1. Introduction
Potatoes (
Solanum tuberosum L.) are one of the most important crops globally, serving as a fundamental source of nutrition for millions of people. The potato is native to the Andean region of South America, but numerous varieties of the genus Solanum have evolved, leading to the selection of the varieties known today [
1]. More than 2000 species of Solanum are known, of which only 160–180 produce tubers. Most of these are used for food, with
S. tuberosum being the most cultivated worldwide. Today, potatoes are grown in more than a hundred countries. According to the Food and Agriculture Organization (FAO), potatoes are the most widely grown crop after cereals (wheat, rice, and corn) and are part of the diet in almost all countries. In 2023, global potato production reached 375 million tons, growing on more than 19 million hectares. Of this production, 50.4% will come from Asia (China and India), 31.4% from Europe, 11.4% from the Americas, and 6.4% from Africa. In recent years, the potato cultivation area in Italy has been around 50,000 hectares, with an average total national production of around 1,400,000 tons per year and an average yield of around 30 tons per hectare, with the Piemonte, Emilia-Romagna, and Lombardia regions being the most productive [
2]. The number of potato varieties is impossible to define, although some authors estimate a list close to 7000. Cultivated species are well documented, including several such as
Solanum tuberosum L.,
Solanum curtilobum,
Solanum ajanhuiri, and
Solanum juzepczukii, along with
S. tuberosum,
Chilotanum, and
Andigenum. These species are characterized by different evolutionary trajectories and biogeographic distributions [
1].
This diversity within cultivated potato species reflects the broader genetic diversity of crops, which is critical for agricultural sustainability and resilience to climate change. Crop genetic diversity allows for the development of varieties that are resistant to a variety of environmental conditions. As a result, agrobiodiversity serves as an important genetic reservoir for both agriculture and humans. In the plant domain, genetic resources include wild species (plants that have not been domesticated and are used directly or indirectly), wild relatives (wild plants closely related to domesticated plants that can be used in genetic improvement programs), ecotypes (spontaneous populations that have adapted to specific environments independently of human influence), local varieties (traditional varieties that reproduce sexually or asexually and represent variable populations identifiable by local names), and improved varieties (derived from genetic improvement programs) [
3].
Local varieties, or landraces, are not subject to genetic improvement programs because they are specifically adapted to the environmental conditions and agricultural practices of specific areas, thanks to reproduction/conservation by farmers. In addition, the conservation of potato germplasm can facilitate the incorporation of traits from wild relatives into cultivated varieties to restore lost genetic diversity or to introduce new traits to improve breeding qualities. This includes the identification and introduction of desirable traits, such as disease resistance and processing quality, with the aim of identifying and transferring beneficial alleles, thereby accelerating the genetic improvement of potato [
4]. Therefore, in an era of global production and trade, the rediscovery and valorization of landraces is increasingly necessary. These landraces benefit production economies, help to prevent depopulation in agricultural regions, and contribute to their revitalization. They also help mitigate climate change caused by factors such as increased CO
2 levels and deforestation due to intensive farming practices. Furthermore, valuing agrobiodiversity reduces diet homogeneity, which is beneficial to human health, and makes agrifood production less susceptible to pathogens and environmental stresses. Landraces can play an important role in agriculture because they possess distinct characteristics that make them valuable for sustainable agriculture. Genetic, physiological, and heat stress response characterization of these landraces is essential for their conservation and future use [
5,
6].
Given that the agrobiodiversity reservoir is also tested for tolerance to abiotic stressors, we attempted to differentiate potato varieties based on their tolerance to heat stress, which is widely regarded as one of the most important abiotic stressors for plants worldwide. It is also widely acknowledged that potato crops are extremely sensitive to high temperatures. At the physiological level, photosynthetic rates are already significantly impaired at temperatures of 31/22 °C (day/night) [
7] and 35 °C [
8], with Havaux [
9] identifying 38 °C as the threshold temperature above which photosystem II function is rapidly and permanently lost. High temperatures also accelerate respiration rates [
10,
11], resulting in a significant reduction in the photosynthesis/respiration ratio, depletion of stored carbon, and decreased plant biomass. The optimal day temperature for potato photosynthesis is typically around 24 °C, and keeping the night temperature below 22 °C is critical for tuber growth [
12]. Higher nighttime temperatures inhibit tuber growth, lowering yield and quality [
13]. Given the significance of photosynthetic impairment as a primary cause of reduced growth and productivity, investigating gas exchange and chlorophyll fluorescence parameters is critical for understanding the physiological responses of potato plants to heat stress. These parameters are useful tools for assessing the impact of sublethal temperatures and developing strategies to reduce the effects of thermal stress in potato cultivation. Heat stress caused by climate change is a global issue, but its consequences are being experienced at a local level [
14], as evidenced by numerous studies on the effects of climate change in Italy on crops such as maize [
15], rice [
16], and olive trees [
15].
A small number of studies have investigated the local potato agrobiodiversity in Italy, with a particular emphasis on mountain areas. Giupponi et al. [
17] studied the Ligurian Apennines, characterizing three local varieties (Quarantina Bianca, Quarantina Prugnona, and Rubra Spes) and comparing them to commercial varieties. The results indicated good adaptability to various altitudes. To distinguish local potato accessions from commercial varieties, Di Donato et al. [
18] used ATR-FTIR spectroscopy in conjunction with morphological and molecular analyses. This method proved to be effective for distinguishing between varieties based on pulp composition. Finally, Pacifico [
19] investigated the quality of Italian mountain potatoes, discussing how altitude affects the sensory and nutritional value of tubers, as well as the environmental and socioeconomic implications of mountain farming. All manuscripts emphasized the importance of preserving and valorizing local varieties, as well as the need for additional research to fully understand the interaction of genetics, environment, and product quality.
In this study, three local potato landraces from Mount Amiata (Italy) were investigated: ‘Biancona del Faggeto’ (characterized by its elongated shape and light beige skin), ‘Quarantina delle Macchie’ (with a round shape and yellow skin, medium deep eyes, and cream-colored flesh), and ‘Rossa delle Macchie’ (with an oval shape and reddish-brown skin, shallow red eyes, and pale-yellow pulp). The unique orographic conditions of the lava dome of Mount Amiata have historically created a form of isolation that has favored the development of local species, while the ancient lava flows, rich in minerals, have contributed significantly to the fertility of the soil [
20]. The extensive investigation in the area led to the identification of only these landraces; they have been cultivated despite their lower productivity characteristics, primarily for the superior taste of the pulp and the ability to be stored and used as food for several months after harvesting without any chemical treatment or refrigeration. The three landraces were evaluated for their nutraceutical potential, such as starch concentration, antioxidant capacity, and polyphenol content, in addition to their tolerance to heat stress conditions. We designed the experimental approach with physiological and chemical analyses that were faster, more direct, and measurable, allowing us to gain a comprehensive understanding of the response of the three varieties to heat stress. Systematic measurements of physiological parameters were made during plant growth in controlled environments (Microcosmo) under heat stress conditions. The Microcosmo proved to be an invaluable tool in this study, providing a highly controlled environment that allowed for accurate and reproducible analyses of plant responses to abiotic stress while minimizing the impact of the external environment on genotypes. By utilizing these controlled systems, the study ensures a solid framework for interpreting physiological responses with greater accuracy and relevance; thus, we preferred to study the landraces under well-controlled conditions in a cutting-edge growth chamber like the Microcosmo. The results of this study will provide a deeper understanding of the unique characteristics of these landraces and their potential use for conservation and sustainable agriculture. We anticipate that our findings will help to improve food security, promote agricultural resilience in the face of climate change, and increase cultivation of these ancient landraces in a region in need of economic development.
2. Materials and Methods
2.1. Plant Materials
A long and detailed survey was first carried out in the Mount Amiata area, interviewing farmers and locals to gather evidence of the presence of local potato landraces. In two years of research, only three presumed landraces were found to be present at the local level, cultivated by three different families, preserved by the memory of the ancestors, and grown in small family gardens. Tubers of the three potato landraces were collected from different sources and planted in the same field in the Le Macchie area of Mount Amiata (4285269 N, 1151911 E) during 2023.
Figure 1 shows the temperature (
A) and precipitation (
B) recorded by the nearest weather station in Santa Fiora (4747824 N, 708815 E). The experimental design was carried out using a split-plot design with three plots. The field was 30 square meters divided into three subplots, each containing one of the landraces in an experimental unit of ten plants (three plants per square meter with a row distance of 0.9 m and a plant distance of 0.3 m). The soil was kept weed-free between the rows by two passages with a small harrow and irrigated twice during the season. All the landraces were harvested on the same day, and the nutritional analysis was performed immediately after harvesting. Five tubers from each landrace were selected and planted in a controlled environment (Microcosmo; FOS Spa, Genoa, Italy) with Vigor Plant substrate. Physiological and genetic analyses were performed on the leaves of these grown plants. Microcosmo is an innovative high-tech field simulator developed by FOS and ENEA to simulate plant growth in controlled environments, both indoors and under extreme conditions. The 5 individuals per genotype grown in Microcosmo were the same as those used for phenotypic observations, based on the guidelines of the Germplasm Bank of the Tuscany Region (
http://germoplasma.regione.toscana.it (accessed on 11 April 2025)).
2.2. Nutraceutical Analyses
For bioactive compounds extraction, 0.5 g of peel and flesh from tubers of each landrace were weighed and transferred to tubes containing 3 mL of 70% acetone; samples were homogenized using a homogenizer (Ultra-Turrax® T25-based IKA, Saint Louis, MO, USA) for 3 min. To facilitate more efficient extraction of compounds, all samples were placed in the sonicator (Elma Transsonic T 460/H, Wezikon, Switzerland) for 15 min and vortexed for 30 s. Finally, samples were centrifuged at 2000 g for 5 min, and the supernatant was collected to obtain the extract for subsequent colorimetric assays.
The FRAP method was used to measure the antioxidant capacity [
21]. The following method was applied to three technical replicates for each sample. Each test tube contained 2040 µL acetate buffer pH 3.6, 200 µL TPTZ, 200 µL 0.2 M ferric chloride, and 20 µL extract (diluted 1:20). After incubation for one hour at 37 °C, the absorbance was read at 593 nm using a spectrophotometer (Shimadzu UV-1280; Shimadzu Corporation, Kyoto, Japan). Results were expressed as mmol Fe
2+ equivalents per 100 g of fresh material.
Total polyphenols were determined by the Folin-Ciocalteau assay [
21]. Three replicates were run for each sample. Each test tube contained 5 µL extract (diluted 1:100), 3000 µL H
2O, 250 µL Folin reagent, 750 µL sodium carbonate, and 950 µL distilled H
2O. For blank preparation, the sample volume was replaced with an identical volume of distilled water. Samples were incubated in a thermostatically controlled oven at 37 °C for 30 min. After 30 min of incubation, the absorbances were read at 765 nm using a spectrophotometer (Shimadzu UV-1280). The results were expressed as mg gallic acid per 100 g fresh material.
The spectrophotometric method using Lugol’s reagent was used to quantify the starch present in the peel and pulp of the tubers [
22]. 50 mg of pulp and peel were weighed and separately placed in a tube with 2 mL of dimethyl sulfoxide (DMSO). After two minutes of homogenization, 500 μL of 8 M HCl was then added to the mixture. Samples were incubated at 60 °C for 30 min. Then, 500 μL of 8 M NaOH and 7 mL of distilled water were added. After a centrifugation at 2000 g for 8 min, 0.167 mL of supernatant was collected, to which 0.333 mL of distilled H
2O and 2.5 mL of Lugol’s reagent were added. Absorbances were read at 605 nm with a spectrophotometer (Shimadzu UV-1280).
2.3. DNA Extraction
A pool of leaves taken from 3 plants for each genotype was collected in the field and used for DNA extraction. Upon collection, the samples were frozen in liquid nitrogen and stored at −80 °C. The DNeasy Plant Mini Kit (QIAGEN, Hilden, Germany) was used for DNA extraction and purification. Briefly, leaves of each landrace were pulverized in liquid nitrogen using a mortar and pestle. Next, 100 mg of sample for each landrace was weighed and transferred to 2 mL Eppendorf tubes following the subsequent instructions of the QIAGEN protocol. DNA was quantified using the Eppendorf BIOPHOTOMER spectrophotometer (Eppendorf AG, Hamburg, Germany).
2.4. SSR Genotyping
The characterization of the genotypes of the three potato landraces was carried out using 6 primer pairs for SSR amplification, as shown in
Table 1.
2.5. Amplification by PCR and Detection of Fragments
PCR was performed in triplicate for each of the three potato varieties. The reaction was performed separately for each primer using Taq DNA Polymerase with 25 mM MgCl
2 (Thermofisher Scientific, Waltham, MA, USA), 2 μL of DNA at a concentration of 30 ng/mL, and the primers at 0.3 μM according to the maximum efficiency, in a final volume of 25 μL. The PCR (Eppendorf AG 5341 Mastercycler PCR Thermal Cycler; Eppendorf AG, Hamburg, Germany) was run using a program with the parameters shown in
Table 2. A single PCR protocol was optimized for the simultaneous amplification of the six selected SSR primers. An annealing temperature of 57 °C was determined to be optimal for all primers, based on prior temperature gradient PCR experiments.
For allelic fragment size analysis, PCR products were diluted 1:10. For each sample, 1 μL DNA and 0.5 μL standard (GeneScanTM 600 LIZTM Dye Size Standard v2.0, ThermoFisher, USA) were added to a total volume of 10 μL and brought to volume with formamide. The amplicons were then automatically sequenced using the SeqStudio Genetic Analyzer tool with SeqStudioTM Cartridge v2 (ThermoFisher, Waltham, MA, USA). Next, the amplicons were analyzed using GeneMarker
® V3.0.1 software (
https://softgenetics.com/ (accessed on 11 April 2025); SoftGenetics, State College, Centre County, PA, USA), which allows the determination of allele size. Finally, GeneAlEx version 6.5 (developed by Rod Peakall and Peter Smouse at the Australian National University in Canberra, Australia) was used to calculate genetic similarity coefficients to assess the degree of genetic relationship among the different genotypes. The phylogenetic tree was inferred using the neighbor-joining method with the MEGA7 program (
https://www.megasoftware.net/ (accessed on 11 April 2025)).
2.6. Controlled Growth in Microcosmo Simulators
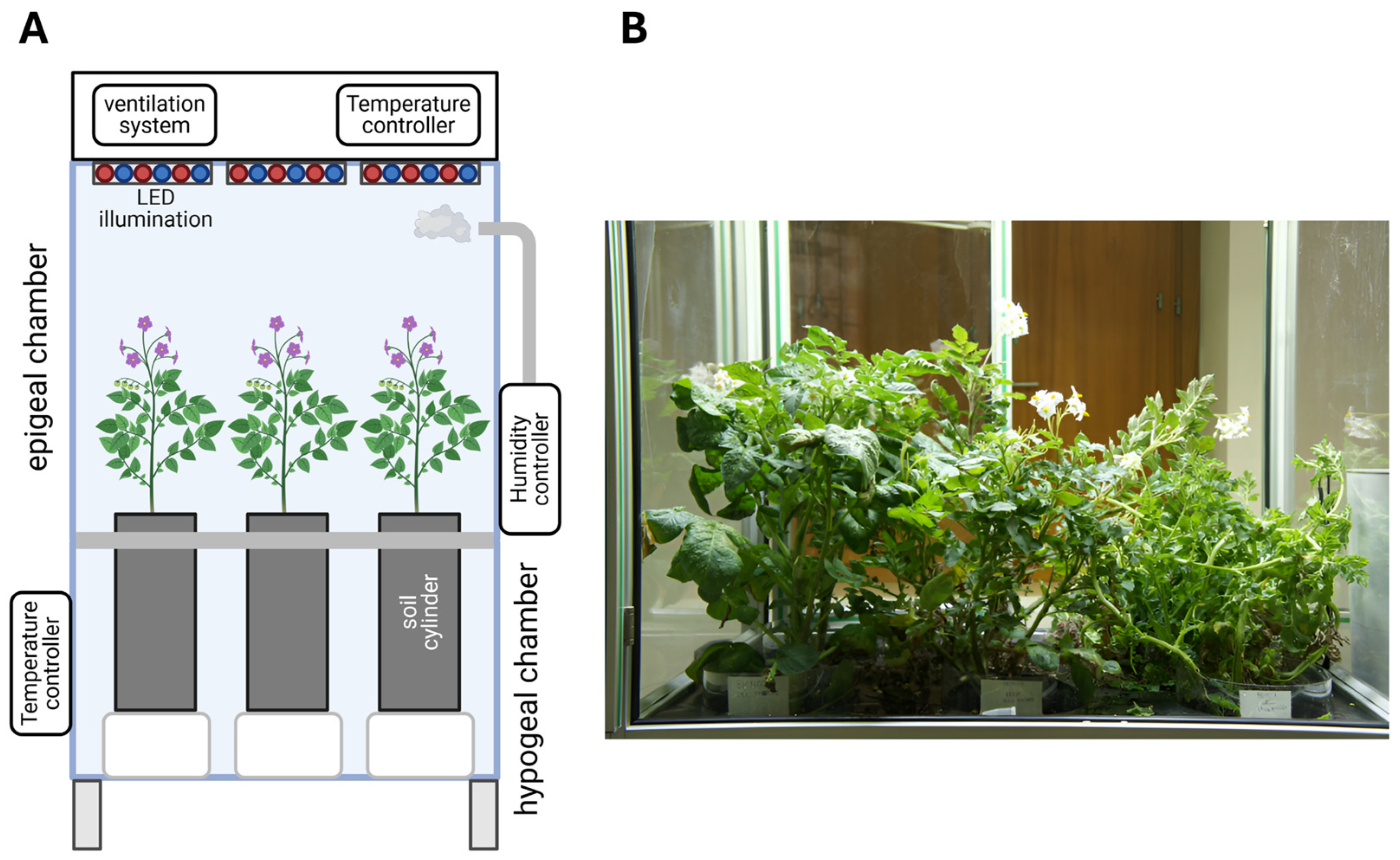
The Microcosmo (FOS S.p.A, Genova, Italy, and ENEA, Rome, Italy) is a mobile closed structure, which can be used as a field simulator for the growth of plants in a controlled environment (
https://pianogreen.com/en/microcosmo/ (accessed on 11 April 2025)). The Microcosmo allows control of environmental parameters such as epigeal temperature, light conditions/photoperiod, humidity, and hypogeal temperature [
25].
Figure 2 shows a schematic of the simulator and an image of the plants grown in Microcosmo.
The light conditions in the chamber were a PPFD of 100 µmol m
−2 s
−1 at canopy level and a red–blue–white ratio of 70:20:10 [
26]. Regarding temperature setting, eight individuals for each landrace were grown under control temperature conditions (CTRL) of 24/20 °C (day/night) in the epigeal chamber and 22/18 °C in the hypogeal chamber, which had been individually determined to be optimal for gas exchange and tuber development [
7,
11,
12,
13]. After 30 days of growth, four individuals were maintained under the same conditions (CTRL), while another four for each landrace were subjected to sublethal heat stress for 4 days (HS). During HS, the temperature in the epigeal and hypogeal chambers reached 35 °C and 24 °C, respectively, during the hottest hours, as this is the temperature at which a reduction in gas exchange should occur [
7,
8] without reaching photosystem impairment [
9]. Temperatures remained unchanged compared to CTRL for the rest of the day. Plots of temperature trends in CTRL and HS conditions are shown in
Figure 3.
2.7. Physiological Analysis
Physiological analyses of mature leaves were performed in vivo under CTRL and HS conditions simulated in Microcosmo using a portable gas exchange analyzer (LI-6800, LICOR Environmental, Lincoln, NE, USA) equipped with a leaf chamber fluorometer. For gas exchange measurements, the following conditions were set: photosynthetically active radiation at 1200 µmol m−2 s−1, reference CO2 at 400 ppm, block temperature at the corresponding Microcosmo temperature, and relative humidity at 60% inside the leaf chamber. In this study, stomatal conductance (gs, mmol H2O/m−2 s−1), net carbon assimilation (A, µmol m−2 s−1), and effective photosynthetic efficiency (phiPSII) were recorded. The water use efficiency (WUE, mm CO2/mmol H2O) was calculated from the parameters A and gs. Finally, the maximum quantum yield of PSII (Fv/Fm) was determined for dark-adapted leaves covered with aluminum foil for at least 20 min. Five measurements were taken on fully expanded mature leaves of each landrace before heat stress and on the fourth and last day of treatment at 10 am when the temperature was 30 °C and at 3 pm when the temperature had reached 35 °C for 4 h. At the same time, the same parameters were recorded on the control plants (at 25 °C).
2.8. Statistical Analysis
Data were analyzed by one-way ANOVA followed by Tukey’s post hoc test for significant differences (p < 0.05), using GraphPad Prism 10.0 (GraphPad Software, San Diego, CA, USA). Differences detected by post hoc comparisons were indicated between pulp and peel or CTRL and HS groups of the same landrace.
4. Discussion
The study investigated the phylogenetic relationships, nutraceutical composition, and physiological response to heat exposure of three potato landraces (Biancona del Faggeto, Rossa delle Macchie, and Quarantina delle Macchie), which are primarily grown in the rural area of Mount Amiata (Grosseto, Tuscany). Our goal was to understand how the distinct genetic characteristics of landraces correlate to their nutritional value and resilience to environmental stressors, thereby providing valuable insights for sustainable agriculture practices in the region.
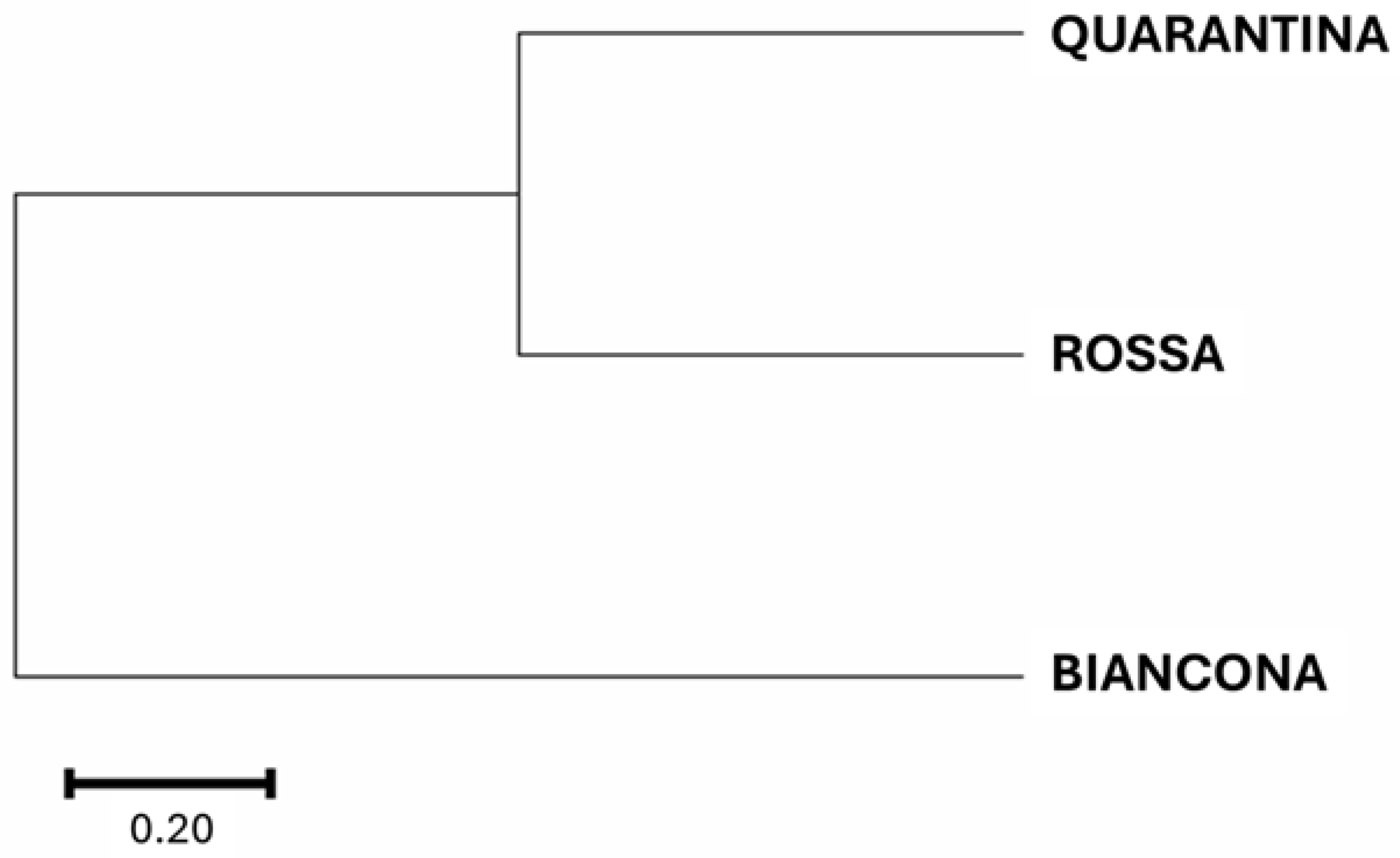
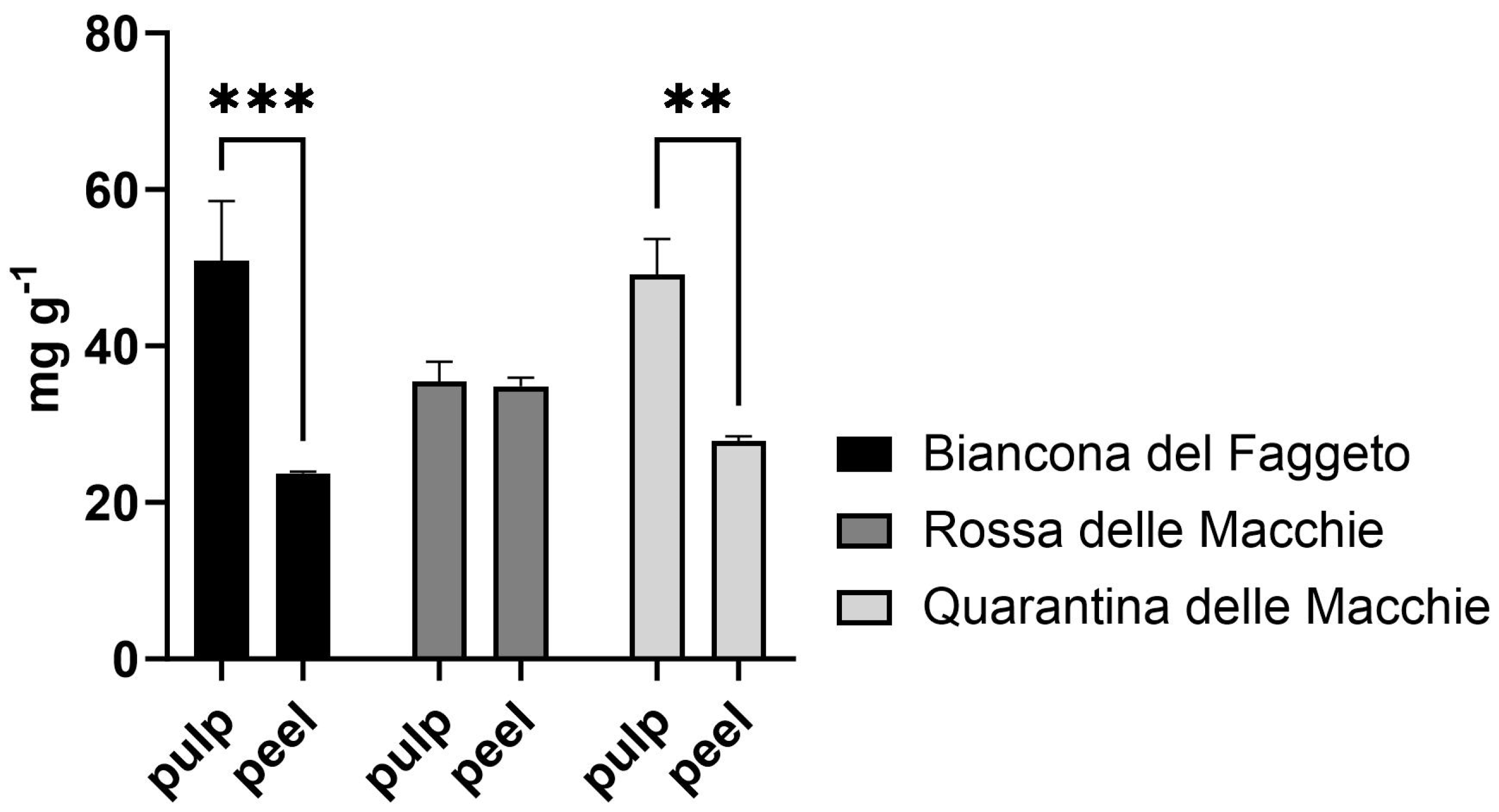
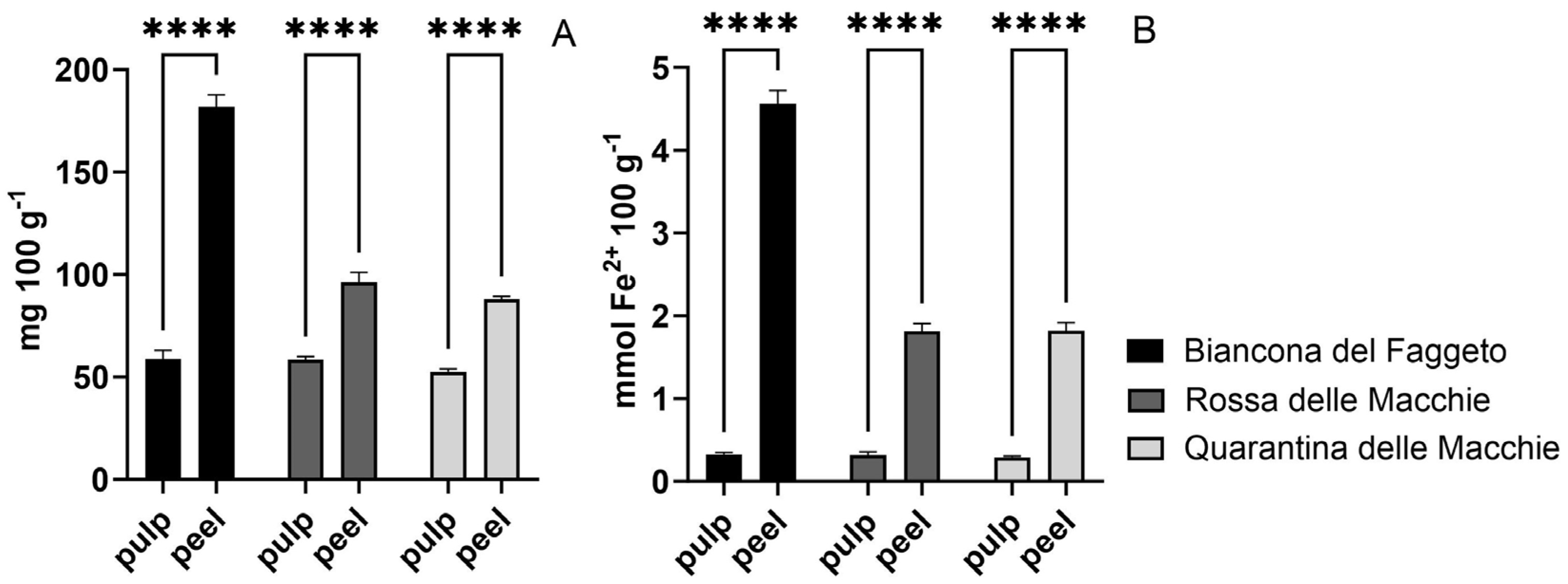
Firstly, phylogenetic analysis revealed the distinct genotype identity of these potato landraces, indicating that ‘Quarantina’ and ‘Rossa’ were more closely related than ‘Biancona’. Because the differences between potato varieties are primarily due to genetic variability, this finding supported the differences found in the nutraceutical content and physiological responses of landraces [
12,
29,
30,
31]. The genetic differences are reflected in the higher starch content of the ‘Biancona del Faggeto’ pulp and the higher total polyphenol content and antioxidant capacity of its peel in comparison to the pulp; however, the other two landraces follow the same content diversity between pulp and peel. The tissue-specific allocation revealed that the higher starch content in the pulp is consistent with the literature, identifying the pulp as the primary tissue for carbohydrate storage in potato tubers [
32]. This resource allocation pattern, which is typical of potato cultivars, suggests metabolic roles and different patterns of starch accumulation that are unique to each potato landrace. Furthermore, the higher total polyphenols and antioxidant capacity in the peel observed across all landraces is consistent with previous research. The peel is known to serve as a protective layer rich in antioxidant compounds, helping to protect against environmental stress and reactive oxygen species [
33]. Consequently, the findings suggest that ‘Biancona’ exhibits a higher concentration of antioxidant compounds, which could potentially result in greater defensive activity. However, it is essential to emphasize that a high antioxidant content does not necessarily associate with an enhanced defensive capability—this is a possibility that requires further corroboration and may or may not occur. Overall, these results underscore the distinct roles played by pulp and peel tissues in nutrient storage and protective functions, respectively. Potato genetic diversity is essential for creating stress-tolerant cultivars, especially against heat and drought [
12,
29,
30,
31]. Different potato genotypes, including wild relatives, exhibit varying stress responses, as evidenced by yield and physiological traits such as chlorophyll content and membrane stability [
12,
34]. Breeding efforts should prioritize genotypes that can withstand both heat and drought stress, reflecting real-world climate change scenarios. Wild potato relatives like
S. chacoense and
S. commersonii have proven to be valuable gene sources for stress resistance breeding [
30]. These diverse responses to abiotic stresses are driven by genetic variability, as revealed by transcriptomic and physiological analyses of stress-related molecular and biochemical reactions. For example, different cultivars exhibit different expression patterns of stress-related genes, such as the histone modifiers StPRMT and StHDMA [
35], microRNAs [
31,
36], and heat shock proteins [
12,
37]. Aside from genetic mechanisms of diversification, geographical differentiation of potato varieties must be considered. Studies in South Africa, Ethiopia, and Peru have shown that landraces adapt to specific environmental conditions, giving them advantages in drought or heat tolerance. These adaptations are critical for ensuring potato crop survival and productivity in harsh climates, ultimately contributing to food security and agricultural sustainability [
36,
38]. Statistical methods such as restricted maximum likelihood (REML)/best linear unbiased predicted (BLUP), relative performance of predicted genotypic values (RPGV), harmonic mean of relative performance of genotypic values (HMRPGV) and harmonic mean of genotypic values (HMGV) help to identify and select superior, stable and adaptable genotypes for breeding programs [
30]. These tools allow breeders to make informed decisions based on the performance and genetic potential of various genotypes, ultimately leading to the development of more resilient and high-yielding crop varieties, making agriculture more adaptable to changing environments.
Regarding high temperature exposure and physiological responses, the main results showed a reduced stomatal conductance and, possibly, a reduction in CO
2 uptake, which is consistent with the extensive literature showing that plants often close their stomata to limit water loss during heat stress, thereby also limiting CO
2 uptake for photosynthesis [
39]. These findings are consistent with the well-established link between stomatal closure and decreased CO
2 assimilation, which is a common occurrence under various abiotic stresses. In response to stress, plants clearly prioritize water conservation over carbon uptake, which can have a significant impact on overall plant growth and productivity [
40]. These effects were more pronounced in the landrace ‘Quarantina delle Macchie’, making it the most vulnerable. However, not all parameters were affected by temperature. The consistent and stable values of both actual and maximum quantum yield of photosystem II suggest that temperature did not affect photosystem II functionality, as damage typically occurs under more extreme or prolonged conditions [
30]. The observed PSII stability suggests that the primary limitation to photosynthesis in this study was stomatal conductance and CO
2 diffusion, rather than light capture. This is consistent with previous research highlighting how stomatal closure creates a bottleneck for carbon assimilation [
40]. It should be noted that the temperature of 35 °C is below the critical threshold identified for providing biochemical impairments in potato leaves, which was found to be 38 °C [
9].
The agreement between the phylogenetic tree and the observed physiological responses supports the idea that evolutionary history influences a plant’s ability to cope with environmental stress, as discussed in Koornneef et al. [
41]. However, it is important to note that physiological responses to heat stress are complex and can be influenced by a variety of genes. The phylogenetic tree is based on a small number of genetic markers, which may not include all relevant genetic variation. As a result, the phylogenetic tree and the physiological responses to heat stress in these potato landraces are somewhat comparable. The genetically close landraces (Quarantina and Rossa) showed more similar reductions in CO
2 uptake and stomatal conductance, implying more conservative water management, whereas the more genetically distinct ‘Biancona’, which was less affected by the stress, may have evolved a better photosynthesis maintenance mechanism. This suggests that genetic background influences the various responses to heat stress. It is also possible that similar responses are the result of different processes. For example, Quarantina and Rossa may share a common genetic pathway that leads to their similar responses, while Biancona may have developed a unique mechanism to cope with heat stress.