Viroid GYSVd1 Exhibited Different Regulations on the Qualities of Berries and Wines from 6 Grape Varieties
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Viroid GYSVd1 Identification
2.3. Determination of General Physical and Chemical Indexes
2.4. Determination of Total Phenolic Compounds
2.5. Determination of Anthocyanin
2.6. Determination of Tannin
2.7. Determination of Soluble Sugar
2.8. Data Analysis
3. Results
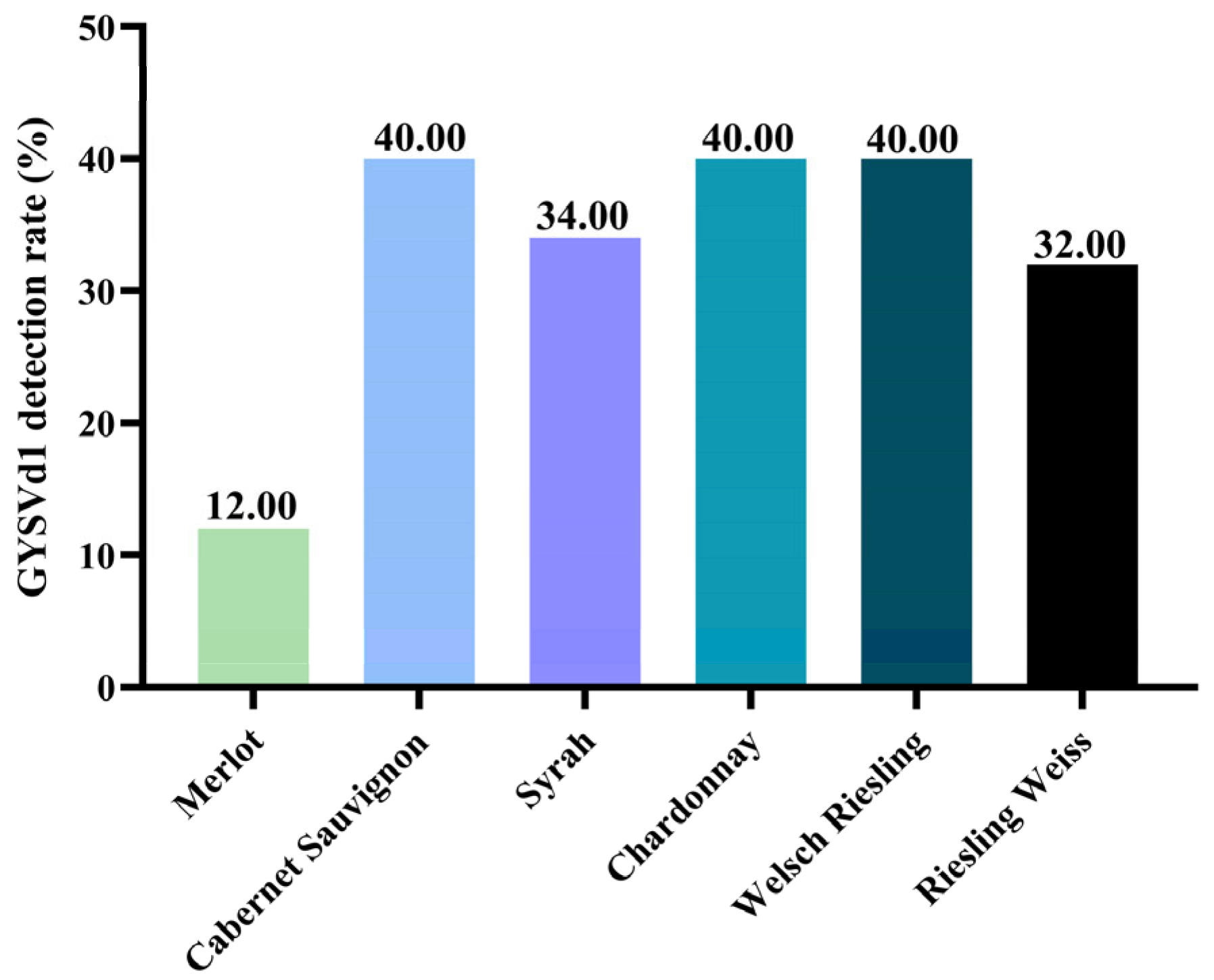
3.1. Detection of GYSVd1 of Six Grape Varieties
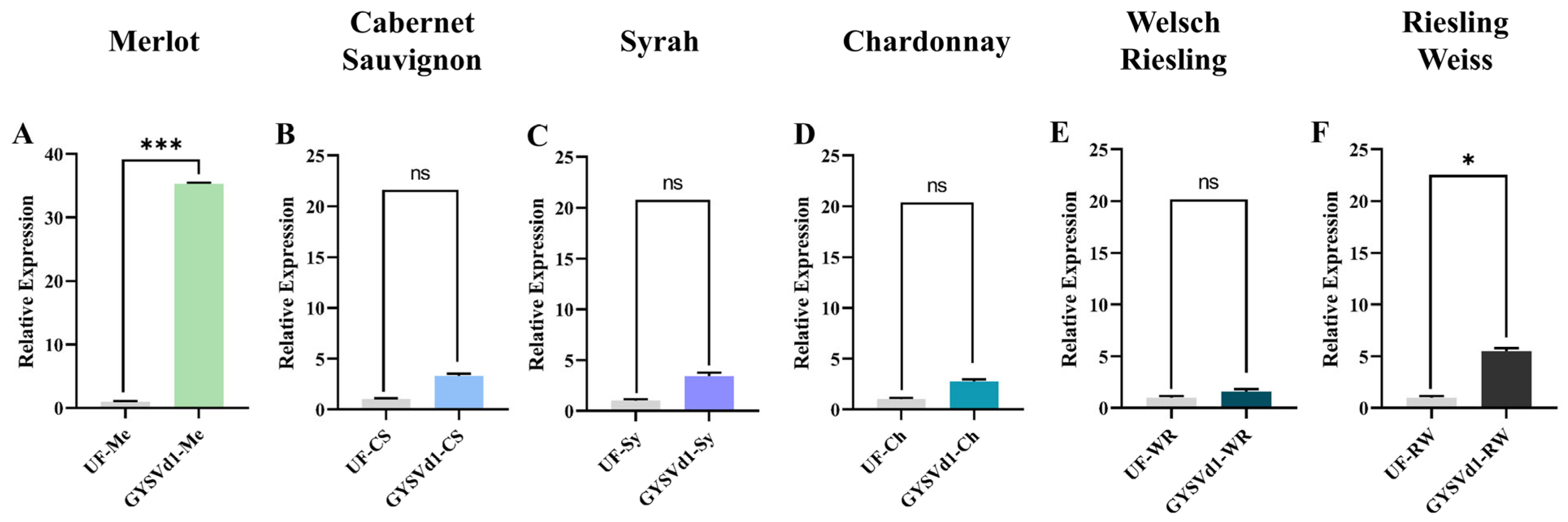
3.2. Viroid Accumulation of GYSVd1 in 6 Grape Varieties
3.3. GYSVd1 Modulated the Changes in General Physicochemical Indexes of Grape Berries and Wine
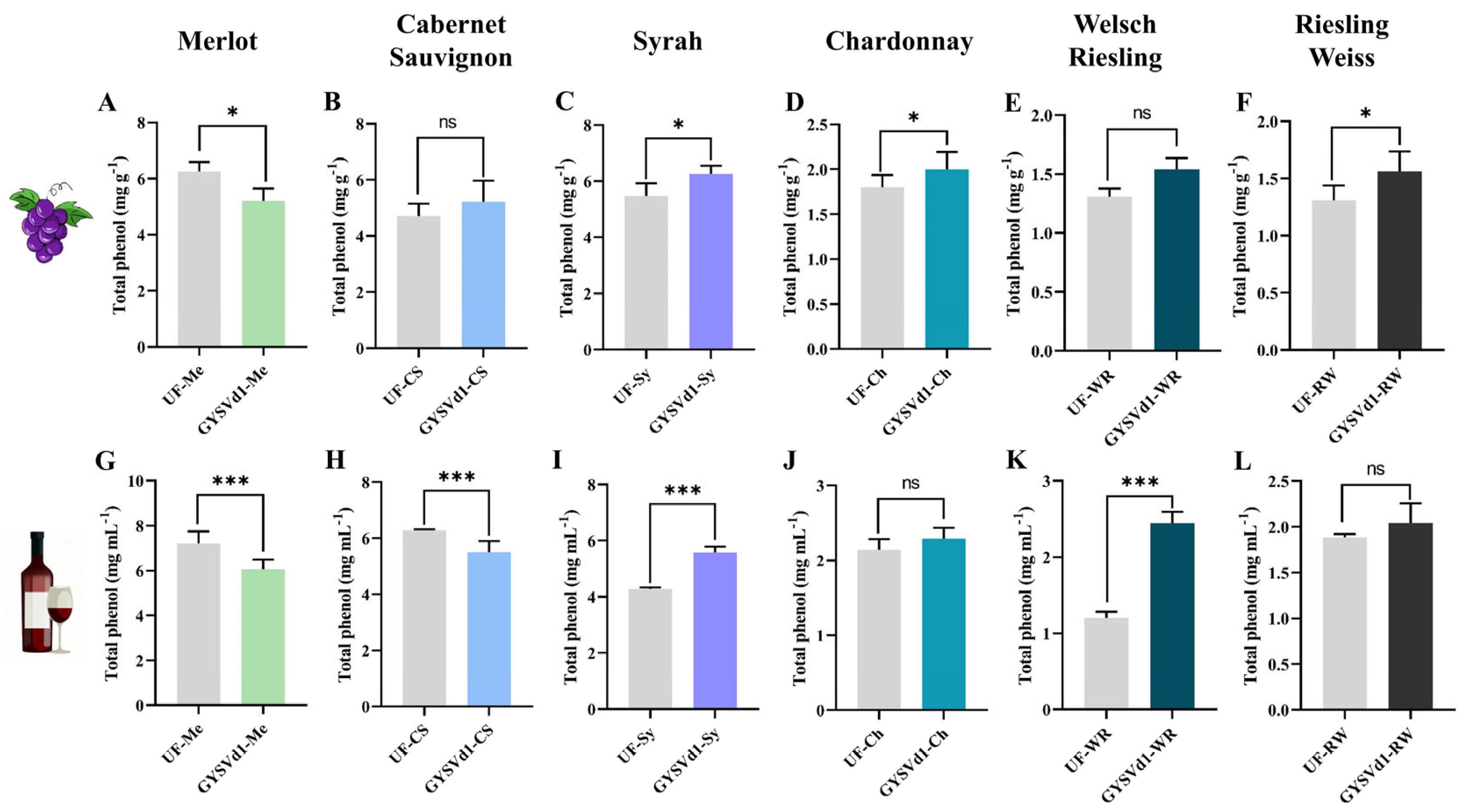
3.4. GYSVd1 Modulated the Total Phenolic Compounds Content of Grape Berries and Wine
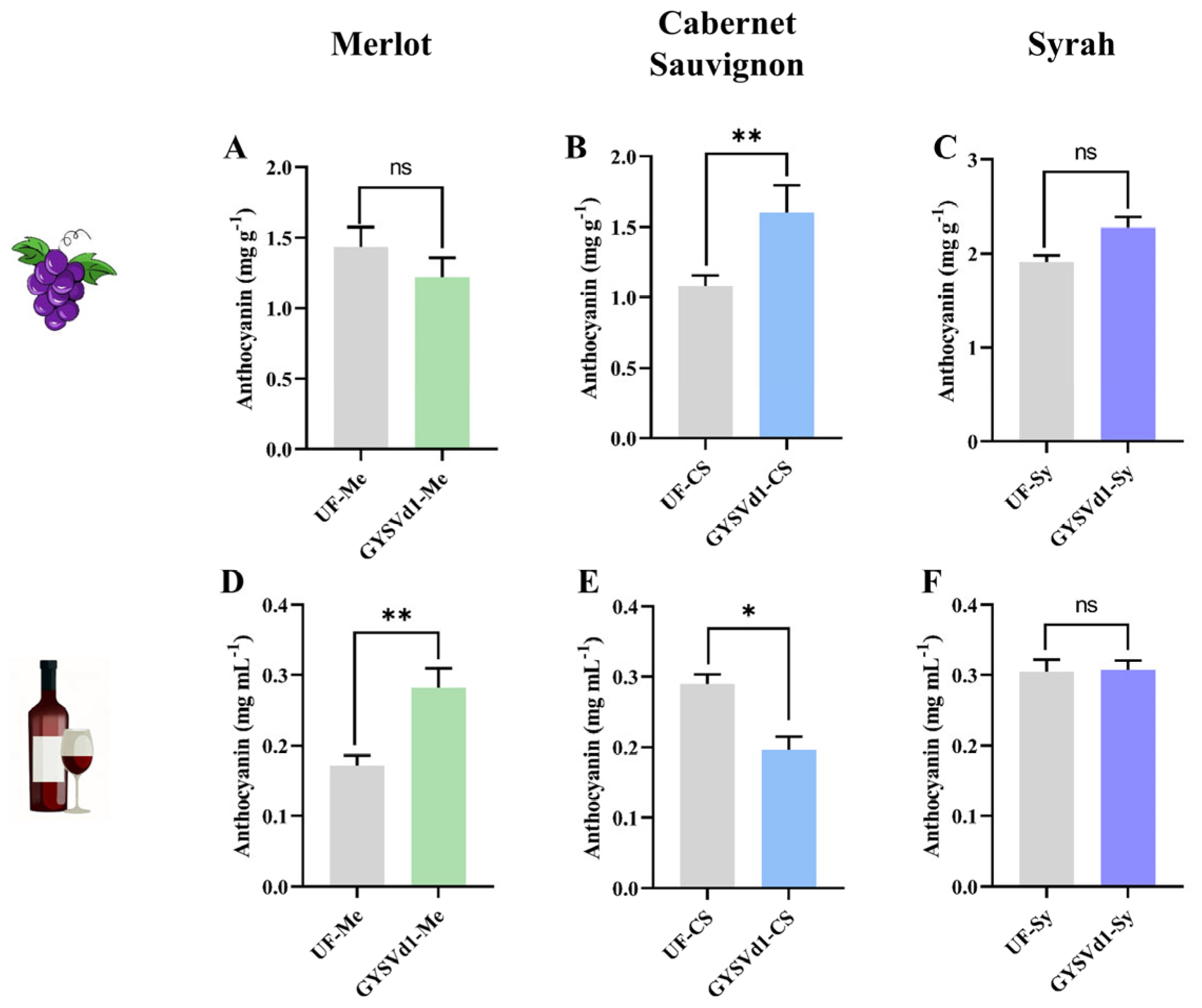
3.5. GYSVd1-Modulated Anthocyanin Content of Grape Berries and Wine
3.6. GYSVd1-Modulated Tannin Content of Grape Berries and Wine
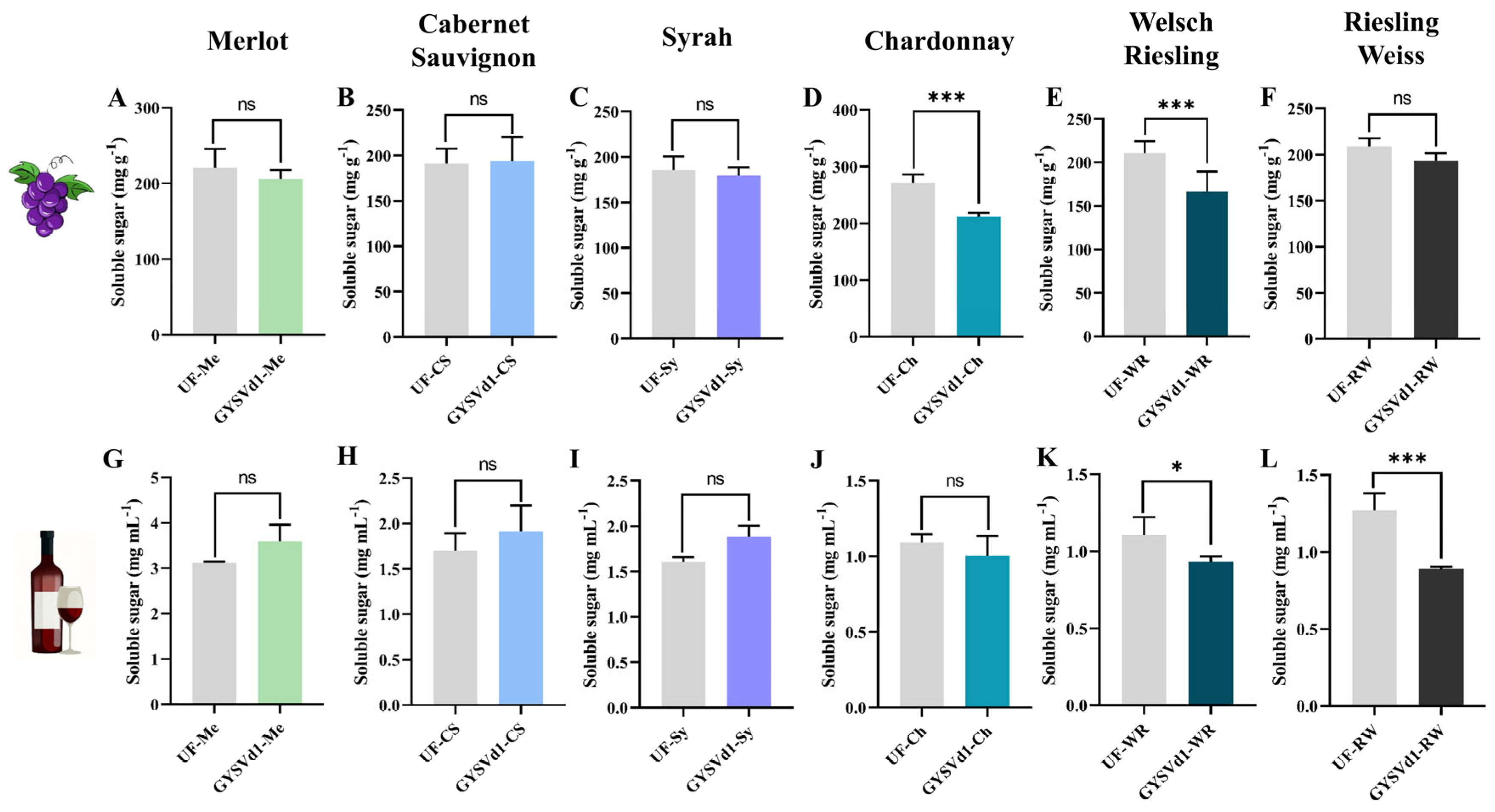
3.7. GYSVd1-Modulated Soluble Sugar Content of Grape Berries and Wine
3.8. Principal Component Analysis (PCA)
4. Discussion
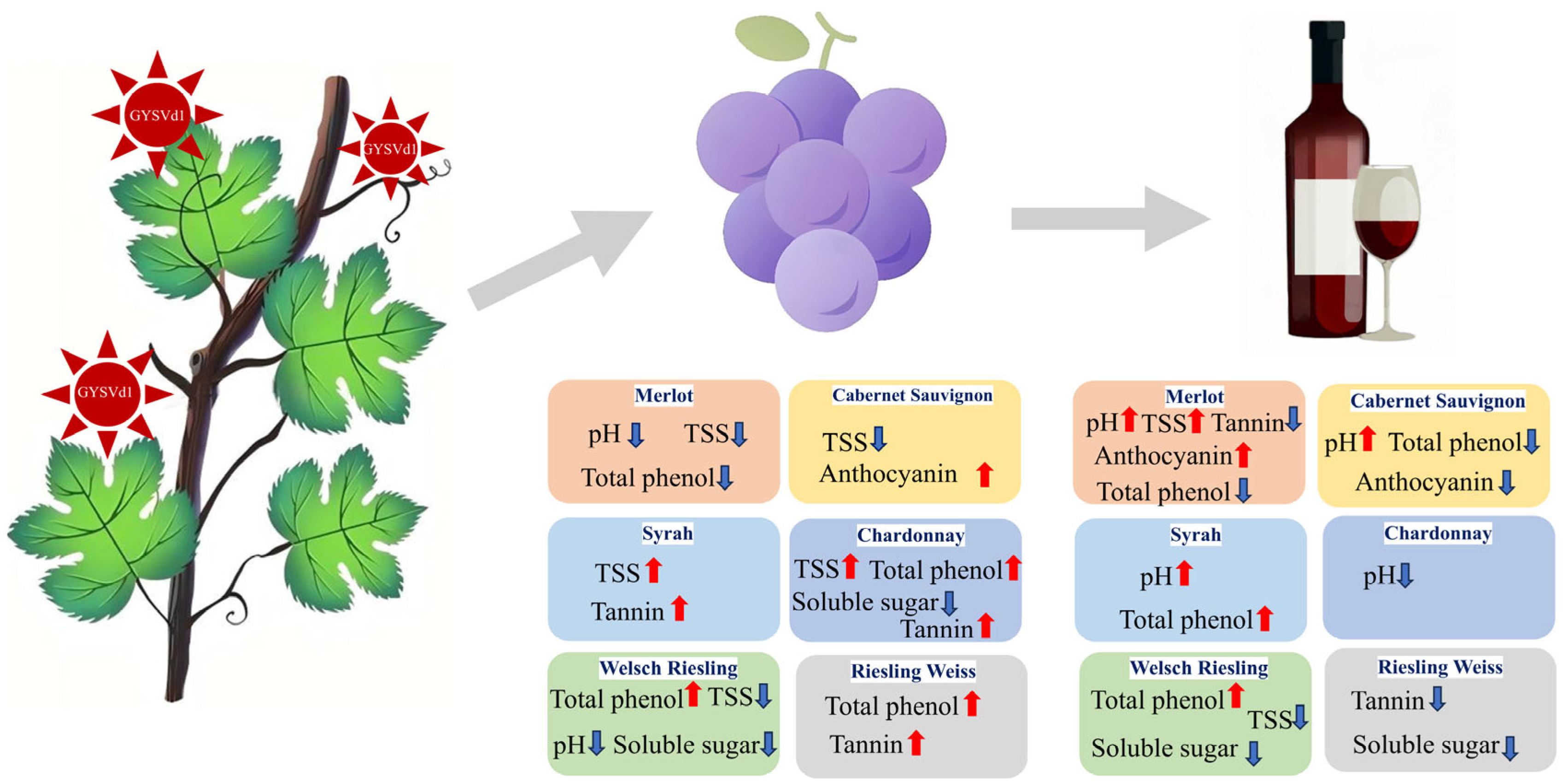
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yue, X.; Ju, Y.; Zhang, T.; Yu, R.; Xu, H.; Zhang, Z. Application of salicylic acid to cv. Muscat Hamburg grapes for quality improvement: Effects on typical volatile aroma compounds and anthocyanin composition of grapes and wines. LWT 2025, 182, 1067–1080. [Google Scholar] [CrossRef]
- Karimi, R.; Koulivand, M.; Ollat, N. Soluble sugars, phenolic acids and antioxidant capacity of grape berries as affected by iron and nitrogen. Acta Physiol. Plant. 2019, 41, 974–985. [Google Scholar] [CrossRef]
- Ren, F.; Qiao, S.; Fan, X.; Hu, G.; Zhou, Z.; Dong, Y. Impacts of three virus combinations on grape composition and fruit quality of Vitis vinifera ‘Kyoho’. Hortic. Plant J. 2024, 45, 98–109. [Google Scholar] [CrossRef]
- Fuchs, M.; Rwahnih, M.A.; Blouin, A.G.; Burger, J.; Chooi, K.M.; Constable, F.; Várallyay, É. A list of eclectic viruses, virus-like diseases and viroids of grapevines that should not be considered for regulatory oversight: A global plea from virologists. J. Plant Pathol. 2025, 25, 876–882. [Google Scholar] [CrossRef]
- Umer, M.; Liu, J.; You, H.; Xu, C.; Dong, K.; Luo, N.; Kong, L.; Li, X.; Hong, N.; Wang, G.; et al. Genomic, Morphological and Biological Traits of the Viruses Infecting Major Fruit Trees. Viruses 2019, 11, 515. [Google Scholar] [CrossRef]
- Chiaki, Y.; Ito, T.; Sato, A.; Sugiura, H.; Nishimura, R. Dwarfing caused by viral pathogens and leaf malformations in ‘Shine Muscat’ grapevine. J. Gen. Plant Pathol. 2019, 86, 34–38. [Google Scholar] [CrossRef]
- Lee, J.; Rennaker, C.D.; Thompson, B.D.; Dahan, J.; Karasev, A.V. Idaho ‘Cabernet Sauvignon’ grape composition altered by grapevine leafroll-associated virus 3. NFS J. 2023, 31, 1–6. [Google Scholar]
- Vega, A.; Gutiérrez, R.A.; Peña-Neira, A.; Cramer, G.R.; Arce-Johnson, P. Compatible GLRaV-3 viral infections affect berry ripening decreasing sugar accumulation and anthocyanin biosynthesis in Vitis vinifera. Plant Mol. Biol. 2011, 77, 261–274. [Google Scholar]
- Blanco-Ulate, B.; Hopfer, H.; Figueroa-Balderas, R.; Ye, Z.; Rivero, R.M.; Albacete, A.; Pérez-Alfocea, F.; Koyama, R.; Anderson, M.M.; Smith, R.J.; et al. Red blotch disease alters grape berry development and metabolism by interfering with the transcriptional and hormonal regulation of ripening. J. Exp. Bot. 2017, 68, 1225–1238. [Google Scholar]
- Pereira, G.E.; Padhi EM, T.; Sudarshana, M.R.; Fialho, F.B.; Medina-Plaza, C.; Girardello, R.C.; Tseng, D.; Bruce, R.C.; Erdmann, J.N.; Slupsky, C.M.; et al. Impact of grapevine red blotch disease on primary and secondary metabolites in ‘Cabernet Sauvignon’ grape tissues. Food Chem. 2021, 342, 1278–1284. [Google Scholar]
- Martínez, L.; Miranda, C.; Royo, J.B.; Urrestarazu, J.; Martínez de Toda, F.; Balda, P.; Santesteban, L.G. Direct and indirect effects of three virus infections on yield and berry composition in grapevine (Vitis vinifera L.) cv. ‘Tempranillo’. Sci. Hortic. 2016, 212, 20–28. [Google Scholar] [CrossRef]
- Matoušek, J.; Siglová, K.; Jakše, J.; Radišek, S.; Brass JR, J.; Tsushima, T.; Guček, T.; Duraisamy, G.S.; Sano, T.; Steger, G. Propagation and some physiological effects of Citrus bark cracking viroid and Apple fruit crinkle viroid in multiple infected hop (Humulus lupulus L.). J. Plant Physiol. 2017, 213, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Palukaitis, P. What has been happening with viroids? Virus Genes 2014, 49, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Tang, J.; Perez-Egusquiza, Z.; Thompson, J.R. Development of TaqMan RT-qPCR for the detection of regulated citrus viruses and viroids in Aotearoa New Zealand. J. Virol. Methods 2024, 327, 56–62. [Google Scholar] [CrossRef]
- Salman, T.M.; Habili, N.; Shi, B. Effect of temperature on symptom expression and sequence polymorphism of grapevine yellow speckle viroid 1 in grapevine. Virus Res. 2014, 189, 243–247. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, Y.; Zhong, R.; Tang, J.; Pervaiz, T.; Zhou, S.; Liu, J.; Wang, B.; Jia, H. Brassinolide and gibberellin promote grape fruit development and quality. Sci. Hortic. 2024, 338, 76–84. [Google Scholar] [CrossRef]
- Algul, B.E.; Al Shoffe, Y.; Park, D.; Cheng, L.; Watkins, C.B. Preharvest 1-methylcyclopropene and aminoethoxyvinylglycine treatment effects on ‘NY2’ (RubyFrost®) apple fruit quality and postharvest watercore dissipation at different temperatures. Postharvest Biol. Technol. 2025, 220, 2674–2689. [Google Scholar] [CrossRef]
- Owoyemi, A.; Balaklav, M.; Kochanek, B.; Porat, R.; Koenigstein, N.; Salzer, Y.; Lichter, A. Deviations from optimal storage temperature and its impact on postharvest quality of table grape cv. Scarlotta Seedless. Postharvest Biol. Technol. 2024, 215, 3401–3412. [Google Scholar] [CrossRef]
- Palma-López, J.; Sánchez-Rodríguez, A.R.; del Campillo, M.C.; León-Gutiérrez, J.M.; Ramírez-Pérez, P. Influence of soil properties on grape and must quality in the Montilla − Moriles protected designation of origin (southern Spain). Catena 2024, 241, 67–79. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Z.; Wu, Y.; Wu, W.; Lyu, L.; Li, W. Combined transcriptomic and metabolomic analysis of the effect of different nitrogen application levels on soluble sugar and organic acid accumulation in blackberry fruit. Sci. Hortic. 2024, 338, 3485–3495. [Google Scholar] [CrossRef]
- Maliogka, V.I.; Olmos, A.; Pappi, P.G.; Lotos, L.; Efthimiou, K.; Grammatikaki, G.; Candresse, T.; Katis, N.I.; Avgelis, A.D. A novel grapevine badnavirus is associated with the Roditis leaf discoloration disease. Virus Res. 2015, 203, 47–55. [Google Scholar] [PubMed]
- Su, J.; Li, M.; Yang, H.; Shu, H.; Yu, K.; Cao, H.; Xu, G.; Wang, M.; Zhu, Y.; Zhu, Y.; et al. Enrichment of grape berries and tomato fruit with health-promoting tartaric acid by expression of the Vitis vinifera transketolase VvTK2 gene. Int. J. Biol. Macromol. 2024, 257, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ji, H.; Jiang, Q.; Liu, T.; Cao, H.; Zhang, Z. Effects of full shading of clusters from véraison to ripeness on fruit quality and volatile compounds in Cabernet Sauvignon grapes. Food Chem. X 2024, 21, 1256–1263. [Google Scholar]
- Chen, M.; Zhang, S.; Ren, Y.; Le, Z.; Li, L.; Sun, B. Effects of Different Brewing Technologies on Polyphenols and Aroma Components of Black Chokeberry Wine. Foods 2023, 12, 868. [Google Scholar] [CrossRef]
- Sultanova, N.; Bayramova, N.; Aliyeva, D.; Rastgou, M.; Huseynova, I. Induced changes in metabolic constituents of grapevine (Vitis vinifera L.) leaves infected with grapevine leafroll-associated virus-3. Physiol. Mol. Plant Pathol. 2019, 106, 57–63. [Google Scholar]
- Zhang, B.; Zhang, C.; Chen, J.; Zhao, C.; Du, Y.; Yang, Y.; Xie, X.; He, L.; Liu, S.; Shi, K. On-vine drying (passérillage) improves the quality of “Hutai No. 8” table grape wine: Focusing on phenolics, aromas, color and sensory attributes. Food Chem. 2025, 463, 975–986. [Google Scholar] [CrossRef]
- Dravie, E.E.; Kortei, N.K.; Essuman, E.K.; Tettey, C.O.; Boakye, A.A.; Hunkpe, G. Antioxidant, phytochemical and physicochemical properties of sesame seed (Sesamum indicum L). Sci. Afr. 2020, 8, 451–463. [Google Scholar]
- Shi, M.; Zhang, Y.; Zhang, T.; Zhang, W.; Wang, S.; Wei, M.; Wang, S.; Zhao, L. The NAC activator, MdNAC77L, regulates anthocyanin accumulation in red flesh apple. Hortic. Plant J. 2024, 45, 1221–1231. [Google Scholar]
- Shang, Y.-F.; Cao, H.; Ma, Y.-L.; Zhang, C.; Ma, F.; Wang, C.-X.; Ni, X.-L.; Lee, W.-J.; Wei, Z.-J. Effect of lactic acid bacteria fermentation on tannins removal in Xuan Mugua fruits. Food Chem. 2019, 274, 118–122. [Google Scholar]
- Xiao, Q.; Ye, S.; Wang, H.; Xing, S.; Zhu, W.; Zhang, H.; Zhu, J.; Pu, C.; Zhao, D.; Zhou, Q.; et al. Soluble sugar, organic acid and phenolic composition and flavor evaluation of plum fruits. Food Chem. X 2024, 24, 232–239. [Google Scholar]
- Hadidi, A.; Randles, J.W. Viroids, and the Legacy of Ricardo Flores (1947–2020). Cells 2021, 10, 2570. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.D.; Koonin, E.V. Viroids and Viroid-like Circular RNAs: Do They Descend from Primordial Replicators? Life 2022, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Pallas, V.; García, J.A. How do plant viruses induce disease? Interactions and interference with host components. J. Gen. Virol. 2011, 92, 2691–2705. [Google Scholar] [CrossRef] [PubMed]
- Owens, R.A.; Tech, K.B.; Shao, J.Y.; Sano, T.; Baker, C.J. Global Analysis of Tomato Gene Expression During Potato spindle tuber viroid Infection Reveals a Complex Array of Changes Affecting Hormone Signaling. e-Xtra 2011, 25, 583–595. [Google Scholar] [CrossRef]
- Leng, X.; Li, J.; Miao, W.; Liu, Y.; Haider, M.S.; Song, M.; Li, Q. Comparison of physicochemical characteristics, antioxidant and immunomodulatory activities of polysaccharides from wine grapes. Int. J. Biol. Macromol. 2023, 239, 589–595. [Google Scholar] [CrossRef]
- Montero, R.; Mundy, D.; Albright, A.; Grose, C.; Trought MC, T.; Cohen, D.; Chooi, K.M.; MacDiarmid, R.; Flexas, J.; Bota, J. Effects of Grapevine Leafroll associated Virus 3 (GLRaV-3) and duration of infection on fruit composition and wine chemical profile of Vitis vinifera L. cv. Sauvignon blanc. Food Chem. 2016, 197, 1177–1183. [Google Scholar]
- Saenphon, C.; Ditcharoen, S.; Malai, C.; Saengprachatanarug, K.; Wongpichet, S.; Sirisomboon, P.; Saechua, W.; Khurnpoon, L.; Phuphaphud, A.; Maraphum, K.; et al. Total soluble solids, dry matter content prediction and maturity stage classification of durian fruit using long-wavelength NIR reflectance. J. Food Compos. Anal. 2023, 124, 3421–3436. [Google Scholar]
- White, A.C.; Rogers, A.; Rees, M.; Osborne, C.P. How can we make plants grow faster? A source–sink perspective on growth rate. J. Exp. Bot. 2016, 67, 31–45. [Google Scholar] [CrossRef]
- Lee, J.; Rennaker, C.D.; Thompson, B.D.; Karasev, A.V. Influence of Grapevine red blotch virus (GRBV) on Idaho ‘Syrah’ grape composition. Sci. Hortic. 2021, 282, 548–557. [Google Scholar] [CrossRef]
- DeBolt, S.; Cook, D.R.; Ford, C.M. L-Tartaric acid synthesis from vitamin C in higher plants. PNAS 2006, 103, 5608–5613. [Google Scholar]
- Borsani, J.; Budde, C.O.; Porrini, L.; Lauxmann, M.A.; Lombardo, V.A.; Murray, R.; Andreo, C.S.; Drincovich, M.F.; Lara, M.V. Carbon metabolism of peach fruit after harvest: Changes in enzymes involved in organic acid and sugar level modifications. J. Exp. Bot. 2009, 60, 1823–1837. [Google Scholar]
- Wang, Y.; Zhang, Z.; Wang, X.; Yuan, X.; Wu, Q.; Chen, S.; Zou, Y.; Ma, F.; Li, C. Exogenous dopamine improves apple fruit quality via increasing flavonoids and soluble sugar contents. Sci. Hortic. 2021, 280, 346–361. [Google Scholar]


| Grape Variety | Berry | Wine | |||||
|---|---|---|---|---|---|---|---|
| Weight | Shape Index | pH | TSS | pH | Alcoholic Strength | ||
| Merlot | UF-Me | 1.21 ± 0.33 | 1.04 ± 0.05 | 4.22 ± 0.08 | 23.13 ± 0.48 | 3.80 ± 0.05 | 10.00 ± 0.00 |
| GYSVd1 | 1.09 ± 0.26 ns | 1.01 ± 0.03 ns | 3.96 ± 0.08 *** | 22.05 ± 0.92 * | 3.95 ± 0.07 *** | 11.04 ± 0.83 ns | |
| Cabernet Sauvignon | UF-CS | 1.11 ± 0.31 | 0.99 ± 0.02 | 4.07 ± 0.01 | 24.07 ± 0.13 | 3.96 ± 0.01 | 8.80 ± 0.10 |
| GYSVd1 | 1.35 ± 0.20 ns | 1.01 ± 0.03 ns | 4.03 ± 0.13 ns | 21.42 ± 2.15 *** | 4.03 ± 0.06 ** | 9.92 ± 0.50 ns | |
| Syrah | UF-Sy | 1.91 ± 0.26 | 0.97 ± 0.04 | 3.77 ± 0.01 | 21.60 ± 0.26 | 3.70 ± 0.01 | 11.57 ± 0.15 |
| GYSVd1 | 1.66 ± 0.38 ns | 0.95 ± 0.04 ns | 3.76 ± 0.05 ns | 23.10 ± 1.07 *** | 3.77 ± 0.05 *** | 10.82 ± 0.67 ns | |
| Chardonnay | UF-Ch | 1.43 ± 0.19 | 1.00 ± 0.04 | 3.54 ± 0.01 | 20.66 ± 0.34 | 3.92 ± 0.01 | 11.03 ± 0.25 |
| GYSVd1 | 1.44 ± 0.26 ns | 0.99 ± 0.02 ns | 3.88 ± 0.15 ns | 23.16 ± 0.05 *** | 3.90 ± 0.18 ns | 9.42 ± 1.17 ns | |
| Welsch Riesling | UF-WR | 1.05 ± 0.39 | 0.97 ± 0.05 | 4.35 ± 0.03 | 25.90 ± 0.68 | 4.24 ± 0.02 | 9.97 ± 0.25 |
| GYSVd1 | 1.16 ± 0.25 ns | 0.95 ± 0.04 ns | 4.30 ± 0.06 *** | 20.80 ± 0.79 *** | 4.27 ± 0.12 ns | 7.42 ± 0.64 ns | |
| Riesling Weiss | UF-RW | 1.14 ± 0.31 | 0.98 ± 0.04 | 4.33 ± 0.09 | 20.57 ± 0.30 | 3.95 ± 0.01 | 11.57 ± 0.15 |
| GYSVd1 | 1.22 ± 0.31 ns | 1.00 ± 0.03 ns | 4.21 ± 0.12 ns | 19.85 ± 1.70 ns | 3.93 ± 0.08 ns | 10.82 ± 0.67 ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Liu, S.; Wang, P.; Li, Z.; Zhang, J.; Du, Y.; Zhu, S. Viroid GYSVd1 Exhibited Different Regulations on the Qualities of Berries and Wines from 6 Grape Varieties. Horticulturae 2025, 11, 345. https://doi.org/10.3390/horticulturae11040345
Wu M, Liu S, Wang P, Li Z, Zhang J, Du Y, Zhu S. Viroid GYSVd1 Exhibited Different Regulations on the Qualities of Berries and Wines from 6 Grape Varieties. Horticulturae. 2025; 11(4):345. https://doi.org/10.3390/horticulturae11040345
Chicago/Turabian StyleWu, Menghuan, Shuo Liu, Ping Wang, Zhaotan Li, Junbo Zhang, Yejuan Du, and Shuhua Zhu. 2025. "Viroid GYSVd1 Exhibited Different Regulations on the Qualities of Berries and Wines from 6 Grape Varieties" Horticulturae 11, no. 4: 345. https://doi.org/10.3390/horticulturae11040345
APA StyleWu, M., Liu, S., Wang, P., Li, Z., Zhang, J., Du, Y., & Zhu, S. (2025). Viroid GYSVd1 Exhibited Different Regulations on the Qualities of Berries and Wines from 6 Grape Varieties. Horticulturae, 11(4), 345. https://doi.org/10.3390/horticulturae11040345







