Characterization of Plant Defensin (PDF) Genes in Banana (Musa acuminata) Reveals the Antifungal Ability of MaPDF2.2 to Fusarium Wilt Pathogens
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Identification of Banana PDF Genes
2.3. Cloning and Bioinformatic Analysis of MaPDFs
2.4. Phylogenetic Analysis of MaPDFs and Prediction of Cis-Acting Elements in Promoters of MaPDFs
2.5. Expression Analysis of MaPDFs
2.6. Overexpression Vector Construction and Tobacco Leaf Transient Overexpression
2.7. Prokaryotic Expression and Purification of MaPDF2.2
2.8. Antifungal Activity Assays of His-MaPDF2.2 Proteins
3. Results
3.1. Identification, Cloning, and Characterization of MaPDFs
3.2. Gene Structure, Conserved Motifs, and Phylogenetic Analysis of MaPDFs
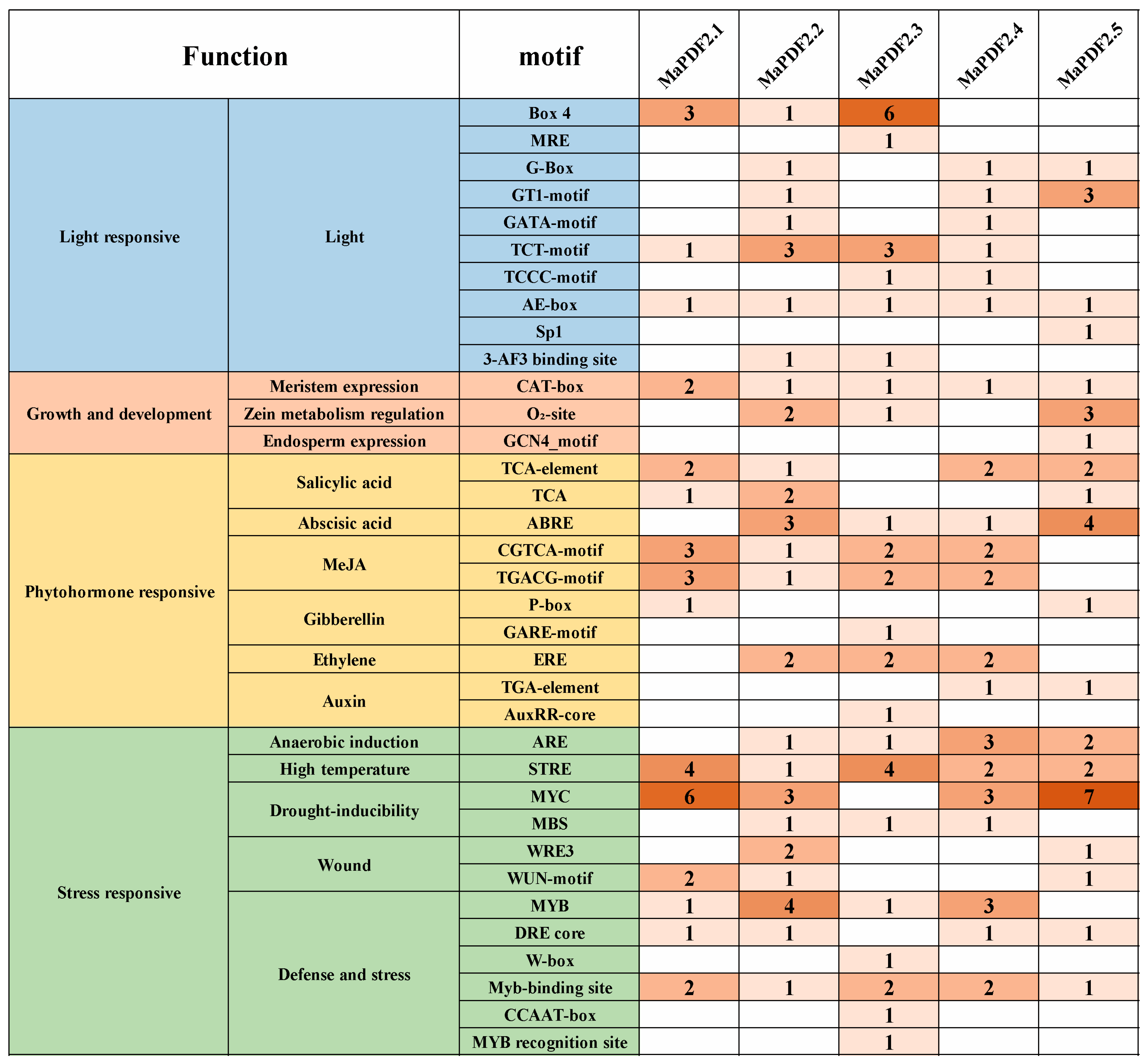
3.3. Analysis of Cis-Acting Elements in MaPDFs Promoters
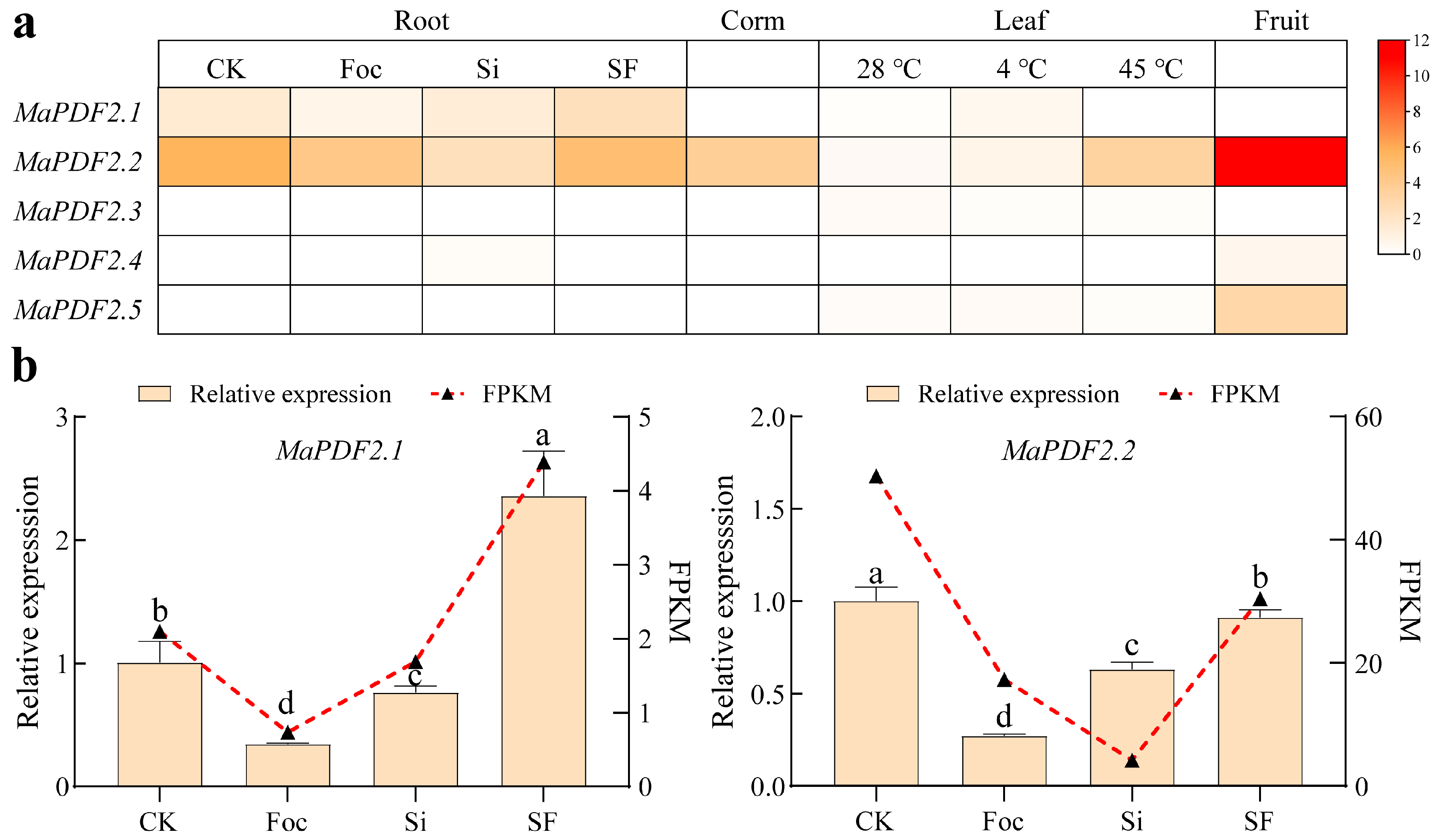
3.4. Expression Pattern Analysis of MaPDFs in Different Organs and Under Various Stress Conditions
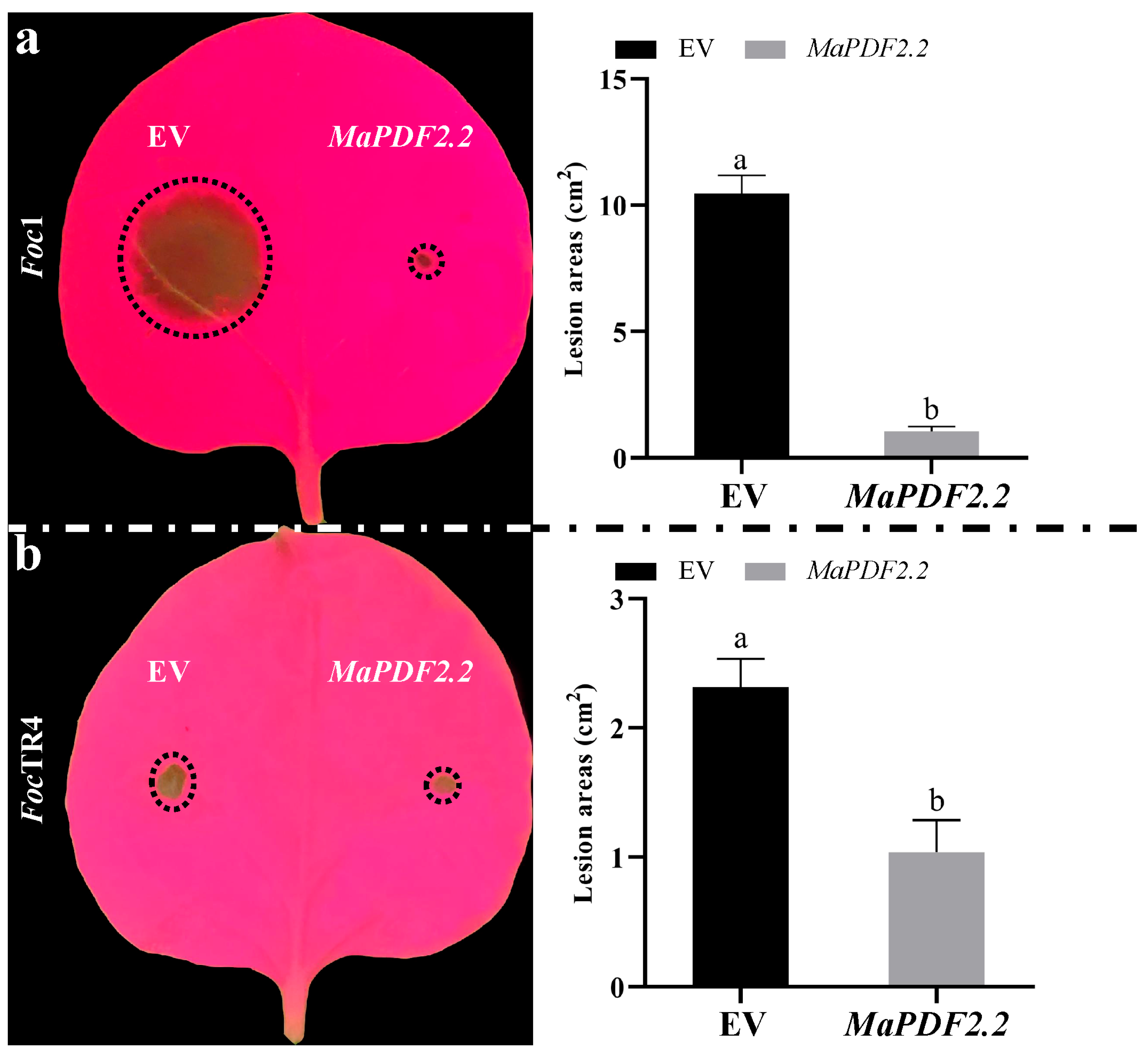
3.5. Transient Overexpression of MaPDF2.2 Can Inhibit the Growth of Foc1 and FocTR4 in Tobacco Leaves
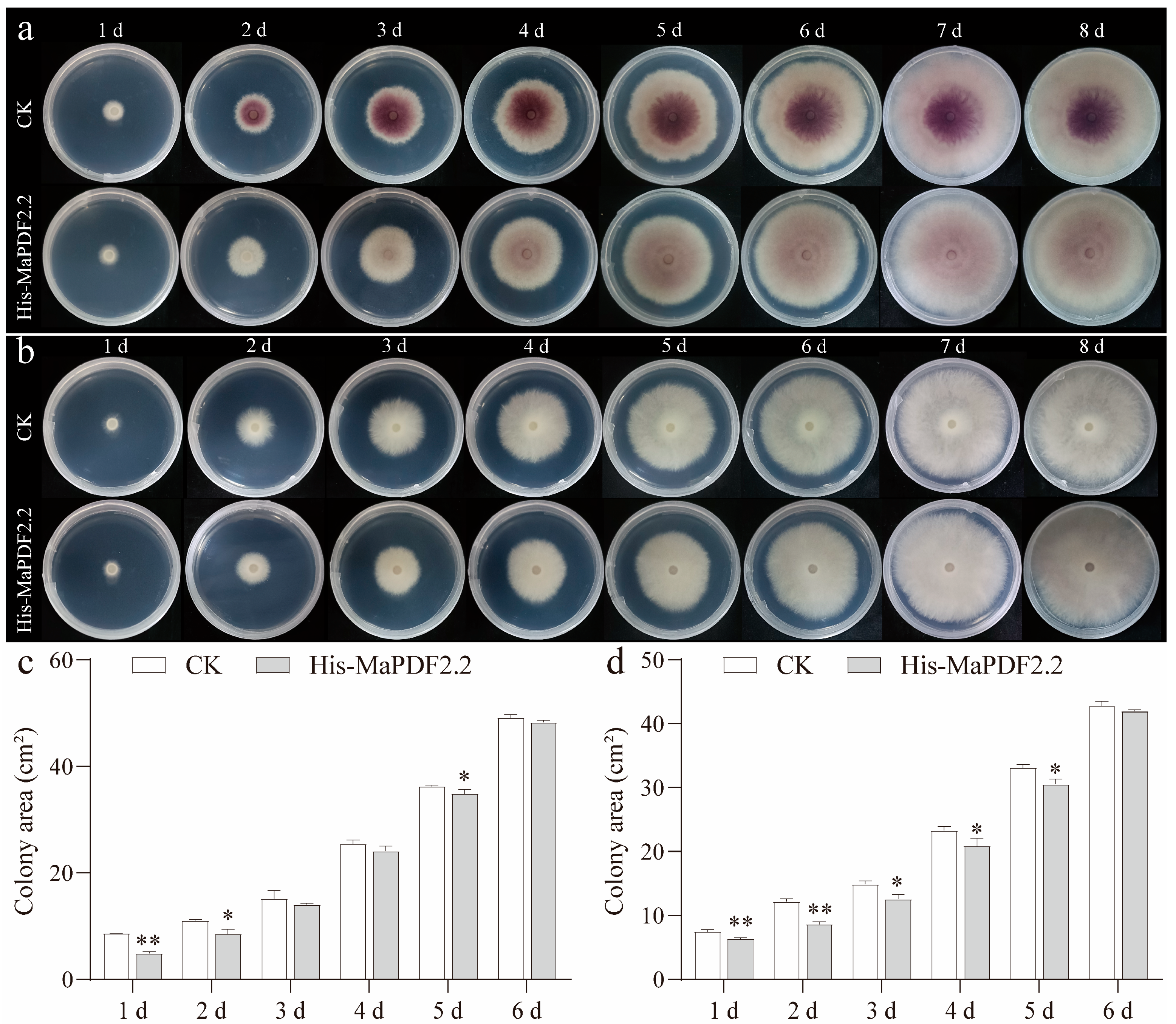
3.6. Prokaryotic Expressed Recombinant His-MaPDF2.2 Can Inhibit the Growth and Development of Both Foc1 and FocTR4 In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| Dpi | Days post inoculation |
| ET | Ethylene |
| GA | Gibberellin |
| FPKM | Fragments Per Kilobase of transcript per Million mapped reads |
| Foc1 | Fusarium oxysporum f. sp. cubense 1 |
| FocTR4 | Fusarium oxysporum f. sp. cubense Tropical Race 4 |
| FW | Fusarium wilt |
| PR | Pathogenesis-related proteins |
| Plant defensin | |
| SA | Salicylic acid |
References
- De Oliveira, S.S.S.; Cherene, M.B.; Taveira, G.B.; De Oliveira Mello, É.; De Oliveira Carvalho, A.; Gomes, V.M. Plant antimicrobial peptides and their main families and roles: A review of the literature. Curr. Issues Mol. Biol. 2024, 47, 1. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liang, Z.; Yang, A.; Yan, J.; Cong, P.; Han, X.; Zhang, C. Comparative transcriptome analysis reveals key genes and pathways in response to Alternaria alternata apple pathotype infection. Hortic. Plant J. 2024, 10, 641–656. [Google Scholar] [CrossRef]
- Mirouze, M.; Sels, J.; Richard, O.; Czernic, P.; Loubet, S.; Jacquier, A.; François, I.E.J.A.; Cammue, B.P.A.; Lebrun, M.; Berthomieu, P.; et al. A putative novel role for plant defensins: A defensin from the zinc hyper-accumulating plant, Arabidopsis halleri, confers zinc tolerance. Plant J. 2006, 47, 329–342. [Google Scholar] [CrossRef]
- Guan, L.; Cui, Q.; Li, S.; Dong, C.; Li, H.; Peng, J.; Li, X. Research advances on the structure, function and regulation of defensins gene family in plants. Biotechnol. Bull. 2018, 34, 33–39. (In Chinese) [Google Scholar] [CrossRef]
- Sher Khan, R.; Iqbal, A.; Malak, R.; Shehryar, K.; Attia, S.; Ahmed, T.; Ali Khan, M.; Arif, M.; Mii, M. Plant defensins: Types, mechanism of action and prospects of genetic engineering for enhanced disease resistance in plants. 3 Biotech 2019, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wu, H.; Zhang, Y. Characterization and functional analysis of gerbera plant defensin (PDF) genes reveal the role of GhPDF2.4 in defense against the root rot pathogen Phytophthora cryptogea. aBiotech 2024, 5, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Hazlett, L.; Wu, M. Defensins in innate immunity. Cell Tissue Res. 2011, 343, 175–188. [Google Scholar] [CrossRef]
- Lay, F.T.; Poon, S.; McKenna, J.A.; Connelly, A.A.; Barbeta, B.L.; McGinness, B.S.; Fox, J.L.; Daly, N.L.; Craik, D.J.; Heath, R.L.; et al. The C-terminal propeptide of a plant defensin confers cytoprotective and subcellular targeting functions. BMC Plant Biol. 2014, 14, 41. [Google Scholar] [CrossRef]
- Vi, T.X.T.; Le, H.D.; Nguyen, V.T.T.; Le, V.S.; Chu, H.M. Expression of the ZmDEF1 gene and α-amylase inhibitory activityof recombinant defensin against maize weevils. Turk. J. Biol. 2017, 41, 98–104. [Google Scholar] [CrossRef]
- Vi, T.X.T.; Nguyen, T.N.L.; Pham, T.T.N.; Nguyen, H.Q.; Nguyen, T.H.Y.; Tu, Q.T.; Le, V.S.; Chu, H.M. Overexpression of the ZmDEF1 gene increases the resistance to weevil larvae in transgenic maize seeds. Mol. Biol. Rep. 2019, 46, 2177–2185. [Google Scholar] [CrossRef]
- Liu, Q.; Yan, H.; Zhang, Z.; Zheng, L.; Zhou, Y.; Gao, F. AmDEF2.7, a tandem duplicated defensin gene from Ammopiptanthus mongolicus, activated by AmWRKY14, enhances the tolerance of Arabidopsis to low temperature and osmotic stress. Environ. Exp. Bot. 2024, 227, 105956. [Google Scholar] [CrossRef]
- Zhao, K.; Ren, R.; Ma, X.; Zhao, K.; Qu, C.; Cao, D.; Ma, Q.; Ma, Y.; Gong, F.; Li, Z.; et al. Genome-wide investigation of defensin genes in peanut (Arachis hypogaea L.) reveals AhDef2.2 conferring resistance to bacterial wilt. Crop J. 2022, 10, 809–819. [Google Scholar] [CrossRef]
- Kaur, J.; Fellers, J.; Adholeya, A.; Velivelli, S.L.S.; El-Mounadi, K.; Nersesian, N.; Clemente, T.; Shah, D. Expression of apoplast-targeted plant defensin MtDef4.2 confers resistance to leaf rust pathogen Puccinia triticina but does not affect mycorrhizal symbiosis in transgenic wheat. Transgenic Res. 2017, 26, 37–49. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Y.; Tang, M.; Chen, W.; Chai, Y.; Wang, W. Bioinformatic analysis of wheat defensin gene family and function verification of candidate genes. Front. Plant Sci. 2023, 14, 1279502. [Google Scholar] [CrossRef]
- Cheng, C.; Wu, S.; Deng, G.; Sheng, O.; Yi, G.; Yang, Q. Recent advances and future directions in banana molecular biology and breeding. Mol. Hortic. 2024, 4, 42. [Google Scholar] [CrossRef]
- Li, X.; Yu, S.; Cheng, Z.; Chang, X.; Yun, Y.; Jiang, M.; Chen, X.; Wen, X.; Li, H.; Zhu, W.; et al. Origin and evolution of the triploid cultivated banana genome. Nat. Genet. 2024, 56, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Abd Murad, N.B.; Mohamed Nor, N.M.I.; Shohaimi, S.; Mohd Zainudin, N.A.I. Genetic diversity and pathogenicity of Fusarium species associated with fruit rot disease in banana across peninsular malaysia. J. Appl. Microbiol. 2017, 123, 1533–1546. [Google Scholar] [CrossRef]
- Uribe-Palacio, S.; Ramírez-Sánchez, M.; Umaña-Rojas, G.; Sáenz- Murillo, M.V. Studies on the potential for treatment with short wave ultraviolet light (UV-C) to reduce postharvest diseases in banana fruit crown (Musa sp., Group AAA, subgroup Cavendish). Fruits 2022, 77, 1–7. [Google Scholar] [CrossRef]
- Conti Taguali, S.; Riolo, M.; Dopazo, V.; Meca, G.; Cacciola, S.O. Characterization of mycotoxins produced by two Fusarium species responsible for postharvest rot of banana fruit. J. Plant Pathol. 2024, 106, 1785–1800. [Google Scholar] [CrossRef]
- Chen, A.; Sun, J.; Matthews, A.; Armas-Egas, L.; Chen, N.; Hamill, S.; Mintoff, S.; Tran-Nguyen, L.T.T.; Batley, J.; Aitken, E.A.B. Assessing variations in host resistance to Fusarium oxysporum f sp. Cubense Race 4 in Musa species, with a focus on the subtropical Race 4. Front. Microbiol. 2019, 10, 1062. [Google Scholar] [CrossRef]
- Maymon, M.; Sela, N.; Shpatz, U.; Galpaz, N.; Freeman, S. The origin and current situation of Fusarium Oxysporum f. sp. Cubense Tropical Race 4 in Israel and the Middle East. Sci. Rep. 2020, 10, 1590. [Google Scholar] [CrossRef]
- Radhajeyalakshmi, R.; Xia, Y.; Shah, D. Antifungal defensins in controlling panama wilt of banana Fusarium Oxysporum f. sp. Cubense. Ann. Biol. Sci. 2020, 8, 38–43. [Google Scholar]
- Lay, F.T.; Brugliera, F.; Anderson, M.A. Isolation and properties of floral defensins from ornamental tobacco and petunia. Plant Physiol. 2003, 131, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Ghag, S.B.; Shekhawat, U.K.S.; Ganapathi, T.R. Petunia floral defensins with unique prodomains as novel candidates for development of fusarium wilt resistance in transgenic banana plants. PLoS ONE 2012, 7, e39557. [Google Scholar] [CrossRef] [PubMed]
- Ghag, S.B.; Shekhawat, U.K.S.; Ganapathi, T.R. Transgenic banana plants expressing a Stellaria media defensin gene (Sm-AMP-D1) demonstrate improved resistance to Fusarium Oxysporum. Plant Cell Tissue Organ Cult. 2014, 119, 247–255. [Google Scholar] [CrossRef]
- Yun, Y.; Song, A.; Bao, J.; Chen, S.; Lu, S.; Cheng, C.; Zheng, W.; Wang, Z.; Zhang, L. Genome data of Fusarium Oxysporum f. Sp. Cubense Race 1 and tropical race 4 isolates using long-read sequencing. Mol. Plant-Microbe Interact. 2019, 10, 1270–1272. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, F.; Sun, X.; Wang, B.; Liu, J.; Ni, X.; Hu, C.; Deng, G.; Tong, Z.; Zhang, Y.; et al. Genome-wide identification of FAD gene family and their contributions to the temperature stresses and mutualistic and parasitic fungi colonization responses in banana. Int. J. Biol. Macromol. 2022, 204, 661–676. [Google Scholar] [CrossRef]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview version 2—A multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef]
- Crooks, G.E.; Hon, G.; Chandonia, J.-M.; Brenner, S.E. WebLogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xu, Y.; Xu, S.; Wu, H.; Qu, P.; Tong, Z.; Lü, P.; Cheng, C. Characterization of banana SNARE genes and their expression analysis under temperature stress and mutualistic and pathogenic fungal colonization. Plants 2023, 12, 1599. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.; Fu, P.; Liu, Y.; Xiang, J.; Lu, J. Molecular characterization and overexpression of VpRPW8s from Vitis pseudoreticulata enhances resistance to Phytophthora capsici in Nicotiana benthamiana. Int. J. Mol. Sci. 2018, 19, 839. [Google Scholar] [CrossRef]
- Kovaleva, V.; Bukhteeva, I.; Kit, O.Y.; Nesmelova, I.V. Plant defensins from a structural perspective. Int. J. Mol. Sci. 2020, 21, 5307. [Google Scholar] [CrossRef] [PubMed]
- De-Paula, V.S.; Razzera, G.; Medeiros, L.; Miyamoto, C.A.; Almeida, M.S.; Kurtenbach, E.; Almeida, F.C.L.; Valente, A.P. Evolutionary relationship between defensins in the poaceae family strengthened by the characterization of new sugarcane defensins. Plant Mol. Biol. 2008, 68, 321–335. [Google Scholar] [CrossRef]
- Costa, L.S.M.; Pires, Á.S.; Damaceno, N.B.; Rigueiras, P.O.; Maximiano, M.R.; Franco, O.L.; Porto, W.F. In Silico characterization of Class II plant defensins from Arabidopsis thaliana. Phytochemistry 2020, 179, 112511. [Google Scholar] [CrossRef] [PubMed]
- De Coninck, B.M.A.; Sels, J.; Venmans, E.; Thys, W.; Goderis, I.J.W.M.; Carron, D.; Delauré, S.L.; Cammue, B.P.A.; De Bolle, M.F.C.; Mathys, J. Arabidopsis thaliana plant defensin AtPDF1.1 is involved in the plant response to biotic stress. New Phytol. 2010, 187, 1075–1088. [Google Scholar] [CrossRef]
- Gaudet, D.A.; Laroche, A.; Frick, M.; Huel, R.; Puchalski, B. Cold induced expression of plant defensin and lipid transfer protein transcripts in winter wheat. Physiol. Plant. 2003, 117, 195–205. [Google Scholar] [CrossRef]
- Domingo, G.; Locato, V.; Cimini, S.; Ciceri, L.; Marsoni, M.; De Gara, L.; Bracale, M.; Vannini, C. A Comprehensive characterization and expression profiling of defensin family peptides in Arabidopsis thaliana with a focus on their abiotic stress-specific transcriptional modulation. Curr. Plant Biol. 2024, 39, 100376. [Google Scholar] [CrossRef]
- Koike, M.; Okamoto, T.; Tsuda, S.; Imai, R. A novel plant defensin-like gene of winter wheat is specifically induced during cold acclimation. Biochem. Biophys. Res. Commun. 2002, 298, 46–53. [Google Scholar] [CrossRef]
- Sasaki, K.; Kuwabara, C.; Umeki, N.; Fujioka, M.; Saburi, W.; Matsui, H.; Abe, F.; Imai, R. The cold-induced defensin TAD1 confers resistance against snow mold and Fusarium head blight in transgenic wheat. J. Biotechnol. 2016, 228, 3–7. [Google Scholar] [CrossRef]
- Cerrudo, I.; Keller, M.M.; Cargnel, M.D.; Demkura, P.V.; de Wit, M.; Patitucci, M.S.; Pierik, R.; Pieterse, C.M.J.; Ballaré, C.L. Low red/far-red ratios reduce arabidopsis resistance to Botrytis cinerea and jasmonate responses via a COI1-JAZ10-dependent, salicylic acid-independent mechanism. Plant Physiol. 2012, 158, 2042–2052. [Google Scholar] [CrossRef]
- Czékus, Z.; Kukri, A.; Hamow, K.Á.; Szalai, G.; Tari, I.; Ördög, A.; Poór, P. Activation of local and systemic defence responses by Flg22 is dependent on daytime and ethylene in intact tomato plants. Int. J. Mol. Sci. 2021, 22, 8354. [Google Scholar] [CrossRef]
- Nandi, A.; Kachroo, P.; Fukushige, H.; Hildebrand, D.F.; Klessig, D.F.; Shah, J. Ethylene and jasmonic acid signaling affect the npr1-independent expression of defense genes without impacting resistance to Pseudomonas syringae and Peronospora parasitica in the Arabidopsis ssi1 mutant. Mol. Plant-Microbe Interact. 2003, 16, 588–599. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tian, H.; Xu, L.; Li, X.; Zhang, Y. Salicylic acid: The roles in plant immunity and crosstalk with other hormones. J. Integr. Plant Biol. 2024, 67, 773–785. [Google Scholar] [CrossRef]
- Zarei, A.; Körbes, A.P.; Younessi, P.; Montiel, G.; Champion, A.; Memelink, J. Two GCC boxes and AP2/ERF-domain transcription factor ORA59 in jasmonate/ethylene-mediated activation of the PDF1.2 promoter in Arabidopsis. Plant Mol. Biol. 2011, 75, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Kaewklom, S.; Wongchai, M.; Petvises, S.; Hanpithakphong, W.; Aunpad, R. Structural and biological features of a novel plant defensin from Brugmansia x candida. PLoS ONE 2018, 13, e0201668. [Google Scholar] [CrossRef]
- Batool, R.; Umer, M.J.; Wang, Y.; He, K.; Shabbir, M.Z.; Zhang, T.; Bai, S.; Chen, J.; Wang, Z. Myco-synergism boosts herbivory-induced maize defense by triggering antioxidants and phytohormone signaling. Front. Plant Sci. 2022, 13, 790504. [Google Scholar] [CrossRef] [PubMed]
- Tetorya, M.; Li, H.; Djami-Tchatchou, A.T.; Buchko, G.W.; Czymmek, K.J.; Shah, D.M. Plant defensin MtDef4-derived antifungal peptide with multiple modes of action and potential as a bio-inspired fungicide. Mol. Plant Pathol. 2023, 24, 896–913. [Google Scholar] [CrossRef]
- Gaspar, Y.M.; McKenna, J.A.; McGinness, B.S.; Hinch, J.; Poon, S.; Connelly, A.A.; Anderson, M.A.; Heath, R.L. Field resistance to Fusarium oxysporum and Verticillium dahliae in transgenic cotton expressing the plant defensin NaD1. J. Exp. Bot. 2014, 65, 1541–1550. [Google Scholar] [CrossRef]
- Fernández, A.; Colombo, M.L.; Curto, L.M.; Gómez, G.E.; Delfino, J.M.; Guzmán, F.; Bakás, L.; Malbrán, I.; Vairo-Cavalli, S.E. Peptides derived from the α-core and γ-core regions of a putative Silybum marianum flower defensin show antifungal activity against Fusarium graminearum. Front. Microbiol. 2021, 12, 632008. [Google Scholar] [CrossRef]
- Al Kashgry, N.A.T.; Abulreesh, H.H.; El-Sheikh, I.A.; Almaroai, Y.A.; Salem, R.; Mohamed, I.; Waly, F.R.; Osman, G.; Mohamed, M.S.M. Utilization of a recombinant defensin from Maize (Zea Mays L.) as a potential antimicrobial peptide. AMB Express 2020, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo Dos Santos, L.; Taveira, G.B.; da Silva, M.S.; da Silva Gebara, R.; da Silva Pereira, L.; Perales, J.; Teixeira-Ferreira, A.; de Oliveira Mello, É.; de Oliveira Carvalho, A.; Rodrigues, R.; et al. Antimicrobial peptides from Capsicum chinense fruits: Agronomic alternatives against phytopathogenic fungi. Biosci. Rep. 2020, 40, BSR20200950. [Google Scholar] [CrossRef]
- Bell, A.A.; Wheeler, M.H.; Liu, J.; Stipanovic, R.D.; Puckhaber, L.S.; Orta, H. United states department of agriculture—Agricultural research service studies on polyketide toxins of Fusarium oxysporum f sp vasinfectum: Potential targets for disease control. Pest Manag. Sci. 2003, 59, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Rajendar, G.; Rehman, M. A short approach to cytogenin and first total synthesis of aspergisocoumarin A and fusarimarin C. Tetrahedron 2024, 168, 134343. [Google Scholar] [CrossRef]
- Lebeau, J.; Petit, T.; Clerc, P.; Dufossé, L.; Caro, Y. Isolation of two novel purple naphthoquinone pigments concomitant with the bioactive red bikaverin and derivates thereof produced by Fusarium Oxysporum. Biotechnol. Prog. 2019, 35, e2738. [Google Scholar] [CrossRef]
- Wiemann, P.; Willmann, A.; Straeten, M.; Kleigrewe, K.; Beyer, M.; Humpf, H.; Tudzynski, B. Biosynthesis of the red pigment bikaverin in Fusarium fujikuroi: Genes, their function and regulation. Mol. Microbiol. 2009, 72, 931–946. [Google Scholar] [CrossRef]
- Amuzu, P.; Pan, X.; Hou, X.; Sun, J.; Jakada, M.A.; Odigie, E.; Xu, D.; Lai, D.; Zhou, L. Recent updates on the secondary metabolites from Fusarium fungi and their biological activities (covering 2019 to 2024). J. Fungi 2024, 10, 778. [Google Scholar] [CrossRef]
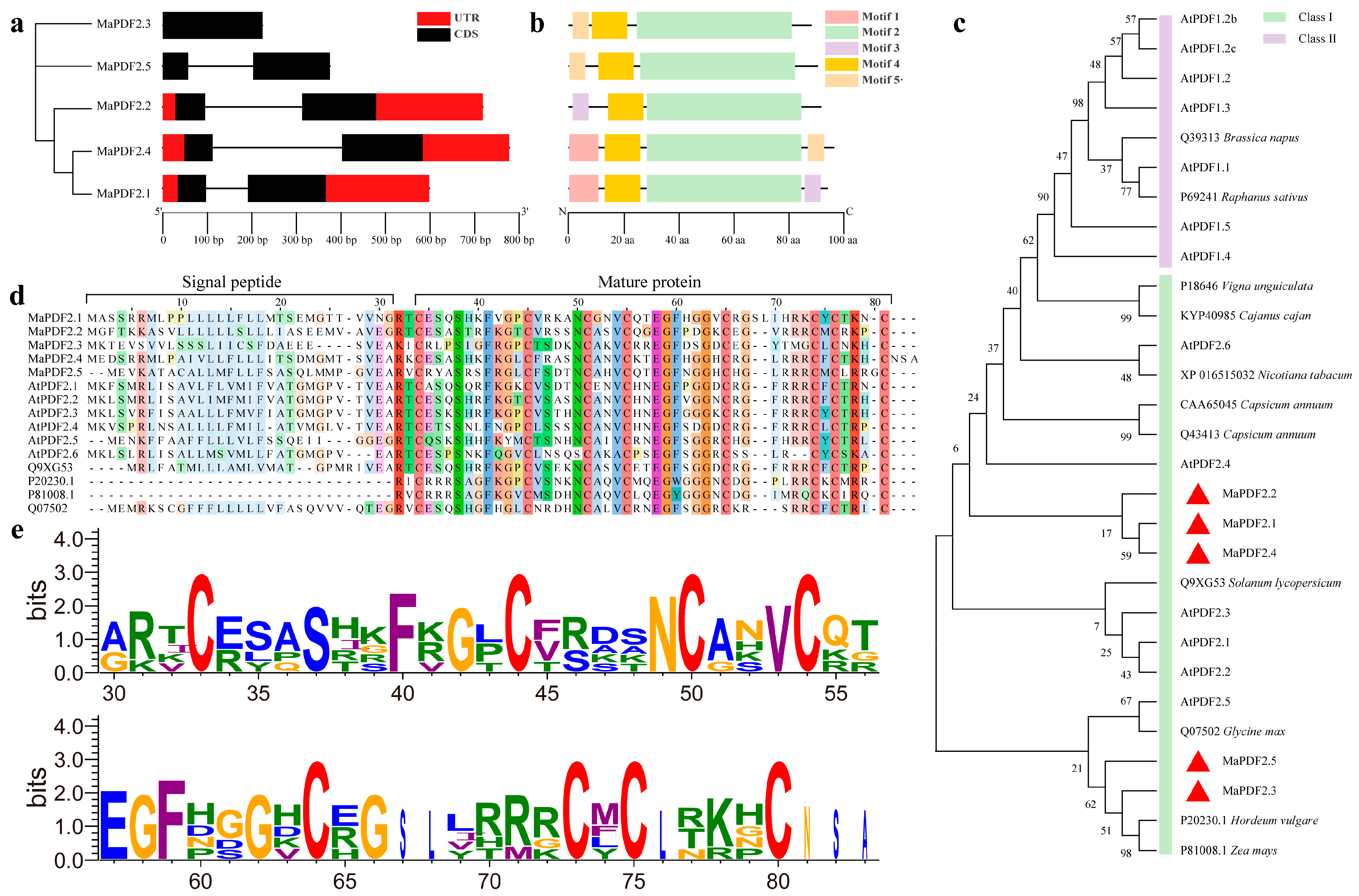
| Gene ID | Gene Name | Chromosome Location | Protein Size/aa | Molecular Weight/kD | PI | Instability Coefficient | Aliphatic Index | GRAVY | Subcellular Location |
|---|---|---|---|---|---|---|---|---|---|
| Ma02_g12840 | MaPDF2.1 | chr02: 21473861…21474459(−) | 79 | 8.67928 | 9.27 | 42.53 | 73.92 | 0.019 | Extracellular |
| Ma04_g36140 | MaPDF2.2 | chr04: 34629849…34630567(−) | 77 | 8.32086 | 8.92 | 37.42 | 79.74 | 0.121 | Extracellular |
| Ma06_g21420 | MaPDF2.3 | chr06: 15679425…15679649(+) | 74 | 8.04623 | 5.26 | 49.89 | 65.81 | −0.109 | Extracellular |
| Ma08_g13660 | MaPDF2.4 | chr08: 10784713…10785491(−) | 81 | 9.04163 | 9.27 | 51.59 | 66.3 | −0.105 | Extracellular |
| Ma11_g12930 | MaPDF2.5 | chr11: 16886961…16887336(−) | 76 | 8.46802 | 9.06 | 64.58 | 65.53 | 0.158 | Chloroplast |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Wang, B.; Wu, H.; Cheng, C. Characterization of Plant Defensin (PDF) Genes in Banana (Musa acuminata) Reveals the Antifungal Ability of MaPDF2.2 to Fusarium Wilt Pathogens. Horticulturae 2025, 11, 513. https://doi.org/10.3390/horticulturae11050513
Li R, Wang B, Wu H, Cheng C. Characterization of Plant Defensin (PDF) Genes in Banana (Musa acuminata) Reveals the Antifungal Ability of MaPDF2.2 to Fusarium Wilt Pathogens. Horticulturae. 2025; 11(5):513. https://doi.org/10.3390/horticulturae11050513
Chicago/Turabian StyleLi, Ruide, Bin Wang, Huan Wu, and Chunzhen Cheng. 2025. "Characterization of Plant Defensin (PDF) Genes in Banana (Musa acuminata) Reveals the Antifungal Ability of MaPDF2.2 to Fusarium Wilt Pathogens" Horticulturae 11, no. 5: 513. https://doi.org/10.3390/horticulturae11050513
APA StyleLi, R., Wang, B., Wu, H., & Cheng, C. (2025). Characterization of Plant Defensin (PDF) Genes in Banana (Musa acuminata) Reveals the Antifungal Ability of MaPDF2.2 to Fusarium Wilt Pathogens. Horticulturae, 11(5), 513. https://doi.org/10.3390/horticulturae11050513






