Alginate Oligosaccharide Promoted the Nutrient Uptake and Growth of Cucumber Seedlings Under Suboptimal Temperature Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.3. Measurement of Growth Parameters
2.4. Measurement of Photosynthetic Characteristics
Measurement of Root Morphology
2.5. Macronutrient Content and Accumulation Analysis
2.6. Biochemical Analysis Assays
2.7. Data Analysis
3. Results
3.1. Effects of Different Nutrient Solution Concentrations with AOS on the Growth of Cucumber Seedlings Under Suboptimal Temperatures
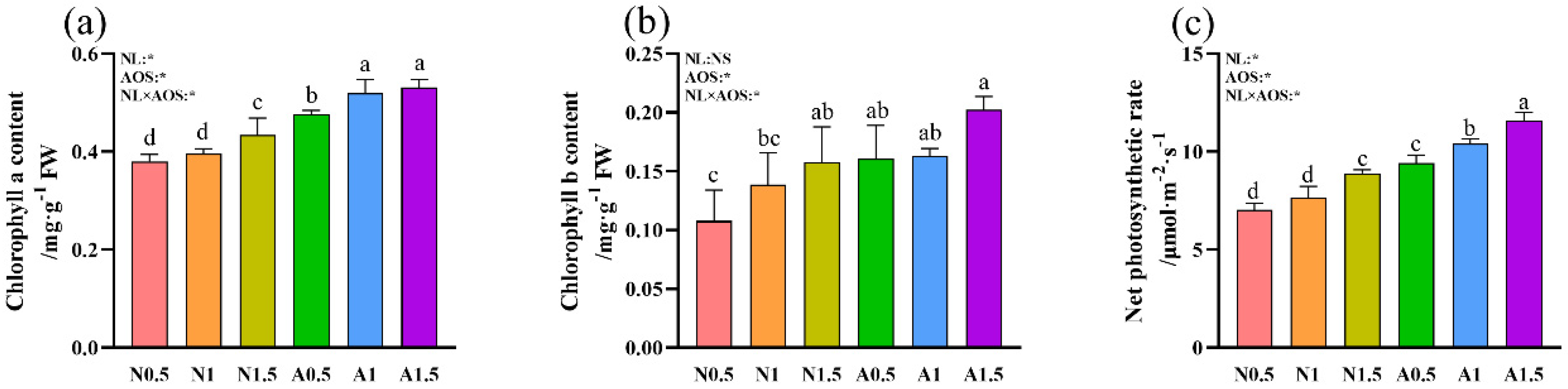
3.2. Effects of AOS and Different Nutrient Solution Levels on the Chlorophyll Content and Pn of Cucumber Seedlings Under Suboptimal Temperatures
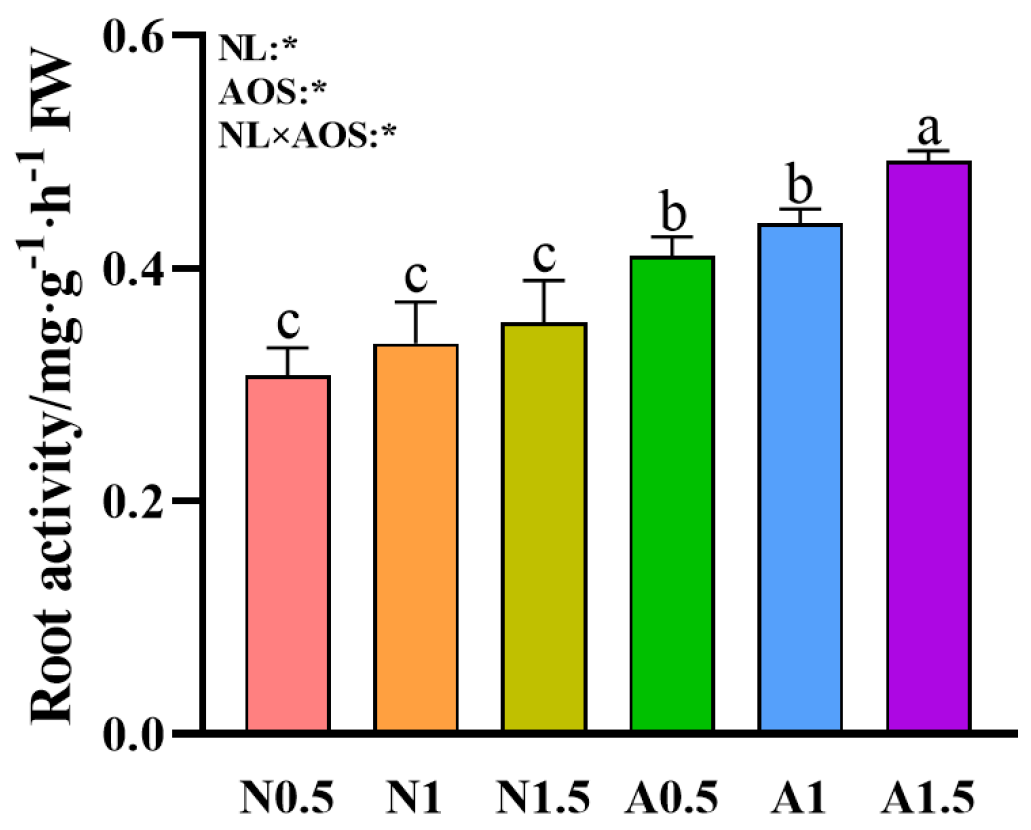
3.3. Effects of AOS and Different Nutrient Solution Levels on the Root Activity of Cucumber Seedlings Under Suboptimal Temperatures
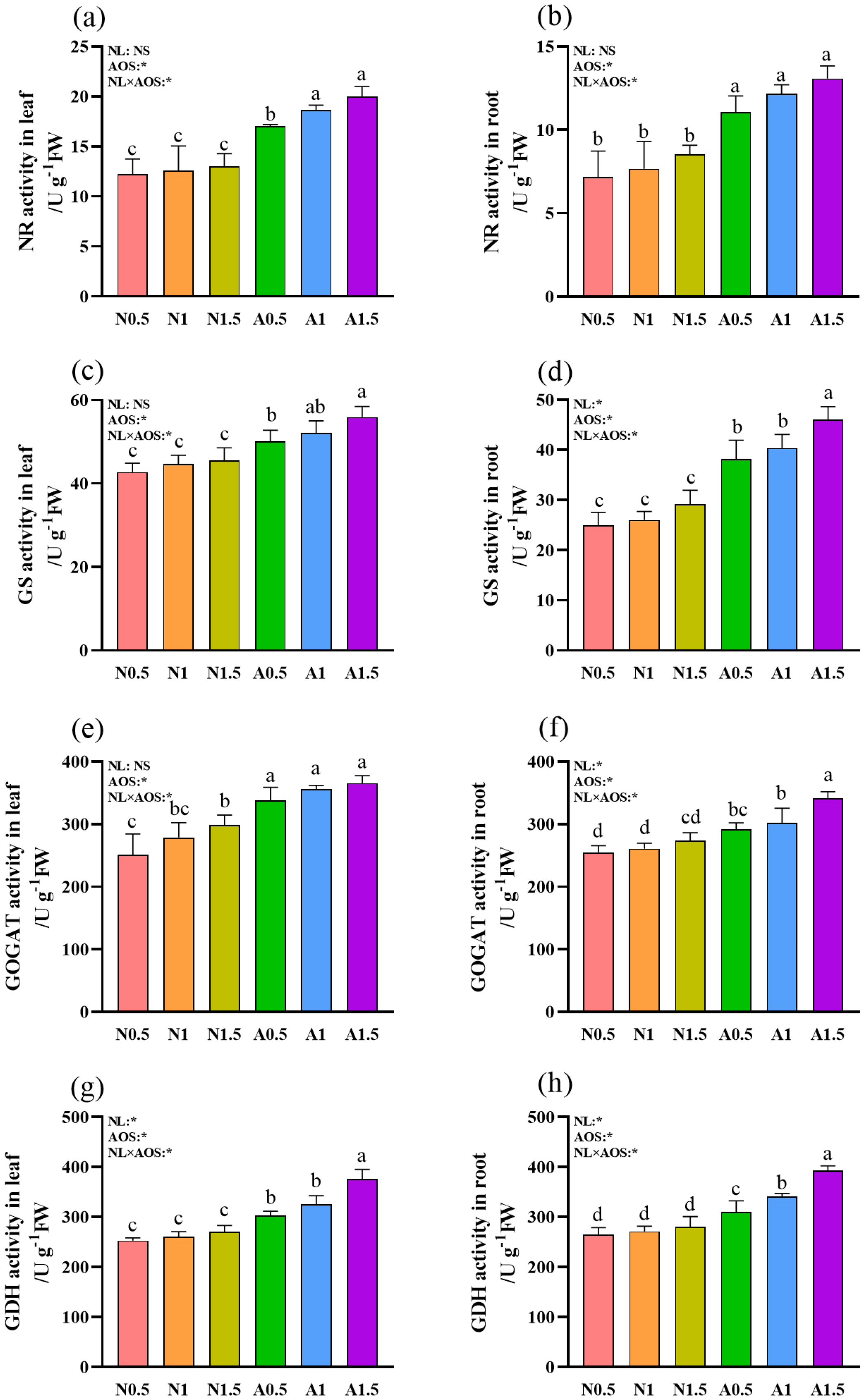
3.4. Effects of AOS and Different Nutrient Solution Levels on the N Metabolism Enzymes Activities in Cucumber Seedlings Under Suboptimal Temperatures
3.5. Effects of AOS and Different Nutrient Solution Levels on N Content, Accumulation, and Distribution in Different Cucumber Organs Under Suboptimal Temperatures
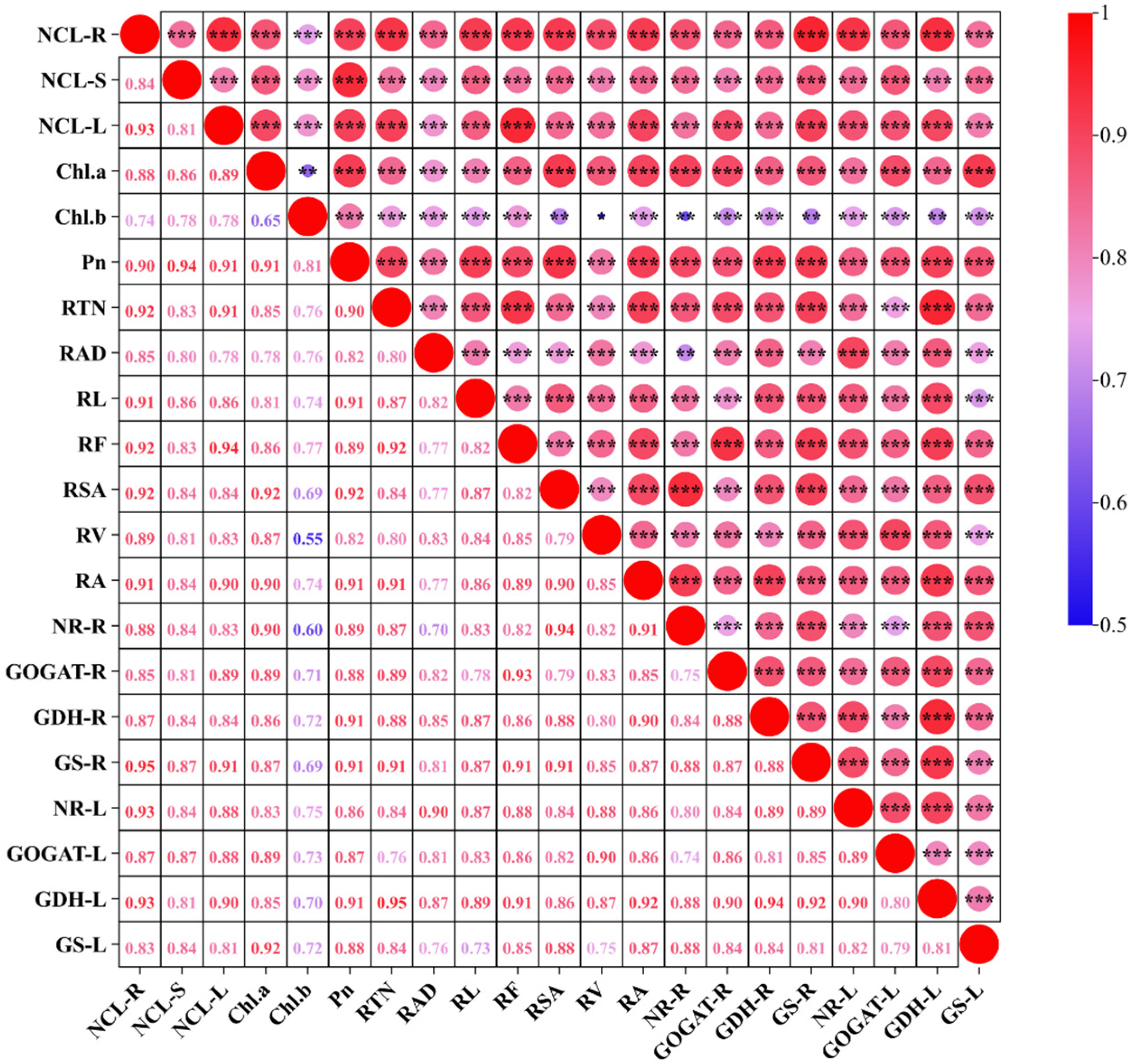
3.6. Correlation Analysis Between Different Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AOS | Alginate oligosaccharide |
| N | Nitrogen |
| P | Phosphorus |
| K | Potassium |
| NR | Nitrate reductase |
| GOGAT | Glutamate synthase |
| GS | Glutamine synthetase |
| GDH | Glutamate dehydrogenase |
| NL | Nutrient level |
| NCL-R | N content in root |
| NCL-S | N content in stem |
| NCL-L | N content in leaf |
| Chl. a | Chlorophyll a content |
| Chl. b | Chlorophyll b content |
| Pn | Net photosynthetic rate |
| RTN | Root tip number |
| RAD | Root average diameter |
| RL | Total root length |
| RF | Root forks |
| RSA | Root surface area |
| RV | Root volume |
| RA | Root activity |
| NR-R | Nitrate reductase activity in root |
| GOGAT-R | Glutamate synthase activity in root |
| GDH-R | Glutamate dehydrogenase activity in root |
| GS-R | Glutamine synthetase in root. |
| NR-L | Nitrate reductase activity in leaf |
| GOGAT-L | Glutamate synthase activity in leaf |
| GDH-L | Glutamate dehydrogenase activity in leaf |
| GS-L | Glutamine synthetase in leaf |
| NUE | N use efficiency |
| GA | Gibberellin |
References
- Shibaeva, T.G.; Sherudilo, E.G.; Titov, A.F. Response of cucumber (Cucumis sativus L.) plants to prolonged permanent and short-term daily exposures to chilling temperature. Russ. J. Plant Physiol. 2018, 65, 286–294. [Google Scholar] [CrossRef]
- Bai, L.; Deng, H.; Zhang, X.; Yu, X.; Li, Y. Gibberellin is involved in inhibition of cucumber growth and nitrogen uptake at suboptimal root-zone temperatures. PLoS ONE 2016, 11, e0156188. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Ban, T.; Yu, H.; Li, Q.; Li, X.; Jiang, W.; Xie, J. Increased ammonium enhances suboptimal-temperature tolerance in cucumber seedlings. Plants 2023, 12, 2243. [Google Scholar] [CrossRef]
- Gao, H.; Yang, W.; Li, C.; Zhou, X.; Gao, D.; Khashi u Rahman, M.; Li, N.; Wu, F. Gene expression and K+ uptake of two tomato cultivars in response to sub-optimal temperature. Plants 2020, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Li, Y.; He, C.; Yu, X. 24-epibrassinolide promotes NO3− and NH4+ ion flux rate and NRT1 gene expression in cucumber under suboptimal root zone temperature. BMC Plant Biol. 2019, 19, 225. [Google Scholar] [CrossRef]
- Yan, Q.; Duan, Z.; Mao, J.; Li, X.; Dong, F. Effects of root-zone temperature and N, P, and K supplies on nutrient uptake of cucumber (Cucumis sativus L.) seedlings in hydroponics. Soil. Sci. Plant Nutr. 2012, 58, 707–717. [Google Scholar] [CrossRef]
- Haghighi, M.; Abdolahipour, B. Rootzone temperature on nitrogen absorption and some physiological traits in cucumber. J. Plant Process Funct. 2020, 8, 51–59. [Google Scholar]
- Yan, Q.; Duan, Z.; Mao, J.; Li, X.; Dong, F. Low root zone temperature limits nutrient effects on cucumber seedling growth and induces adversity physiological response. J. Integr. Agric. 2013, 12, 1450–1460. [Google Scholar] [CrossRef]
- Wang, Z.; Li, D.; Gruda, N.S.; Duan, Z.; Li, X. Fertilizer application rate and nutrient use efficiency in Chinese greenhouse vegetable production. Resour. Conserv. Recycl. 2024, 203, 107431. [Google Scholar] [CrossRef]
- D’Addabbo, T.; Laquale, S.; Perniola, M.; Candido, V. Biostimulants for plant growth promotion and sustainable management of phytoparasitic nematodes in vegetable crops. Agronomy 2019, 9, 616. [Google Scholar] [CrossRef]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Khan, E.A. 24-epibrassinolide application in plants: An implication for improving drought stress tolerance in plants. Plant Physiol. Biochem. 2019, 135, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.; Kurepin, L.V.; Catto, W.; Pharis, R.P. Enhancing crop yield with the use of N-based fertilizers co-applied with plant hormones or growth regulators. J. Sci. Food Agric. 2015, 95, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, L.; Zhang, H.; Yi, B.; Everaert, N. Alginate oligosaccharides preparation, biological activities and their application in livestock and poultry. J. Integr. Agric. 2021, 20, 24–34. [Google Scholar] [CrossRef]
- Zhang, C.; Li, M.; Rauf, A.; Khalil, A.A.; Shan, Z.; Chen, C.; Rengasamy, K.R.R.; Wan, C. Process and applications of alginate oligosaccharides with emphasis on health beneficial perspectives. Crit. Rev. Food Sci. Nutr. 2023, 63, 303–329. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Iwamoto, Y.; Kitamura, Y.; Oda, T.; Muramatsu, T. Root growth-promoting activity of unsaturated oligomeric uronates from alginate on carrot and rice plants. Biosci. Biotechnol. Biochem. 2003, 67, 2022–2025. [Google Scholar] [CrossRef]
- Sarfaraz, A.; Naeem, M.; Nasir, S.; Idrees, M.; Aftab, T.; Hashmi, N.; Khan, M.M.; Moinuddin; Varshney, L. An evaluation of the effects of irradiated sodium alginate on the growth, physiological activities and essential oil production of fennel (Foeniculum vulgare Mill.). J. Med. Plant Res. 2011, 5, 15–21. [Google Scholar]
- Zhang, Y.; Yin, H.; Zhao, X.; Wang, W.; Du, Y.; He, A.; Sun, K. The promoting effects of alginate oligosaccharides on root development in Oryza sativa L. mediated by auxin signaling. Carbohydr. Polym. 2014, 113, 446–454. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.; Yin, H.; Wang, W.; Zhao, X.; Du, Y. Nitric oxide mediates alginate oligosaccharides-induced root development in wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2013, 71, 49–56. [Google Scholar] [CrossRef]
- Aftab, T.; Khan, M.M.A.; Idrees, M.; Naeem, M.; Moinuddin; Hashmi, N.; Varshney, L. Enhancing the growth, photosynthetic capacity and artemisinin content in Artemisia annua L. by irradiated sodium alginate. Radiat. Phys. Chem. 2011, 80, 833–836. [Google Scholar] [CrossRef]
- Naeem, M.; Idrees, M.; Aftab, T.; Khan, M.M.A.; Varshney, L. Irradiated sodium alginate improves plant growth, physiological activities and active constituents in Mentha arvensis L. J. Appl. Pharm. Sci. 2012, 2, 28–35. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Lin, X.; Yan, G.; Liu, L.; Zheng, H.; Zhao, B.; Tang, J.; Guo, Y.D. Alginate-derived oligosaccharides promote water stress tolerance in cucumber (Cucumis sativus L.). Plant Physiol. Biochem. 2018, 130, 80–88. [Google Scholar] [CrossRef]
- Salachna, P.; Grzeszczuk, M.; Meller, E.; Soból, M. Oligo-alginate with low molecular mass improves growth and physiological activity of eucomis autumnalis under salinity stress. Molecules 2018, 23, 812. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-J.; Chen, Y.; Hu, Z.; Ma, S.; Zhang, J.; Shen, H. Alginate oligosaccharides alleviate the damage of rice leaves caused by acid rain and high temperature. Agronomy 2021, 11, 500. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, H.; Liu, H.; Wang, W.; Wu, L.; Zhao, X.; Du, Y. Alginate oligosaccharides regulate nitrogen metabolism via calcium in Brassica campestris L. Var. utilis Tsen et Lee. J. Hortic. Sci. Biotechnol. 2013, 88, 502–508. [Google Scholar] [CrossRef]
- Ali, A.; Khan, M.M.A.; Uddin, M.; Naeem, M.; Idrees, M.; Hashmi, N.; Dar, T.A.; Varshney, L. Radiolytically depolymerized sodium alginate improves physiological activities, yield attributes and composition of essential oil of Eucalyptus citriodora Hook. Carbohydr. Polym. 2014, 112, 134–144. [Google Scholar] [CrossRef]
- Idrees, M.; Nasir, S.; Naeem, M.; Aftab, T.; Khan, M.M.A.; Moinuddin; Varshney, L. Gamma irradiated sodium alginate induced modulation of phosphoenolpyruvate carboxylase and production of essential oil and citral content of lemongrass. Ind. Crops Prod. 2012, 40, 62–68. [Google Scholar] [CrossRef]
- Li, H.; Cheng, Z. Hoagland nutrient solution promotes the growth of cucumber seedlings under light-emitting diode light. Acta Agric. Scand. Sect. B-Soil. Plant Sci. 2015, 65, 74–82. [Google Scholar] [CrossRef]
- Thomas, R.L.; Sheard, R.W.; Moyer, J.R. Comparison of conventional and automated procedures for nitrogen, phosphorus, and potassium analysis of plant material using a single digestion. Agron. J. 1967, 59, 240–243. [Google Scholar] [CrossRef]
- Sumanta, N.; Choudhury, I.; Haque; Nishika, J.; Suprakash, R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014, 4, 63–69. [Google Scholar]
- Clemensson-Lindell, A. Triphenyltetrazolium chloride as an indicator of fine-root vitality and environmental stress in coniferous forest stands: Applications and limitations. Plant Soil. 1994, 159, 297–300. [Google Scholar] [CrossRef]
- Ding, F.; Hu, Q.; Wang, M.; Zhang, S. Knockout of SlSBPASE suppresses carbon assimilation and alters nitrogen metabolism in tomato plants. Int. J. Mol. Sci. 2018, 19, 4046. [Google Scholar] [CrossRef] [PubMed]
- Imsande, J.; Touraine, B. N demand and the regulation of nitrate uptake. Plant Physiol. 1994, 105, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Ruffel, S.; Krouk, G.; Ristova, D.; Shasha, D.; Birnbaum, K.D.; Coruzzi, G.M. Nitrogen economics of root foraging: Transitive closure of the nitrate-cytokinin relay and distinct systemic signaling for N supply vs. demand. Proc. Natl. Acad. Sci. USA 2011, 108, 18524–18529. [Google Scholar] [CrossRef]
- Kiba, T.; Kudo, T.; Kojima, M.; Sakakibara, H. Hormonal control of nitrogen acquisition: Roles of auxin, abscisic acid, and cytokinin. J. Exp. Bot. 2011, 62, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, Y.; Xu, J.; Qi, J.; Liu, X.; Guo, L.; Zhang, H. Research on the mechanisms of phytohormone signaling in regulating root development. Plants 2024, 13, 3051. [Google Scholar] [CrossRef]
- Li, X.; Zeng, R.; Liao, H. Improving crop nutrient efficiency through root architecture modifications. J. Integr. Plant Biol. 2016, 58, 193–202. [Google Scholar] [CrossRef]
- Iwasaki, K.; Matsubara, Y. Purification of alginate oligosaccharides with root growth-promoting activity toward lettuce. Biosci. Biotechnol. Biochem. 2000, 64, 1067–1070. [Google Scholar] [CrossRef]
- Yang, J.; Shen, Z.; Sun, Z.; Wang, P.; Jiang, X. Growth stimulation activity of alginate-derived oligosaccharides with different molecular weights and mannuronate/guluronate ratio on Hordeum vulgare L. J. Plant Growth Regul. 2021, 40, 91–100. [Google Scholar] [CrossRef]
- Zheng, B.; Zhang, X.; Chen, P.; Du, Q.; Zhou, Y.; Yang, H.; Wang, X.; Yang, F.; Yong, T.; Yang, W. Improving maize’s N uptake and N use efficiency by strengthening roots’ absorption capacity when intercropped with legumes. Peer J. 2021, 9, e11658. [Google Scholar] [CrossRef]
- Xie, X.; Weng, B.; Cai, B.; Dong, Y.; Yan, C. Effects of arbuscular mycorrhizal inoculation and phosphorus supply on the growth and nutrient uptake of Kandelia obovata (Sheue, Liu & Yong) seedlings in autoclaved soil. Appl. Soil. Ecol. 2014, 75, 162–171. [Google Scholar] [CrossRef]
- Liu, X.; Hu, B.; Chu, C. Nitrogen assimilation in plants: Current status and future prospects. J. Genet. Genom. 2022, 49, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.Q.; Crawford, N.M. Identification and characterization of a chlorate-resistant mutant of Arabidopsis thaliana with mutations in both nitrate reductase structural genes NIA1 and NIA2. Mol. Gen. Genet. 1993, 239, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wang, Y.; Chen, G.; Zhang, A.; Yang, S.; Shang, L.; Wang, D.; Ruan, B.; Liu, C.; Jiang, H.; et al. The indica nitrate reductase gene OsNR2 allele enhances rice yield potential and nitrogen use efficiency. Nat. Commun. 2019, 10, 5207. [Google Scholar] [CrossRef] [PubMed]
- Redinbaugh, M.G.; Campbell, W.H. Glutamine synthetase and ferredoxin-dependent glutamate synthase expression in the maize (Zea mays) root primary response to nitrate (evidence for an organ-specific response). Plant Physiol. 1993, 101, 1249–1255. [Google Scholar] [CrossRef]
- Prinsi, B.; Espen, L. Mineral nitrogen sources differently affect root glutamine synthetase isoforms and amino acid balance among organs in maize. BMC Plant Biol. 2015, 15, 96. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, X.; Zhang, Z.; Xiong, S.; Meng, X.; Zhang, J.; Wang, L.; Zhang, X.; Yu, M.; Ma, X. Nitrogen regulating the expression and localization of four glutamine synthetase isoforms in wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2020, 21, 6299. [Google Scholar] [CrossRef] [PubMed]
- Melo-Oliveira, R.; Oliveira, I.C.; Coruzzi, G.M. Arabidopsis mutant analysis and gene regulation define a nonredundant role for glutamate dehydrogenase in nitrogen assimilation. Proc. Natl. Acad. Sci. USA 1996, 93, 4718–4723. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiao, B.; Wang, J.; Zhao, P.; Dong, F.; Yang, F.; Ma, C.; Guo, P.; Zhou, S. Identification of wheat glutamate synthetase gene family and expression analysis under nitrogen stress. Genes 2024, 15, 827. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, J.; Gao, W.; Chen, Y.; Li, H.; Lawlor, D.W.; Paul, M.J.; Pan, W. Exogenous trehalose improves growth under limiting nitrogen through upregulation of nitrogen metabolism. BMC Plant Biol. 2017, 17, 247. [Google Scholar] [CrossRef]
- Kchikich, A.; Roussi, Z.; Krid, A.; Nhhala, N.; Ennoury, A.; Benmrid, B.; Kounnoun, A.; El Maadoudi, M.; Nhiri, N.; Mohamed, N. Effects of mycorrhizal symbiosis and Ulva lactuca seaweed extract on growth, carbon/nitrogen metabolism, and antioxidant response in cadmium-stressed sorghum plant. Physiol. Mol. Biol. Plants 2024, 30, 605–618. [Google Scholar] [CrossRef]
- Moussa, H.R.; Taha, M.A.; Dessoky, E.S.; Selem, E. Exploring the perspectives of irradiated sodium alginate on molecular and physiological parameters of heavy metal stressed Vigna radiata L. Plants. Physiol. Mol. Biol. Plants 2023, 29, 447–458. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Li, Y.; Fan, K.; Guo, L.; Yang, Y.; Cheng, C.; Hou, L.; Miao, Y.; Sun, M.; Li, Y.; et al. Alginate Oligosaccharide Promoted the Nutrient Uptake and Growth of Cucumber Seedlings Under Suboptimal Temperature Conditions. Horticulturae 2025, 11, 501. https://doi.org/10.3390/horticulturae11050501
Guo X, Li Y, Fan K, Guo L, Yang Y, Cheng C, Hou L, Miao Y, Sun M, Li Y, et al. Alginate Oligosaccharide Promoted the Nutrient Uptake and Growth of Cucumber Seedlings Under Suboptimal Temperature Conditions. Horticulturae. 2025; 11(5):501. https://doi.org/10.3390/horticulturae11050501
Chicago/Turabian StyleGuo, Xu, Yun Li, Kai Fan, Lingru Guo, Yongzhao Yang, Chunming Cheng, Leiping Hou, Yanxiu Miao, Meihua Sun, Yaling Li, and et al. 2025. "Alginate Oligosaccharide Promoted the Nutrient Uptake and Growth of Cucumber Seedlings Under Suboptimal Temperature Conditions" Horticulturae 11, no. 5: 501. https://doi.org/10.3390/horticulturae11050501
APA StyleGuo, X., Li, Y., Fan, K., Guo, L., Yang, Y., Cheng, C., Hou, L., Miao, Y., Sun, M., Li, Y., & Bai, L. (2025). Alginate Oligosaccharide Promoted the Nutrient Uptake and Growth of Cucumber Seedlings Under Suboptimal Temperature Conditions. Horticulturae, 11(5), 501. https://doi.org/10.3390/horticulturae11050501





