Identification of Cost-Relevant Factors in the Production of a Triterpenoid Saponin in Hydroponically Grown Soapwort: A Case Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Cuttings
2.3. Root Harvest and Saponin Analysis
2.4. Lighting
2.5. Cost Data Collection and Cost Analysis Model
3. Results
3.1. Cost Data Collection
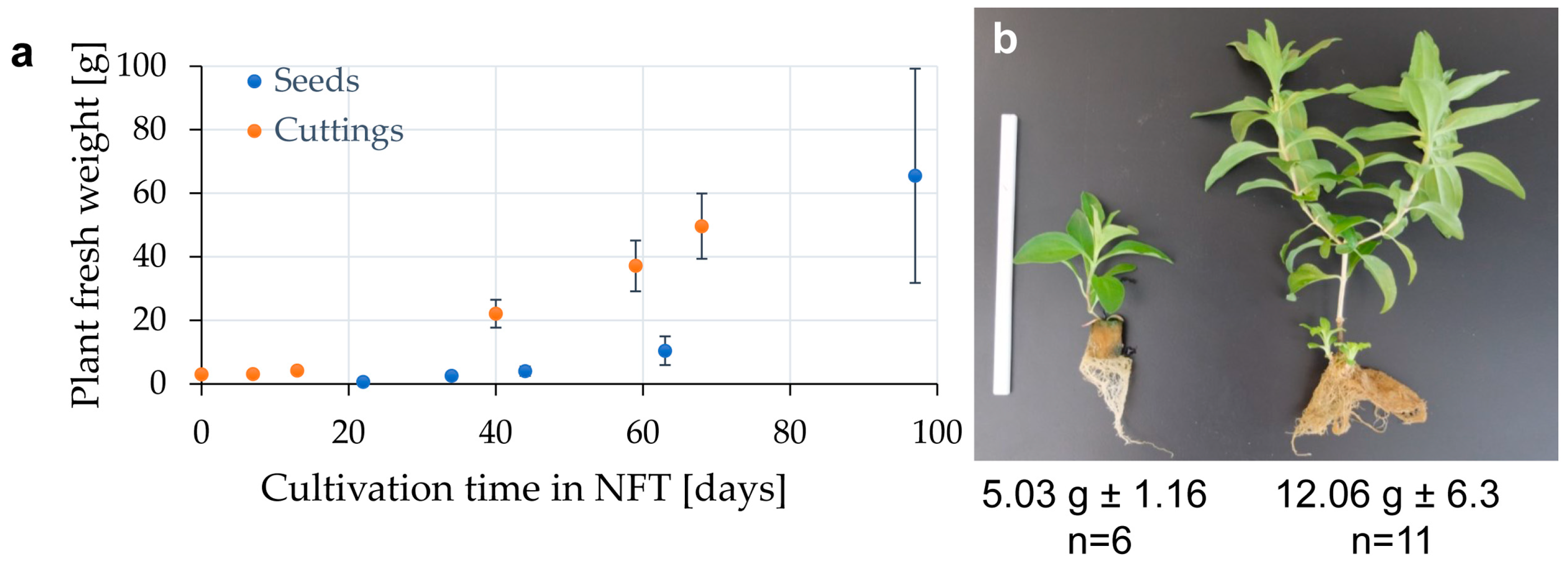
3.2. Cuttings or Seeds
3.3. Root Biomass Accumulation
3.4. SO1861 Content
3.5. Light Comparison
3.6. Combination of Cost Factors and Cost Calculation Tool
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CoG | Cost of goods |
| HPS | High-pressure sodium lamps |
| LED | Light-emitting diodes |
| NFT | Nutrient film technology |
| SO1861 | Triterpenoid saponin SO1861 |
References
- Rates, S.M. Plants as source of drugs. Toxicon 2001, 39, 603–613. [Google Scholar] [CrossRef]
- Chen, S.L.; Yu, H.; Luo, H.M.; Wu, Q.; Li, C.F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin. Med. 2016, 11, 37. [Google Scholar] [CrossRef]
- Khan, F.A. A review on hydroponic greenhouse cultivation for sustainable agriculture. Int. J. Agric. Environ. Food Sci. 2018, 2, 59–66. [Google Scholar] [CrossRef]
- Pereira, C.G.; Gualtieri, I.P.; Maia, N.B.; Meireles, M.A. Supercritical extraction to obtain vetiver (Vetiveria zizanioides L. Nash) extracts from roots cultivated hydroponically. J. Agric. Sci. Technol. 2008, 2, 44–50. Available online: https://repositorio.ufrn.br/bitstream/123456789/32547/1/SupercriticalExtractionObtain%20vetiver_PEREIRA_2008.pdf (accessed on 17 March 2025).
- Chandra, S.; Rawat, D.S.; Bhatt, A. Phytochemistry and pharmacological activities of Saponaria officinalis L.: A review. Not. Sci. Biol. 2021, 13, 10809. [Google Scholar] [CrossRef]
- Rai, S.; Acharya-Siwakoti, E.; Kafle, A.; Devkota, H.P.; Bhattarai, A. Plant-derived saponins: A review of their surfactant properties and applications. Sci 2021, 3, 44. [Google Scholar] [CrossRef]
- Sama, S.; Jerz, G.; Schmieder, P.; Woith, E.; Melzig, M.F.; Weng, A. Sapofectosid–Ensuring non-toxic and effective DNA and RNA delivery. Int. J. Pharm. 2017, 534, 195–205. [Google Scholar] [CrossRef]
- Anderson, J. Determining manufacturing costs. Chem. Eng. Prog. 2009, 105, 27–31. Available online: https://my.che.utah.edu/~ring/Design%20I/Articles/CostEstn.pdf (accessed on 17 March 2025).
- Drury, C.M. Management and Cost Accounting, 3rd ed.; Springer Science + Business Media: Berlin/Heidelberg, Germany, 2013; p. 16. [Google Scholar]
- Velazquez-Gonzalez, R.S.; Garcia-Garcia, A.L.; Ventura-Zapata, E.; Barceinas-Sanchez, J.D.O.; Sosa-Savedra, J.C. A review on hydroponics and the technologies associated for medium-and small-scale operations. Agriculture 2022, 12, 646. [Google Scholar] [CrossRef]
- Thapa, U.; Hansda, N.N.; Kundu, S.; Giri, A.; Tamang, D.; Rahaman, A.O. Advancements in Hydroponic Systems: A Comprehensive Review. Arch. Curr. Res. Int. 2024, 24, 317–328. [Google Scholar]
- Maucieri, C.; Nicoletto, C.; Junge, R.; Schmautz, Z.; Sambo, P.; Borin, M. Hydroponic systems and water management in aquaponics: A review. Ital. J. Agron. 2018, 13, 1012. [Google Scholar] [CrossRef]
- Czitrom, V. One-factor-at-a-time versus designed experiments. Am. Stat. 1999, 53, 126–131. [Google Scholar] [CrossRef]
- Michalis, E.; Giatra, C.E.; Skordos, D.; Ragkos, A. Assessing the different economic feasibility scenarios of a hydroponic tomato greenhouse farm: A case study from Western Greece. Sustainability 2023, 15, 14233. [Google Scholar] [CrossRef]
- Souza, V.; Gimenes, R.M.T.; de Almeida, M.G.; Farinha, M.U.S.; Bernardo, L.V.M.; Ruviaro, C.F. Economic feasibility of adopting a hydroponics system on substrate in small rural properties. Clean Technol. Environ. Policy 2023, 25, 2761–2775. [Google Scholar] [CrossRef]
- Zaynab, M.; Sharif, Y.; Abbas, S.; Afzal, M.Z.; Qasim, M.; Khalofah, A.; Ansari, M.J.; Khan, K.A.; Tao, L.; Li, S. Saponin toxicity as key player in plant defense against pathogens. Toxicon 2021, 193, 21–27. [Google Scholar] [CrossRef]
- Jo, S.; El-Demerdash, A.; Owen, C.; Srivastava, V.; Wu, D.; Kikuchi, S.; Reed, J.; Hodgson, H.; Harkess, A.; Shu, S.; et al. Unlocking saponin biosynthesis in soapwort. Nat. Chem. Biol 2025, 21, 215–226. [Google Scholar] [CrossRef]
- Bafort, F.; Kohnen, S.; Maron, E.; Bouhadada, A.; Ancion, N.; Crutzen, N.; Jijakli, M.H. The agro-economic feasibility of growing the medicinal plant Euphorbia peplus in a modified vertical hydroponic shipping container. Horticulturae 2022, 8, 256. [Google Scholar] [CrossRef]
- Atherton, H.R.; Li, P. Hydroponic Cultivation of Medicinal Plants—Plant Organs and Hydroponic Systems: Techniques and Trends. Horticulturae 2023, 9, 349. [Google Scholar] [CrossRef]
- Kowalczyk, K.; Olewnicki, D.; Mirgos, M.; Gajc-Wolska, J. Comparison of selected costs in greenhouse cucumber production with LED and HPS supplemental assimilation lighting. Agronomy 2020, 10, 1342. [Google Scholar] [CrossRef]
- de Costa, F.; Alves Yendo, A.C.; Fleck, J.D.; Gosmann, G.; Fett-Neto, A.G. Accumulation of a bioactive triterpene saponin fraction of Quillaja brasiliensis leaves is associated with abiotic and biotic stresses. Plant Physiol. Biochem. 2013, 66, 56–62. [Google Scholar] [CrossRef]
- Schippmann, U.; Leaman, D.; Cunningham, A.B. A comparison of cultivation and wild collection of medicinal and aromatic plants under sustainability aspects. In Medicinal and Aromatic Plants, 1st ed.; Bogers, R.J., Craker, L.E., Lange, D., Eds.; Springer: Dordrecht, The Netherlands, 2006; Volume 17, pp. 75–95. [Google Scholar]
- Holzem, A. (Gartenbau Achim Holzem, Mönchengladbach, Germany). Personal communication, 2023.
- Jiang, J.; Liao, M.; Lin, T.; Huang, C.; Chou, C.; Yeh, S.; Lin, T.T.; Fang, W. Toward a higher yield: A wireless sensor network-based temperature monitoring and fan-circulating system for precision cultivation in plant factories. Precis. Agric. 2018, 19, 929–956. [Google Scholar] [CrossRef]
| Experiment LE1 (July–August 22) 1 | Experiment LE2 (September 22–January 23) 2 | |||
|---|---|---|---|---|
| HPS | LED | HPS | LED | |
| Plant number | 30 each | 72 each | ||
| Plant age at beginning [days] | 70–90 | 0 | ||
| Plant cultivation [days] | 62 | 147 | ||
| Cultivation size [m2] | 0.83 | 1.66 | ||
| Artificial light [h] | 337.2 | 1962.6 | ||
| Cost Element | Unit | Unit Costs (EUR) | Units Required | Total Cost (EUR) |
|---|---|---|---|---|
| Labor (start and end) 1 | hour | 9.55 | 10 | 95.50 |
| Labor during cultivation | hour | 9.55 | 84 | 802.20 |
| Fertilizers | month | 1.20 | 7 | 8.40 |
| Water/waste water (beginning and end) 1 | m3 | 6.60 | 0.5 | 3.30 |
| Water/waste water during cultivation | m3 | 6.60 | 0.7 | 4.62 |
| Lighting | kwh | 0.17 | 1477 | 1757.63 |
| Heating | kwh | 0.08 | 10,304 | 772.80 |
| Cooling | kwh | 0.17 | 10,399 | 1035.30 |
| Water pump | kwh | 0.17 | 63 | 10.71 |
| Total costs | 4490.06 | |||
| 6 Months’ Cultivation (E2) | 5 Months’ Cultivation HPS (LE2) | 5 Months’ Cultivation LED (LE2) | |
|---|---|---|---|
| Line 3 | 4.76 ± 1.72 | 4.55 ± 1.42 | 4.07 ± 1.81 |
| Line 4 * | 5.82 ± 1.24 | 5.09 ± 1.54 | 5.08 ± 1.72 |
| Line 9 * | 4.27 ± 0.98 | 4.16 ± 1 | 3.59 ± 1.34 |
| Experiment LE1 (July–August 22) 1 | Experiment LE2 (September 22–January 23) 2 | |||
|---|---|---|---|---|
| HPS | LED | HPS | LED | |
| Root fresh weight [g] | 902.8 | 885.8 | 1406 | 1847.5 |
| Root dry weight [g] | 83.9 | 82.3 | 330 | 317.8 |
| SO1861 content [mg] | 162 | 182 | 634 | 626 |
| Energy used [kWh] | 318.9 | 44.9 | 2783.8 | 522.8 |
| Scenario | A | B | C | D | E | F | G | H | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Observed Costs [E1] | Seeds | Large Biomass | 75% Producing | Large Concentration | LED | Best Case | Commercial (LED) | Commercial (HPS) | |||
| Assumptions | Propagation | cuttings | seeds | cuttings | cuttings | cuttings | cuttings | cuttings | cuttings | cuttings | |
| Cultivation [months] | 7 | 8 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | ||
| Dry roots [g] | 1270 | 1270 | 1480 | 1270 | 1270 | 1270 | 1480 | 3810 | 3810 | ||
| SO1861 content [g] | 12.7 | 12.7 | 14.8 | 9.5 | 15.9 | 12.7 | 18.5 | 38.1 | 38.1 | ||
| Cost elements | Start | Labor | EUR 95.50 | EUR 95.50 | EUR 95.50 | EUR 95.50 | EUR 95.50 | EUR 95.50 | EUR 95.50 | EUR 286.50 | EUR 286.50 |
| Seeds and support | EUR 24.70 | ||||||||||
| Water and waste | EUR 3.30 | EUR 3.30 | EUR 3.30 | EUR 3.30 | EUR 3.30 | EUR 3.30 | EUR 3.30 | EUR 3.30 | EUR 3.30 | ||
| Cultivation | Labor | EUR 802.20 | EUR 916.80 | EUR 802.20 | EUR 802.20 | EUR 802.20 | EUR 802.20 | EUR 802.20 | EUR 2406.60 | EUR 2406.60 | |
| Fertilizer | EUR 8.40 | EUR 9.60 | EUR 8.40 | EUR 8.40 | EUR 8.40 | EUR 8.40 | EUR 8.40 | EUR 25.20 | EUR 25.20 | ||
| Water and waste | EUR 4.62 | EUR 5.28 | EUR 4.62 | EUR 4.62 | EUR 4.62 | EUR 4.62 | EUR 4.62 | EUR 13.86 | EUR 13.86 | ||
| HPS light | EUR 1757.63 | EUR 2008.72 | EUR 1757.63 | EUR 1757.63 | EUR 1757.63 | EUR 5272.89 | |||||
| LED light | EUR 371.28 | EUR 371.28 | EUR 1113.84 | ||||||||
| Heating | EUR 772.80 | EUR 883.20 | EUR 772.80 | EUR 772.80 | EUR 772.80 | EUR 772.80 | EUR 772.80 | EUR 772.80 | EUR 772.80 | ||
| Cooling | EUR 1035.30 | EUR 1183.20 | EUR 1035.30 | EUR 1035.30 | EUR 1035.30 | EUR 1035.30 | EUR 1035.30 | EUR 1035.30 | EUR 1035.30 | ||
| Fertilizer pump | EUR 10.71 | EUR 12.24 | EUR 10.71 | EUR 10.71 | EUR 10.71 | EUR 10.71 | EUR 10.71 | EUR 32.13 | EUR 32.13 | ||
| Calculation | Total costs | EUR 4490.46 | EUR 5142.54 | EUR 4490.46 | EUR 4490.46 | EUR 4490.46 | EUR 3,104.11 | EUR 3104.11 | EUR 5689.53 | EUR 9848.58 | |
| Costs per gram of SO1861 | EUR 353.58 | EUR 404.92 | EUR 303.50 | EUR 471.44 | EUR 282.86 | EUR 244.42 | EUR 167.84 | EUR 149.33 | EUR 258.49 | ||
| Difference to observed costs | EUR 51.34 | EUR 50.08 | EUR 117.86 | EUR 70.72 | EUR 109.16 | EUR 185.74 | EUR 204.25 | EUR 95.09 | |||
| Difference in percent | 14.5% | −14.2% | 33.3% | −20.0% | −30.9% | −52.5% | −57.8% | −26.9% | |||
| Cost factor | 1.15 | 0.86 | 1.33 | 0.80 | 0.69 | 0.47 | 0.42 | 0.73 | |||
| Cost Factor | Description | Impact on Costs |
|---|---|---|
| Plant genetics | Plants produce no SO1861 | Decrease in SO1861 production at same price |
| Plants produce different amounts of SO1861 | ||
| Plants produce different amounts of root biomass | ||
| Propagation | Plants grown from seeds need longer to accumulate a given amount of root biomass than plants grown from cuttings | Using seeds lengthens the cultivation period, thus increasing the cost per gram of SO1861 |
| Lights | LEDs use less energy than HPS | Using LEDs decreases energy costs, thus decreasing the cost per gram of SO1861 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoffel, S.T.; de Vaumas, R.; Postel, R.; Schillberg, S.; Schwenkglenks, M.; Schinkel, H. Identification of Cost-Relevant Factors in the Production of a Triterpenoid Saponin in Hydroponically Grown Soapwort: A Case Study. Horticulturae 2025, 11, 353. https://doi.org/10.3390/horticulturae11040353
Stoffel ST, de Vaumas R, Postel R, Schillberg S, Schwenkglenks M, Schinkel H. Identification of Cost-Relevant Factors in the Production of a Triterpenoid Saponin in Hydroponically Grown Soapwort: A Case Study. Horticulturae. 2025; 11(4):353. https://doi.org/10.3390/horticulturae11040353
Chicago/Turabian StyleStoffel, Sandro T., René de Vaumas, Ruben Postel, Stefan Schillberg, Matthias Schwenkglenks, and Helga Schinkel. 2025. "Identification of Cost-Relevant Factors in the Production of a Triterpenoid Saponin in Hydroponically Grown Soapwort: A Case Study" Horticulturae 11, no. 4: 353. https://doi.org/10.3390/horticulturae11040353
APA StyleStoffel, S. T., de Vaumas, R., Postel, R., Schillberg, S., Schwenkglenks, M., & Schinkel, H. (2025). Identification of Cost-Relevant Factors in the Production of a Triterpenoid Saponin in Hydroponically Grown Soapwort: A Case Study. Horticulturae, 11(4), 353. https://doi.org/10.3390/horticulturae11040353







