Insight into the Sulforaphane Content and Glucosinolate Profile of Broccoli Stems After Heat Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials and Treatment
2.3. Determination of Sulforaphane Content
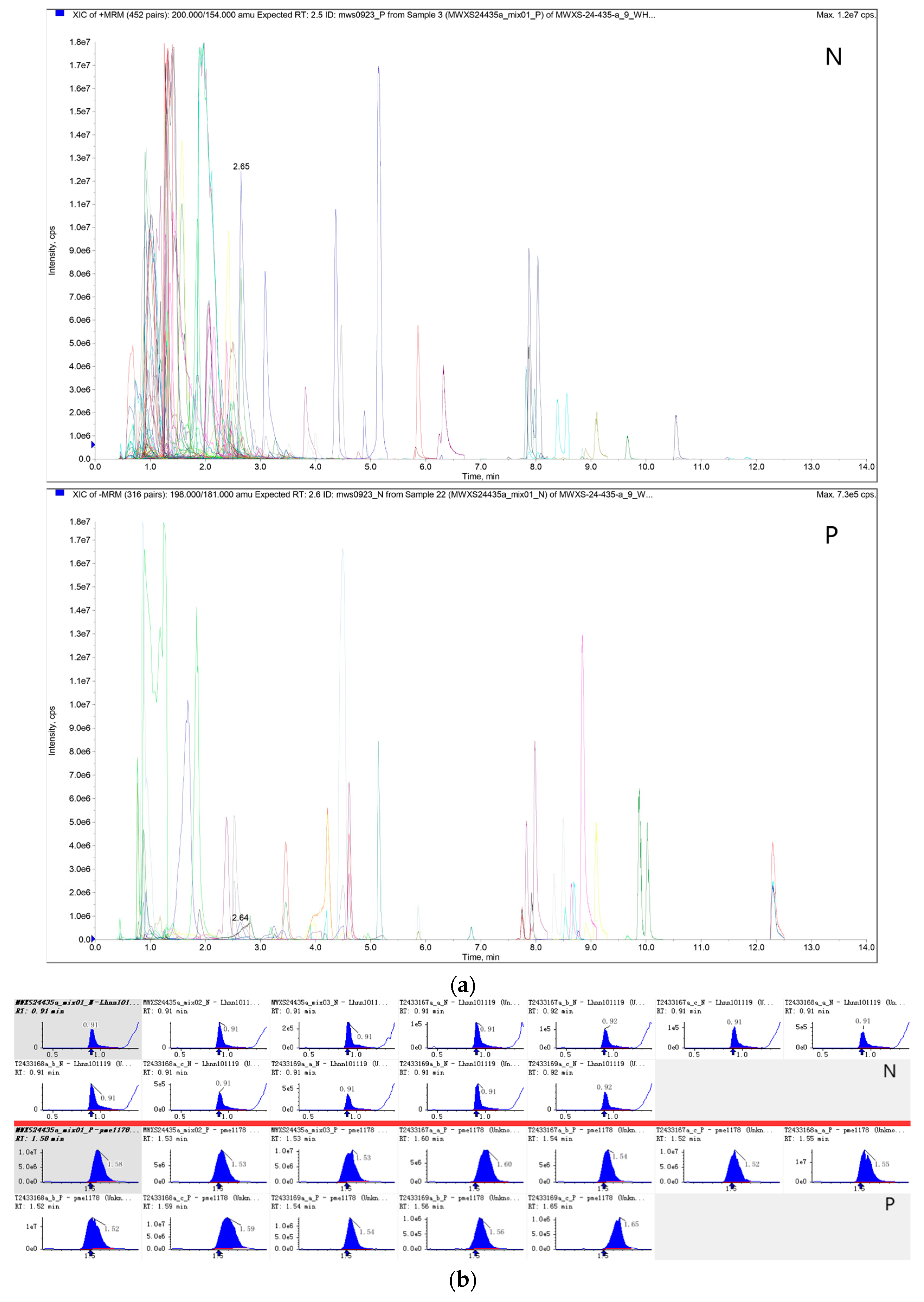
2.4. Identification of GSL Using UPLC-MS/MS
2.5. Data Analysis
3. Results and Discussion
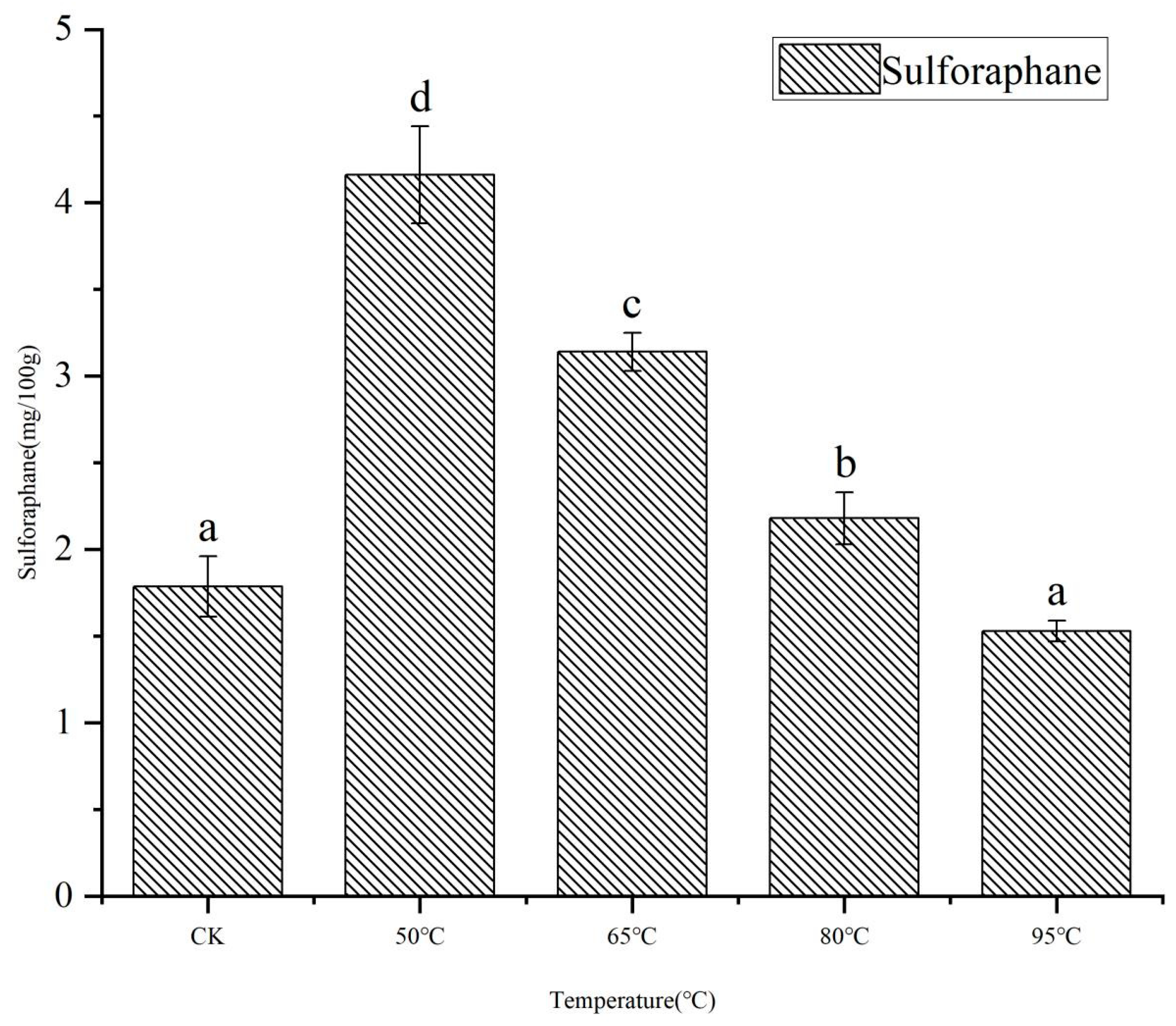
3.1. Content of Sulforaphane
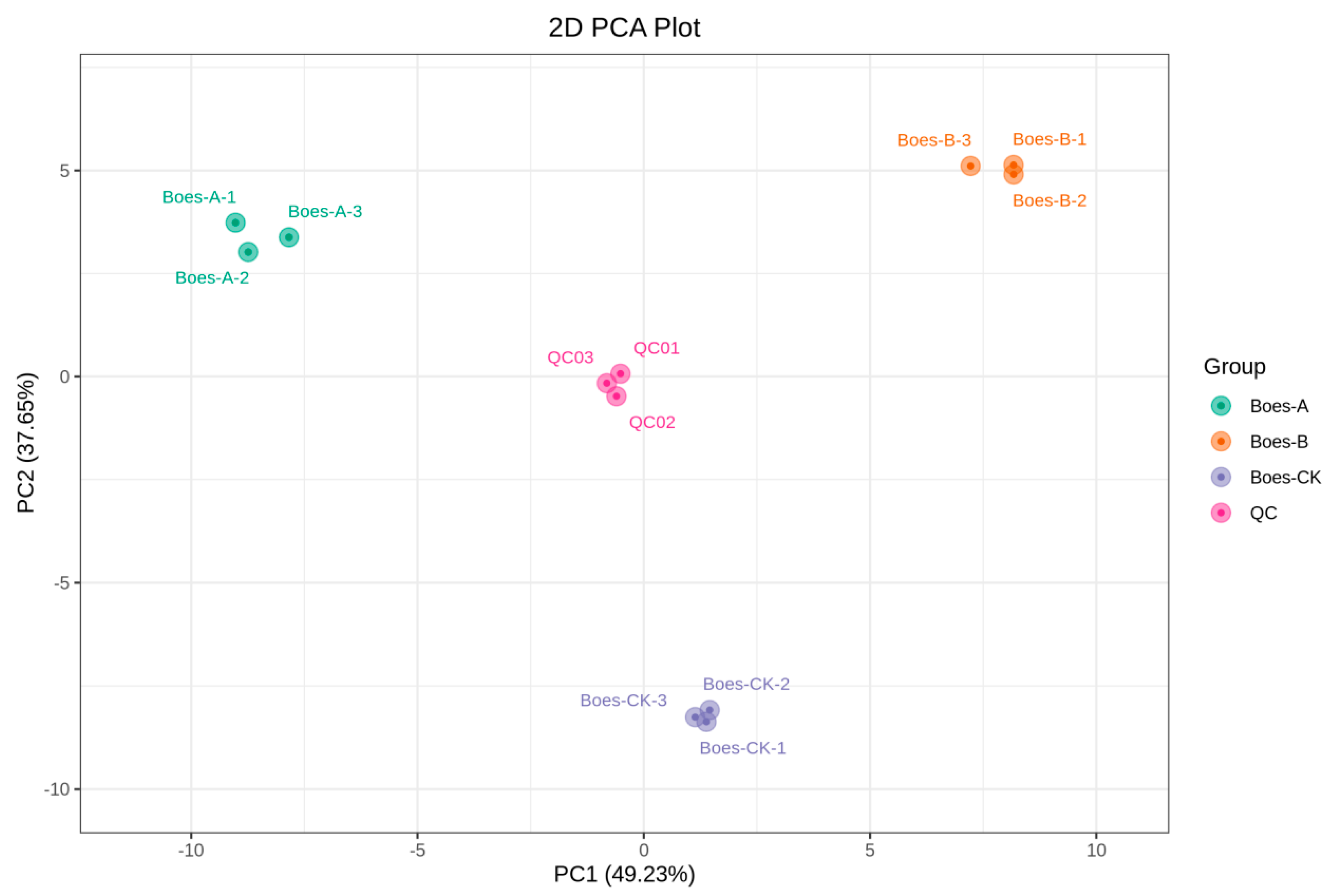
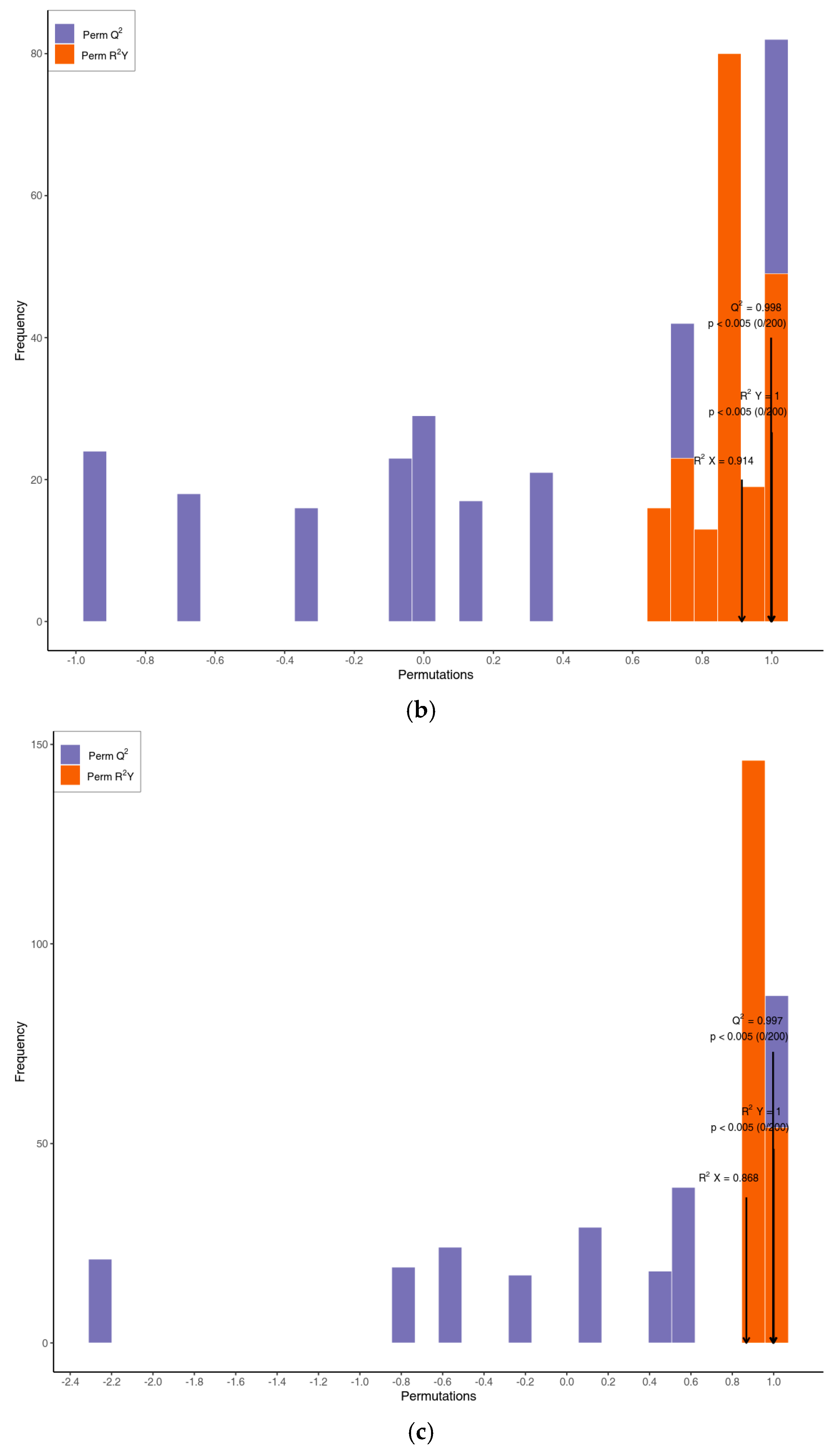
3.2. Multivariate Statistical Analysis
3.3. GSLs in Broccoli Stems
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.-J. Dietary Broccoli Impedes Western Diet-Enhanced Fatty Liver and Hepatocellular Carcinoma Development; University of Illinois at Urbana-Champaign: Champaign-Urbana, IL, USA, 2015. [Google Scholar]
- Beaver, L.M.; Löhr, C.V.; Clarke, J.D.; Glasser, S.T.; Watson, G.W.; Wong, C.P.; Zhang, Z.; Williams, D.E.; Dashwood, R.H.; Shannon, J.; et al. Broccoli Sprouts Delay Prostate Cancer Formation and Decrease Prostate Cancer Severity with a Concurrent Decrease in HDAC3 Protein Expression in Transgenic Adenocarcinoma of the Mouse Prostate (TRAMP) Mice. Curr. Dev. Nutr. 2018, 2, nzy002. [Google Scholar] [CrossRef] [PubMed]
- Ballance, S.; Knutsen, S.H.; Fosvold, O.W.; Wickham, M.; Trenado, C.D.-T.; Monro, J. Glyceamic and insulinaemic response to mashed potato alone, or with broccoli, broccoli fibre or cellulose in healthy adults. Eur. J. Nutr. 2018, 57, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Holman, J.; McKinstry, D.; Trindade, B.C.; Eaton, K.A.; Mendoza-Castrejon, J.; Ho, S.; Wells, E.; Yuan, H.; Wen, B.; et al. A steamed broccoli sprout diet preparation that reduces colitis via the gut microbiota. J. Nutr. Biochem. 2023, 112, 109215. [Google Scholar] [CrossRef]
- Blazevic, I.; Montaut, S.; Burcul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef]
- Ding, Y.H.; He, H.J.; Song, S.H.; Jian, Y.C.; Zhao, X.Z.; Wang, X.Q. Glucosinolate Component and Content Analysis of Different Broccoli Varieties. J. Change Veg. 2015, 20, 70–74. [Google Scholar]
- Yan, M.; Song, C.; Su, S.; Li, J.; Hu, Z.; Lin, S.; Zou, H.; Tang, Z.; Yan, X. Quantification and Diversity Analyses of Glucosinolates in 191 Broccoli Genotypes Highlight Valuable Genetic Resources for Molecular Breeding. Agron.-Basel 2023, 13, 2928. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, H.; Chen, S.; Li, Y.; Hamouda, H.I.; Balah, M.A.; Xue, C.; Mao, X. Strategy for preparing of glucosinolate derivatives with outstanding functional activities based on myrosinase. Food Chem. 2025, 479, 143778. [Google Scholar] [CrossRef]
- Williams, D.E. Indoles Derived From Glucobrassicin: Cancer Chemoprevention by Indole-3-Carbinol and 3,3′-Diindolylmethane. Front. Nutr. 2021, 8, 734334. [Google Scholar] [CrossRef]
- Vo, Q.V.; Nam, P.C.; Dinh, T.N.; Mechler, A.; Tran, T.T.V. Anti-inflammatory activity of synthetic and natural glucoraphanin. J. Serbian Chem. Soc. 2019, 84, 445–453. [Google Scholar] [CrossRef]
- Xu, X.; Dai, M.; Lao, F.; Chen, F.; Hu, X.; Liu, Y.; Wu, J. Effect of glucoraphanin from broccoli seeds on lipid levels and gut microbiota in high-fat diet-fed mice. J. Funct. Foods 2020, 68, 103858. [Google Scholar] [CrossRef]
- Sun, W.; Sun, J.; Hu, W.; Luo, C.; Lu, Z.; He, F.; Zhao, H.; Zeng, X.; Cao, D.; Li, J.; et al. Sulforaphane inhibits multiple myeloma cell-induced osteoclast differentiation and macrophage proliferation by elevating ferroportin1. Cancer Chemother. Pharmacol. 2025, 95, 3. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, B.; Ma, Z.; Chen, T.; Zou, H.; Chen, Y.; Dong, Z.; Chen, J.; Zhang, H.; Ding, Y.; et al. Sulforaphane promotes diabetic wound healing by regulating macrophage efferocytosis and polarization. Int. Immunopharmacol. 2025, 150, 114243. [Google Scholar] [CrossRef] [PubMed]
- Ruhee, R.T.; Ma, S.; Suzuki, K. Effects of Sulforaphane Treatment on Skeletal Muscle from Exhaustive Exercise-Induced Inflammation and Oxidative Stress Through the Nrf2/HO-1 Signaling Pathway. Antioxidants 2025, 14, 210. [Google Scholar] [CrossRef]
- Wei, R.; Pan, X.; Cai, D.; Pan, L. Synergistic Inhibition of Breast Carcinoma Cell Proliferation by Quercetin and Sulforaphane via Activation of the ERK/MAPK Pathway. Cell Biochem. Biophys. 2025. [Google Scholar] [CrossRef]
- Moreira-Rodriguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velazquez, D.A. UVA, UVB Light Doses and Harvesting Time Differentially Tailor Glucosinolate and Phenolic Profiles in Broccoli Sprouts. Molecules 2017, 22, 1065. [Google Scholar] [CrossRef]
- Sun, J.; Kou, L.; Geng, P.; Huang, H.; Yang, T.; Luo, Y.; Chen, P. Metabolomic Assessment Reveals an Elevated Level of Glucosinolate Content in CaCl2 Treated Broccoli Microgreens. J. Agric. Food Chem. 2015, 63, 1863–1868. [Google Scholar] [CrossRef]
- Tian, M.; Yang, Y.; Avila, F.W.; Fish, T.; Yuan, H.; Hui, M.; Pan, S.; Thannhauser, T.W.; Li, L. Effects of Selenium Supplementation on Glucosinolate Biosynthesis in Broccoli. J. Agric. Food Chem. 2018, 66, 8036–8044. [Google Scholar] [CrossRef]
- Guo, L.; Yang, R.; Zhou, Y.; Gu, Z. Heat and hypoxia stresses enhance the accumulation of aliphatic glucosinolates and sulforaphane in broccoli sprouts. Eur. Food Res. Technol. 2016, 242, 107–116. [Google Scholar] [CrossRef]
- Eun-Sun, H.; Lee, S. Comparative study on the bioactive compound contents and antioxidant activity of broccoli cooked with different methods. Food Sci. Preserv. 2024, 31, 579–589. [Google Scholar]
- Lu, Y.; Pang, X.; Yang, T. Microwave cooking increases sulforaphane level in broccoli. Food Sci. Nutr. 2020, 8, 2052–2058. [Google Scholar] [CrossRef]
- Matusheski, N.V.; Juvik, J.A.; Jeffery, E.H. Heating decreases epithiospecifier protein activity and increases sulforaphane formation in broccoli. Phytochemistry 2004, 65, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.-h.; Yang, C.-x.; Fang, C.-r.; Qiu, N.-n.; Xu, J.-j.; Bai, S.-s.; Li, D.; Cui, Y.-j. Determination of sulforaphane in cruciferous vegetables by HPLC. Food Ferment. Ind. 2021, 47, 218–223. [Google Scholar]
- Forsgren, E.; Bjorkblom, B.; Trygg, J.; Jonsson, P. OPLS-Based Multiclass Classification and Data-Driven Interclass Relationship Discovery. J. Chem. Inf. Model. 2025, 65, 1762–1770. [Google Scholar] [CrossRef] [PubMed]
- Hanschen, F.S.; Platz, S.; Mewis, I.; Schreiner, M.; Rohn, S.; Kroh, L.W. Thermally Induced Degradation of Sulfur-Containing Aliphatic Glucosinolates in Broccoli Sprouts (Brassica oleracea var. italica) and Model Systems. J. Agric. Food Chem. 2012, 60, 2231–2241. [Google Scholar] [CrossRef]
- Cai, Y.X.; Augustin, M.A.; Jegasothy, H.; Wang, J.H.; Terefe, N.S. Mild heat combined with lactic acid fermentation: A novel approach for enhancing sulforaphane yield in broccoli puree. Food Funct. 2020, 11, 779–786. [Google Scholar] [CrossRef]
- Triska, J.; Balik, J.; Houska, M.; Novotna, P.; Magner, M.; Vrchotova, N.; Hic, P.; Jilek, L.; Thorova, K.; Snurkovic, P.; et al. Factors Influencing Sulforaphane Content in Broccoli Sprouts and Subsequent Sulforaphane Extraction. Foods 2021, 10, 1927. [Google Scholar] [CrossRef]
- Wu, Y.; Lv, C.; Zou, L.; Sun, J.; Song, X.; Zhang, Y.; Mao, J. Approaches for enhancing the stability and formation of sulforaphane. Food Chem. 2021, 345, 128771. [Google Scholar] [CrossRef]
- Kushad, M.M.; Brown, A.F.; Kurilich, A.C.; Juvik, J.A.; Klein, B.P.; Wallig, M.A.; Jeffery, E.H. Variation of glucosinolates in vegetable crops of Brassica oleracea. J. Agric. Food Chem. 1999, 47, 1541–1548. [Google Scholar] [CrossRef]
- Ciska, E.; Verkerk, R.; Honke, J. Effect of boiling on the content of ascorbigen, indole-3-carbinol, indole-3-acetonitrile, and 3,3′-diindolylmethane in fermented cabbage. J. Agric. Food Chem. 2009, 57, 2334–2338. [Google Scholar] [CrossRef]
- Verkerk, R.; Dekker, M. Glucosinolates and myrosinase activity in red cabbage (Brassica oleracea L. var. Capitata f. rubra DC.) after various microwave treatments. J. Agric. Food Chem. 2004, 52, 7318–7323. [Google Scholar] [CrossRef]
- Oerlemans, K.; Barrett, D.M.; Suades, C.B.; Verkerk, R.; Dekker, M. Thermal degradation of glucosinolates in red cabbage. Food Chem. 2006, 95, 19–29. [Google Scholar]
- Hanschen, F.S.; Rohn, S.; Mewis, I.; Schreiner, M.; Kroh, L.W. Influence of the chemical structure on the thermal degradation of the glucosinolates in broccoli sprouts. Food Chem. 2012, 130, 1–8. [Google Scholar] [CrossRef]

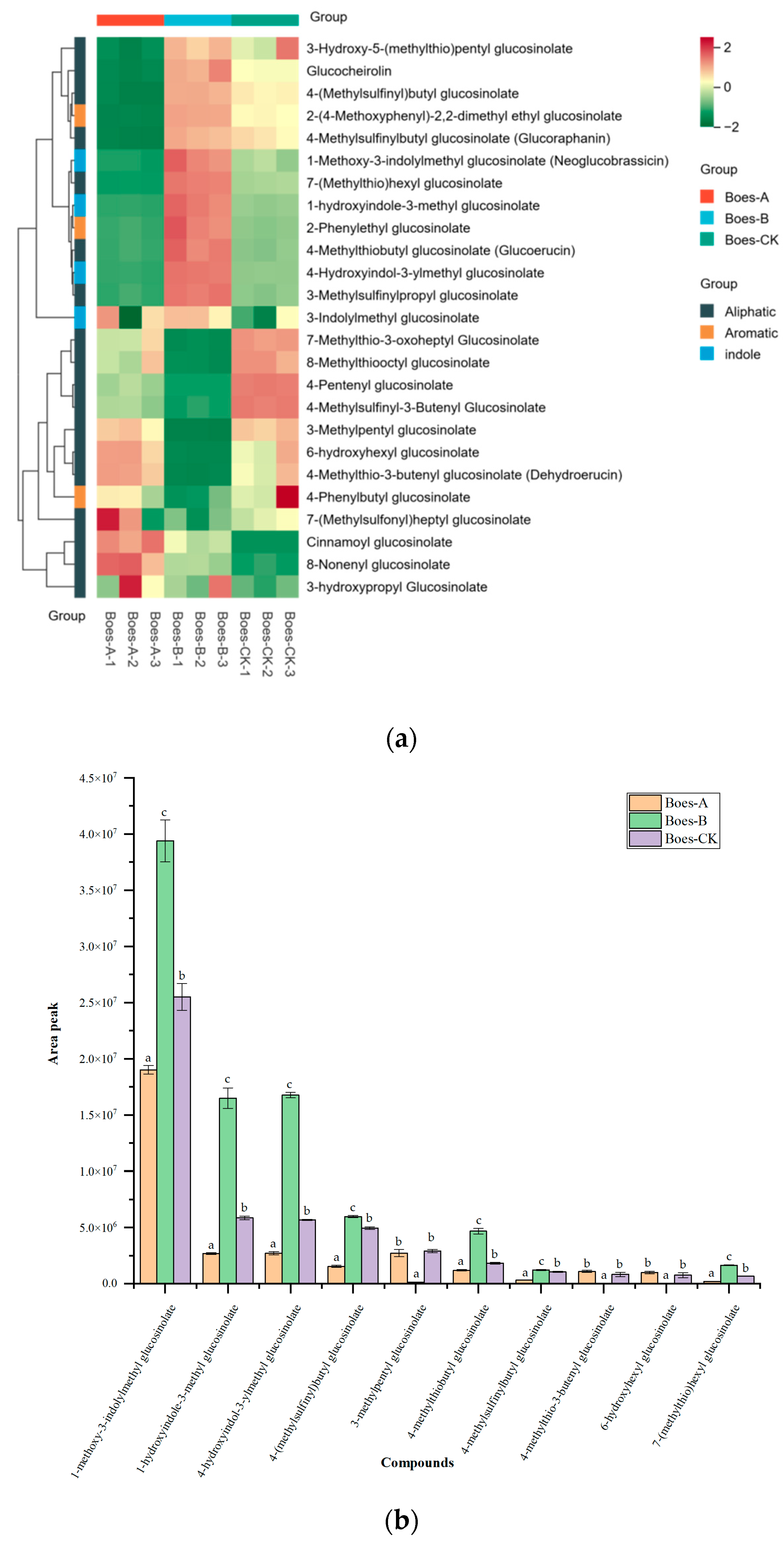
| Boes-A | Boes-B | Boes-CK | |
|---|---|---|---|
| Total GLSs content | 3.37 × 107 | 8.88 × 107 | 5.20 × 107 |
| Aliphatic | 6.40 × 106 | 1.58 × 107 | 1.23 × 107 |
| Indole | 2.45 × 107 | 7.27 × 107 | 3.59 × 107 |
| Aromatic | 2.94 × 106 | 3.07 × 105 | 3.36 × 106 |
| Percentage of aliphatic | 18.972% | 17.746% | 23.600% |
| Percentage of indole | 72.645% | 81.908% | 69.095% |
| Percentage of aromatic | 8.734% | 0.345% | 6.455% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-H.; Liao, X.-Y.; Li, Z.-H.; Guo, Y.-F.; Ma, M.-X.; Zhang, G.-Q. Insight into the Sulforaphane Content and Glucosinolate Profile of Broccoli Stems After Heat Treatment. Horticulturae 2025, 11, 383. https://doi.org/10.3390/horticulturae11040383
Zhang Y-H, Liao X-Y, Li Z-H, Guo Y-F, Ma M-X, Zhang G-Q. Insight into the Sulforaphane Content and Glucosinolate Profile of Broccoli Stems After Heat Treatment. Horticulturae. 2025; 11(4):383. https://doi.org/10.3390/horticulturae11040383
Chicago/Turabian StyleZhang, Yu-Hong, Xue-Yi Liao, Zheng-Hong Li, Yu-Feng Guo, Ming-Xin Ma, and Guo-Qiang Zhang. 2025. "Insight into the Sulforaphane Content and Glucosinolate Profile of Broccoli Stems After Heat Treatment" Horticulturae 11, no. 4: 383. https://doi.org/10.3390/horticulturae11040383
APA StyleZhang, Y.-H., Liao, X.-Y., Li, Z.-H., Guo, Y.-F., Ma, M.-X., & Zhang, G.-Q. (2025). Insight into the Sulforaphane Content and Glucosinolate Profile of Broccoli Stems After Heat Treatment. Horticulturae, 11(4), 383. https://doi.org/10.3390/horticulturae11040383





