Abstract
The global landscape is marked by climatic, socioeconomic, and demographic complexities, and enhancing food production through byproducts has emerged as a powerful strategy to address these challenges. This review aimed to analyze the potential impacts of VIUSID® agro, an amino acid–based growth promoter, on plant growth, productivity, and tolerance to salt stress. A quantitative systematic review was conducted utilizing databases such as Web of Science, Scopus, and Google Scholar. The research papers highlighted the positive effects of VIUSID® agro on growth and production, as well as on physiological and biochemical indices related to salt stress across various crops. Furthermore, this biostimulant can be administered in different doses and through various application methods. The review also examined its effects during the ex vitro acclimatization phase and in tissue culture. The results demonstrated enhanced crop growth, increased biomass accumulation, modulation of photosynthesis, improved enzymatic antioxidant defenses, and maintenance of ionic homeostasis, all contributing to superior crop performance. These findings suggest that VIUSID® agro is beneficial for a wide range of crops, with its effectiveness primarily attributed to its rich amino acid composition, which influences and modulates various physiological and biochemical processes within plant cells.
1. Introduction
Anthropogenic activity, particularly agriculture, is a major contributor to greenhouse gas emissions and significantly impacts climate change globally [1]. Approximately 40% of the Earth’s surface is dedicated to food production [2]. The intensive use of soil and its resources, in combination with high water demand, has led to negative impacts on nitrogen (N) cycles and biodiversity. Human activities have also contributed to climate change. Meanwhile, there is an urgent need to increase food production to meet the growing food demand of the world’s population [3].
Unit Nations projections estimate that the population will grow from about 7.5 billion today to over 9.7 billion in 2050, highlighting the need for sustainable and efficient food production in modern agriculture [4]. This highlights the complex relationship between human population and agriculture [5], especially the impact of climate change on the most vulnerable populations [6], which has led to a reduction in both the quality and the yield of crops [7,8,9].
Climate change is expected to impact food availability for humans and livestock owing to rising temperatures, uneven rainfall distribution, and soil salinization [10,11,12,13]. These challenges require sustainable alternatives such as bioproducts, biostimulants, and biofertilizers, which enhance plant growth and productivity while mitigating issues such as salinity and drought [14]. Biostimulants are essential in modern agriculture, as they have been shown to enhance plant growth and crop productivity [15]. Additionally, they help crops develop resistance to adverse weather conditions [16,17,18]. Nonetheless, how they confer these advantages has not been entirely clarified [19].
Biostimulants are defined as products made from biological components that enhance plant productivity through the unique properties of their constituents rather than merely through the presence of nutrients or growth regulators [20]. However, biostimulants, especially those rich in amino acids, have been developed and recognized for their positive effects on crops [21]. Likewise, VIUSID® agro (a commercial mixture of aspartic acid, arginine, glycine, and tryptophan) serves as a plant growth promoter that influences and regulates various physiological and biochemical processes within plant cells [22,23,24]. Consequently, it is a significant innovation in the modern agricultural landscape.
This review aims to analyze the effects of VIUSID® agro as a biostimulant on plant growth and agricultural productivity, while also exploring its potential role in mitigating the impacts of abiotic stress. The study focuses on two key research questions: (i) which crops have been most significantly treated with VIUSID® agro, and how have they responded in terms of morphology, physiology, and yield?; and (ii) what are the advantages and limitations of using VIUSID® agro as a biostimulant across various agricultural applications?
2. Methods
To identify and analyze the literature concerning the impacts of VIUSID® agro on agriculture, potential crops, doses, application methods, and main findings, the quantitative systematic review method was used [25]. This methodology reduces publication bias, adopts a protocol-based approach to identify potential research opportunities, and is clear and reproducible [25,26].
2.1. Literature Review
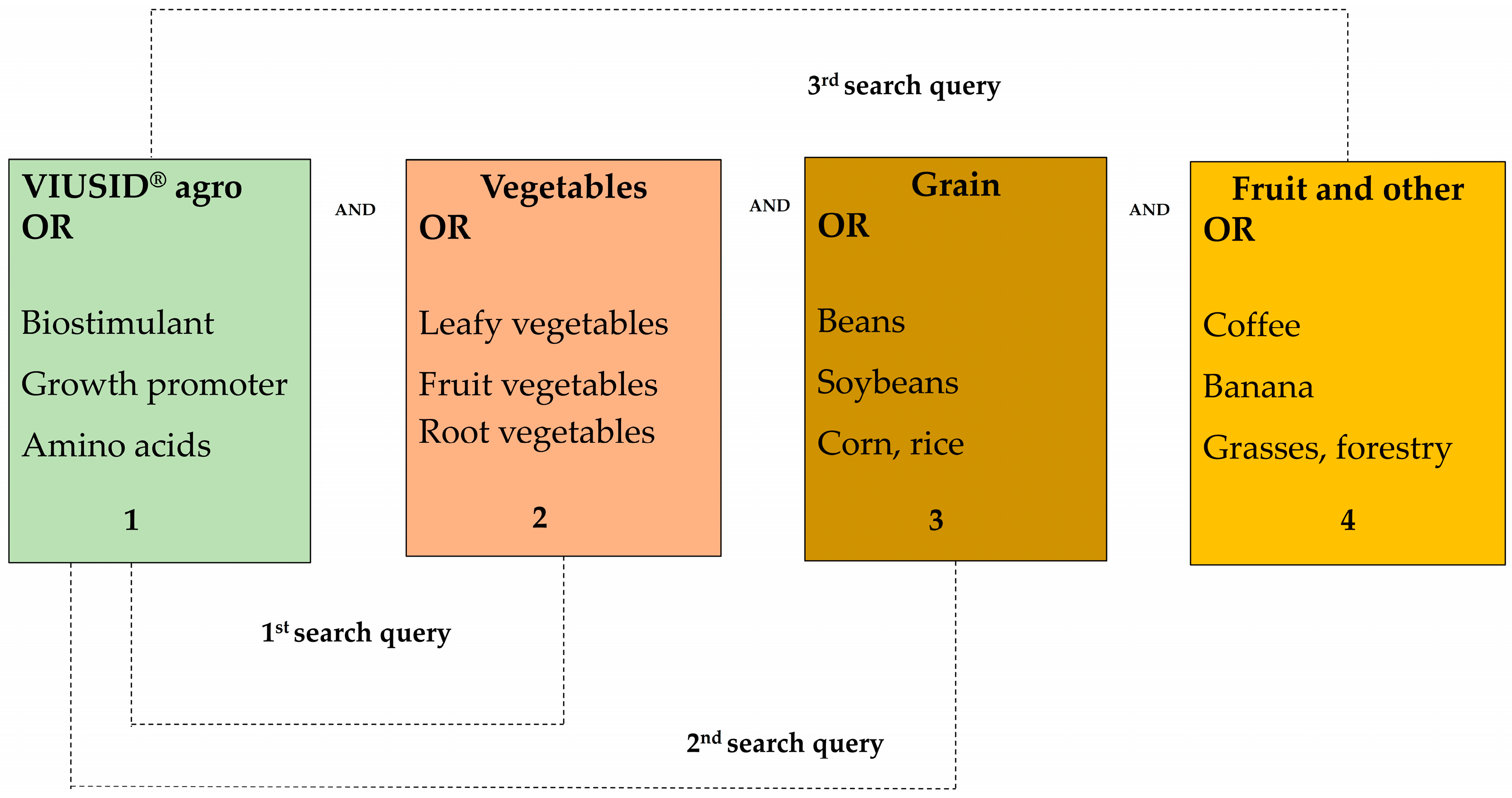
The databases consulted for this research included Web of Science, SCOPUS, Google Scholar, Springer, Frontiers, and Multidisciplinary Digital Publishing Institute (MDPI). Specific keywords were used to identify relevant literature. Search terms were distributed into four groups as follows: VIUSID® agro, vegetables, grains, and various crops (Figure 1). The search was carried out using keywords, and three combined queries were performed for each database. The first search query contained keywords for VIUSID® agro AND vegetables clusters; the second for VIUSID® agro AND grains clusters; and the third for VIUSID® agro AND fruit trees (Figure 1).
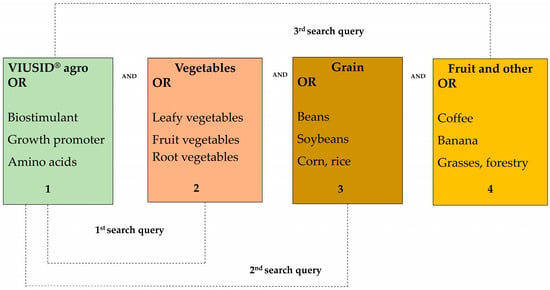
Figure 1.
Keywords used in the literature search.
2.2. Paper Selection
An initial selection of potential articles for inclusion was made based on their titles and abstracts, followed by a more thorough evaluation of those that passed this preliminary phase. Only peer-reviewed articles were selected. Reference lists of articles and reviews were used to ensure the comprehensiveness of the review and to identify additional studies. This process involved a detailed analysis of the objectives, materials and methods, results, conclusions, and future perspectives sections of each document.
The selection of articles was subject to the following conditions: (1) year of publication from 2015 to 2025; taking into account that the first report of the product’s use was in 2015; (2) original scientific research works published in peer-reviewed scientific journals, review articles, books, and book chapters; (3) thematic areas: agriculture, environmental sciences, and agricultural and forestry sciences; and (4) geographic location.
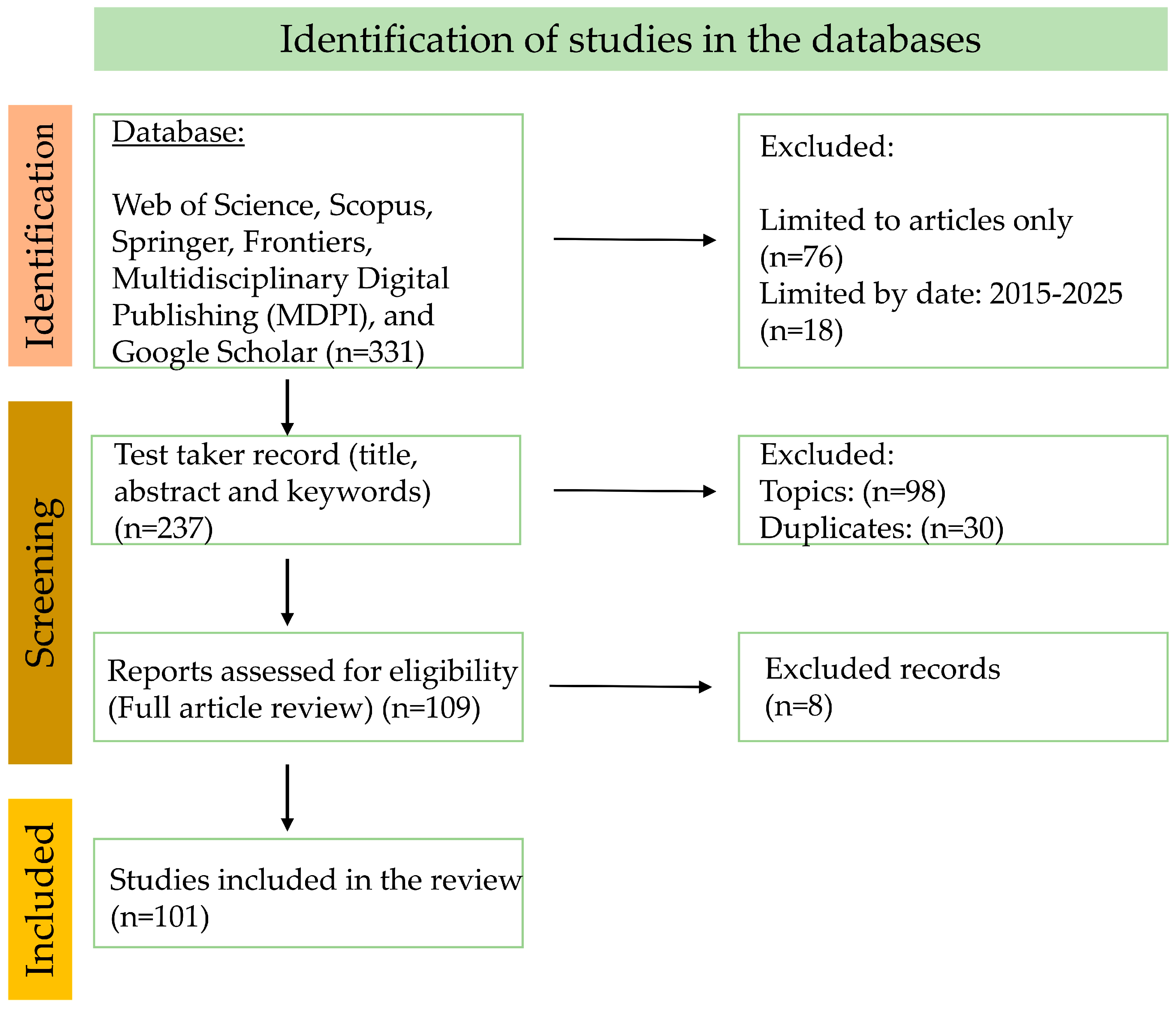
No languages were excluded, although the selected works were required to present a summary in English, and grey literature was not included. From Section 5 onwards we only papers associated with the product under analysis are included; however, in the introduction and methods, papers pertaining to contextualization and methods are cited, provided that they were related to the thematic area addressed. The total volume of information consulted was 331 documents; however, after analyzing each of them, several exclusion processes were carried out, and finally 101 were integrated into this review (Figure 2).
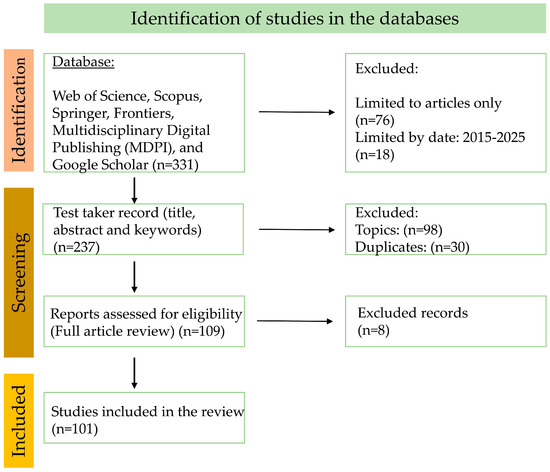
Figure 2.
Flowchart (PRISMA) of the research methodology.
2.3. Data Extraction
To control selection bias, the established inclusion and exclusion criteria were followed, and the quality of the articles was assessed by means of critical analysis using the GRADE (Grading of Recommendations Assessment Development and Evaluation) adapted System for qualitative studies and literature reviews [27]. Data were extracted from the included articles (where the product was used) according to the following variables of interest: (1) location; (2) species studied; (3) treatments evaluated (dose, factors, control, number); (4) variables under study; and (5) main findings.
2.4. Synthesis and Data Analysis
All data were synthesized using Microsoft Excel® software. In addition, VOSviewer software (version 1.6.20) was used to analyze the literature published during the last decade and to extract critical points and possible gaps or most and least explored points. The available variables in each article were compiled in a single table to compare them and create the figures for their interpretation. Furthermore, due to the division by crops and to facilitate the results analysis, the articles from Section 5 onwards were first grouped according to the type of crop (a) and type of farming system (b) and treated as discrete variables. The increase or lack of increase in yields and the variables that contributed to this were also taken into account.
3. Mechanism of Action of VIUSID® Agro Constituent Amino Acids
Amino acids act as biologically active substances and play various roles in promoting plant growth [28]. In addition, when applied exogenously, they mainly function as plant growth regulators, leading to improved crop resistance to stress, increased yield, and optimized quality of agricultural products [24]. Moreover, they have a chelating influence on macronutrients such as magnesium, which more easily regulate the uptake and transfer of all types of nutrients within the plant, as a result of the impact on the permeability of cell membranes [29].
Amino acids play a fundamental role in various enzymatic processes within plant cells, including both primary and secondary metabolism [30]. Also, they have a notable influence on numerous physiological and biochemical processes, including germination, vegetative growth, fruit ripening, and the signaling and activation of defense systems against biotic and abiotic stress [31]. Lastly, they contribute to osmoregulation, help in the inactivation of reactive oxygen species (ROS), and act as an important N source [32].
Aspartic acid plays a fundamental role in metabolic activation and the biosynthesis of several metabolites. It also acts as a precursor to the tricarboxylic acid cycle and participates in the synthesis of other essential amino acids, such as lysine, threonine, isoleucine, and methionine, in addition to being an essential component for nucleotide synthesis and for the production of nicotinamide adenine dinucleotide [33]. Likewise, aspartic acid is also involved in the synthesis of asparagine, an essential compound for N transport and storage, improves enzymatic activity, and plays a fundamental role in the mobilization of energy reserves during seed formation [29].
Exogenous application of arginine improves carbon utilization, activates essential metabolic pathways, and upregulates carotenoid- and lipid-related genes, such as those encoding arginine decarboxylase, carnitine transporter, arginine succinate synthase, nitric oxide synthase, and ornithine aminotransferase [34]. In addition, arginine favorably influences the transcription of genes involved in crucial processes such as carbon fixation and pyruvate metabolism, which are essential for energy production in plants, as occurs in the Krebs cycle [35]. Likewise, arginine is the main substance for the synthesis of osmolytes and signaling components, such as proline, nitric oxide, and polyamines, and is known to efficiently increase the activity of antioxidant enzymes [36].
Glycine is associated with plant growth optimization, nutrient uptake and translocation, photosynthetic activity, and signaling, has semi-hormonal effects, and is involved in N metabolism and osmotic regulation [34]. It is also an intermediary of phytohormone biosynthesis and acts as an antioxidant, protecting plant cells from damage caused by ROS [37,38].
Tryptophan is one of the nine essential amino acids and acts as a bioactive precursor of auxin in plants, and its exogenous application can increase the auxin content in plant tissues and directly benefit growth and productivity [39]. Moreover, this amino acid can be applied in different ways, such as by foliar spraying (it is directly metabolized as auxin), by adding it to the soil, or by immersing seeds in it, which leads to positive effects on seedlings’ initial growth [40]. It has been demonstrated that foliar application of this amino acid benefits seed germination, promotes the accumulation of dry mass in plant organs, increases plant height, increases the chlorophyll content in leaves and the dry mass of reserve organs, increases the root/aerial part ratio, and consequently increases yield more than 40%, depending on the crop [41]. Furthermore, high applied concentrations increase the content of total proteins and alkaloids, as well as the activities of gibberellin, cytokinin, and abscisic acid [40,41], and also result in beneficial abiotic stress regulation [42].
VIUSID® agro is composed of several amino acids (i.e., aspartic acid, arginine, glycine, and tryptophan) that mitigate salt stress [23]. In this context, aspartic acid promotes proline synthesis, maintaining osmotic balance. Arginine regulates the production of nitric oxide and polyamines, improving photosynthetic efficiency and reducing oxidative damage [28]. Glycine facilitates potassium (K+) uptake, thereby decreasing sodium (Na+) content and maintaining ionic homeostasis [23,34]. Together with tryptophan, these amino acids optimize osmoregulation, photosynthesis, and the antioxidant defense, improving tolerance to salt stress [43].
Recent studies have shown that exogenous application of amino acids is equally beneficial when combined or formulated into an amino acid–based growth promoter. This strategy is effective, both individually or combined, leading to significant promotion of stress tolerance and increasing plant growth [23] and performance [31]. Furthermore, foliar application of amino acid mixtures favors osmolyte overaccumulation, maintains the photosynthetic machinery, and upregulates secondary metabolites and antioxidant systems [16,44]. VIUSID® agro is composed of a mixture of amino acids, each of which plays an important role in plant metabolism, leading to its impact on growth, productivity, and salt stress remediation [22,23], which will be summarized below.
4. Bibliography and Distribution Characterization by Year and Crop Group
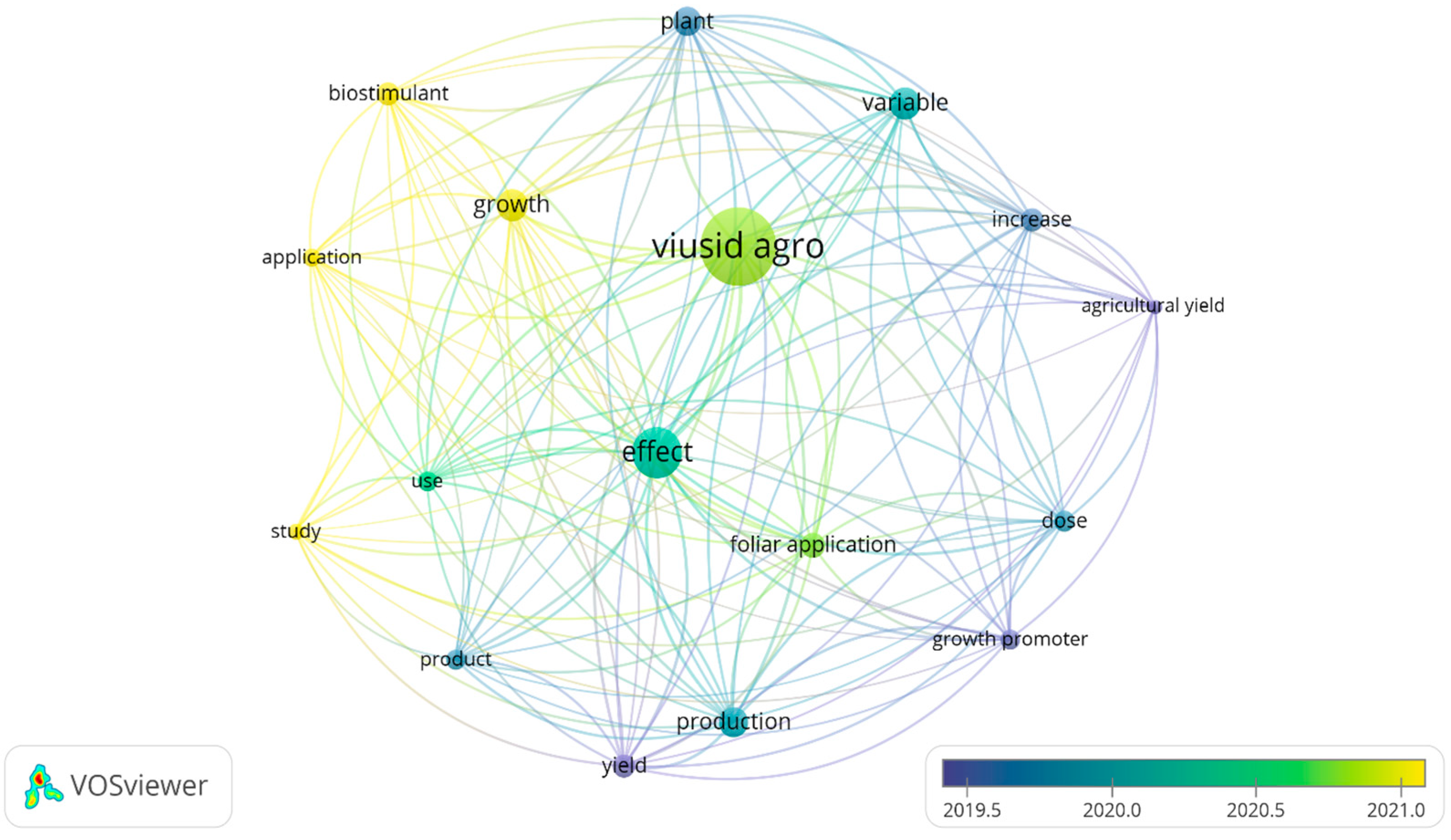
VOSviewer software was used to analyze the literature published during the last decade in the selected databases. This tool was also applied to connect the keywords associated with the following terms: “VIUSID® agro”, “growth”, “productivity” and “abiotic stress”, in order to generate a map highlighting the critical points of the research reports (Figure 3). In the map generated, 11 research hotspots were identified, including “plant”, “biostimulant”, “yield”, “treatment”, “effect”, “dose”, “growth”, “growth promoter”, “production”, “foliar application”, and “use”, all of which were closely related to “VIUSID® agro”, as well as to growth and productivity.
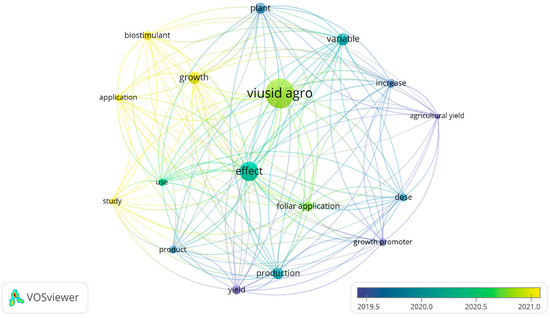
Figure 3.
Analysis of publication hotspots regarding “VIUSID® agro”, “growth”, “productivity”, and “saline stress” in the last 10 years. The size of each point represents the focal weight of each keyword in the literature, and the lines between two points indicate their coupling relationships.
Regarding the application of VIUSID® agro, most published articles focus on plant growth and productivity responses under normal and saline stress conditions. While salt stress was the only abiotic factor investigated, it was been found to be infrequent and is not directly represented on the map. Additionally, no studies explored the potential effects of VIUSID® agro on other abiotic stresses, such as drought, extreme temperatures, flooding, and heavy metals.
Furthermore, concerning the effects on plant growth and productivity associated with VIUSID® agro, key research areas that have emerged in recent years include “leaf area”, “biomass production”, “fruit production per plant”, “fruit mass”, and “yield”. These variables have also been the most extensively studied in the past two years (Table 1, Table 2 and Table 3).
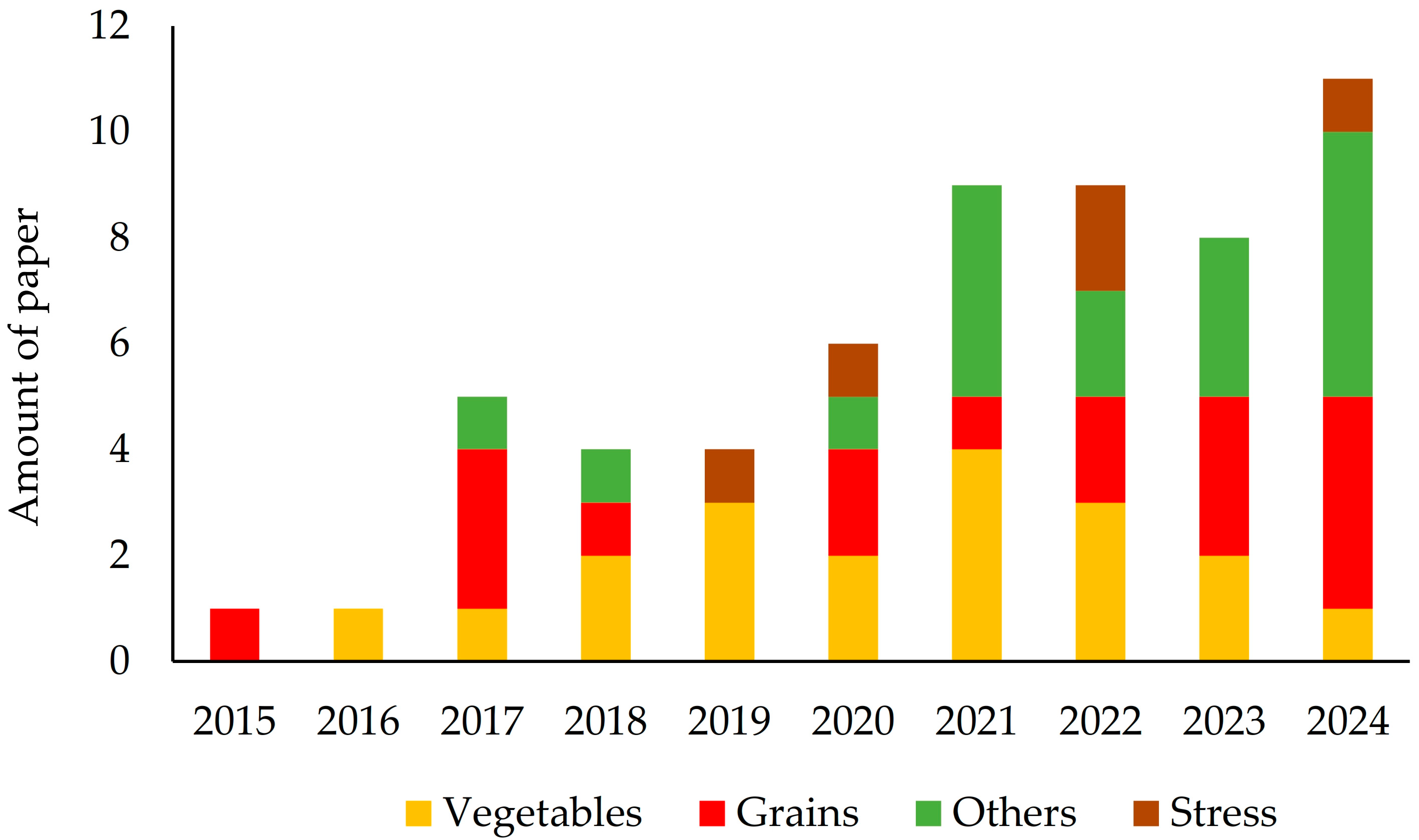
Scientific articles related to VIUSID® agro and crop groups published from 2015 to 2024 (the year each study ended) in journals indexed in the databases mentioned above were evaluated (Figure 4). A notable increase in research and dissemination of results was observed throughout this period. The first reports on the use of the product date back to 2015, focusing on grain crops, followed by studies on vegetables in 2016. From 2017 onwards, there was steady growth in the number of publications, expanding the verification of beneficial effects of VIUSID® agro on various crops, including not only vegetables and grains, but also roots and tubers, fruit trees, pastures and forages, and forest crops. Moreover, since 2019, reports have highlighted the benefits of VIUSID® agro on plant growth and productivity, as well as the anti-stress effects on crops exposed to soil salinity. Furthermore, in 2025, a research article was published that is mentioned in Table 2, but it was not included in the analysis because it used information from previous years (the study was completed before 2025) (Figure 4).
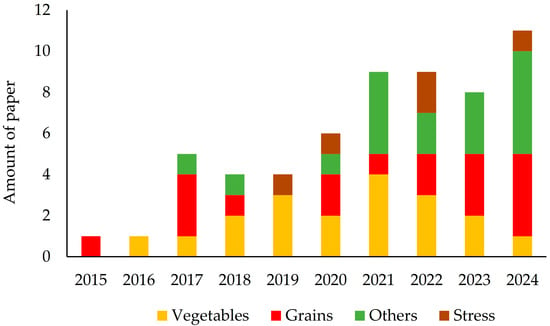
Figure 4.
Research distribution by year and crop group.
5. Effects of the Application of an Amino Acid–Based Growth Promoter on Horticultural Crops
The effects of the amino acid–based growth promoter (VIUSID® agro) on various horticultural crops have been evaluated (Table 1). The most common application method was foliar spraying, using doses varying between 0.1 and 1.0 L ha−1. The parameters most evaluated in the research were morphophysiological, growth, and production variables. In horticultural crops, notable effects were achieved, particularly in garlic (Allium sativum L.) (Table 1), which showed a 65% increase in yield with a dose of 0.25 L ha−1 compared to the control [45,46]. Another vegetable studied was radish (Raphanus sativus L.), which achieved a 50% increase in yield with the application of the 0.7 L ha−1 dose compared to the control [47]. In lettuce (Lactuca sativa L.), a 33% increase in fresh leaf biomass and a yield greater than 80% were achieved under hydroponic conditions [48,49]. On the other hand, in beets (Beta vulgaris L.) (Table 1), a stimulation of the physiological response was observed, resulting in a 34% increase in production [50]. VIUSID® agro was also used to enrich culture media with solutions between 1.0 and 2.5 mL L−1 (Table 1). The benefits of in vitro propagation were in bulb development and shelf life [46,51]. It is important to note that the variability in the applied doses is related to the species evaluated, as well as to the characteristics of the soil or substrate and to the climatic conditions or season of the year during which evaluation of the product was carried out.

Table 1.
Effect of the application of VIUSID® agro on various horticultural crops.
Table 1.
Effect of the application of VIUSID® agro on various horticultural crops.
| Crops | Mode or Interval of Application | Dose or Concentration | Morphophysiological and Production Responses | References |
|---|---|---|---|---|
| Solanum lycopersicum (L). | Foliar spraying at 7, 14, and 21 days. | 0.10, 0.20, and 0.30 L ha−1 | Productivity increased (fruits per plant, fruit mass and yield) | [52] |
| L. sativa; B. vulgaris; R. sativus | Foliar spraying every 7 days. | 0.20, 0.50, 0.70, and 1.00 L ha−1 | Plant growth as leaf number, diameter and mass of commercial root and stem, and significantly increased yield. | [47] |
| R. sativus | Foliar spraying every 7 days. | 0.20, 0.50, 0.70, and 1.00 L ha−1 | Improved morphological variables, growth rates, and performance. | [53] |
| R. sativus | Foliar spraying every 7 days. | 0.20, 0.50, 0.70, and 1.00 L ha−1 | Improved morphological variables, growth rates, and performance. | [54] |
| A. sativum | Foliar spraying every 7 days. | 0.25, 0.50, and 0.75 L ha−1 | Improved plant height, number of leaves, bulb diameter, and yield. | [45] |
| A. sativum | Culture medium. | 0.5, 1.0, 1.5, 2.0, and 2.5 mL L−1 | Beneficial effects on in vitro propagation. | [46] |
| Cucumis sativus (L.) | Foliar spraying every 7 days. | 1.0, 2.0, and 3.0 mL L−1 | Improved fruit per plant and mass, increased seed yield per area. | [55] |
| Allium cepa (L.) | Foliar spraying every 7 days. | 30 mL ha−1 | Increased yield and post-harvest life. | [56] |
| L. sativa | Foliar spraying every 7 days (starting 16 days after sowing). | 0.20, 0.40, 0.60-, and 0.80-mL L−1 | Under hydroponic conditions, plant growth, plant length, number of leaves, leaf area, and the fresh and dry mass of plants were benefited. | [48] |
| A. sativum | Culture medium enrichment. | 1.0, 1.5, 2.0, and 2.5 mL L−1 | Under in vitro conditions it stimulated the development and formation of bulbs. | [51] |
| Daucus carota (L.) | Foliar spraying every 7 days. | 0.10, 0.20, and 0.30 L ha−1 | Increased number of leaves, root diameter and length, greater mass accumulation (dry biomass), and yield. | [57] |
| A. sativum | Foliar spraying after sprouting every 7 days. | 200 mL ha−1 | Increased yields and post-harvest life. | [58] |
| B. vulgaris var. Cicla | Foliar spraying every 7 days. | 0.9, 1.2, and 1.5 mL L−1 | Beneficial effects on growth (number of leaves, stem diameter, fresh and dry mass of leaves) and yield. | [59] |
| L. sativa | Foliar spraying every 7 days (three applications). | 0.15, 0.20, and 0.25 mL L−1 | The number of commercial leaves, root length, plant mass, and yield were improved. | [49] |
| A. sativum | Foliar spraying every 15 days. | 0.25 L ha−1 | Net assimilation rate, relative growth rate, and leaf area ratio were enhanced. | [60] |
| A. sativum | Culture medium enrichment. | 2.00 and 2.50 mL L−1 | Beneficial effects on induced mutagenesis in garlic (prebasic seed can be started directly in the acclimatization phase). | [61] |
| B. vulgaris var. Cicla | Foliar spraying every 7 days. | 0.90, 1.20, and 1.50 mL L−1 | Improved growth (i.e., number of leaves, stem diameter, fresh and dry mass of leaves) and yields. | [62] |
| D. carota | Foliar spraying every 7 days. | 0.10, 0.20, and 0.30 L ha−1 | Greater number of leaves, greater root diameter and length, greater accumulation of dry biomass, and greater yield. | [63] |
| B. vulgaris | Foliar spraying every 7 days. | 0.30, 0.60, 0.90, and 1.20 mL L−1 | Improvement of morphological variables, growth rates, total chlorophyll, and yield. | [50] |
6. Effects of Applying an Amino Acid–Based Growth Promoter on Grain Production
Various grain crops respond to the application of VIUSID® agro (Table 2). The most frequent application mode was foliar spraying, with an interval of 7 days, and it was typically applied in the physiological phases of greatest nutrient demand based on crop type. In addition, beans (Phaseolus vulgaris L.) were most frequently evaluated, and the doses ranged from 0.25 to 1.0 L ha⁻1, which resulted in an increase in production between 11% and 30%, depending on the variety [64]. In addition, an evaluation was carried out without any fertilizer addition, in which foliar spraying at a 0.25 L ha⁻1 dose achieving a yield increase of 42% compared to the control [65]. Hence, this dose is the best option for bean production due to its contribution to reducing the amount applied, even with soils poor in nutrients.
Another of the most evaluated crops was maize (Zea mays L.), where the product demonstrated significant benefits in crop growth and production [66,67]. An increase in protein percentage was also observed, as well as in the levels of oils, carbohydrates, and grain yield [68], with foliar application at doses of 0.3 and 0.5 L ha−1 that did not differ from each other and significantly exceeded the control, suggesting that these doses may be considered for use in culture (Table 2).
In the case of rice (Oryza sativa L.), the product favored various variables of growth and production [69]. Likewise, yield increases of more than 20% were recorded for sesame (Sesamum indicum L.) and peanuts (Arachis hypogaea L.) with a dose of 0.25 L ha−1, which was the one with the best results compared to the rest of the treatments [22,70], and in the protein content of wheat (Triticum aestivum L.), among other important variables [71].

Table 2.
Effect of the application of VIUSID® agro on grain production.
Table 2.
Effect of the application of VIUSID® agro on grain production.
| Crops | Mode or Interval of Application | Dose or Concentration | Morphophysiological and Production Responses | References |
|---|---|---|---|---|
| P. vulgaris | Foliar spraying every 7, 14, and 21 days after sowing. | 0.10 L ha−1 | Increased growth, grains per pods, and grains per plants, as well as agricultural yield. | [72] |
| Z. mays | Foliar spraying at 5, 7, and 10 days after sowing. | 0.96, 1.44, and 2.0 L ha−1 | Improved agronomic characteristics and the relationship between grain yield and different doses. | [66] |
| P. vulgaris | Foliar spraying every 7 days during three physiological phases: growth, flowering, and fruiting. | 0.50, 0.80, and 1.0 L ha−1 | Favored plant growth and production through an increase in legumes per plant, increased the mass of grains, and favored production per plant and yield. | [73] |
| P. vulgaris | Foliar spraying every 7 days | 0.50, 0.80, and 1.00 L ha−1 | Favored the growth of plants and production through an increase un legumes per plant and increased mass of grains, production per plant, and yield. | [74] |
| Z. mays | Foliar spraying at 7, 10, and 14 days. | 0.96, 1.44, and 2.00 L ha−1 | The percentage of protein, fat, and carbohydrates and grain yield benefited. | [68] |
| P. vulgaris | Foliar spraying every 7 days. | 0.5, 0.8, and 1.0 L ha−1 | Increased plant growth, increased pods per plant, and increased production. | [64] |
| Oryza sativa (L.) | Foliar spraying at maximum tillering and rearing. | 150 mL every 0.42 ha−1 | Growth and production benefited. | [69] |
| Z. mays | Foliar spraying at 15, 30, and 45 days after germination. | 0.10, 0.30, and 0.50 L ha−1 | Stimulated growth by 24.55% and increased yield by 45.06%. | [67] |
| Glycine max (L.) | Foliar spraying weekly. | 0.40, 0.80, and 1.20 mL L−1 | Enhanced photosynthesis, transpiration, stomatal conductance, quantum and photochemical efficiency, relative water and proline content, Na+/K+ ratio, and dry mass accumulation and decreased lipid peroxidation. | [23] |
| Triticum aestivum L. | Foliar spraying combined with compost and N fertilizer. | 0.75, 1.13, and 1.50 L ha−1 | Increased grain yield and quality. Increased grain content of proteins, carbohydrates, total sugars, phosphorous, K, calcium, copper, and zinc and reduced the need for chemical fertilizers. | [71] |
| Z. mays (forage) | Foliar spraying combined with N levels. | 0.90 L ha−1 | Improved fresh biomass and yield. | [75] |
| O. sativa | Foliar spraying at maximum tillering, panicle initiation, and grain formation. | 0.70, 1.40, and 2.10 mL L−1 | Benefited chlorophyll content, plant height, and grain yield per hectare. Higher relative efficiency of the area. | [76] |
| P. vulgaris | Foliar spraying every 7, 14, and 21 days after planting. | 0.10 L ha−1 | Improved number of legumes per plant, production per plant, and agricultural yield. | [77] |
| P. vulgaris | Foliar spraying every 10 days after germination. | 0.25, 0.50, and 0.75 L ha−1 | More legumes per plant, grains per plant, grains per legume, and agricultural yield. | [78] |
| P. vulgaris | Foliar spraying at phenological phases V4, R5, and R7. | 0.50, 0.75, and 1.00 L ha−1 | Plant height and stem diameter were favored, as well as legumes per plant, grains per legume, seed mass, and yield. | [79] |
| P. vulgaris | Foliar spraying weekly, starting at 10 days after germination. | 0.25, 0.50, and 0.75 L ha−1 | Improved the number of legumes per plant, grains per plant, and agricultural yield. | [65] |
| S. indicum | Foliar spraying in vegetative stages V2–V6 and reproductive stages R6–R8. | 0.25 and 0.50 L ha−1 | Enhanced plant height, number of leaves per plant, leaf area index, flowering onset, capsules per plant, seed mass, and yield. | [22] |
| A. hypogaea | Foliar spraying weekly at phenological phases V1, V2, V3, V4, V5, and R1. | 0.25 L ha−1 | Better plant length, number of stems per plant, chlorophyll content, fruit per plant, grains per plant, and yield. | [70] |
| A. hypogaea | Foliar spraying in physiological phases demanding nutrition. | 0.25 L ha−1 | Enhanced plant growth, yield, and components. | [80] |
7. Effects of the Application of a Growth Promoter on Other Crops of Economic Interest
The growth promoter has been evaluated in various crops of economic and agricultural interest, such as sugarcane (Saccharum spp.), coffee (Coffea arabica L.), and several species of grasses and forage plants, among others (Table 3). In the case of sugarcane, the product favored the acclimatization process of the seedlings, which resulted in an increase in tillering and pigment content [81]. In addition, an improvement in seedling survival and in various morphological characteristics, as well as in other quality-related variables, was observed [82].
On the other hand, in coffee cultivation, evaluations have focused on seed germination, where significant improvements were observed in several key indicators. A 58% increase in leaf area and a 66% increase in dry biomass accumulation were achieved compared to untreated plants [83]. In addition, in vitro somatic embryogenesis obtained a 100% survival rate when using the product [84]. In the nursery phase, more vigorous seedlings were produced, which is crucial for this crop, as having quality seedlings facilitates their successful establishment in the field [85].
In crops such as tobacco (Nicotiana tabacum L.), increases in leaf area of more than 50% were observed in nursery seedlings when doses of 0.50 and 0.70 L ha−1 were applied. However, for the nursery, it is recommended to use a dose of 0.20 L ha−1, as this favors a better proportion of seedlings and increases survival during transplanting [86]. On the other hand, in acacia nurseries, it was possible to reduce the time the seedlings remained in the nursery and improve their quality compared to not using the product [87] (Table 3).

Table 3.
Effect of the application of VIUSID® agro on other crops.
Table 3.
Effect of the application of VIUSID® agro on other crops.
| Crops | Mode or Interval of Application | Dose or Concentration | Morphophysiological and Production Responses | References |
|---|---|---|---|---|
| Colocasia esculenta (L.) Schott | Foliar spraying during the acclimatization phase. | 0.50, 0.70, and 1.00 L ha−1 | Increased growth, plant height, and root length, and provided better conditions in the acclimatization phase. | [88] |
| N. tabacum | Foliar spraying every 7 days. | 0.2, 0.5, 0.7, and 1.0 L ha−1 | Seedling growth and quality increased, and growth rates benefited. | [86] |
| Saccharum spp. | Daily for the first 3 days and then weekly. | 0.50 and 0.80 mL L−1 | Increased plant survival multiplication coefficient ex vitro regardless of the planting season and improved morphophysiological variables. In the rainy season, 0.50 mL L−1 had a greater effect on plant variables in vitro. | [82] |
| Saccharum spp. | Immersion before planting the buds. | 0.80 mL L−1 | It benefited from the establishment of the sugarcane mother plant bank under semi-controlled conditions. | [89] |
| Saccharum spp. | Two foliar sprays daily the first 3 days and then once a week (ex vitro acclimatization). | 0.80 mL L−1 | The survival percentage of seedlings, height, leaf length, total chlorophyll, and dry mass of the aerial part increased. | [90] |
| Gossypium hirsutum (L.) cv. BRS 286 | Independent fertigation and in mixtures with biofertilizers. | 1 mL L−1 | Improved plant height, leaf area, number of flower buds, and bud mass. Also enhanced soil characteristics, macronutrients, cation exchange capacity, and saturation percentage. | [91] |
| Coffea arabica L. cv. Caturra rojo-884 | Foliar spraying twice daily for the first 3 days and then once daily application from day 7 to day 90 after transplantation. | 0.50 and 0.80 mL L−1 | High survival rate in the in vitro propagation protocol via somatic embryogenesis compared to control. Morphophysiological variables were significantly improved with use of the product. | [84] |
| Morus alba (L.); Cratylia argentea (Desv) O. Kuntze Tithonia diversifolia (Hemsl.) A. Gray | Immersion of the seed for 12 h. | 0.80 mL L−1 | Benefited bud sprouting and favored rooting. | [92] |
| Cucumis melo (L.) | Seed immersion and foliar applications, fertigation. | 100, 150, and 200 mL ha−1 | Increased number of leaves, stem diameter, and fresh and dry mass of the aerial part, and the rate of CO2 assimilation and fruiting increased. | [93] |
| Musa spp. | Foliar application during the first, third, and fifth weeks after transplantation. Ex vitro acclimatization. | 0.20 mL L−1 | Basal diameter (mm), height (cm), number of leaves, leaf area (cm2), radial length of roots emerging directly from the corm (cm), biomass of the leaf, and underground area improved with respect to the control treatment. | [94] |
| C. arabica | Foliar spraying monthly from the appearance of the second to the fifth pair of leaves. A total fourth applications were performed. | 0, 0.20, 0.40, 0.60, 0.80, and 1.00 mL L−1 | Increase in stem length and diameter, dry mass, quality index, and leaf area of coffee seedlings. | [85] |
| C. arabica | Seed immersion and foliar applications when seedlings grew the second and fourth pair of leaves. | 0.50 mL L−1 | Improved seed germination, seedling length, stem diameter, leaf area, total dry mass, and efficiency index. | [83] |
| Zingiber officinale Roscoe | Two foliar applications were performed daily the first 3 days and then once a week. Acclimatization ex vitro up to 45 days. | 0.5- and 0.8-mL L−1 | Significantly increased survival. In addition, after 90 days of cultivation, the highest growth and quality of the plants was achieved. | [95] |
| Saccharum spp. | Foliar spraying twice a day (9:00 am and 4:00 pm) for the first 3 days and, from 7 days, weekly application up to 45 days. | 0.50 and 0.80 mL L−1 | Increased plant height, stem diameter, total chlorophyll content, and fresh and dry mass of the plants. After 60 days of cultivation, acclimatized in vitro plants were obtained with a height of more than 15 cm. Also increased the survival of sugarcane plants by more than 20%. Tillering increased in vitro under ex vitro acclimatization conditions in both planting seasons. | [81] |
| C. arabica | Foliar spraying once a month from the appearance of the second pair of levels to the fifth pair of leaves. Two applications to the second, fourth, and sixth pair of leaves and three applications to the third and fifth pairs of leaves. | 0.60 mL L−1 | Favored morpho-agronomic characteristics and efficiency. | [96] |
| Moringa oleifera L. | Inoculation of the seeds with single and mixed bioproducts. | 1.00 mL L−1 | Increased germination speed and uniformity of seed vigor. | [97] |
| Acacia mangium Willd. | Weekly foliar spraying of seedlings (nurseries). | 1.20, 1.50, and 1.80 mL L−1 | Increased plant length and stem diameter, improved the Dickson Quality Index (DCI), improved growth rates, and reduced time in nursery. | [87] |
8. Effects of Biostimulant Application on Crops Subjected to Salinity Stress
The use of biostimulants such as VIUSID® agro has been studied to mitigate the effects of salt stress in various crops. In a study on lettuce cultivation (L. sativa) under salinity conditions, it was concluded that biostimulant application at a dose of 150 mL ha⁻1, both in soils with an electrical conductivity of 1.60 dS m⁻1 and in soils without salinity, did not significantly affect the leaf development and root volume of plants. However, the results suggest that the biostimulant may be a useful tool for promoting healthy growth under adverse conditions, indicating its potential as a mitigator of salt stress [98].
According to these findings, the application of VIUSID® agro to watermelon plants (Citrullus lanatus (Thunb.) cv. Crimson Sweet) exposed to a salinity level of 3.60 dS m⁻1 resulted in an increase in the number of leaves, as well as improvements in the fresh and dry biomass of the aerial parts, along with better dry mass distribution. Furthermore, under moderate salinity conditions (2.60 dS m⁻1), the biostimulant positively affected several growth parameters. Notably, even under low salinity conditions (0.60 dS m⁻1), a significant increase in total fresh and dry biomass was observed. These findings support the notion that the biostimulant has the potential to improve plant development in saline soils [99].
In addition, in a study on beans in sandy soils affected by combined abiotic stress (saline water, saline soil, and lack of nutrients), organic amendments were used in conjunction with VIUSID® agro to increase bean seed yield and protein content. The results showed that the combination of vegetable compost and 1.5 L ha⁻1 of foliar-applied VIUSID® agro significantly increased seed yield in cultivars Sakha-4, Sakha-1, and Giza-843 by 17.2%, 33.0%, and 19.8%, respectively, compared to the control. This study suggests that the combination of organic amendments and biostimulants may be an effective strategy for improving productivity under conditions of salt stress [100].
In a study on soybean plants under salt stress, foliar application of VIUSID® agro showed positive results. Using a factorial design (4 × 3) with different biostimulant concentrations and salinity levels (50 and 100 mmol L−1 NaCl), it was observed that the application of VIUSID® agro significantly improved plant growth, K+ accumulation, relative water content, photosynthesis, transpiration, and stomatal conductance. The highest concentration of biostimulant (1.2 mL L⁻1) proved to be the most effective, reducing Na+ accumulation and oxidative biomarkers. These findings support the idea that the amino acids present in the biostimulant can mitigate the effects of salt stress in soybeans, promoting tolerance and sustainability [23].
However, not all studies have found significant benefits with the application of VIUSID® agro. In recent research on watermelon seedlings, it was observed that the application of the biostimulant at a level of 300 mL ha⁻1 failed to mitigate the toxic effects of salinity in the substrate. Seedlings exposed to salt concentrations between 0.6 and 4.6 dS m⁻1 showed limited growth and development, suggesting that the recommended dose was not sufficient to counteract salt stress in this species. This finding highlights the need to investigate higher or alternative doses of biostimulants to improve seedling resilience under saline conditions [101].
9. Conclusions and Future Perspectives
VIUSID® agro has proven to be highly effective in various horticultural and grain crops, showing significant increases in yield and improvements in morphological variables. In garlic, the yield increased by 65%, while in radishes it increased by 50%. In lettuce, a notable increase in fresh and dry biomass was observed, especially under hydroponic conditions. In grain crops such as beans, corn, and rice, improvements were also recorded, highlighting a 42% increase in beans with a dose of 0.25 L ha⁻1, without additional fertilizers.
In addition, VIUSID® agro showed benefits in sugarcane, coffee, and tobacco, improving seedling acclimatization and nursery vigor. It is also suggested that it may be useful in increasing the productivity of basic grains, which are essential for food security. Regarding mitigation of abiotic stress, the biostimulant was effective against salt stress, improving growth under conditions of moderate salinity, although the results were less consistent with high salinity. It is important to investigate areas that have not been explored such as other stresses, gene expression, physiological and biochemical mechanisms, and variability in crop responses.
The future of foliar spraying of amino acid mixtures holds promise for enhancing sustainable agriculture. Further research is essential for a deeper understanding of mechanisms involved in abiotic stress mitigation, biotic stress resistance, improved nutrient use efficiency (including synergistic effects, chelation, and bioavailability), reduced reliance on synthetic inputs, enhanced crop quality (nutritional content and organoleptic properties), integration with other bio-stimulants (combined formulations), and a comprehensive understanding of molecular mechanisms and the specific roles of amino acids. Therefore, new research on the long-term effects of VIUSID® agro on soil health, ecosystem balance, and crop quality is needed.
Author Contributions
Conceptualization, K.P.-C. and A.C.-H.; methodology, K.P.-C., J.F.M.-R. and J.C.R.-F.; software, O.G.G.-C. and M.T.G.-G.; validation, K.P.-C., L.M.-C. and A.J.-M.; formal analysis, K.P.-C. and A.C.-H.; research, K.P.-C., A.C.-H. and J.F.M.-R.; resources, K.P.-C. and A.C.-H.; data curation, K.P.-C. and L.M.-C.; Drafting: preparation of the original draft, K.P.-C.; writing: revision and editing, K.P.-C.; visualization, O.G.G.-C. and M.T.G.-G.; supervision, K.P.-C. and A.C.-H.; project management, K.P.-C.; acquisition of financing, K.P.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the project University, urban agriculture and rural communities: an interdisciplinary approach to promote food sovereignty and gender equality (AgroFuturo), grant number: NA223SS500-035, and the APC was funded by Catalysis S.A., Spain.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank the institutional project AgroFuturo, grant number: NA223SS500-035 and the doctoral program Sustainable Agricultural and Industrial Processes (PAIS).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ROS | Reactive oxygen species |
| N | Nitrogen |
| K+ | Potassium |
| Na+ | Sodium |
References
- Filho, W.L.; Setti, A.F.F.; Azeiteiro, U.M.; Lokupitiya, E.; Donkor, F.K.; Etim, N.A.N.A.; Matandirotya, N.; Olooto, F.M.; Sharifi, A.; Nagy, G.J.; et al. An overview of the interactions between food production and climate change. Sci. Total Environ. 2022, 838, 156438. [Google Scholar] [CrossRef] [PubMed]
- Crist, E.; Mora, C.; Engelman, R. The interaction of human population, food production, and biodiversity protection. Science 2017, 356, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Majeed, A.; Muhammad, Z. Salinity: A major agricultural problem-causes, impacts on crop productivity and management strategies. In Plant Abiotic Stress Tolerance: Agronomic, Molecular and Biotechnological Approaches; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 83–99. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef]
- Wani, S.H.; Mohan, A.; Singh, G.P. Physiological, Molecular, and Genetic Perspectives of Wheat Improvement; Springer Nature: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Ayyildiz, M.; Erdal, G. The relationship between carbon dioxide emission and crop and livestock production indexes: A dynamic common correlated effects approach. Environ. Sci. Pollut. Res. 2021, 28, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Mirón, I.J.; Linares, C.; Díaz, J. The influence of climate change on food production and food safety. Environ. Res. 2023, 216, 114674. [Google Scholar] [CrossRef]
- Peña-Calzada, K.; Toledo, C.; Garciga, J.P.; Barrera-Cardoso, E.L.; Iriondo-Pérez, M.E.; Sotolongo-Hernández, E.; Scognamiglio, A. Advances and challenges of agrivoltaic in the americas: A look at its current situation. Agrofor. Syst. 2025, 99, 8. [Google Scholar] [CrossRef]
- Ajila-Celi, G.E.; Lata-Tenesaca, L.F.; Peña-Calzada, K.; de Cassia Alves, R.; da Cruz, M.C.P.; Junior, J.S.P.; Carrega, W.C.; Gratão, P.L. Exogenous ascorbic acid mitigates salt-induced damage in soybean by modulating photosynthesis, antioxidant defense, and ionic homeostasis. Acta Physiol. Plant. 2025, 47, 26. [Google Scholar] [CrossRef]
- Li, L.; Huang, Z.; Zhang, Y.; Mu, Y.; Li, Y.; Nie, L. Regulation of 2-Acetyl-1-Pyrroline (2-AP) biosynthesis and grain quality in fragrant rice under salt stress. Field Crop Res. 2025, 322, 109747. [Google Scholar] [CrossRef]
- Olivera Viciedo, D.; de Mello Prado, R.; Martinez, C.A.; Habermann, E.; Branco, R.B.F.; de Cássia Piccolo, M.; Calero Hurtado, A.; Peña Calzada, K.; Lata Tenesaca, L.F. Water stress and warming impact nutrient use efficiency of mombasa grass (Megathyrsus Maximus) in tropical conditions. J. Agron. Crop Sci. 2021, 207, 128–138. [Google Scholar] [CrossRef]
- Hurtado, A.C.; Chiconato, D.A.; Prado, R.d.M.; Sousa, G.d.S.S., Jr.; Gratão, P.L.; Felisberto, G.; Viciedo, D.O.; dos Santos, D.M.M. Different methods of silicon application attenuate salt stress in sorghum and sunflower by modifying the antioxidative defense mechanism. Ecotoxicol. Environ. Saf. 2020, 203, 110964. [Google Scholar] [CrossRef]
- Olivera-Viciedo, D.; Salas Aguilar, D.; de Mello Prado, R.; Peña Calzada, K.; Calero Hurtado, A.; de Cássia Piccolo, M.; Bomfim Soares, M.; Lizcano Toledo, R.; Alves, G.R.; Ferreira, D.; et al. Silicon-mediated adjustments in C:N:P ratios for improved beetroot yield under ammonium-induced stress. Agronomy 2024, 14, 1104. [Google Scholar] [CrossRef]
- Funes Aguilar, F. Reseña sobre el estado actual de la agroecología en Cuba. Agroecología 2017, 12, 7–18. [Google Scholar]
- Al-Karaki, G.N.; Othman, Y. Effect of foliar application of amino acid biostimulants on growth, macronutrient, total phenol contents and antioxidant activity of soilless grown lettuce cultivars. South Afr. J. Bot. 2023, 154, 225–231. [Google Scholar] [CrossRef]
- Del Buono, D. Can biostimulants be used to mitigate the effect of anthropogenic climate change on agriculture? It is time to respond. Sci. Total Environ. 2021, 751, 141763. [Google Scholar] [CrossRef]
- Mandal, S.; Anand, U.; López-Bucio, J.; Radha; Kumar, M.; Lal, M.K.; Tiwari, R.K.; Dey, A. Biostimulants and environmental stress mitigation in crops: A novel and emerging approach for agricultural sustainability under climate change. Environ. Res. 2023, 233, 116357. [Google Scholar] [CrossRef] [PubMed]
- Zamljen, T.; Medic, A.; Veberic, R.; Hudina, M.; Grohar, M.C.; Slatnar, A. Influence of hydrolyzed animal protein-based biostimulant on primary, soluble and volatile secondary metabolism of genovese and greek-type basil grown under salt stress. Sci. Hortic. 2023, 319, 112178. [Google Scholar] [CrossRef]
- Li, G.; Wei, J.; Li, C.; Fu, K.; Li, C.; Li, C. Amino acid metabolism response to post-anthesis drought stress during critical periods of elite wheat (Triticum aestivum L.) endosperm development. Environ. Exp. Bot. 2024, 218, 105577. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Priyanka, B.; Ramesh, T.; Rathika, S.; Balasubramaniam, P. Foliar application of fish amino acid and egg amino acid to improve the physiological parameters of rice. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 3005–3009. [Google Scholar] [CrossRef]
- Pérez Díaz, Y.; Calero Hurtado, A.; Peña Calzada, K.; Gutiérrez Díaz, J.L.; Rodríguez González, V. Densidades de plantas y aplicación foliar de aminoácidos incrementan el rendimiento del ajonjolí. Temas Agrar. 2024, 29, 100–112. [Google Scholar] [CrossRef]
- Peña Calzada, K.; Olivera Viciedo, D.; Habermann, E.; Calero Hurtado, A.; Lupino Gratão, P.; De Mello Prado, R.; Lata-Tenesaca, L.F.; Martinez, C.A.; Ajila Celi, G.E.; Rodríguez, J.C. Exogenous application of amino acids mitigates the deleterious effects of salt stress on soybean plants. Agronomy 2022, 12, 2014. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Liu, W.; Ji, J.; Zhang, K.; Li, H.; Feng, Y.; Xue, J.; Ji, C.; Zhang, L.; et al. Mechanisms of branched chain amino acids promoting growth and lipid accumulation in camelina sativa seedlings under drought and salt stress. Sustain. Energy Technol. Assess. 2025, 75, 104201. [Google Scholar] [CrossRef]
- Pickering, C.; Byrne, J. The benefits of publishing systematic quantitative literature reviews for PhD candidates and other early-career researchers. High. Educ. Res. Dev. 2014, 33, 534–548. [Google Scholar] [CrossRef]
- Pickering, C.; Grignon, J.; Steven, R.; Guitart, D.; Byrne, J. Publishing not perishing: How research students transition from novice to knowledgeable using systematic quantitative literature reviews. Stud. High. Educ. 2014, 40, 1756–1769. [Google Scholar] [CrossRef]
- Sanabria, A.J.; Rigau, D.; Rotaeche, R.; Selva, A.; Marzo-Castillejo, M.; Alonso-Coello, P. Sistema GRADE: Metodología para la realización de recomendaciones para la práctica clínica. Aten. Primaria 2015, 47, 48–55. [Google Scholar] [CrossRef]
- Trovato, M.; Funck, D.; Forlani, G.; Okumoto, S.; Amir, R. Editorial: Amino acids in plants: Regulation and functions in development and stress defense. Front. Plant Sci. 2021, 12, 772810. [Google Scholar] [CrossRef]
- Sadak, M.S.; Sekara, A.; Al-ashkar, I.; Habib-ur-Rahman, M.; Skalicky, M.; Brestic, M.; Kumar, A.; Sabagh, A.E.; Abdelhamid, M.T. Exogenous aspartic acid alleviates salt stress-induced decline in growth by enhancing antioxidants and compatible solutes while reducing reactive oxygen species in wheat. Front. Plant Sci. 2022, 13, 987641. [Google Scholar] [CrossRef]
- Sohail, M.; Wills, R.B.H.; Bowyer, M.C.; Pristijono, P. Multiple amino acids inhibit postharvest senescence of broccoli. Horticulturae 2021, 7, 71. [Google Scholar] [CrossRef]
- Utgés-Minguell, L.; Sierras-Serra, N.; Marín, C.; Pintó-Marijuan, M. Enhanced production by terra-sorb® symbiotic biostimulant in two model species under nitrogen stress. Plants 2025, 14, 1087. [Google Scholar] [CrossRef]
- El Sayed, S.; Bakry, A.B.; Nofal, O.A.; Horish, M.A.A. Effectiveness of biofertilizers foliar application on yield and quality traits of flax (Linum usitatissimum L.). Oil Crop Sci. 2024, 9, 91–101. [Google Scholar] [CrossRef]
- Han, M.; Zhang, C.; Suglo, P.; Sun, S.; Wang, M.; Su, T. L-aspartate: An essential metabolite for plant growth and stress acclimation. Molecules 2021, 26, 1887. [Google Scholar] [CrossRef]
- Almutairi, K.F.; Saleh, A.A.; Ali, M.M.; Sas-Paszt, L.; Abada, H.S.; Mosa, W.F.A. Growth performance of guava trees after the exogenous application of amino acids glutamic acid, arginine, and glycine. Horticulturae 2022, 8, 1110. [Google Scholar] [CrossRef]
- Acheampong, A.; Wang, R.; Elsherbiny, S.M.; Bondzie-Quaye, P.; Huang, Q. Exogenous arginine promotes the coproduction of biomass and astaxanthin under high-light conditions in Haematococcus pluvialis. Bioresour. Technol. 2024, 393, 130001. [Google Scholar] [CrossRef] [PubMed]
- Malekzadeh, P.; Hatamnia, A.A.; Tiznado-Hernández, M.E. Arginine catabolism induced by exogenous arginine treatment reduces the loss of green color rate in broccoli florets. Physiol. Mol. Plant Pathol. 2023, 124, 101973. [Google Scholar] [CrossRef]
- Zargar Shooshtari, F.; Souri, M.K.; Hasandokht, M.R.; Jari, S.K. Glycine mitigates fertilizer requirements of agricultural crops: Case study with cucumber as a high fertilizer demanding crop. Chem. Biol. Technol. Agric. 2020, 7, 19. [Google Scholar] [CrossRef]
- Noroozlo, Y.A.; Souri, M.K.; Delshad, M. Stimulation effects of foliar applied glycine and glutamine amino acids on lettuce growth. Open Agric. 2019, 4, 164–172. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, Y.; Yi, W.; Zhang, S.; Zhang, Q.; Xing, P.; Tang, X. Foliar application of phenylalanine, tryptophan, and tyrosine in fragrant rice production: Aroma, yield, grain quality, and economic return. Eur. J. Agron. 2024, 155, 127117. [Google Scholar] [CrossRef]
- Mustafa, A.; Imran, M.; Ashraf, M.; Mahmood, K. Perspectives of using L-tryptophan for Improving productivity of agricultural crops: A review. Pedosphere 2018, 28, 16–34. [Google Scholar] [CrossRef]
- Sanada, A.; Agehara, S. Characterizing root morphological responses to exogenous tryptophan in soybean (Glycine max) seedlings using a scanner-based rhizotron system. Plants 2023, 12, 186. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Z.; Kong, X.; Chen, Y.; Li, J. Exogenous tryptophan application improves cadmium tolerance and inhibits cadmium upward transport in broccoli (Brassica oleracea Var. Italica). Front. Plant Sci. 2022, 13, 969675. [Google Scholar] [CrossRef]
- Godoy, F.; Olivos-Hernández, K.; Stange, C.; Handford, M. Abiotic stress in crop species: improving tolerance by applying plant metabolites. Plants 2021, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A.; Zahra, N.; Tahir, H.; Hafeez, M.B.; Abbas, W.; Raza, A. Modulation of growth and biochemical responses in spinach (Spinacia Oleracea L.) through foliar application of some amino acids under drought conditions. South Afr. J. Bot. 2023, 158, 243–253. [Google Scholar] [CrossRef]
- Carmona, L.M.; Melendrez, J.F. Efecto de dosis de VIUSID agro en el cultivo del ajo (Allium sativum L.) en el municipio Taguasco. InfoCiencia 2019, 23, 60–70. [Google Scholar]
- Guerra, D.G.; Juan, S.; Morales, R.; Dominguez, K. VIUSID Agro®en la propagación in vitro del ajo (Allium satium L.). Rev. Agric. Trop. 2019, 5, 34–44. [Google Scholar]
- Peña Calzada, K.; Rodríguez, J.C.; Olivera, D.; Meléndrez, J.; Rodríguez, L.; García, R.; Rodríguez, L. Effects of a growth promoter on different vegetable crops. Int. J. Dev. Res. 2017, 7, 11737–11743. [Google Scholar]
- Pordeus, A.V.; Moraes, L.D.A.; Medeiros, D.D.O.; Benitez, L.C. Growth response of hydroponic Lactuca sativa L. to application of fertilizer organic VIUSID Agro®. J. Agric. Sci. 2020, 12, 268. [Google Scholar] [CrossRef]
- Pérez-Fernández, N.; Gutiérrez-Gevara, O.; Fonseca-Pérez, M. Efecto de VIUSID® agro en el cultivo de lechuga (Lactuca sativa, L.) en condiciones de organoponía. Cultiv. Trop. 2022, 43, 819–4087. [Google Scholar]
- Peña Calzada, K.; Calero Hurtado, A.; Peistrup, V.; Mühlmann, I.; Rodríguez Miranda, D.; Rodríguez Coca, L.I.; Rodríguez González, M.; Rodríguez Fernández, J.C. Respuestas fisiológicas y productivas de plantas de remolacha tratadas con una solución de aminoácidos. Temas Agrar. 2024, 29, 113–125. [Google Scholar] [CrossRef]
- Galvez-Guerra, D.; Juan, S.; Morales, R.; Vázquez, D.; Medina, A.J.; Kosky, R.G.; Pérez, P.; Calzada, K.P.; Kukurtcu, B. Somatic embryogenesis and regeneration of plants in blanco criollo garlic (Allium sativum L.) cultivar. IOSR J. Biotechnol. Biochem. 2021, 7, 14–23. [Google Scholar]
- Peña, K.; Rodríguez, J.C.; Meléndrez, J.F. El VIUSID agro una alternativa en el incremento de la producción de tomate (Solanum lycopersicum L.). Rev. Caribeña Cienc. Soc. 2016, 15, 1–10. [Google Scholar]
- Peña-Calzada, K.; Rodríguez, J.C.; Viciedo, D.O.; Hurtado, A.C.; Meléndrez, J.F.; Valdez, R.G. Efecto de dosis de VIUSID Agro® en el comportamiento morfo-fisiológico y productivo del rábano (Raphanus sativus L.). Rev. La Fac. Agrononomia 2018, 35, 293–317. [Google Scholar]
- Peña Calzada, K.; Carlos Rodríguez, J.; León, N.; Valle, C.D.; Cristo, M. Efecto de un promotor del crecimiento en características morfofisiológicas y productivas del rábano (Raphanus sativus L.). Av. Investig. Agropecu. 2018, 22, 29–45. [Google Scholar]
- Maza, N.E.; Álvarez, M.W.C.; Alvarado, C.M.R.; Hernández, G.T.; Instituto, A.B.A. Influencia de VIUSID agro en la producción de semillas de pepino (Cucumis sativus L.). Agric. Trop. 2019, 5, 1–11. [Google Scholar]
- Vazquez, D.; Galvez; Morales; Gómez. Robaina efecto del viusid Agro® en la producción y conservación postcosecha en el cultivo de la cebolla (Allium cepa L.) agricultura tropical. Rev. Agric. Trop. 2020, 6, 48–53. [Google Scholar]
- Cabrera-Julien, N.; Peña-Calzada, K. Efecto de dosis de viusid agro en el cultivo de la zanahoria (Daucus carota L.). Rev. Infociencias 2021, 25, 48–58. [Google Scholar]
- Galvez Guerra, D.; Juan, S.; Morales, R.; Domínguez Vázquez, K.; Beovides García, Y.; Gómez Kosky, R.; Posada Pérez, L.; Peña Calzada, K.; Robaina Jiménez, A.; Daniels, D.; et al. Impact of the use of viusid Agro® on the production and post-harvest conservation of garlic (Allium sativum L.). J. Agric. Vet. Sci. 2021, 14, 9–12. [Google Scholar]
- Rodríguez Coca, L.I.; Peña-Calzada, K. Efecto del VIUSID agro en el comportamiento morfofisiológico de la remolacha (Beta Vulgaris L.). Rev. Infociencia 2021, 25, 23–35. [Google Scholar]
- Rodríguez-Coca, L.I.; García González, M.T.; Rodríguez, C.M.C. Comportamiento fisiológico del ajo (Allium sativum L.) bajo el efecto de bioestimulantes de usos agrícolas. Rev. Infociencia 2022, 26, 36–48. [Google Scholar]
- Galvez Guerra, D.; Juan, S.; Morales, R.; Domínguez Vázquez, K.; Medina, A.J.; Gómez Kosky, R.; Posada Pérez, L.; Calzada, K.P.; Kukurtcu, B. Induced mutagenesis in garlic cloves (Allium sativum l.) in the cultivar “blanco criollo”. J. Biotechnol. Immunol. 2022, 4. [Google Scholar] [CrossRef]
- Peña, K.; Rodríguez, L.; Olivera-Viciedo, D.; Martínez, N.; Estrada, Y. Aplicación foliar de solución de aminoácidos beneficia respuesta morfológica y productiva de la acelga. In Soberanía Alimentaria y Desarrollo Agropecuario y Forestal Sostenible. Aportes Desde la Universidad de Sancti Spirítus José Martí Pérez; Valdivia, Y.C., Peña, C.K., Eds.; Editorial Feijóo: Sancti Spíritus, Cuba, 2023; pp. 164–175. ISBN 978-959-312-608-3. [Google Scholar]
- Julien, N.C.; Castro, M.L.; Jiménez, Y.A.; Pérez, B. Efecto de Diferentes Dosis de VIUSID Agro En La Respuesta Morfológica y Productiva de La Zanahoria. In Soberanía Alimentaria y Desarrollo Agropecuario y Forestal Sostenible: Aportes desde la Universidad de Sancti Spíritus José Marí Pérez; Valdivia, Y.C., Peña, C.K., Eds.; Editorial Feijóo: Sancti Spíritus, Cuba, 2023; pp. 176–187. ISBN 978-959-312-608-3. [Google Scholar]
- Valle, C.D. Efecto Del Bioestimulante VIUSID Agro En El Comportamiento Productivo De Cuatro Variedades De Frijol (Phaseolus Vulgaris L.); Española, E.A., Ed.; Editorial Académica Española: London, UK, 2020; p. 56. ISBN 6202810955. [Google Scholar]
- Melendrez, J.F.; Peña, K.; Rdriguez Miranda, D. Efectos del VIUSID agro en la respuesta agroporductiva del frijol sin aplicación de fertilizantes. In Soberanía Alimentaria y Desarrollo Agropecuario y Forestal Sostenible II; Valdivia, Y.C., Peña, K., Eds.; Editorial Feijóo: Sancti Spíritus, Cuba, 2024; pp. 145–157. ISBN 978-959-312-650-2. [Google Scholar]
- Mohamed, M.; Atta, M.; Abdel-lattif, H.M. Yield and agronomic traits. Biosci. Res. 2017, 14, 604–615. [Google Scholar]
- Peña, K.; Calero-Hurtado, A.; Olivera-Viciedo, D.; Rodríguez, J.C.; Fernandes, T.; García, R.; Ajila, G. Agroproductive response of Zea mayz L. with the foliar application of VIUSID Agro®. Rev. Fac. Agron. Univ. Zulia 2021, 38, 573–584. [Google Scholar] [CrossRef]
- Absy, R.; Abdel-Lattif, H.; Atta, M. Effect of growth promoter supplement on yield and grain quality of maize (Zea mays L.). Egypt. J. Agron. 2018, 40, 165–180. [Google Scholar] [CrossRef]
- Talha, I.A.; Gomma, M.A.; Nada, A.M.; Tabl, D.M. Enhancement the productivity of some rice varieties by using some growth promoter supplement. Alex. Sci. Exch. J. 2020, 41, 553–572. [Google Scholar] [CrossRef]
- González, Y.A.; Pérez Díaz, Y.; Calero Hurtado, A. Prácticas agrícolas sostenibles que benefician la productividad del maní. In Soberanía Alimentaria y Desarrollo Agropecuario y Forestal Sostenible II; Valdivia, Y.C., Peña Calzada, K., Eds.; Editorial Feijóo: Sancti Spíritus, Cuba, 2024; pp. 97–114. ISBN 978-959-312-650-2. [Google Scholar]
- Abbas, M.; Abdel-Lattif, H.; Badawy, R.; El-Wahab, M.A.; Shahba, M. Compost and biostimulants versus mineral nitrogen on productivity and grain quality of two wheat cultivars. Agriculture 2022, 12, 699. [Google Scholar] [CrossRef]
- Peña Calzada, K.; Carlos Rodríguez Fernández, J.; Sotolongo, M.S. Comportamiento productivo del frijol (Phaseolus vulgaris L.) ante la aplicación de un promotor del crecimiento activado molecularmente. Avances 2015, 17, 327–337. [Google Scholar]
- Peña Calzada, K.; Rodríguez Fernández, J.C.; Santana Sotolongo, M.; Viciedo, D.O.; Valle Expósito, C.D.; Hernández, R.D. Effects of a growth promoter on bean (Phaseolus vulgaris L.) crops in Sancti Spíritus province, Cuba. Acta Agron. 2017, 66, 360–366. [Google Scholar] [CrossRef]
- Peña, K.; Olivera, D.; León, N.; Rodríguez, J.; Lugones, Y. Effect of a growth promoter in the production behavior of bean (Phaseolus vulgaris L.). Av. En Investig. Agropecu. 2017, 21, 35–45. [Google Scholar]
- Van Nguyen, L.; Nguyen, D.A.; Dinh, H.T.; Rumanzi, M.S.; Nguyen, V.L. Effect of growth promoter VIUSID® on performance of fodder maize under different levels of nitrogen. Vegetos 2022, 35, 558–563. [Google Scholar] [CrossRef]
- Hawash, A.; Abo-yousef, M. Improvement the productivity of some rice varieties (Oryza sativa L.) by using growth regulator foliar application. Al-Azhar J. Agric. Res. 2023, 48, 83–96. [Google Scholar] [CrossRef]
- Rodríguez, M.A.; Cabrera, L.R.; Madrigal, Y.E. Respuesta productiva del frijol ante la aplicación de un promotor del crecimiento activado molecularmente. In Soberanía Alimentaria y Desarrollo Agropecuario y Forestal Sostenible. Aportes Desde la Universidad de Sancti Spirítus José Martí Pérez; Valdivia, Y.C., Kolima, P., Eds.; Editorial Feijóo: Sancti Spíritus, Cuba, 2023; pp. 199–210. [Google Scholar]
- Melendrez, J.F.; Peña Calzada, K.; Gómez, L.M.C. Efecto del VIUSID agro en la respuesta agroproductiva del frijol. In Soberanía Alimentaria y Desarrollo Agropecuario y Forestal Sostenible II; Valdivia, Y.C., Peña, K., Eds.; Editorial Feijóo: Sancti Spíritus Cuba, 2023; pp. 188–198. ISBN 978-959-312-608-3. [Google Scholar]
- Betancourt, V.M.H.; Hernández, O.H.; Chaviano, G.F. Efecto de tres dosis de VIUSID agro en el comportamiento productivo del frijol (Phasoelus vulgaris L.) variedad buenaventura. In Soberanía Alimentaria y Desarrollo Agropecuario y Forestal Sostenible II; Valdivia, Y.C., Peña Calzada, K., Eds.; Editorial Feijóo: Sancti Spíritus, Cuba, 2024; pp. 158–170. ISBN 978-959-312-650-2. [Google Scholar]
- Acevedo González, Y.; Pérez Díaz, Y.; Calero Hurtado, A.; Peña Calzada, K. Densidades de plantas y fertilizantes en la producción sostenible de maní. Rev. Fac. Cienc. Univ. Nac. Colomb. 2025, 14, 23–38. [Google Scholar] [CrossRef]
- Bernal-villegas, A.; Núñez-jaramillo, D.; Toledo-rodríguez, E.A.; Delgado-mora, I.; Gómez-kosky, R. Empleo de bioproductos en la aclimatización ex vitro de la caña de azúcar (Saccharum Spp.). Icidca Sobre Los Deriv. Caña Azúcar 2024, 58, 22–30. [Google Scholar]
- Gómez-Kosky, R.; Jaramillo, D.N.; Esquiro, C.R.; Villegas, A.B.; Calimano, M.B.; Armas, P.M.; Ferreiro, J.Á.; Pineda, E.; Kukurtcu, B.; Daniels, D.D. Effect of VIUSID Agro® and FitoMas-E® on the ex vitro acclimatization of sugarcane plants (Saccharum Spp.) cultivar C90-469. Sugar Tech 2020, 22, 42–51. [Google Scholar] [CrossRef]
- Díaz Medina, A.; Carrillo González, A.; Suárez Pérez, C. Efecto de bioproductos sobre el desarrollo de posturas de café en vivero. Rev. Mex. Cienc. Agrícolas 2023, 14, 495–505. [Google Scholar] [CrossRef]
- Posada-Pérez, L.; Barbón Rodríguez, R.; Capote Pérez, A.; Pérez Pérez, A.; Padrón Montesino, Y.; Kukurtcu, B.; Dion, D.D.; Guillermo, R.; Gómez-Kosky, R. Effect of VIUSID-agro on the conversion of somatic embryos of coffee (Coffea arabica L.) cv. red caturra rojo-884. Afr. J. Biotechnol. 2021, 20, 229–236. [Google Scholar] [CrossRef]
- Bustamante González, C.A.; Vázquez-Osorio, Y.; Fernández-Rosales, I.; Ferrás-Negrín, Y. Effects of different VIUSID Agro® concentrations on the growth of Coffea arabica L. seedlings. Agro Product. 2023, 7, 79–87. [Google Scholar] [CrossRef]
- Peña, K.; Rodríguez, J.C.; Olivera, D.; Calero, A.; Dorta, R.; Meléndrez, J.; Veloso, F.Y.; Kukurtcu, B. Effect of the growth promoter VIUSID agro on the morphophysiological and productive performance of tobacco growth (Nicotiana tabacum L.). J. Agric. Sci. Technol. B 2018, 8, 157–167. [Google Scholar] [CrossRef]
- Peña Calzada, K.; Trocones Boggiano, A.; Delgado Fernández, L.; Martínez Alonso, Y.; Martín Conesa, Y.; Calero Hurtado, A.; Carlos Rodríguez Fernández, J. Growth promoter in Acacia mangium willd. Improves quality and reduces permanence in nursery. Rev. Mex. Cienc. For. 2024, 15, 52–76. [Google Scholar] [CrossRef]
- Beovides García, Y.; Pérez, D.R.; Peña, K.C.; Diosdada Guerra, G. Efecto del VIUSID Agro® en plantas de malanga colocasia ‘INIVIT Mc-2012’ producidas in vitro durante la fase de aclimatización. Agric. Trop. 2017, 3, 57–61. [Google Scholar]
- Bernal Villegas, A.; Armas, P.M.; Jaramillo, D.N.; Rodríguez, E.A.T. Establecimiento de un banco de plantas madre de caña de azúcar en condiciones semicontroladas para la propagación in vitro. Biotecnol. Veg. 2021, 21, 53–61. [Google Scholar]
- Becerra, B.H.; Gómez-kosky, R.; Villegas, A.B.; Alejandro, E.; Rodríguez, T.; Ferreiro, J.A.; Calimano, M.B. Efecto del bioestimulante Enerplant® en la aclimatizaciónex vitro deplantas propagadasin vitro de caña de azúcar cv. C97-445. Biotecnol. Veg. 2021, 21, 119–127. [Google Scholar]
- Magna, M.; Nunes, M.; De Cassia, R.; Saboya, C.; Grande, C. Crescimento, produtividade e fertilidade do solo na cultura do algodoeiro sob o uso de biofertilizantes e adubação NPK. Rev. Agroecol. No Semiárido 2021, 5, 1–15. [Google Scholar]
- Gómez-Kosky, R.; Delgado-Mora, I.; del Carmen, H.L.M.; Toledo-Rodriguez, E.A. Desarrollo de estrategias para la propagación de especies de plantas proteicas y forrajeras. Icidca Sobre Deriv. Caña Azúcar 2022, 56, 22–29. [Google Scholar]
- Júnio, E.; Caetano, M.; Costa, C.C.; Batista, J.D.; Armando, E.; Arielly, C.; De Sousa, A.; De Medeiros, A.C.; Maracajá, P.B. Aplicação de bioestimulante e número de frutos sobre a alocação de fitomassa em meloeiro. Rev. Bras. Gestão Ambient. 2022, 16, 27–36. [Google Scholar]
- Romero, L.M.; Estrada, N.M.; Gálvez, J.R.; Concepción, O.M.; Chávez, E.; Kukurtcu, B. Effect of viusid Agro® on the growth of banana (Musa Spp.) seedlings under nursery conditions. Int. J. Agric. Res. Environ. Sci. 2023, 4, 4. [Google Scholar] [CrossRef]
- Posada-Pérez, L.; Padrón, Y.; La, M.; Gómez-Kosky, R.; Roque, B.; Rivero, L. Efecto de VIUSID-agro en la aclimatización ex vitro de zingiber officinale. Biotecnol. Veg. 2024, 24, 10–24. [Google Scholar]
- Bustamante González, C.A.; Vázquez-Osorio, Y.; Álvarez-Morales, R. Effect of the application timing of VIUSID Agro® on the growth of Coffea arabica L. seedlings. Agro Product. 2024, 12, 173–179. [Google Scholar] [CrossRef]
- Bequer, C.; Puertas, A. Germination of Moringa oleifera L. with the application of Ecomic® and two synthetic biostimulants under controlled conditions. Cuba. J. Agric. Sci. 2024, 58, 1–12. [Google Scholar]
- Sousa, C.A.; Armando, E.C.; Cavalcanti, C.C.; Caetano, E.J.; Sousa, A.T.; Sena, A.C. Acúmulo de Biomassa Em Plantas de Alface Em Funçao Da Salinidade Do Solo e Aplicação de Bioestimulante. Rev. Craibeira Agroecol. 2019, 4, 58. [Google Scholar]
- Sousa, C.A.A.; Caciana, C.; Santos, J.B.; Medeiros, A.C. Use of biostimulant in the initial development of watermelon in saline soil. Res. Soc. Dev. 2020, 9, e92996837. [Google Scholar] [CrossRef]
- Abbas, M.S.; Badawy, R.A.; Abdel-Lattif, H.M.; El-Shabrawi, H.M. Synergistic effect of organic amendments and biostimulants on faba bean grown under sandy soil conditions. Sci. Agric. 2022, 79, e20200300. [Google Scholar] [CrossRef]
- de Aquino Gomes, M.M.; Costa, C.C.; dos Santos Pereira, U.; de Sousa, M.E.; de Sousa, C.A.A.; Lopes, K.P.; Diniz, G.L.; da Silva, G.C. da Foliar biostimulant application on the growth and development of citrullus lanatus seedlings grown in salinized substrate. Cad. Pedagógico 2024, 21, e8350. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).