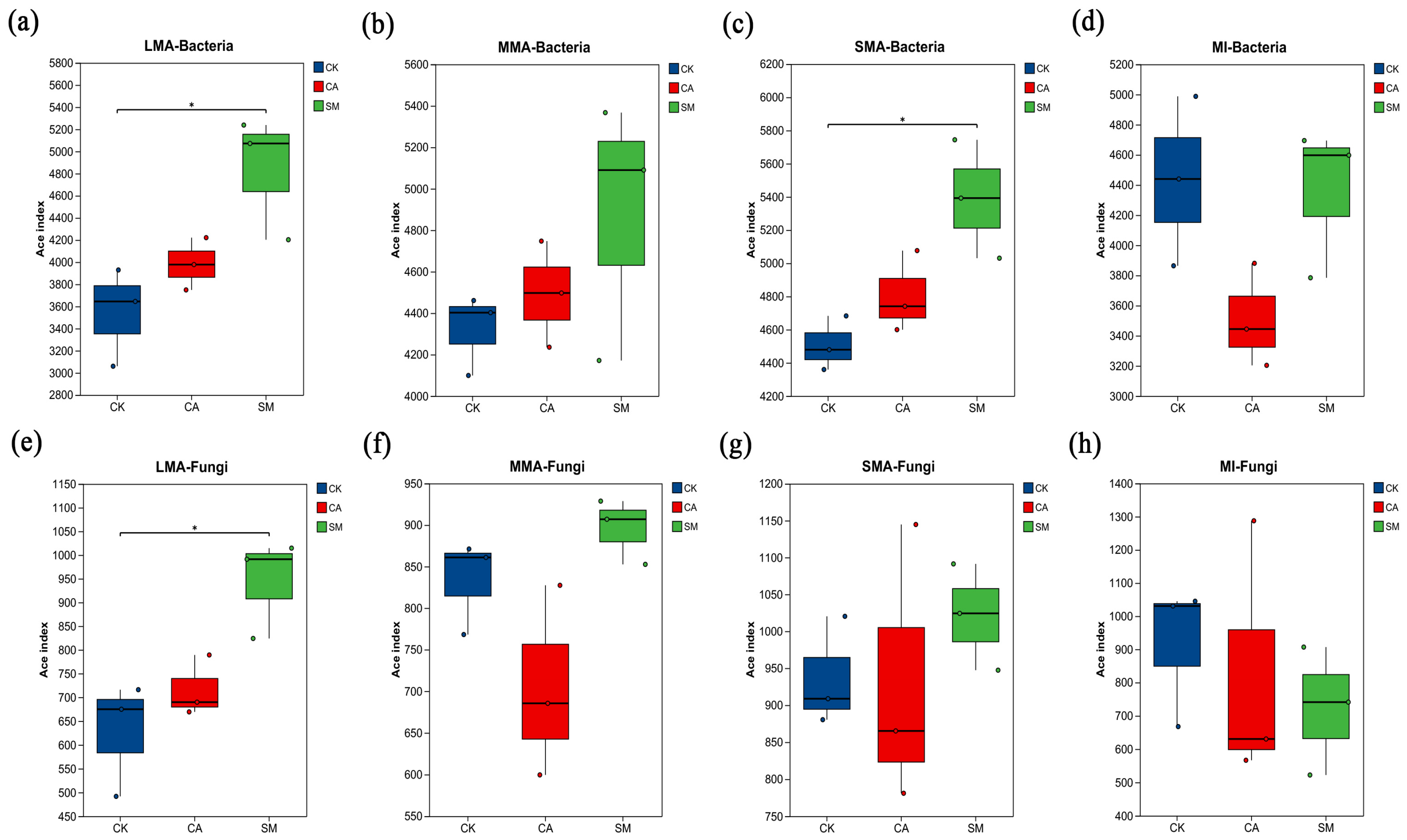
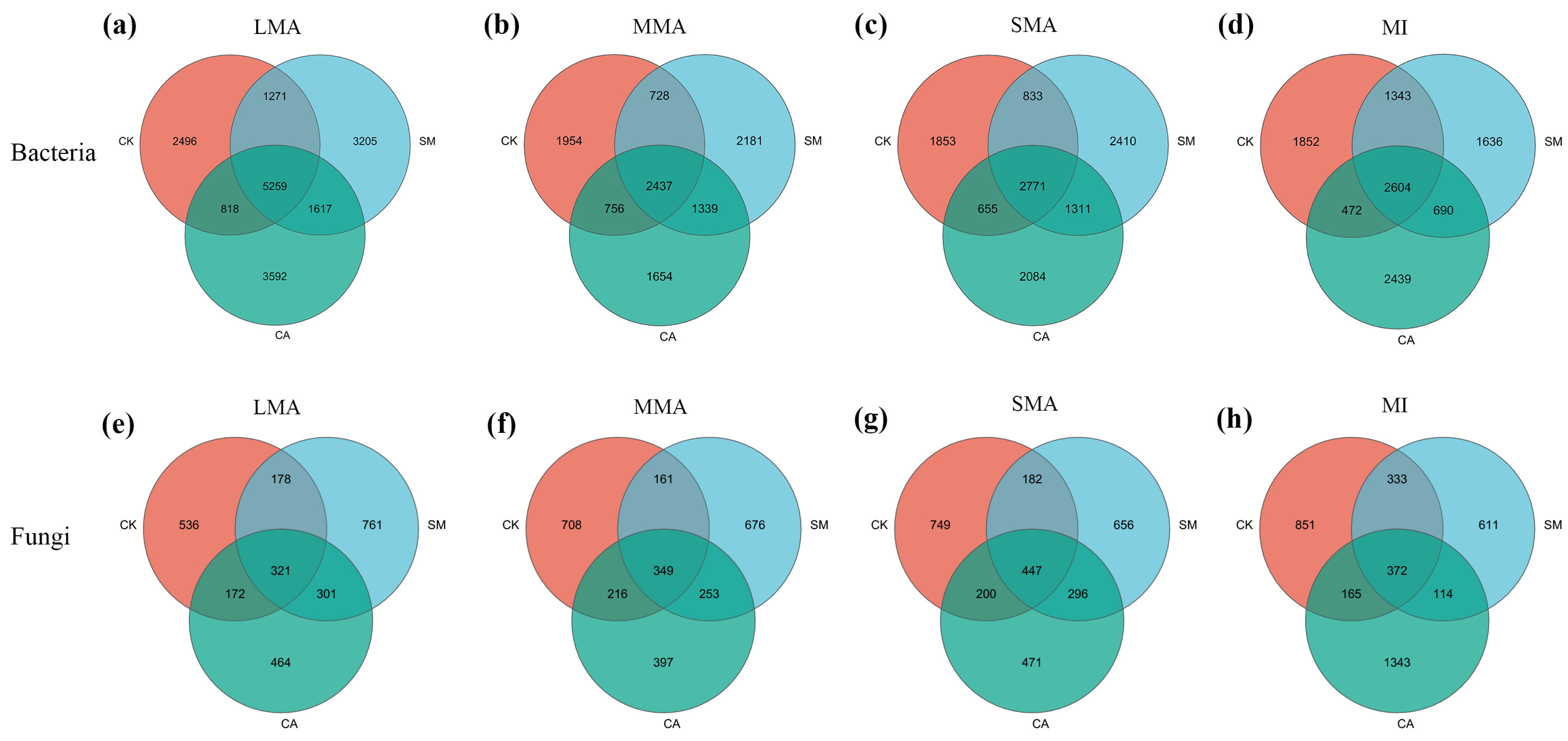
3.3.1. The Characteristics of Microbial Communities
3.3.2. Microbial Functional Group
3.4. Microbial Functional Genes Related to Nutrient Cycling in the Soil Aggregates
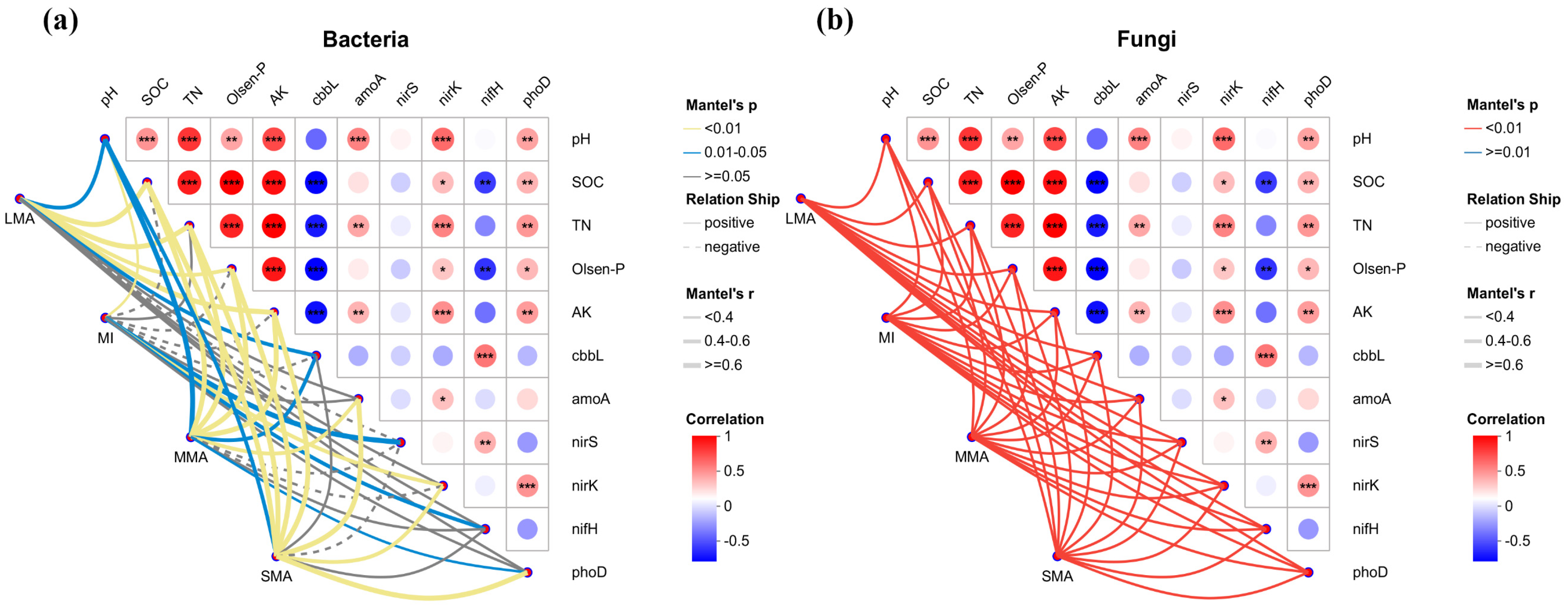
3.5. The Correlation Between Microbial Community and Environmental Factors and Gene Abundance in the Soil Aggregates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Treeby, M.T.; Henriod, R.E.; Bevington, K.B.; Milne, D.J.; Storey, R. Irrigation management and rootstock effects on navel orange [Citrus sinensis (L.) Osbeck] fruit quality. Agric. Water Manag. 2007, 91, 24–32. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, X.; Zhou, J.; Zhang, H.; Yang, C. Linear and nonlinear multivariate regressions for determination sugar content of intact Gannan navel orange by Vis–NIR diffuse reflectance spectroscopy. Math. Comput. Model. 2009, 51, 1438–1443. [Google Scholar] [CrossRef]
- Li, P.; Li, Y.; Xu, L.; Zhang, H.; Shen, X.; Xu, H.; Hu, F. Crop yield-soil quality balance in double cropping in China’s upland by organic amendments: A meta-analysis. Geoderma 2021, 403, 115197. [Google Scholar] [CrossRef]
- Xiao, D.; Huang, Y.; Feng, S.; Ge, Y.; Zhang, W.; He, X.; Wang, K. Soil organic carbon mineralization with fresh organic substrate and inorganic carbon additions in a red soil is controlled by fungal diversity along a pH gradient. Geoderma 2018, 321, 79–89. [Google Scholar] [CrossRef]
- Tan, H.; Fang, F.; Lin, Y.; Zhi, J.; Yao, Y.; Liu, Y.; Wang, J. Multidimensional effects of arable soil organic carbon distribution: A comparison among terrains. J. Soil. Sediment. 2025, 25, 207–221. [Google Scholar] [CrossRef]
- Ou, Y.; Rousseau, A.N.; Wang, L.X.; Yan, B.X. Spatio-temporal patterns of soil organic carbon and pH in relation to environmental factors—A case study of the black Soil region of Northeastern China. Agric. Ecosyst. Environ. 2017, 245, 22–31. [Google Scholar] [CrossRef]
- Teng, M.J.; Zeng, L.X.; Xiao, W.F.; Huang, Z.L.; Zhou, Z.X.; Yan, Z.G.; Wang, P.C. Spatial variability of soil organic carbon in Three Gorges Reservoir area, China. Sci. Total Environ. 2017, 599, 1308–1316. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, H.; Fu, L.; Yin, M.; Wang, Z.; Li, Y.; Cao, W. Green manuring enhances soil multifunctionality in tobacco field in southwest China. Microorganisms 2024, 12, 949. [Google Scholar] [CrossRef]
- Zhang, F.; Shen, J.; Zhang, J.; Zuo, Y.; Li, L.; Chen, X. Rhizosphere processes and management for improving nutrient use efficiency and crop productivity: Implications for China. Adv. Agron. 2010, 107, 1–22. [Google Scholar]
- Liang, H.; Li, S.; Zhou, G.; Fu, L.; Hu, F.; Gao, S.; Cao, W. Targeted regulation of the microbiome by green manuring to promote tobacco growth. Biol. Fert. Soils 2023, 60, 69–85. [Google Scholar] [CrossRef]
- Asghar, W.; Kataoka, R. Green manure incorporation accelerates enzyme activity, plant growth, and changes in the fungal community of soil. Arch. Microbiol. 2021, 204, 7. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.; Santos, S. Sustainable approach to weed management: The role of precision weed management. Agronomy 2022, 12, 118. [Google Scholar] [CrossRef]
- Gao, S.; Li, S.; Zhou, G.; Cao, W. The potential of green manure to increase soil carbon sequestration and reduce the yield-scaled carbon footprint of rice production in southern China. J. Integr. Agric. 2023, 22, 2233–2247. [Google Scholar] [CrossRef]
- Xu, Q.; Yao, Z.; Chen, Y.; Liu, N.; Teng, Z.; Huang, D.; Gao, Y. Priming and balance of soil organic carbon differ with additive C:N ratios and long-term green manuring. Appl. Soil Ecol. 2024, 201, 105495. [Google Scholar] [CrossRef]
- Liao, Q.; Li, T.; Liu, D. Evolutionary patterns and influencing factors of relationships among ecosystem services in the hilly red soil region of Southern China. Environ. Monit. Assess. 2024, 196, 360. [Google Scholar] [CrossRef]
- Bach, E.M.; Baer, S.G.; Meyer, C.K.; Six, J. Soil texture affects soil microbial and structural recovery during grassland restoration. Soil Biol. Biochem. 2010, 42, 2182–2191. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agrochemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Al-Shammary, A.A.G.; Kouzani, A.Z.; Kaynak, A.; Khoo, S.Y.; Norton, M.; Gates, W. Soil bulk density estimation methods: A review. Pedosphere 2018, 28, 581–596. [Google Scholar] [CrossRef]
- Wang, B.; Xue, S.; Liu, G.B.; Zhang, G.H.; Li, G.; Ren, Z.P. Changes in soil nutrient and enzyme activities under different vegetations in the Loess Plateau area, Northwest China. Catena 2012, 92, 186–195. [Google Scholar] [CrossRef]
- Covaleda, S.; Pajares, S.; Gallardo, J.F.; Etchevers, J.D. Short-term changes in C and N distribution in soil particle size fractions induced by agricultural practices in a cultivated volcanic soil from Mexico. Org. Geochem. 2006, 37, 1943–1948. [Google Scholar] [CrossRef]
- Panagea, I.S.; Apostolakis, A.; Berti, A.; Bussell, J.; Čermak, P.; Diels, J.; Wyseure, G. Impact of agricultural management on soil aggregates and associated organic carbon fractions: Analysis of long-term experiments in Europ. Soil 2022, 8, 621–644. [Google Scholar] [CrossRef]
- Bacha, E.M.; Williams, R.J.; Hargreaves, S.K.; Yang, F.; Hofmockel, K.S. Greatest soil microbial diversity found in micro-habitats. Soil Biol. Biochem. 2018, 118, 217–226. [Google Scholar] [CrossRef]
- Dorodnikov, M.; Blagodatskaya, E.; Blagodatsky, S.; Fangmeier, A.; Kuzyakov, Y. Stimulation of r- vs. K-selected microorganisms by elevated atmospheric CO2 depends on soil aggregate size. FEMS Microbiol. Ecol. 2009, 69, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Fang, L.; Shen, G.; Delgado-Baquerizo, M.; Chen, J.; Zhou, G.; Blagodatskaya, E. New perspectives on microbiome and nutrient sequestration in soil aggregates during long-term grazing exclusion. Glob. Change Biol. 2024, 30, e17027. [Google Scholar] [CrossRef]
- Selesi, D.; Pattis, I.; Schmid, M.; Kandeler, E.; Hartmann, A. Quantification of bacterial RubisCO genes in soils by cbbL targeted real-time PCR. J. Microbiol. Meth. 2007, 69, 497–503. [Google Scholar] [CrossRef]
- Wallenstein, M.D.; Vilgalys, R.J. Quantitative analyses of nitrogen cycling genes in soils. Pedobiologia 2005, 49, 665–672. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef]
- Buee, M.; Reich, M.; Murat, C.; Morin, E.; Nilsson, R.H.; Uroz, S.; Martin, F. Pyrosequencing analyses of forest soils reveal an unexpectedly high fungal diversity. New Phytol. 2009, 184, 449–456. [Google Scholar] [CrossRef]
- Duan, G.; Li, L. Deciphering the mechanism of jujube vinegar on hyperlipoidemia through gut microbiome based on 16S rRNA, BugBase analysis, and the stamp analysis of KEEG. Front. Nutr. 2023, 10, 1160069. [Google Scholar] [CrossRef]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Chang, F.; He, S.; Dang, C. Assisted Selection of Biomarkers by Linear Discriminant Analysis Effect Size (LEfSe) in Microbiome Data. Jove-J. Vis. Exp. 2022, 183, e61715. [Google Scholar] [CrossRef]
- Kudraszow, N.L.; Fasano, M.V. Robust approaches to redundancy analysis. Commun. Stat-Theor. M. 2024, 53, 607–626. [Google Scholar] [CrossRef]
- Mbah, C.N.; Mbah, E.C.; Orji, J.E.; Igberi, C.; Abam, P.; Awere, S.U. Using Gliricidia sepium prunings as green manure in a degraded ultisol; effects on soil physical properties and yield of okra (Abelmuschus esculentus) in Abakaliki, southeast Nigeria. Biol. Agric. Hortic. 2024, 40, 257–266. [Google Scholar] [CrossRef]
- Adekiya, A.O.; Agbede, T.M.; Aboyeji, C.M.; Olaniran, A.F.; Aremu, C.; Ejue, W.S.; Adegbite, K. Poultry and green manures effects on soil properties, and sorghum performance, and quality. Commun. Soil Sci. Plan. 2022, 53, 463–477. [Google Scholar] [CrossRef]
- Li, P.; Jia, L.; Chen, Q.; Zhang, H.; Deng, J.; Lu, J.; Jiao, J. Adaptive evaluation for agricultural sustainability of different fertilizer management options for a green manure-maize rotation system: Impacts on crop yield, soil biochemical properties and organic carbon fractions. Sci. Total Environ. 2024, 908, 168170. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, R.; Li, X.; Hu, G.; Dong, Y.; Liu, Z. Long-term adoption of plow tillage and green manure improves soil physicochemical properties and optimizes microbial communities under a continuous peanut monoculture system. Front. Microbiol. 2024, 15, 1513528. [Google Scholar] [CrossRef]
- Özbolat, O.; Sánchez-Navarro, V.; Zornoza, R.; Egea-Cortines, M.; Cuartero, J.; Ros, M.; Martínez-Mena, M. Long-term adoption of reduced tillage and green manure improves soil physicochemical properties and increases the abundance of beneficial bacteria in a Mediterranean rainfed almond orchard. Geoderma 2023, 429, 116218. [Google Scholar] [CrossRef]
- Cantarella, H.; Otto, R.; Soares, J.R.; de Brito Silva, A.G. Agronomic efficiency of NBPT as a urease inhibitor: A review. J. Adv. Res. 2018, 13, 19–27. [Google Scholar] [CrossRef]
- Fonseca, A.A.D.; Santos, D.A.; Moura-Junior, C.D.; Passos, R.R.; Rangel, O.J.P. Phosphorus and potassium in aggregates of degraded soils: Changes caused by biochar application. Clean-Soil. Air. Water 2021, 49, 2000366. [Google Scholar] [CrossRef]
- Zhu, D.; Yang, Q.; Zhao, Y.; Cao, Z.; Han, Y.; Li, R.; Wu, Z. Afforestation influences soil aggregate stability by regulating aggregate transformation in karst rocky desertification areas. Forests 2023, 14, 1356. [Google Scholar] [CrossRef]
- Ren, C.; Liu, K.; Dou, P.; Li, J.; Wang, K. The changes in soil microorganisms and soil chemical properties affect the heterogeneity and stability of soil aggregates before and after grassland conversion. Agriculture 2022, 12, 307. [Google Scholar] [CrossRef]
- Qi, J.; Han, S.; Lin, B.; Xiao, X.; Jensen, J.L.; Munkholm, L.J.; Zhang, H. Improved soil structural stability under no-tillage is related to increased soil carbon in rice paddies: Evidence from literature review and field experiment. Environ. Technol. Inno. 2022, 26, 102248. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Chen, H.; Tang, M. Symbiosis of arbuscular mycorrhizal fungi and Robinia Pseudoacacia L. improves root tensile strength and soil aggregate stability. PLoS ONE 2016, 11, e153378. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Zhao, J.S.; Shi, Z.H.; Wang, L. Soil aggregates are key factors that regulate erosion-related carbon loss in citrus orchards of southern China: Bare land vs. grass-covered land. Agric. Ecosyst. Environ. 2021, 309, 107254. [Google Scholar] [CrossRef]
- Conrad, K.; Dalal, R.C.; Fujinuma, R.; Menzies, N.W. Soil organic carbon and nitrogen sequestration and turnover in aggregates under subtropical leucaena–grass pastures. Soil Res. 2018, 56, 632–647. [Google Scholar] [CrossRef]
- LiuSui, Y.; Zhu, X.; Li, D.; Yan, C.; Sun, T.; Jia, H.; Zhao, X. Soil aggregate and intra-aggregate carbon fractions associated with vegetation succession in an alpine wetland of Northwest China. Catena 2019, 181, 104107. [Google Scholar] [CrossRef]
- Kang, L.; Wu, J.; Zhang, C.; Zhu, B.; Chu, G. Alterations of soil aggregates and intra-aggregate organic carbon fractions after soil conversion from paddy soils to upland soils: Distribution, mineralization and driving mechanism. Pedosphere 2024, 34, 121–135. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. The Effect of Crop Rotation on Aggregation in a Red-brown Earth. Aust. J. Soil Res. 1980, 18, 423–433. [Google Scholar] [CrossRef]
- Oades, J.M.; Waters, A.C. Aggregate Hierarchy in Soils. Aust. J. Soil Res. 1991, 29, 815–828. [Google Scholar] [CrossRef]
- Feller, C.; Beare, M.H. Physical control of soil organic matter dynamics in the tropics. Geoderma 1997, 79, 69–116. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, W.; Liang, G.; Sun, J.; Wang, X.; He, P. Distribution of soil nutrients, extracellular enzyme activities and microbial communities across particle-size fractions in a long-term fertilizer experiment. Appl. Soil Ecol. 2015, 94, 59–71. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, W.; Li, Y.; Liu, S.; Khan, A.; Yan, S.; Xiong, Y. Effects of reduced tillage with stubble remaining and nitrogen application on soil aggregation, soil organic carbon and grain yield in maize-wheat rotation system. Eur. J. Agron. 2023, 149, 126920. [Google Scholar] [CrossRef]
- Liu, L.; Liu, D.; Ding, X.; Chen, M.; Zhang, S. Straw incorporation and nitrogen fertilization enhance soil carbon sequestration by altering soil aggregate and microbial community composition in saline-alkali soil. Plant Soil 2023, 498, 341–356. [Google Scholar] [CrossRef]
- Zimmermann, M.; Leifeld, J.; Abiven, S.; Schmidt, M.W.I.; Fuhrer, J. Sodium hypochlorite separates an older soil organic matter fraction than acid hydrolysis. Geoderma 2007, 139, 171–179. [Google Scholar] [CrossRef]
- Leifeld, J.; Koegel-Knabner, I. Soil organic matter fractions as early indicators for carbon stock changes under different land-use? Geoderma 2005, 124, 143–155. [Google Scholar] [CrossRef]
- Schrumpf, M.; Kaiser, K. Large differences in estimates of soil organic carbon turnover in density fractions by using single and repeated radiocarbon inventories. Geoderma 2015, 239–240, 168–178. [Google Scholar] [CrossRef]
- Blaud, A.; Lerch, T.Z.; Chevallier, T.; Nunan, N.; Chenu, C.; Brauman, A. Dynamics of bacterial communities in relation to soil aggregate formation during the decomposition of 13C-labelled rice straw. Appl. Soil Ecol. 2012, 53, 1–9. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; Soon, Y.K. Carbon and nitrogen release from legume crop residues for three subsequent crops. Soil Sci. Soc. Am. J. 2015, 79, 1650–1659. [Google Scholar] [CrossRef]
- Jiang, Y.; Liang, Y.; Li, C.; Wang, F.; Sui, Y.; Suvannang, N.; Sun, B. Crop rotations alter bacterial and fungal diversity in paddy soils across East Asia. Soil Biol. Biochem. 2016, 95, 250–261. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Wang, T. Effects of increasing drip irrigation at different maize growth stages on soil microorganisms. Front. Microbiol. 2024, 15, 1343302. [Google Scholar] [CrossRef]
- Zumsteg, A.; Schmutz, S.; Frey, B. Identification of biomass utilizing bacteria in a carbon-depleted glacier forefield soil by the use of 13C DNA stable isotope probing. Environ. Microbiol. Rep. 2013, 5, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Boubekri, K.; Soumare, A.; Mardad, I.; Lyamlouli, K.; Ouhdouch, Y.; Hafidi, M.; Kouisni, L. Multifunctional role of Actinobacteria in agricultural production sustainability: A revie. Microbiol. Res. 2022, 261, 127059. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Senbayram, M.; Blagodatsky, S.; Myachina, O.; Dittert, K.; Lin, X.; Kuzyakov, Y. Soil C and N availability determine the priming effect: Microbial N mining and stoichiometric decomposition theories. Glob. Change Biol. 2014, 20, 2356–2367. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Rochester, I.J.; Trivedi, C.; Van Nostrand, J.D.; Zhou, J.; Karunaratne, S.; Singh, B.K. Soil aggregate size mediates the impacts of cropping regimes on soil carbon and microbial communities. Soil Biol. Biochem. 2015, 91, 169–181. [Google Scholar] [CrossRef]
- Yuan, H.; Ge, T.; Wu, X.; Liu, S.; Tong, C.; Qin, H.; Wu, J. Long-term field fertilization alters the diversity of autotrophic bacteria based on the ribulose-1,5-biphosphate carboxylase/oxygenase (RubisCO) large-subunit genes in paddy soil. Appl. Microbiol. Biot. 2012, 95, 1061–1071. [Google Scholar] [CrossRef]
- Wu, X.; Ge, T.; Wang, W.; Yuan, H.; Wegner, C.; Zhu, Z.; Wu, J. Cropping systems modulate the rate and magnitude of soil microbial autotrophic CO2 fixation in soil. Front. Microbiol. 2015, 6, 379. [Google Scholar] [CrossRef]
- Lynn, T.M.; Ge, T.; Yuan, H.; Wei, X.; Wu, X.; Xiao, K.; Whiteley, A.S. Soil carbon-fixation rates and associated bacterial diversity and abundance in three natural ecosystems. Microb. Ecol. 2017, 73, 645–657. [Google Scholar] [CrossRef]
- Zhou, Z.; Wei, W.; Shi, X.; Liu, Y.; He, X.; Wang, M. Twenty-six years of chemical fertilization decreased soil RubisCO activity and changed the ecological characteristics of soil cbbL-carrying bacteria in an entisol. Appl. Soil Ecol. 2019, 141, 1–9. [Google Scholar] [CrossRef]
- Ge, T.; Wu, X.; Liu, Q.; Zhu, Z.; Yuan, H.; Wang, W.; Wu, J. Effect of simulated tillage on microbial autotrophic CO2 fixation in paddy and upland soils. Sci. Rep. 2016, 6, 19784. [Google Scholar] [CrossRef]
- Xiao, H.; Li, Z.; Chang, X.; Deng, L.; Nie, X.; Liu, C.; Wang, D. Microbial CO2 assimilation is not limited by the decrease in autotrophic bacterial abundance and diversity in eroded watershed. Biol. Fert. Soils 2018, 54, 595–605. [Google Scholar] [CrossRef]
- Daims, H.; Lebedeva, E.V.; Pjevac, P.; Han, P.; Herbold, C.; Albertsen, M.; Wagner, M. Complete nitrification by Nitrospira bacteria. Nature 2015, 528, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Frostegard, A.; Vick, S.H.W.; Lim, N.Y.N.; Bakken, L.R.; Shapleigh, J.P. Linking meta-omics to the kinetics of denitrification intermediates reveals pH-dependent causes of N2O emissions and nitrite accumulation in soil. ISME J. 2022, 16, 26–37. [Google Scholar] [CrossRef] [PubMed]




| Treatment | Bulk Density (g·cm−3) | Maximum Field Capacity (%) | Total Porosity (%) | Capillary Porosity (%) | Aeration Porosity (%) |
|---|---|---|---|---|---|
| CK | 1.32 ± 0.05 a | 29.50 ± 2.33 c | 50.26 ± 2.01 b | 38.45 ± 3.56 c | 11.81 ± 5.24 a |
| CA | 1.13 ± 0.07 b | 42.81 ± 3.76 a | 57.30 ± 2.50 a | 48.27 ± 2.43 a | 9.03 ± 3.08 a |
| SM | 1.16 ± 0.07 b | 34.14 ± 1.33 b | 56.06 ± 2.77 a | 42.70 ± 2.05 b | 13.35 ± 2.43 a |
| Treatment | pH | SOC (g·kg−1) | TN (g·kg−1) | Olsen-P (mg·kg−1) | AK (mg·kg−1) |
|---|---|---|---|---|---|
| CK | 5.40 ± 0.08 a | 5.74 ± 0.50 c | 0.15 ± 0.02 b | 10.75 ± 1.37 c | 111.27 ± 0.55 b |
| CA | 5.53 ± 0.09 a | 12.54 ± 0.10 b | 0.28 ± 0.05 a | 22.56 ± 1.03 b | 261.39 ± 6.34 a |
| SM | 5.45 ± 0.07 a | 16.33 ± 0.41 a | 0.27 ± 0.04 a | 30.76 ± 1.75 a | 271.61 ± 12.14 a |
| Treatment | Urease (μg·g−1·d−1) | Catalase (mg·g−1·d−1) | Amylase (μg·g−1·d−1) | Sucrase (mg·g−1·d−1) | Acid Phosphatase (mg·g−1·d−1) |
|---|---|---|---|---|---|
| CK | 46.17 ± 2.26 b | 120.08 ± 3.20 c | 25.58 ± 0.92 a | 15.31 ± 2.13 b | 0.55 ± 0.05 a |
| CA | 65.67 ± 0.08 a | 197.06 ± 16.50 b | 25.72 ± 1.23 a | 26.75 ± 1.31 a | 0.57 ± 0.04 a |
| SM | 67.52 ± 6.67 a | 254.87 ± 11.81 a | 25.80 ± 1.08 a | 31.65 ± 3.85 a | 0.60 ± 0.00 a |
| Method | Treatment | >2 mm | 1–2 mm | 0.25–1 mm | <0.25 mm |
|---|---|---|---|---|---|
| CK | 56.05 ± 6.00 b | 17.98 ± 2.48 a | 18.23 ± 3.20 a | 7.74 ± 1.51 a | |
| MSA | CA | 62.65 ± 2.48 b | 15.56 ± 1.76 a | 17.00 ± 1.18 a | 4.79 ± 0.18 b |
| SM | 77.55 ± 6.43 a | 8.93 ± 3.36 b | 9.60 ± 3.01 b | 3.92 ± 0.19 b | |
| CK | 46.29 ± 1.82 b | 17.88 ± 0.65 a | 23.22 ± 2.44 a | 12.61 ± 1.62 a | |
| WSA | CA | 46.81 ± 4.00 b | 17.73 ± 1.60 a | 25.84 ± 4.15 a | 9.62 ± 1.93 ab |
| SM | 72.05 ± 5.62 a | 8.31 ± 3.81 b | 12.90 ± 2.37 b | 6.73 ± 0.44 b |
| Treatment | MWD (mm) | GDM (mm) | D | R0.25 (%) | PAD (%) | ELT (%) |
|---|---|---|---|---|---|---|
| CK | 1.61 ± 0.01 b | 0.79 ± 0.04 b | 2.10 ± 0.05 a | 88.06 ± 2.26 b | 5.23 ± 0.26 a | 15.00 ± 1.60 a |
| CA | 1.68 ± 0.02 b | 0.83 ± 0.03 ab | 2.01 ± 0.07 a | 90.38 ± 1.93 ab | 4.79 ± 0.54 a | 12.93 ± 0.64 b |
| SM | 2.03 ± 0.07 a | 0.88 ± 0.01 a | 2.01 ± 0.05 a | 93.27 ± 0.44 a | 2.93 ± 0.53 b | 9.78 ± 0.37 c |
| Aggregates | Treatment | cbbL (105 Copies·g−1 DNA) | amoA (103 Copies·g−1 DNA) | nirS (104 Copies·g−1 DNA) | nirK (104 Copies·g−1 DNA) | nifH (104 Copies·g−1 DNA) |
|---|---|---|---|---|---|---|
| LMA | CK | 11.70 ± 0.58 a | 0.37 ± 0.36 c | 0.79 ± 0.17 a | 4.46 ± 0.78 a | 4.80 ± 0.40 a |
| CA | 7.95 ± 0.78 b | 27.45 ± 1.99 a | 1.11 ± 0.33 a | 8.40 ± 2.31 a | 8.40 ± 2.31 a | |
| SM | 8.64 ± 0.37 b | 17.49 ± 2.31 b | 1.00 ± 0.42 a | 5.91 ± 1.13 a | 5.91 ± 1.13 a | |
| MMA | CK | 11.49 ± 0.18 a | 0.47 ± 0.16 c | 1.02 ± 0.15 a | 4.77 ± 1.06 b | 4.77 ± 1.06 b |
| CA | 9.23 ± 1.40 b | 34.84 ± 5.14 a | 1.34 ± 0.25 a | 10.05 ± 0.38 a | 10.71 ± 1.53 a | |
| SM | 7.71 ± 0.25 b | 17.18 ± 2.68 b | 1.06 ± 0.32 a | 5.56 ± 1.62 b | 6.23 ± 2.77 ab | |
| SMA | CK | 12.80 ± 0.55 a | 0.43 ± 0.07 b | 1.43 ± 0.09 a | 4.40 ± 0.38 c | 4.40 ± 0.38 c |
| CA | 10.90 ± 1.41 a | 27.11 ± 9.30 a | 1.48 ± 0.40 a | 9.91 ± 1.34 a | 9.91 ± 1.34 a | |
| SM | 7.83 ± 1.08 b | 17.20 ± 3.02 a | 1.46 ± 0.28 a | 6.50 ± 0.99 b | 6.50 ± 0.99 b | |
| MI | CK | 16.65 ± 1.01 a | 12.83 ± 3.03 b | 1.58 ± 0.18 a | 5.19 ± 1.48 b | 5.19 ± 1.48 b |
| CA | 10.45 ± 1.29 b | 40.18 ± 6.82 a | 1.50 ± 0.21 a | 10.19 ± 1.06 a | 10.19 ± 1.06 a | |
| SM | 8.56 ± 0.47 b | 14.85 ± 2.32 b | 1.33 ± 0.10 a | 6.35 ± 0.67 b | 6.35 ± 0.67 b | |
| Aggregates (3) | 13.86 ** | 6.67 ** | 7.86 ** | 1.04 | 0.76 | |
| Treatment (2) | 91.04 ** | 144.20 ** | 1.27 | 52.70 ** | 39.92 ** | |
| Aggregates × Treatment (6) | 6.51 ** | 3.54 * | 0.64 | 0.51 | 0.49 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Ren, Z.; Wang, J.; Dai, Y.; Huang, J.; Yang, Y.; Zhuang, X.; Ye, M.; Yang, Z.; Yao, F.; et al. Native Grasses Enhance Topsoil Organic Carbon and Nitrogen by Improving Soil Aggregates and Microbial Communities in Navel Orange Orchards in China. Horticulturae 2025, 11, 560. https://doi.org/10.3390/horticulturae11050560
Wang W, Ren Z, Wang J, Dai Y, Huang J, Yang Y, Zhuang X, Ye M, Yang Z, Yao F, et al. Native Grasses Enhance Topsoil Organic Carbon and Nitrogen by Improving Soil Aggregates and Microbial Communities in Navel Orange Orchards in China. Horticulturae. 2025; 11(5):560. https://doi.org/10.3390/horticulturae11050560
Chicago/Turabian StyleWang, Wenqian, Zhaoyan Ren, Jianjun Wang, Ying Dai, Jingwen Huang, Yang Yang, Xia Zhuang, Mujun Ye, Zhonglan Yang, Fengxian Yao, and et al. 2025. "Native Grasses Enhance Topsoil Organic Carbon and Nitrogen by Improving Soil Aggregates and Microbial Communities in Navel Orange Orchards in China" Horticulturae 11, no. 5: 560. https://doi.org/10.3390/horticulturae11050560
APA StyleWang, W., Ren, Z., Wang, J., Dai, Y., Huang, J., Yang, Y., Zhuang, X., Ye, M., Yang, Z., Yao, F., & Cheng, C. (2025). Native Grasses Enhance Topsoil Organic Carbon and Nitrogen by Improving Soil Aggregates and Microbial Communities in Navel Orange Orchards in China. Horticulturae, 11(5), 560. https://doi.org/10.3390/horticulturae11050560





