Abstract
Hyperhydricity is a significant challenge in the tissue culture of blueberry plantlets, affecting their propagation, survival and quality, which results in economic losses for industrial blueberry micropropagation. The in vitro liquid propagation of two half-highbush blueberry hybrids, HB1 and HB2, showed that a Growtek stationary bioreactor culture system containing a liquid medium exhibited a higher hyperhydricity percentage than a Sigma glass culture system with a semi-solid medium. The percentage of hyperhydricity (75.21 ± 1.89%) and water content (72%) of HB2 was more than that of HB1. A scanning electron microscopy study revealed that hyperhydric plantlets from both genotypes developed slowly, had closed stomata, and displayed enlarged intercellular spaces between the palisade and spongy parenchyma layers. Disrupted vascular bundles, underdeveloped sieve elements and a weak connection between phloem and xylem tissue were also observed in hyperhydric plantlets. An analysis of mesophyll and stem tissues highlighted a compressed adaxial epidermis, which led to compact palisade parenchyma, with irregularly shaped mesophyll cells. Hyperhydric plants showed strong nuclear magnetic resonance (NMR) signals in the aliphatic, aromatic, and sugar regions, specifically at peaks of 2.0, 2.5, 4.0, 4.5, 6.0, and 6.7 ppm. These signals were attributed to the presence of catechin (C15H14O6), a flavonoid compound, suggesting its significant role or accumulation in these plants under hyperhydric conditions. Despite the negative effects of hyperhydricity on commercial propagation, hyperhydric plants were found to contain higher levels of valuable untargeted metabolites, such as β-P-arbutin, chlorogenic acid, quercetin-3-O-glucoside, epicatechin, 2-O-caffeoyl arbutin, various fatty acids, β-glucose, linolenic acid, and acetyl than both in vitro and ex vitro conditions. The enrichment of bioactive compounds in blueberry enhances its antioxidant properties, nutritional profile, and potential health benefits, making them significant for plant defense mechanisms and stress adaptation.
1. Introduction
Plant tissue culture techniques are now vital in modern plant science, providing great potential to improve crop production and maintain global food security challenges in agriculture [1]. The micropropagation of woody plants, such as blueberry (Vaccinium spp.) [1], huckleberry (V. membranaceum) [2], and lingonberry (V. vitis-idaea) [3], is often time-consuming and difficult compared to in vitro propagation. Blueberries, belonging to the genus Vaccinium within the family Ericaceae [1,2], have significant commercial value and are cultivated globally. However, most of them are produced in the United States and Canada [1]. Among 400–500 species, Vaccinium genus is renowned for its high antioxidant activity and is mainly known for its rich phytochemical content, including anthocyanins [4], particularly flavonoids [2,5] and polyphenolics [2]. Along with this, the leaves contain higher levels of proanthocyanidins than other fruits [6]. A leaf extract has been shown to inhibit the expression of the hepatitis C virus [7] and the growth of human promyelocytic leukemia HL60 cells [8]. Both the products derived from fruits and leaves have demonstrated potential in treating cancers and reducing the risk of cancer development [5], stroke [9], cardiovascular disorders [10], diabetes [11], and aging-related diseases [12].
Although in vitro propagation results in higher yields of rooting and fruiting, it is often affected by hyperhydricity (HH), a severe condition marked by a glassy appearance of leaves and shoots [2,13]. Hyperhydric plantlets exhibit several anatomical alterations, including the discontinuous development of the epidermis and cuticle, irregular stomatal distribution, decreased stomatal density, a less developed cell wall, and increased intercellular space in the mesophyll [13]. Physiologically, these plantlets are characterized by reduced chlorophyll and lignin and high water content [14]. HH can lead to significant economic losses in the micropropagation industry. To predict and control the occurrence of HH, a better understanding of the anatomical and physiological features of this kind of plantlet is essential [13].
Targeted analytical methods are commonly used to investigate metabolic changes during different plant conditions. Due to the high levels of secondary metabolites in blueberry plants, it is essential to characterize their natural compounds. NMR spectroscopy methods provide supplementary information for compound identification and determine the plant’s overall metabolic fingerprint [15]. Anthocyanin derivatives with ethyl-linked (epi)catechin were first discovered in blueberry plants, and their presence was also identified as putative ethyl-linked (epi)catechin in half-highbush blueberry [16]. Tannins extracted from V. ovatum exhibited significant correlations between β-ring hydroxylation and the observed di- and tri-methoxybenzenes in 13C NMR [17]. Through the utilization of 1H NMR spectra on crude extracts of bilberry and lingonberry, various metabolites such as chlorogenic acid, catechin, epicatechin, 2-O-caffeoyl arbutin, quercetin-3-O-glucoside, quercetin-3-O-glucuronide, P-coumaroyl, caffeoyl-glucoside derivatives, and β-P-arbutin were identified [15].
This study aims to detect the potential HH, morphological parameters and water content of two hybrids of half-highbush blueberry plants. Physiological and cellular orientations were examined using scanning electron microscopy (SEM) and histology, and nuclear magnetic resonance (NMR) was used for the first time to analyze alterations in blueberry metabolites induced by HH.
2. Materials and Methods
2.1. Experimental Materials, Culture Conditions
In this experiment, two half-highbush blueberry hybrids were used. They were derived from the cross between highbush (V. corymbosum L.) and lowbush blueberry (V. angustifolium Ait.) plants and were designated as HB1 and HB2 (identity not disclosed). The hybrids were developed at the St. John’s Research and Development Centre, Agriculture and Agri-Food Canada, Newfoundland and Labrador, Canada. The plant materials used in this study comply with relevant institutional, national, and international guidelines and legislation [2]. The in vitro conditions (Figure 1A) utilized nodal segments cultured in 175 mL Sigma glass baby food jars [1] containing 35 mL berry basal medium containing 20 g L−1 sucrose, 1.25 g L−1 Gelrite, and 3.5 g L−1 agar [2]. After 3 weeks of culture, nodal explants were transferred to greenhouse growth conditions, which were maintained at 85% relative humidity, 20 ± 2 °C, and a 16 h photoperiod, with a maximum photosynthetic photon flux (PPF) of 90 μmol m−2 s−1, using a Conviron MTR30 growth chamber (ND, USA) [2].
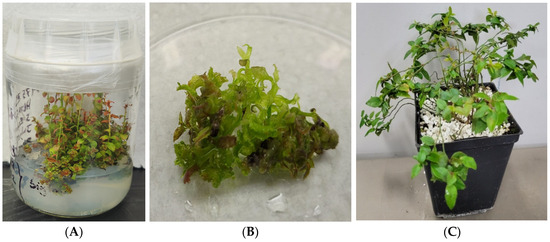
Figure 1.
Photograph of 8-week-old Sigma- (A) and Growtek (B)-cultured explants in in vitro HB2 propagation on a modified berry basal medium supplemented with 4.5 μM zeatin, (C) 24-week-old potted greenhouse plant (peat: perlite 2:1 v/v).
2.2. Shoot Proliferation in Sigma Glass and Growtek Stationary Bioreactor
The in vitro-grown shoots were cultivated using sterilized node cuttings (1–2 inches long) in Sigma glass baby food jars (Sigma-Aldrich, MO, USA) [2] containing 175 mL semi-solid (Figure 1A) media and a Growtek stationary bioreactor (Growtek; Fisher Scientific, Ottawa, ON, Canada; volume: 1.178 L, height: 15 cm, diameter: 5.7 cm) containing 200 mL liquid (Figure 1B) media to produce fully grown shoots. The Sigma glass container is cylindrically shaped borosilicate glass, offering chemical resistance and thermal stability. Its wider airtight lid prevents contamination and helps maintain sterility. The Growtek bioreactor included a rotating explant holder, a medium-exchange side tube, and polycarbonate casing. After 18 weeks, matured plantlets were transferred to pots filled with peat/perlite (2:1) (v/v) (Figure 1C). For each culture, we established subcultures every 8 weeks. Five replications were used per treatment, and the experiment was repeated three times [2].
2.3. Content of Water
Water content was measured using an air oven, following [18] with slight modifications using an air oven. Randomly selected fresh leaf samples from half-highbush blueberry plants were weighed, and their fresh weight (FW) was recorded. Then, they were dried at 70 °C in oven, and their dry weights (DWs) were recorded at intervals over 180 min. Water content was calculated using the following formula:
Water content (%) = 100% × [(FW − DW)/FW]
2.4. Observation of SEM
Following the methodologies described by Picoli et al. [19] and Gao et al. [13], a scanning electron microscope (SEM) study was conducted on 8-week-old proliferated shoots and 24-week-old ex vitro plant samples. Both leaf and stem samples were cut into 1 cm2 and 1 cm pieces and subsequently fixed for 24 h in a 0.1 M phosphate buffer (pH 7.2) containing 2.5% glutaraldehyde. The samples were stained after ultramicrotomy, and images were captured using an SEM with an energy dispersive X-ray (SEM-EDX) (Thermo Fisher Scientific, OR, USA) [2].
2.5. Histology Analysis
Samples from 8-week-old Sigma- and Growtek-cultured plants and 24-week-old greenhouse-grown plants were immersed in an FAA solution (formalin 37%, acetic acid glacial, and ethanol 70%; 1:1:18 v/v) for 48 h at room temperature. These samples underwent drying and wax infiltration using the Tissue-tech VIP Tissue Processor [2]. The tissues were then embedded in wax and sectioned into 5 or 10 mm slices using a rotary microtome (Leica RM2135, Nussloch, Germany). These sections were floated on a water bath at approximately 40 °C to ensure flattening and placed on superfrost slides. The slides were labeled with their section order, thickness, and plant sample code and then affixed by melting the wax on a hot plate at 60 °C for 12 h. The slides were then oven-dried at 38 °C, de-waxed, stained, mounted, and observed using a Lumenera Infinity camera (Teledyne Lumenera, Ottawa, ON, Canada) [20].
2.6. Bioactive Compound Analysis
2.6.1. Reference Compound and Reagents
Catechin (C15H14O6) was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA), deuterium oxide (D2O) was from Millipore Sigma Co. (Rockville, MD, USA). Acetone-d6 and 3-(trimethylsilyl) propionic-2,2,3,3-d4 acid sodium salt (TSP) were from Sigma-Aldrich Co, MO, USA.
2.6.2. Sample Preparation for NMR Analysis
Sample extraction was performed by using the modified method of Liu et al. [15]. Moreover, 8-week-old in vitro young shoot samples and 24-week-old greenhouse-grown young shoots were lyophilized and ground to form leaf powder. Subsequently, 50 mg of leaf powder was poured into 800 μL of a solvent comprising deuterated acetone/D2O/TSP (95:5:0.05, v/v/w). The extraction process occurred over 1 h on a shaker, followed by centrifugation for 5 min at 1650× g. The resulting supernatant was either promptly analyzed or preserved at −80 °C until further analysis [15].
2.6.3. NMR Data Acquisition and Analysis
1H NMR measurements were performed on a Bruker Avance II 600 NMR spectrometer (Bruker Corporation, MA, USA) equipped with a 5 mm TXI probe (Bruker Corporation, MA, USA) at 298 K. Furthermore, 500 μL samples were run for 1H NMR using water suppression pulse (zgpr), with 128 scans were conducted with an acquisition time of 3.2 s, 64 k datapoints and a recycling delay of 5 s [15].
The data were processed using MestReNova (version 15.0.0), with baseline correction (splines) and automatic phase correction, and referenced and normalized (100) to TSP at 0 ppm. The spectra were then stacked and binning analysis was applied from −0.5 to 10 ppm, with a bin width of 0.05 ppm, using the average mode [15]. This analysis investigated catechin (C15H14O6), a flavonoid compound.
2.7. Statistical Analysis
Morphological characteristics and water content were analyzed statistically using the JMP Pro 17.00 software (https://www.jmp.com/en/home), with all data presented as means ± standard error from three replications. The results without zeatin in the medium were excluded, as they showed no significant effects. The statistical data were conducted at p ≤ 0.05 to evaluate the shoot number per explant, HH percentage, and water content across Sigma, liquid bioreactor and greenhouse-grown conditions.
3. Results
3.1. Hyperhydricity in Micropropagated Blueberry
The shoot number per explant varied between the two conditions for each hybrid (Table 1). For HB2, the Sigma culture resulted in 7.25 ± 0.19 shoots per explant, while the Growtek culture had a significantly higher shoot production at 8.20 ± 0.18 shoots per explant. A similar trend was observed for HB1, where Sigma culture produced 6.78 ± 0.14 shoots per explant, while Growtek resulted in 7.00 ± 0.10 shoots per explant. In this experiment, greenhouse-grown plants exhibited zero hyperhydricity.

Table 1.
Impact of HH under varying culture conditions in two half-highbush blueberry varieties.
Regarding HH, a much higher percentage of hyperhydric plantlets was observed in the Growtek culture. For HB2, the percentage of hyperhydric plantlets in Growtek was 75.21 ± 1.89% compared to just 18.67 ± 1.72% in Sigma-cultured plant. Similarly, for HB1, Growtek produced 70.29 ± 1.67% hyperhydric plantlets, while Sigma produced only 15.45 ± 1.59% (Table 1). These results indicate that the Growtek culture significantly increased the percentage of hyperhydricity in plantlets with comparatively less shoot no. per explants in the Sigma condition for both hybrids.
3.2. Water Content of Hyperhydric Plant
In the HB2 hybrid, water content was highest in the Growtek condition at 85%, followed by the greenhouse condition at 75% and the Sigma condition at 70%. Similarly, the highest water content for HB1 was observed in the Growtek condition at 70%, followed by the Sigma condition at 62% and the greenhouse condition at 58% (Figure 2).
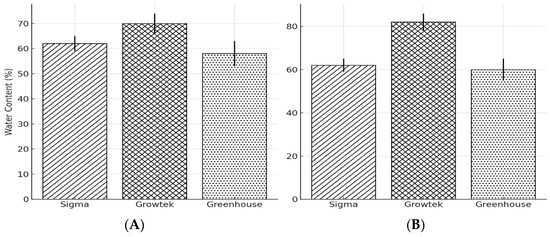
Figure 2.
Water content of three different cultures conditioned of two half-highbush blueberry plants (A) HB1, (B) HB2. Data represent the mean ± standard errors (SEs) derived from three leaf samples collected from three randomly chosen plantlets.
3.3. SEM Analysis of Leaf and Stem
The SEM images revealed distinct differences between the mesophyll tissues, stem surfaces, and stem cross-sections of Sigma, Growtek, and greenhouse-grown half highbush blueberry plants, demonstrating the effects of HH on plant physiology. The mesophyll tissue exhibited a compact arrangement with well-defined cells in Sigma-cultured leaves (Figure 3A,J). But HB1 had a higher quantity of stomata compared to HB2. However, in Growtek-cultured leaves (Figure 3D,M) showing HH, mesophyll tissue was significantly disoriented with enlarged intercellular spaces, indicating abnormal development. Mesophyll tissue exhibited deformed epidermal trichomes and glandular trichomes. The greenhouse-grown leaves (Figure 3G,P) showed structured and intact mesophyll tissue similar to the Sigma-cultured plants but with less stomata density.
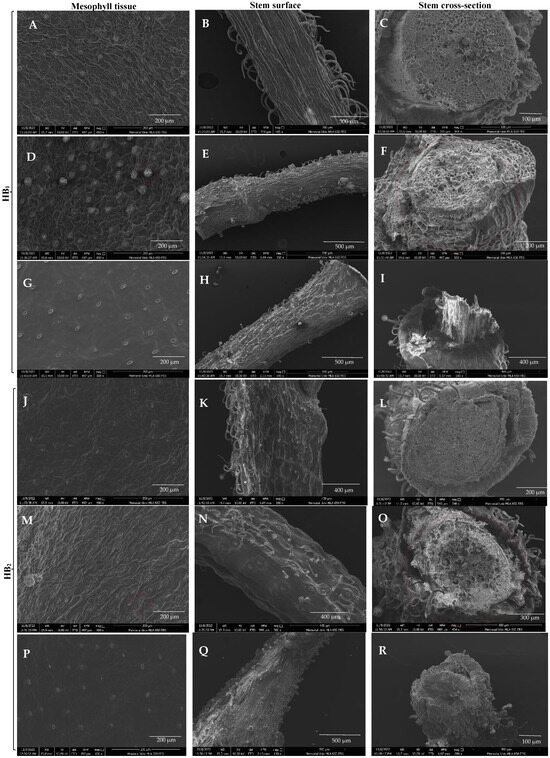
Figure 3.
Scanning electron microscopic view of two half-highbush blueberry hybrids; (A,J) Sigma-cultured leaf mesophyll tissue (8 weeks old); (D,M) Growtek-cultured leaf showing thickness of stomata and disoriented mesophyll tissue in HH condition (8 weeks old). Red circles indicate the hyperhydric stomata; (G,P) greenhouse-grown mesophyll tissue (24 weeks old); (B,K) Sigma-cultured explant’s stem surface (8 weeks old); (E,N) Growtek-cultured explant’s stem surface (8 weeks old) in HH condition; (H,Q) stem surface of greenhouse-grown plant (24 weeks old); (C,L) Sigma-cultured plant’s stem cross-section (8 weeks old); (F,O) Growtek-cultured explant showed irregular xylem and phloem (8 weeks old) in HH condition indicated by red circles; (I,R) greenhouse-grown plant’s stem cross-section (24 weeks old).
Along with this, Sigma-cultured stems (Figure 3B,K) exhibited organized surface structures with visible epidermal cells. In contrast, Growtek-cultured plants (Figure 3E,N) displayed disoriented epidermal cells with an irregular surface texture and fewer non-glandular trichomes. The presence of excessive water in the hyperhydric plants caused the epidermal cells to swell and lose their defined shape, resulting in a less compact surface structure. The greenhouse-grown stems (Figure 3H,Q) showed more defined structures compared to the Growtek-cultured plants, though they were slightly less organized and had less stomata density than Sigma-cultured stems. Sigma-cultured plants (Figure 3C,L) had a well-structured vascular arrangement with distinct xylem and phloem tissues. The Growtek-cultured plants (Figure 3F,O) showed severe vascular disorganization, with irregularly spaced xylem and phloem, thin cell walls, and enlarged intercellular spaces. The intercellular spaces are notably enlarged, contributing to the spongy texture of the tissue, which led to the thin and fragile appearance of cell walls in the vascular bundles. This irregularity indicated abnormal water transport functions due to HH. The greenhouse-grown plants (Figure 3I,R) displayed a more compact and organized vascular bundle structure than those cultured in Growtek. However, they still showed slight disorientation due to the hard stem structure developed when grown outdoors in a natural environment.
3.4. Histology of Hyperhydric and Non-Hyperhydric Plants
The histological analysis (Figure 4) depicts the differences in leaf cross-sections and stem longitudinal sections across the three culture conditions (Sigma, Growtek and greenhouse conditions). The mesophyll tissue of HB1 appears well-organized with clear cell boundaries in Sigma-cultured plants (Figure 4A,B). The stem longitudinal section displays a regular arrangement of vascular tissues with normal cell orientation. Growtek-cultured plants (Figure 4C,D) exhibit disoriented mesophyll tissue, with irregular and enlarged intercellular spaces. In the stem section, cells showing underdeveloped sieve tube elements reflect a rough vascular bundle orientation, indicating developmental abnormalities under hyperhydric conditions. Greenhouse-grown plants (Figure 4E,F) of HB1 demonstrate well-structured mesophyll tissue and organized vascular bundles in the stem section, similar to normal plant growth conditions.
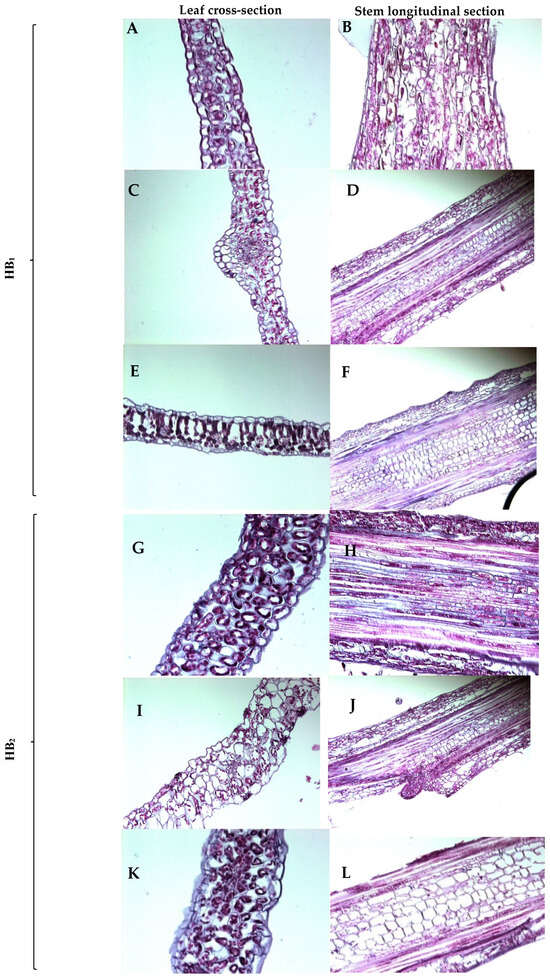
Figure 4.
Histological observation of 8-week-old Sigma- and Growtek-cultured plants and 24-week-old greenhouse-grown plant; (A,G) Sigma-grown organized leaf mesophyll tissue; (C,I) Growtek-grown disoriented leaf mesophyll tissue in HH condition; (E,K) greenhouse-grown leaf mesophyll tissue; (B,H) Sigma-grown stem longitudinal section; (D,J) Growtek-grown underdeveloped cell orientation of stem longitudinal section in HH condition; (F,L) greenhouse-grown stem longitudinal section. Scale bar is 50–100 μM.
In Sigma-grown explants (Figure 4G,H) of the HB2 hybrid, the mesophyll tissue and stem sections displayed normal organization with proper xylem and phloem tissue orientation, similar to the Sigma-cultured HB1. Growtek-cultured plants (Figure 4I,J) showed disoriented mesophyll cells with large intercellular spaces and unclear distance between palisade parenchyma and spongy parenchyma. The stem section presented underdeveloped cell differentiation and irregular vascular tissue alignment with underdeveloped sieve elements, characteristic of HH. Greenhouse-grown plants (Figure 4K,L) exhibited well-structured mesophyll and properly oriented vascular tissues in the stem, consistent with normal growth conditions.
3.5. 1H NMR Analysis of Hyperhydric and Non-Hyperhydric Plants
In the aliphatic region (Figure 4A), catechin signals were found at 2.5 ppm and 2.1–2.05 ppm in all samples, but no samples showed signals of 2.9 ppm. All greenhouse-grown samples of the HB1 hybrid exhibited higher peak intensities at those points compared to the hyperhydric sample, which achieved the second highest signal intensities, where the Sigma-cultured plant showed relatively weaker signals. In the area of the sugar region (Figure 5A), peaks associated with catechin appeared at 4.55–4.5 ppm and 3.95–4.0 ppm. The Sigma-cultured plants had stronger peaks in this region, with the most prominent peak observed at 4.0 ppm. The hyperhydric plant had the highest intensities at 4.55–4.5 ppm. In the aromatic region (Figure 5A), catechin signals were identified both between 6.7 and 7.0 ppm; 5.85 and 6.05 ppm. The Growtek-cultured hyperhydric plant exhibited higher peaks at 6.05 ppm, while the Sigma-cultured non-hyperhydric plant had a stronger signal at 6.9 ppm. Other signals corresponding to fatty acids were found in both hyperhydric and non-hyperhydric plants, with hyperhydric plants showing higher peaks in these regions.
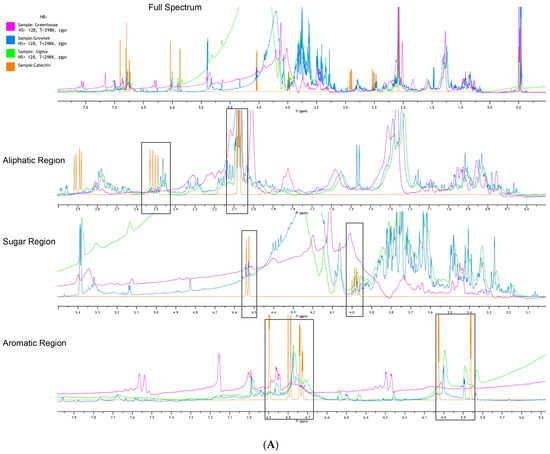
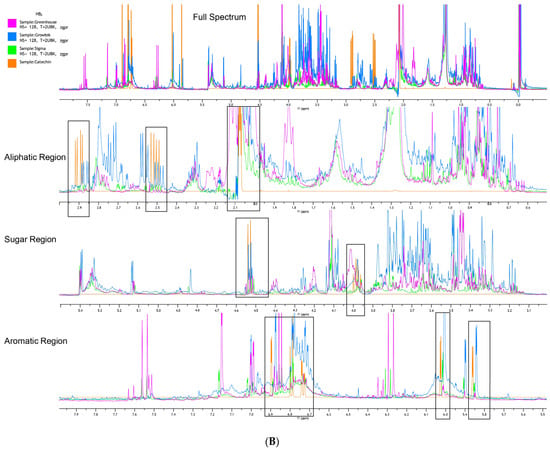
Figure 5.
1H NMR spectra of crude extracts of Growtek-grown hyperhydric (blue) leaf and greenhouse-grown (magenta) leaf and Sigma-grown (green) non-hyperhydric blueberry leaf superimposed with catechin (orange). (A) Half-highbush blueberry hybrid HB1; (B) half-highbush blueberry hybrid HB2. Black boxes denote the intensity of catechin compounds superimposed with plant compounds.
In the investigation of different culture conditions of HB2, catechin signals were detected in aliphatic regions (Figure 5B) around 2.9 ppm, 2.5 ppm, and 2.1 ppm. The hyperhydric extract displayed the highest intensity peaks at 2.5 ppm and 2.9 ppm, indicating a higher presence of catechin and related compounds. The non-hyperhydric samples (greenhouse- and Sigma-cultured) also showed peaks at these points but with lower intensities, particularly in the greenhouse-grown sample. In the sugar region (Figure 4B), catechin peaks were noted between 4.5–4.55 ppm and 4.04–3.95 ppm. The Growtek-cultured hyperhydric plant exhibited the highest peak in both signals, whereas the greenhouse-grown non-hyperhydric sample observed fewer signal intensities. In the aromatic region (Figure 4B), significant peaks were found at 6.02 ppm, 5.85 ppm and between 6.7 ppm and 7.0 ppm, indicating the presence of catechin’s aromatic protons. The hyperhydric sample exhibited stronger peaks at all regions where catechin compound signals were present, while the non-hyperhydric Sigma-cultured plant showed the second highest peak at all points, indicating lower aromatic compound concentrations. Other compounds were identified (Figure 5B) in the aliphatic region; signals at 1.2–1.4 ppm were indicative of fatty acid chains, with hyperhydric plants showing a higher intensity at these points. Peaks related to methylene groups (-CH2-) were also noted in both hyperhydric and non-hyperhydric samples. In the sugar region, peaks for various sugar moieties were found in both groups, with higher intensities in Sigma-cultured non-hyperhydric samples. In the aromatic region, signals suggesting the presence of additional phenolic compounds, such as quercetin derivatives, were observed, with higher peaks in Sigma-cultured non-hyperhydric plants.
4. Discussion
The issue of hyperhydricity (HH) in plantlets and the degree of abnormal morphogenesis typically depend on the plant’s unique physiological responses to culture conditions, the species, and the specific hybrid involved [13]. The physical morphology highlights a clear relationship between normal and hyperhydric explants in half-highbush blueberry hybrids (Figure 6). The Growtek culture system clearly gave rise to HH compared to the Sigma system and greenhouse conditions. HH is often associated with higher humidity and excessive nutrients, which is common in Growtek systems [2,13]. At a concentration of 7.5 mg L−1 kinetin, 33.3% of plantlets were observed to be hyperhydric with a regeneration response of 66.7%. However, at a higher concentration of 10 mg L−1 kinetin, 56.7% of the plantlets displayed HH and a slightly reduced regeneration rate of 56.7% [13]. However, in both hybrids, shoot production was consistently higher in the Growtek condition. This may be due to the more nutrient-dense environment or the controlled humidity, which can stimulate shoot proliferation [2,13]. HH is often characterized by excessive water content in plant tissues, leading to abnormal morphogenesis, as seen in the elevated levels observed here, especially in the HB2 hybrid (75.21% HH) (Table 1). HH was found to increase with higher concentrations of N6-benzyladenine (BA), zeatin, and ammonium (NH4+) [21]. In Olearia microdisca [22,23] and Tagetes patula L. [23], high levels of BA tended to cause HH in in vitro plantlets. In carnations (Dianthus caryophyllus), a rise in plant growth regulators (PGRs) like kinetin was also linked to a higher occurrence of HH [24]. The HH percentage in the Sigma system was significantly lower for both hybrids but higher in the Growtek culture condition. These results are consistent with findings from other studies, where lower HH rates are present in traditional culture systems, emphasizing the optimization of culture conditions to balance growth and avoid physiological disorders [14].
Figure 6.
Overview of catechin compound identification, SEM and histology analysis of plant leaf and stem.
The water content result indicates that plants grown in the Growtek system had the highest water content compared to the Sigma and greenhouse systems for both hybrids, HB2 and HB1 (Figure 2). Previous research on HH has shown that hyperhydric plants generally exhibit higher water retention due to excessive water absorption under in vitro conditions [2,13]. The Growtek system, known for its high humidity and nutrient supply, creates conditions conducive to HH [2,25]. During in vitro culture, explants often face various stresses, including physical injury, high humidity, changes in the osmotic or pH levels of the culture medium, and elevated PGR concentrations. Ethylene is crucial in the plant’s response to these environmental stresses [26]. This is observed in the HB2 hybrid (Figure 2B), which showed the highest water content in the Growtek system at 85%. The greenhouse system exhibited the lowest water content for both hybrids, suggesting that the more natural environmental conditions in the greenhouse might help mitigate HH. Reduced water retention in the greenhouse environment likely prevents the excessive water accumulation seen in in vitro conditions, leading to the better development of vascular tissues and normal morphogenesis [27].
The Growtek-cultured explants showed significant morphological abnormalities caused by HH. Previous studies have shown that HH leads to enlarged intercellular spaces and disorganized mesophyll tissues, similar to the findings in blueberry plants [13]. These changes are likely due to excessive water uptake disrupting normal cell wall formation, resulting in mesophyll tissue disorientation [14]. The stomata in the mesophyll tissue were closed, swollen, and disrupted, creating a barrier to normal respiration and photosynthesis. The stem surfaces of Growtek-cultured explants exhibited significant epidermal disorganization compared to Sigma- and greenhouse-grown plants, along with squeezed vascular tissue found in other studies [2,13]. These changes aligned with characteristics observed in the hyperhydric plantlets of other species, including Tectona grandis L., Handroanthus impetiginosus, and Eucalyptus grandis [28,29]. This disorganization is consistent with previous studies that report a loss of structural integrity in hyperhydric plants due to excessive water content [30]. In normal plants, hair-like structures help reduce water loss and prevent excessive heat buildup. However, in hyperhydric plants, these protective trichomes were less present than in Sigma-cultured explants and greenhouse-grown plants. As observed in the SEM images, the Growtek-cultured stems had less defined epidermal layers, which may hinder the plant’s ability to regulate gas exchange and water transpiration effectively. The vascular bundles in the Growtek-cultured explant were notably loosely organized, with enlarged intercellular spaces and poorly lignified cell walls, particularly in the xylem and phloem tissues (Figure 4). Previous findings depicted that hyperhydric plants exhibited disrupted vascular development, leading to impaired water and nutrient transport [2,31]. In contrast, the Sigma-cultured and greenhouse-grown plants maintained more organized vascular bundles and more non-glandular trichomes in both hybrids, allowing for more efficient transport systems [2].
The leaf and stem tissue analysis significantly indicates tissue organization in hyperhydric plants. The disoriented mesophyll structure and irregular vascular bundles observed in hyperhydric plants (Figure 4C,D,I,J) are consistent with previous studies on HH in vitro, where excessive water uptake disrupts normal tissue development. Ziv [32] found HH caused cellular enlargement and irregular differentiation in plant tissues grown in nutrient-rich environments. There was no distinct layer between spongy parenchyma and palisade parenchyma for both HB1 and HB2. In contrast, both Sigma-cultured and greenhouse-grown plants (Figure 4A,B,E–H,K,L) maintain a well-organized mesophyll and vascular structure, with regular cell orientation, indicating proper growth and development. These findings align with those of [30] who observed that plants grown under controlled greenhouse conditions develop more robust cellular architecture than plants grown under in vitro hyperhydric conditions. A lower frequency of stomata per microscopic field was observed in hyperhydric plantlets compared to normal ones. Additionally, the stomata in hyperhydric plantlets were bigger in size and had a disorganized shape compared to those in normal plantlets [14,30]. The underdevelped cell differentiation observed in the stem sections of hyperhydric plants (Figure 4D,J) suggests that HH impairs vascular development, leading to inefficient nutrient and water transport within the plant. This abnormality can contribute to the overall poor performance and survival of hyperhydric plants when transferred to ex vitro conditions, as reported by [30] In addition, hyperhydric plantlet’s cells in the cortex region appeared disorganized and misshapen, whereas normal plantlets had well-arranged, compact cells with a clear shape and size in the cortex region [31]. The non-glandular trichomes were less present in the Growtek-cultured hyperhydric explants than in the Sigma-cultured and greenhouse-grown plants [2].
For the first time, 1H NMR spectra analysis was used to discover clear metabolic differences between hyperhydric and non-hyperhydric half-highbush blueberry plants (Figure 6). In the aliphatic region (2.5 ppm, 2.1 ppm) (Figure 5A), the hyperhydric plant exhibited higher peak intensities, which suggests a significant accumulation of catechin and potentially other fatty acid derivatives in the plant. These findings indicate that increased catechin production acts as a stress response to mitigate oxidative stress, characteristic of phenolic compounds. In contrast, the non-hyperhydric plants, particularly the Sigma-cultured plant, displayed lower peaks in this region, indicating a more stable metabolic state with lower fatty acid accumulation. In the sugar region (4.5 ppm and 4.0 ppm), non-hyperhydric plants, especially the hyperhydric plant, showed more intense peaks in the catechin signal area [32]. The weaker signals in greenhouse-grown non-hyperhydric plants point to less phenolic compound accumulation during metabolism. In the aromatic region, catechin signals were found in three culture conditions, but the hyperhydric plant showed a stronger peak at 5.85 ppm, while the Sigma-cultured non-hyperhydric plants had a more intense peak at 6.7 ppm. This indicates that while both plant types produce catechin, the hyperhydric plant may overproduce catechin and other aromatic compounds as a result of stress conditions, leading to higher aromatic content. The aromatic region of lingonberry leaves had stronger signals than bilberry leaves. However, the red bilberry leaves showed spectra more similar to lingonberry leaves, particularly in the aromatic region. Upon observing the HB2 hybrid (Figure 5B), the hyperhydric sample showed higher intensity peaks at all catechin signals in the aliphatic region. In contrast, non-hyperhydric plants, particularly those grown in the greenhouse, exhibited the lowest catechin level. The hyperhydric plant displayed higher catechin signal peaks in the sugar region than non-hyperhydric plants. All plant types exhibited catechin-related peaks in the aromatic region, but the hyperhydric plant also showed a stronger catechin signal. This indicates hyperhydric plants accumulate higher levels of catechin and related aromatic compounds, likely due to the stress conditions imposed by HH. Overall, greenhouse-grown plants showed low signal intensities among aliphatic, aromatic and sugar regions. This might suggest that they lacked a nutrient-rich environment compared to high concentration of both macro- and micronutrients with PGRs in in vitro conditions. In this study, the following is clearly observed:
Hyperhydric plant ∝ Catechin signal
Compared to normal plants, hyperhydric plants showed the highest peak in catechin signals in NMR.
Various untargeted metabolic compounds in the plant samples were characterized based on their signals in different ppm regions. In the NMR analysis of HB1 under three culture conditions, β-P-arbutin was present in both hyperhydric and non-hyperhydric plants, while Quercetin-3-O-glucuronide was missing in the greenhouse-grown plant but was found in both bilberry and lingonberry [15]. Chlorogenic acid showed the highest signal in the greenhouse-grown plant but was absent in the Sigma-cultured plant. 2-O-caffeoyl arbutin was detected in both the hyperhydric Growtek-cultured plant and the normal greenhouse plant. The -CH=CH- of fatty acids had the highest intensity in the hyperhydric plant compared to the others. However, β-glucose was not present in the Sigma-cultured plant. In the aliphatic region, signals from fatty acid chains (-CH2-) and the acetyl group were strongest in the greenhouse-grown plant, similar to lingonberry and bilberry. Both the greenhouse- and Growtek-cultured plants showed strong signals for CH3- of alpha-linolenic acid, as seen in lingonberry and green bilberry [15]. In the HB2 hybrid, β-P-arbutin was found in all culture conditions, but the Growtek-cultured hyperhydric plant showed the strongest signal for this compound, similar to lingonberry [15]. Chlorogenic acid and 2-O-caffeoyl arbutin were most abundant in Growtek-cultured plants. Chlorogenic acid and lipid content were the most abundant phenolic compounds in bilberry leaves. Additionally, Quercetin-3-O-glucoside and Quercetin-3-O-glucuronide were prominent in the greenhouse-grown plant, similar to lingonberry and green bilberry. Compounds like 2-O-caffeoyl arbutin, β-glucose, and β-coumaroyl were only detected in the hyperhydric plant, similar to lingonberry and green bilberry. The -CH=CH- of fatty acids was found in all three conditions, just like in lingonberry and green bilberry [15]. The greenhouse-grown plant had the strongest signals for most fatty acids and other untargeted metabolites.
5. Conclusions
HH is a significant challenge in in vitro micropropagation. This study presents the first observation of the 1H NMR analysis of blueberry plantlets for phenolic compound detection. Remarkably, hyperhydric plantlets exhibited strong peaks corresponding to catechin and other important metabolites. For the first time, leaf and stem tissue analyses in hyperhydric blueberry plants were observed and revealed underdeveloped sieve elements, loosely organized xylem and phloem tissues, and a reduced presence of non-glandular trichomes. SEM analysis highlighted disorganized mesophyll tissue and closed and disoriented stomata, hampering photosynthesis. Additionally, both palisade parenchyma and spongy parenchyma were misaligned, with the palisade layer appearing more compact.
Author Contributions
The study’s material was conceived of and organized by R.B. and S.C.D. R.B. wrote the original draft. S.C.D. conceptualized, designed, managed and supervised the study, interpreted the results, and played a role in funding acquisition, manuscript writing, reviewing and editing. S.K. assisted in compound analysis and SEM analysis. A.U.I. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by St. John’s Research and Development Centre, Agriculture and Agri-Food Canada, St. John’s, Newfoundland and Labrador, Canada A1E 6J5.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors thank lab technician Darryl Martin for his significant technical help and support and the former lab student Arindam Sikdar for his valuable suggestions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ghosh, A.; Igamberdiev, A.U.; Debnath, S.C. Thidiazuron induced somatic embryogenesis and changes of antioxidant properties in tissue cultures of half-high blueberry plants. Sci. Rep. 2018, 8, 16978. [Google Scholar] [CrossRef] [PubMed]
- Barua, R.; Kundu, S.; Majumder, S.; Marshall, H.D.; Igamberdiev, A.U.; Debnath, S.C. Exploring two bioreactor systems for micropropagation of Vaccinium membranaceum and the antioxidant enzyme profiling in tissue culture-raised plants. Plant Growth Regul. 2025, 105, 1–15. [Google Scholar] [CrossRef]
- Sikdar, A.; Sharma, U.; Barua, R.; Igamberdiev, A.U.; Debnath, S.C. Epigenomic insight of lingonberry: Health promoting traits during micropropagation. Sci. Rep. 2021, 12, 12487. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; Mcewen, J.; O’Brien, C.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G.; et al. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J. Agric. Food Chem. 1998, 46, 2686–2693. [Google Scholar] [CrossRef]
- Smith, G.J.; Thomsen, S.J.; Markham, K.R.; Andary, C.; Cardon, D. The photostabilities of naturally occurring 5-hydroxyflavones, flavonols, their glycosides and their aluminium complexes. J. Photochem. Photobiol. A Chem. 2000, 136, 87–91. [Google Scholar] [CrossRef]
- Riihinen, K.; Jaakola, L.; Kärenlampi, S.; Hohtola, A. Organ specific distribution of phenolic compounds in bilberry (Vaccinium myrtillus) and “northblue” blueberry (Vaccinium rymbosum x V. angustifolium). Food Chem. 2008, 110, 156–160. [Google Scholar] [CrossRef]
- Takeshita, M.; Ishida, Y.I.; Akamatsu, E.; Ohmori, Y.; Sudoh, M.; Oto, H.; Tsubouchi, H.; Kataoka, H. Proanthocyanidin from blueberry leaves suppresses expression of subgenomic hepatitis C virus RNA. J. Biol. Chem. 2009, 284, 21165–21176. [Google Scholar] [CrossRef]
- Skupien, K. Evaluation of chemical composition of fresh and frozen blueberry fruit (Vaccinium corymbosum L.). Acta Sci. Pol. Hortorum Cultus 2006, 5, 19–25. [Google Scholar]
- Sweeney, M.I.; Kalt, W.; MacKinnon, S.L.; Ashby, J.; Gottschall-Pass, K.T. Feeding rats diets enriched in lowbuch blueberries for six weeks decreases ischemia-induced brain damage. Nutr. Neurosci. 2002, 5, 427–431. [Google Scholar] [CrossRef]
- Neto, C.C. Cranberry and Blueberry: Evidence for protective effects against cancer and vascular diseases. Mol. Nutr. Food Res. 2007, 51, 652–664. [Google Scholar] [CrossRef]
- Martineau, L.C.; Couture, A.; Spoor, D.; Benhaddou-Andaloussi, A.; Harris, C.; Meddah, B.; Leduc, C.; Burt, A.; Vuong, T.; Mai Le, P.; et al. Anti-Diabetic properties of the canadian lowbush blueberry Vaccinium angustifolium Ait. Phytomedicine 2006, 13, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, M.A.; Dimakopoulou, A.; Linardaki, Z.I.; Cordopatis, P.; Klimis-Zacas, D.; Margarity, M.; Lamari, F.N. Effect of a polyphenol-rich wild blueberry extract on cognitive performance of mice, brain antioxidant markers and acetylcholinesterase activity. Behav. Brain Res. 2009, 198, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Li, J.; Ji, H.; An, L.; Xia, X. Hyperhydricity induced ultrastructural and physiological changes in blueberry (Vaccinium spp.). Plant Cell Tissue Organ Cult. 2018, 133, 65–76. [Google Scholar] [CrossRef]
- Olmos, E.; Hellin, E. Ultrastructural differences of hyperhydric and normal leaves from regenerated carnation plants. Sci. Hortic. 1998, 75, 91–101. [Google Scholar] [CrossRef]
- Liu, P.; Lindstedt, A.; Markkinen, N.; Sinkkonen, J.; Suomela, J.P.; Yang, B. Characterization of metabolite profiles of leaves of bilberry (Vaccinium myrtillus L.) and lingonberry (Vaccinium vitis-idaea L.). J. Agric. Food Chem. 2014, 62, 12015–12026. [Google Scholar] [CrossRef]
- Brown, E.; Gill, C.; Stewart, D.; McDougall, G. Lingonberries (Vaccinium vitis-idaea L.) and blueberries (Vaccinium crymbosum) contain discrete epicatechin anthocyanin derivatives linked by ethyl bridges. J. Berry Res. 2016, 6, 13–23. [Google Scholar] [CrossRef]
- Nierop, K.G.J.; Preston, C.M.; Kaal, J. Thermally Assisted Hydrolysis and Methylation of Purified Tannins from Plants. Anal. Chem. 2005, 77, 5604–5614. [Google Scholar] [CrossRef]
- Gao, H.; Xia, X.; An, L.; Xin, X.; Liang, Y. Reversion of hyperhydricity in Pink (Dianthus chinensis L.) plantlets by AgNO3 and its associated mechanism during in vitro culture. Plant Sci. 2017, 254, 1–11. [Google Scholar] [CrossRef]
- Picoli, E.A.T.; Paiva, E.A.S.; Xavier, A.; Aguiar, R.M.; Carolino, S.M.B.; Fari, M.G.; Otoni, W.C. Ultrastructural and biochemical aspects of normal and hyperhydric eucalypt. Int. J. Hortic. Sci. 2008, 14, 61–69. [Google Scholar] [CrossRef]
- de Almeida, M.; de Almeida, C.V.; Graner, E.M.; Brondani, G.E.; de Abreu-Tarazi, M.F. Pre-Procambial Cells Are Niches for Pluripotent and Totipotent Stem-like Cells for Organogenesis and Somatic Embryogenesis in the Peach Palm: A Histological Study. Plant Cell Rep. 2012, 31, 1495–1515. [Google Scholar] [CrossRef]
- Ivanova, M.; Van Staden, J. Effect of ammonium ions and cytokinins on hyperhydricity and multiplication rate of in vitro regenerated shoots of Aloe polyphylla. Plant Cell Tissue Organ Cult. 2008, 92, 227–231. [Google Scholar] [CrossRef]
- Williams, R.R.; Taji, A.M. Effect of temperature, gel concentration and cytokinins on vitrffication of Olearia microdisca (J.M. Black) in vitro shoot cultures. Plant Cell Tissue Organ Cult. 1991, 26, 1–6. [Google Scholar] [CrossRef]
- Modi, P.; Sinha, A.; Kothari, S.L. Floriculture and ornamental biotechnology reduction of hyperhydricity in micropropagated french marigold (Tagetes patula L.) plants by modified medium parameters. Floric. Ornam. Biotechnol. 2009, 3, 40–45. [Google Scholar]
- Yadav, M.K.; Gaur, A.K.; Garg, G.K. Development of suitable protocol to overcome hyperhydricity in carnation during micropropagation. Plant Cell Tissue Organ Cult. 2003, 72, 153–156. [Google Scholar] [CrossRef]
- Debnath, S.C. Temporary Immersion and Stationary Bioreactors for Mass Propagation of True-to-Type Highbush, Half-High, and Hybrid Blueberries (Vaccinium spp.). J. Hortic. Sci. Biotechnol. 2017, 92, 72–80. [Google Scholar] [CrossRef]
- Acharya, B.R.; Assmann, S.M. Hormone interactions in stomatal function. Plant Mol. Biol. 2009, 69, 451–462. [Google Scholar] [CrossRef]
- Mojica, F.J.M.; Díez-Villaseñor, C.; García-Martínez, J.; Soria, E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J. Mol. Evol. 2005, 60, 174–182. [Google Scholar] [CrossRef]
- Louro, R.P.; Dos Santos, A.V.; Machado, R.D. Ultrastructure of Eucalyptus grandis × Eucalyptus europhylla I. shoots cultivated in vitro in multiplication and elongation rooting media. Int. J. Plant Sci. 1999, 160, 217–227. [Google Scholar] [CrossRef]
- Jausoro, V.; Llorente, B.E.; Apóstolo, N.M. Structural differences between hyperhydric and normal in vitro shoots of Handroanthus impetiginosus (Mart. Ex DC) Mattos (Bignoniaceae). Plant Cell Tissue Organ Cult. 2010, 101, 183–191. [Google Scholar] [CrossRef]
- Kevers, C.; Franck, T.; Strasser, R.J.; Dommes, J.; Gaspar, T. Hyperhydricity of micropropagated shoots: A typically stress induced change of physiological state. Plant Cell Tissue Organ Cult. 2004, 77, 181–191. [Google Scholar] [CrossRef]
- Gantait, S.; Mahanta, M. Hyperhydricity-induced changes among in vitro regenerants of Gerbera. S. Afr. J. Bot. 2022, 149, 496–501. [Google Scholar] [CrossRef]
- Chobot, V.; Huber, C.; Trettenhahn, G.; Hadacek, F. (±)-Catechin: Chemical weapon, antioxidant, or stress regulator? J. Chem. Ecol. 2009, 35, 980–996. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).