Fruit Characteristics of In Situ Collected Sweet Cherry (Prunus avium L.) Genotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Protocol and Research Area
2.2. Physical Traits of the Fruit and Stone
2.3. Chemical Composition
2.4. Statistical Analysis
3. Results and Discussion
3.1. Physical Traits of the Fruit and Stone
3.2. Chemical Composition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ducci, F.; De Cuyper, B.; De Rogatis, A.; Dufour, J.; Santi, F. Wild cherry breeding (Prunus avium L.). In Forest Tree Breeding in Europe: Current State-of-the-Art and Perspectives; Pâques, L., Ed.; Managing Forest Ecosystems; Springer: Dordrecht, The Netherlands, 2013; pp. 463–511. [Google Scholar]
- Dangi, G.; Singh, D.; Chauhan, N.; Dogra, R.; Verma, P.; Sharma, S. Characterization of Selected Sweet Cherry (Prunus avium L.) Varieties using DUS Test Guidelines. Indian J. Plant Genet. Resour. 2021, 34, 290–294. [Google Scholar] [CrossRef]
- Karlidag, H.; Ercisli, S.; Sengul, M.; Tosun, M. Physico-Chemical Diversity in Fruits of Wild-Growing Sweet Cherries (Prunus Avium L.). Biotechnol. Biotechnol. Equip. 2009, 23, 1325–1329. [Google Scholar]
- Sansavini, S.; Lugli, S. Sweet Cherry Breeding Programs in Europe and Asia. Acta Hortic. 2008, 795, 41–58. [Google Scholar]
- Pérez Sánchez, R.; Gómez Sánchez, M.A.; Morales Corts, R. Agromorphological characterization of traditional Spanish sweet cherry (Prunus avium L.), sour cherry (Prunus cerasus L.) and duke cherry (Prunus × gondouinii Rehd.) cultivars. Span. J. Agric. Res. 2008, 6, 42–55. [Google Scholar]
- Benková, M.; Čičová, I.; Benedikova, D.; Mendel, L.; Glasa, M. Variability of Old Sweet Cherries Found in Slovak Regions and Their Preservation. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2017, 71, 184–189. [Google Scholar]
- El Baji, M.; Hanine, H.; En-Nahli, S.; Socias I Company, R.; Kodad, O. Morphological and Pomological Characteristics of Sweet Cherry (Prunus Avium L.) Grown In-situ under South Mediterranean Climate in Morocco. Int. J. Fruit Sci. 2021, 21, 52–65. [Google Scholar]
- Budan, S.; Petre, L.; Gradinariu, G. Evaluation of Some Native Sweet Cherry Genotypes Collected Ex Situ into Romanian National Germplasm. Acta Hortic. 2009, 814, 157–160. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. 2023. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 16 January 2025).
- Quero-García, J.; Lezzoni, A.; Puławska, J.; Lang, G. Cherries: Botany, Production and Uses; CABI: Wallingford, UK, 2017; Available online: https://www.cabi.org/bookshop/book/9781780648378 (accessed on 16 January 2025).
- Karaat, F.E.; Gündüz, K.; Saraçoğlu, O.; Yıldırım, H. Pomological and phytochemical evaluation of different cherry species: Mahaleb (Prunus mahaleb L.), wild sweet cherry (Prunus avium L.) and wild sour cherry (Prunus cerasus L.), sweet and sour cherry cultivars. Acta Sci. Pol. Hortorum Cultus 2019, 18, 181–191. [Google Scholar]
- Rakonjac, V.; Mratinic, E.; Jovkovic, R.; Fotiric, A.M. Analysis of morphological variability in wild cherry (Prunus avium L.) genetic resources from Central Serbia. J. Agric. Sci. Technol. 2014, 16, 151–162. [Google Scholar]
- Gregorius, H.R.; Kownatzki, D.; Höltken, A.M. Spatial patterns of mating relations in wild cherry (Prunus avium L.). Perspect. Plant Ecol. Evol. Syst. 2011, 13, 37–45. [Google Scholar]
- Webster, A.; Looney, N. Cherries: Crop Physiology, Production and Uses; CAB International: Wallingford, UK, 1995. [Google Scholar]
- Jensen, M. Processing for Industrial Uses. In Cherries: Botany, Production and Uses; Quero-García, J., Lezzoni, A., Puławska, J., Lang, G., Eds.; CABI: Boston, MA, USA, 2017; pp. 485–505. [Google Scholar]
- Ferraj, B.; Hodaj, B.; Shahini, Z.; Kukali, E.; Susaj, L. Evaluation of autochthonous cherry cultivars ‘Zhitome’ and ‘Red Belice’. Nat. Montenegrina 2010, 9, 1007–1012. [Google Scholar]
- Di Matteo, A.; Russo, R.; Graziani, G.; Ritieni, A.; Di Vaio, C. Characterization of autochthonous sweet cherry cultivars (Prunus avium L.) of southern Italy for fruit quality, bioactive compounds and antioxidant activity. J. Sci. Food Agric. 2016, 97, 2782–2794. [Google Scholar] [CrossRef] [PubMed]
- Perju, I.; Mineață, I.; Sîrbu, S.; Golache, I.E.; Ungureanu, I.V.; Jităreanu, C.D. Fruit Quality and Production Parameters of Some Bitter Cherry Cultivars. Horticulturae 2025, 11, 87. [Google Scholar] [CrossRef]
- Dulloo, M.E.; Hunter, D.; Borelli, T. Ex situ and in situ conservation of agricultural biodiversity: Major advances and research needs. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 123–135. [Google Scholar]
- Oprea, A.; Sîrbu, C.; Goia, I. The vegetation of the natural reserve Valea Fagilor-Luncaviţa (Tulcea County, Romania). Contrib. Bot. 2011, 46, 17–32. [Google Scholar]
- Ricardo-Rodrigues, S.; Laranjo, M.; Agulheiro-Santos, A.C. Methods for quality evaluation of sweet cherry. J. Sci. Food Agric. 2022, 103, 463–478. [Google Scholar] [PubMed]
- Dziadek, K.; Kopeć, A.; Piątkowska, E.; Leszczyńska, T.; Pisulewska, E.; Witkowicz, R.; Bystrowska, B.; Francik, R. Identification of polyphenolic compounds and determination of antioxidant activity in extracts and infusions of buckwheat leaves. Eur. Food Res. Technol. 2018, 244, 333–343. [Google Scholar]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Rop, O.; Jurikova, T.; Mlcek, J.; Kramarova, D. Sengee Antioxidant activity and selected nutritional values of plums (Prunus domestica L.) typical of the White Carpathian Mountains. Sci. Hortic. 2009, 122, 545–549. [Google Scholar] [CrossRef]
- Landau, S.; Chis Ster, I. Cluster Analysis: Overview. In International Encyclopedia of Education; Elsevier: Amsterdam, The Netherlands, 2010; pp. 72–83. [Google Scholar]
- Mratinic, E.; Fotiric-Aksic, M.; Jovkovic, R. Analysis of wild sweet cherry (Prunus avium L.) germplasm diversity in south-east Serbia. ABI Genet. 2012, 44, 259–268. [Google Scholar]
- Ganopoulos, I.; Moysiadis, T.; Xanthopoulou, A.; Ganopoulou, M.; Avramidou, E.; Aravanopoulos, F.A.; Tani, E.; Madesis, P.; Tsaftaris, A.; Kazantzis, K. Diversity of morpho-physiological traits in worldwide sweet cherry cultivars of GeneBank collection using multivariate analysis. Sci. Hortic. 2015, 197, 381–391. [Google Scholar] [CrossRef]
- Bujdosó, G.; Hrotkó, K.; Feldmane, D.; Giovannini, D.; Demirsoy, H.; Tao, R.; Malchev, S. What kind of sweet cherries do the final consumers prefer. S.-West. J. Hortic. Biol. Environ. 2020, 11, 37–48. [Google Scholar]
- Chockchaisawasdee, S.; Golding, J.B.; Vuong, Q.V.; Papoutsis, K.; Stathopoulos, C.E. Sweet cherry: Composition, postharvest preservation, processing and trends for its future use. Trends Food Sci. Technol. 2016, 55, 72–83. [Google Scholar] [CrossRef]
- Garcia Montiel, F.; Serrano, M.; Martinez-Romero, D.; Alburquerque, N. Factors influencing fruit set and quality in different sweet cherry cultivars. Span. J. Agric. Res. 2010, 8, 1118–1128. [Google Scholar] [CrossRef]
- Hayaloglu, A.A.; Demir, N. Phenolic compounds, volatiles, and sensory characteristics of twelve sweet cherry (Prunus avium L.) cultivars grown in Turkey. J. Food Sci. 2016, 81, 7–18. [Google Scholar] [CrossRef]
- Perju, I.; Mineaţă, I.; Ungureanu, I.V.; Sîrbu, S.; Golache, I.E.; Iurea, E. Agroproductive Evaluation of Some Sweet Cherry Cultivars in the Pedoclimatic Conditions of NE Romania. Sci. Pap. Ser. B. Hortic. 2024, 68, 105–110. [Google Scholar]
- Gonçalves, B.; Landbo, A.K.; Knudsen, D.; Silva, A.P.; Moutinho-Pereira, J.; Rosa, E.; Meyer, A.S. Effect of Ripeness and Postharvest Storage on the Phenolic Profiles of Cherries (Prunus avium L.). J. Agric. Food Chem. 2004, 52, 523–530. [Google Scholar]
- Ferretti, G.; Bacchetti, T.; Belleggia, A.; Neri, D. Cherry Antioxidants: From Farm to Table. Molecules 2010, 15, 6993–7005. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Craciunescu, O.; Seciu-Grama, A.-M.; Mihai, E.; Utoiu, E.; Negreanu-Pirjol, T.; Lupu, C.E.; Artem, V.; Ranca, A.; Negreanu-Pirjol, B.-S. The Chemical Profile, Antioxidant, and Anti-Lipid Droplet Activity of Fluid Extracts from Romanian Cultivars of Haskap Berries, Bitter Cherries, and Red Grape Pomace for the Management of Liver Steatosis. Int. J. Mol. Sci. 2023, 24, 16849. [Google Scholar] [CrossRef]
- Sirbu, S.; Oprica, L.; Poroch, V.; Iurea, E.; Corneanu, M.; Grigore, M.N. Physical Parameters, Total Phenolics, Flavonoids and Vitamin C Content of Nine Sweet Cherry Cultivars. Rev. Chim. 2018, 69, 125–129. [Google Scholar] [CrossRef]
- Usenik, V.; Fabčič, J.; Štampar, F. Sugars, organic acids, phenolic composition and antioxidant activity of sweet cherry (Prunus avium L.). Food Chem. 2008, 107, 185–192. [Google Scholar] [CrossRef]
- Cosmulescu, S.N.; Trandafir, I.; Cornescu, F. Antioxidant Capacity, Total Phenols, Total Flavonoids and Colour Component of Cornelian Cherry (Cornus mas L.) Wild Genotypes. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 47, 390–394. [Google Scholar]
- Farsad, A.; Esna-Ashari, M. Genetic diversity of some Iranian sweet cherry (Prunus avium) cultivars using microsatellite markers and morphological traits. Cytol. Genet. 2016, 50, 8–19. [Google Scholar] [CrossRef]
- Rodrigues, L.C.; Morales, M.R.; Fernandes, A.J.B.; Ortiz, J.M. Morphological characterization of sweet and sour cherry cultivars in a germplasm bank at Portugal. Genet. Resour. Crop Evol. 2007, 55, 593–601. [Google Scholar] [CrossRef]
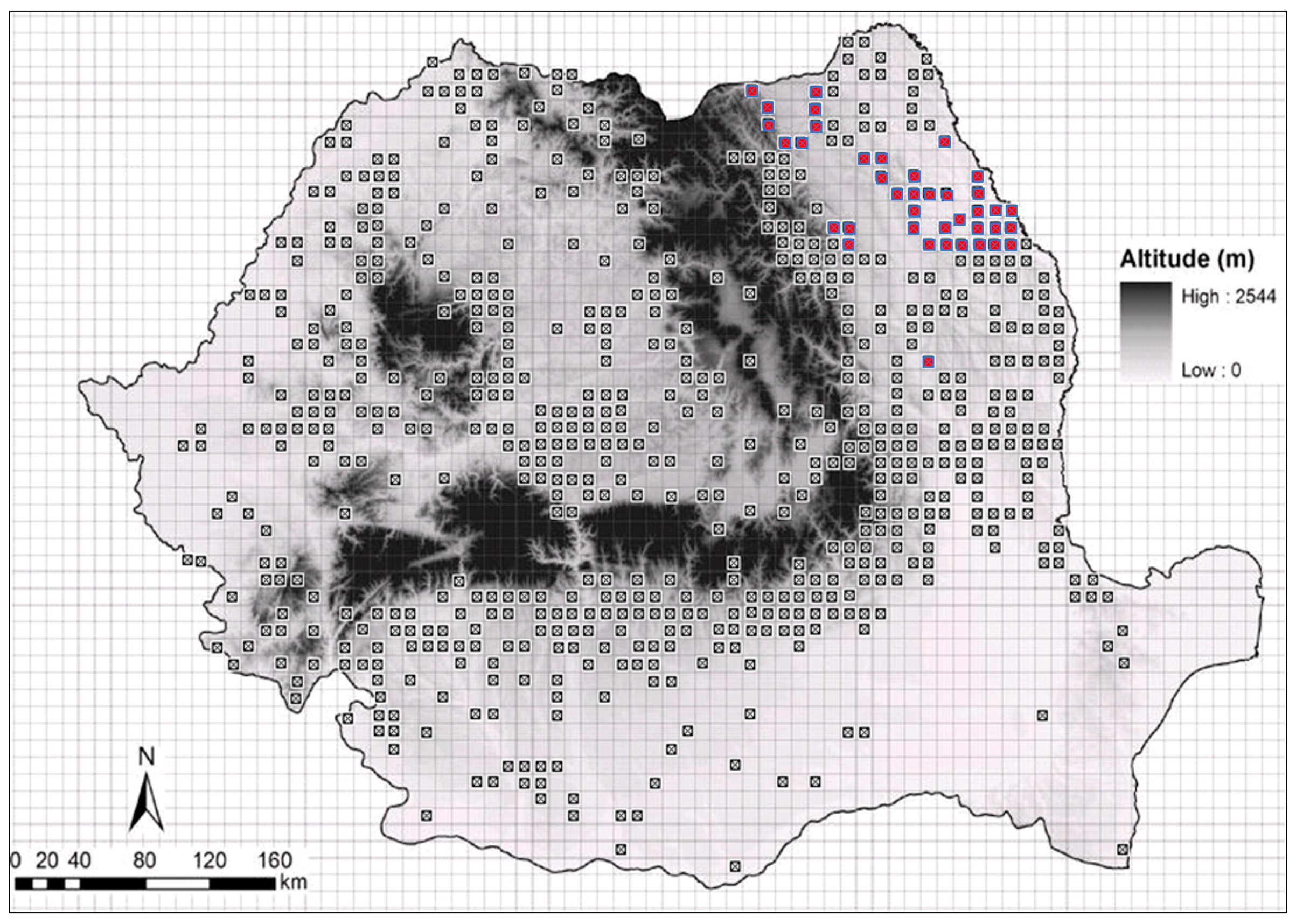
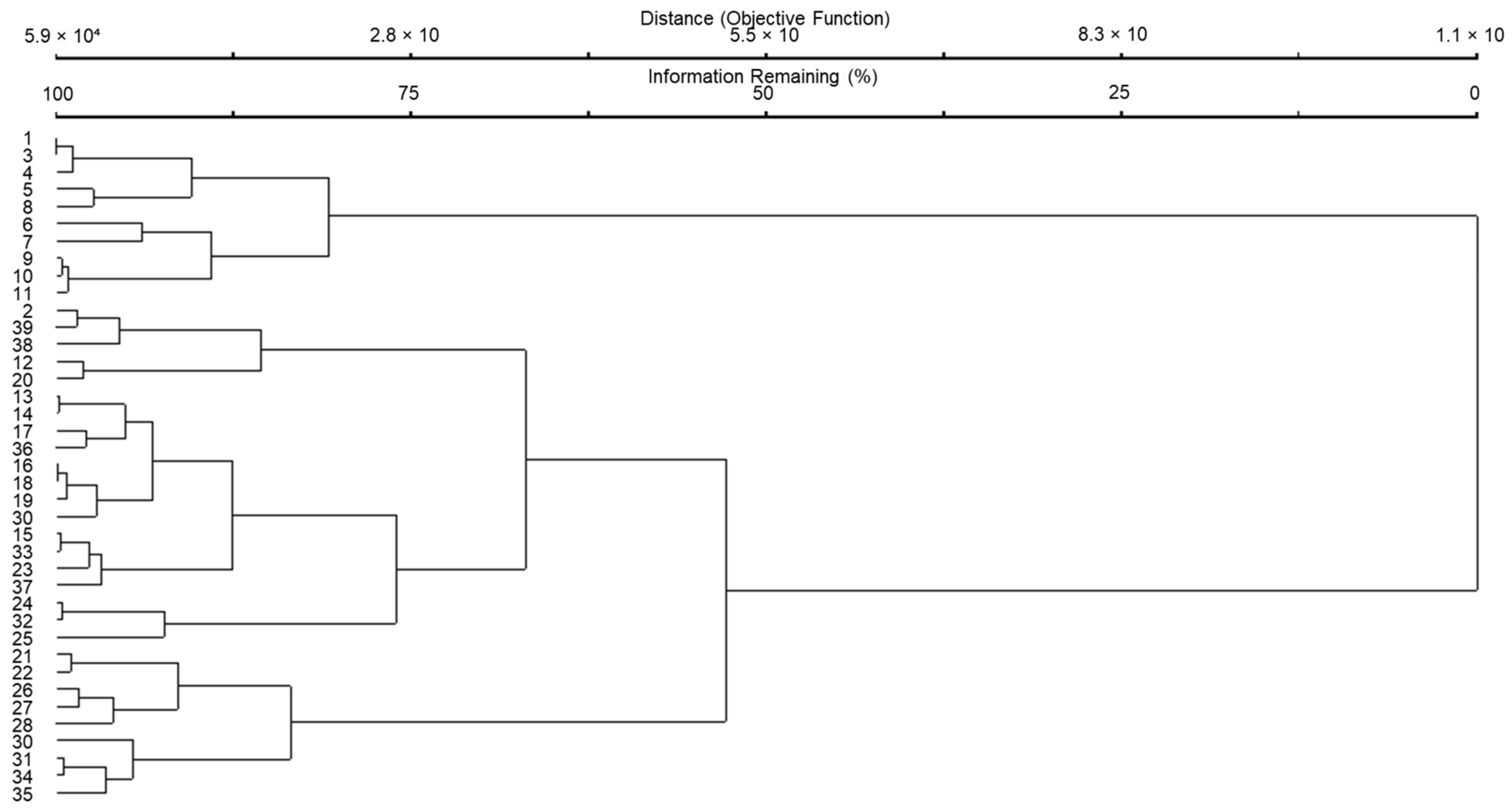
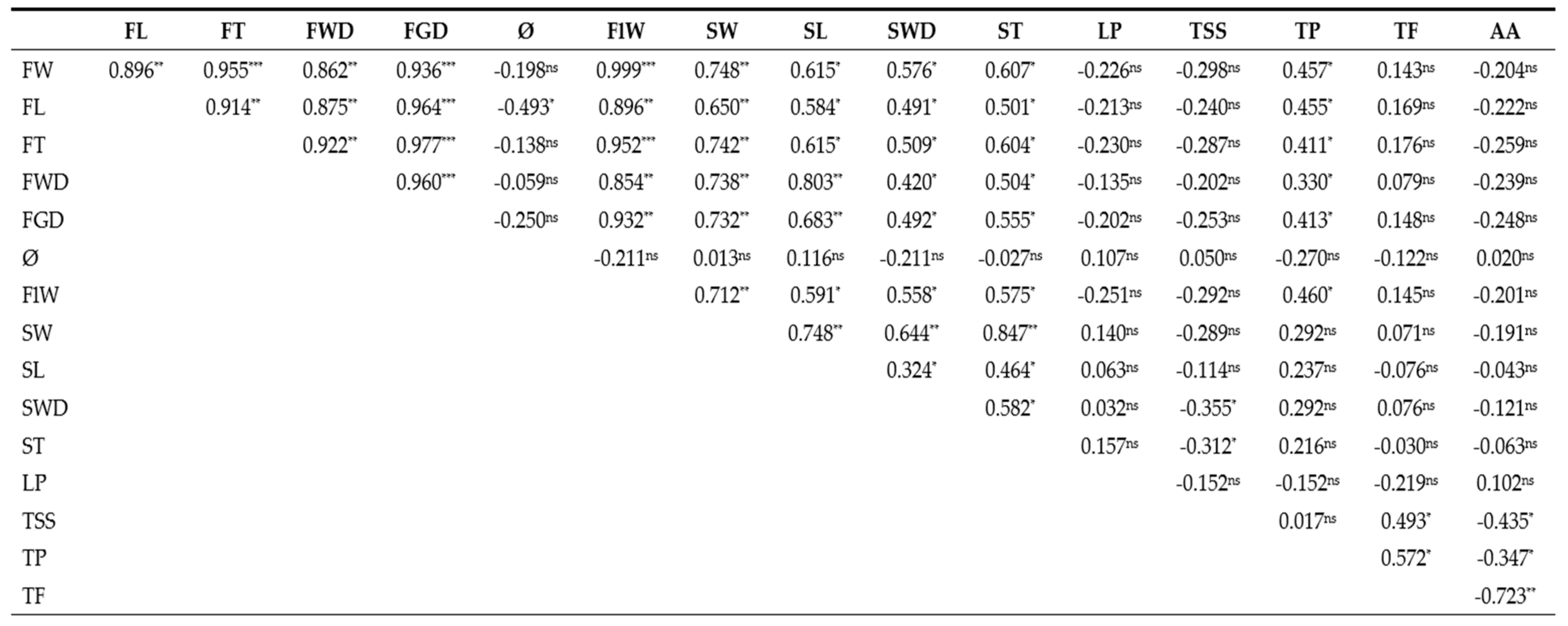
| Genotype | Location | Genotype | Location | Genotype | Location |
|---|---|---|---|---|---|
| G1 | Vaslui-Hupca (46°24′ N, 27°42′ E) | G14 | Suceava-Mariței (47°45′ N, 26°08′ E) | G27 | Iasi-Miroslava (47°07′ N, 27°31′ E) |
| G2 | Vaslui-Hupca (46°24′ N, 27°42′ E) | G15 | Suceava-Șerbăuți (47°49′ N, 26°08′ E) | G28 | Iasi-Miroslava (47°07′ N, 27°31′ E) |
| G3 | Vaslui-Hupca (46°24′ N, 27°42′ E) | G16 | Suceava-Dărmănești (47°44′ N, 26°06′ E) | G29 | Iasi-Miroslava (47°07′ N, 27°31′ E) |
| G4 | Vaslui-Hupca (46°24′ N, 27°42′ E) | G17 | Suceava (47°39′ N, 26°15′ E) | G30 | Suceava-Roșiori (47°21′ N, 26°24′ E) |
| G5 | Vaslui-Bîrlad (46°13′ N, 27°40′ E) | G18 | Suceava-Putna (47°52′ N, 25°36′ E) | G31 | Iași-Breazu (47°13′ N, 27°31′ E) |
| G6 | Vaslui-Bîrlad (46°13′ N, 27°40′ E) | G19 | Suceava-Călinești (47°46′ N, 26°08′ E) | G32 | Iași-Breazu 4 (47°13′ N, 27°31′ E) |
| G7 | Neamț-Căciulești (46°57′ N, 26°28′ E) | G20 | Vaslui-Bunești (46°49′ N, 27°58′ E) | G33 | Iași-Breazu 3 (47°13′ N, 27°31′ E) |
| G8 | Vrancea-Dragosloveni (45°33′ N, 27°04′ E) | G21 | Iași-Dacia 1 (47°09′ N, 27°35′ E) | G34 | Iasi-Breazu 1 (47°13′ N, 27°31′ E) |
| G9 | Neamt-Piatra Neamt (46°55′ N, 26°22′ E) | G22 | Iași-Dacia 2 (47°09′ N, 27°35′ E) | G35 | Vaslui-Floreni (46°14′ N, 27°58′ E) |
| G10 | Neamt-Piatra Neamt (46°55′ N, 26°22′ E) | G23 | Iasi-Miroslava (47°07′ N, 27°31′ E) | G36 | Iasi-Breazu 4.1 (47°13′ N, 27°31′ E) |
| G11 | Iași-Prisăcani (47°04′ N, 27°52′ E) | G24 | Iasi-Miroslava (47°07′ N, 27°31′ E) | G37 | Iasi-GB Iasi (47°04′ N, 27°37′ E) |
| G12 | Iași-Comarna (47°04′ N, 27°48′ E) | G25 | Iasi-Miroslava (47°07′ N, 27°31′ E) | G38 | Iasi-Miroslava R1PII (47°07′ N, 27°31′ E) |
| G13 | Suceava-Călinești (47°46′ N, 26°08′ E) | G26 | Iasi-Miroslava (47°07′ N, 27°31′ E) | G39 | Iasi-Maxut (47°26′ N, 26°53′ E) |
| Genotype | FW | FL | FT | FWD | FGD | Ø | FlW | SW | SL | SWD | ST | LP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (g) | (mm) | (mm) | (mm) | (mm) | (mm2) | (g) | (g) | (mm) | (mm) | (mm) | (mm) | |
| G1 | 1.97 | 15.92 | 13.56 | 15.03 | 14.76 | 0.93 | 1.79 | 0.18 | 9.45 | 7.24 | 5.38 | 45.00 |
| G2 | 2.56 | 15.72 | 15.06 | 15.82 | 15.49 | 0.99 | 2.31 | 0.25 | 9.33 | 7.99 | 6.36 | 45.10 |
| G3 | 1.97 | 16.52 | 12.91 | 15.34 | 14.81 | 0.90 | 1.77 | 0.20 | 9.82 | 7.20 | 5.51 | 43.00 |
| G4 | 2.15 | 12.07 | 13.99 | 14.33 | 13.39 | 1.11 | 1.96 | 0.19 | 8.58 | 7.42 | 6.32 | 45.00 |
| G5 | 1.40 | 16.56 | 11.61 | 12.54 | 13.37 | 0.81 | 1.23 | 0.17 | 8.37 | 7.18 | 5.68 | 45.67 |
| G6 | 2.60 | 18.51 | 14.89 | 15.45 | 16.16 | 0.87 | 2.40 | 0.20 | 8.74 | 7.56 | 5.98 | 45.20 |
| G7 | 3.04 | 18.51 | 16.07 | 15.27 | 16.51 | 0.89 | 2.75 | 0.29 | 9.48 | 8.70 | 6.87 | 40.72 |
| G8 | 1.69 | 13.79 | 13.10 | 13.22 | 13.33 | 0.97 | 1.45 | 0.24 | 8.70 | 7.92 | 6.57 | 48.14 |
| G9 | 2.93 | 16.55 | 15.19 | 15.89 | 15.82 | 0.96 | 2.61 | 0.32 | 9.52 | 8.46 | 6.94 | 45.28 |
| G10 | 3.20 | 18.52 | 16.58 | 15.05 | 16.61 | 0.90 | 2.93 | 0.27 | 8.88 | 8.51 | 6.51 | 45.00 |
| G11 | 3.20 | 18.12 | 16.28 | 16.57 | 16.92 | 0.93 | 2.94 | 0.26 | 9.60 | 8.24 | 6.17 | 44.90 |
| G12 | 2.32 | 17.06 | 14.28 | 14.06 | 15.03 | 0.88 | 2.13 | 0.19 | 8.22 | 7.54 | 6.00 | 36.89 |
| G13 | 1.49 | 13.26 | 12.53 | 12.25 | 12.64 | 0.95 | 1.27 | 0.22 | 8.45 | 7.95 | 6.06 | 45.00 |
| G14 | 1.79 | 13.54 | 13.14 | 13.69 | 13.42 | 0.99 | 1.59 | 0.20 | 8.65 | 7.75 | 5.84 | 45.00 |
| G15 | 1.15 | 11.97 | 11.23 | 11.84 | 11.65 | 0.97 | 0.99 | 0.16 | 8.45 | 6.99 | 5.30 | 36.28 |
| G16 | 1.28 | 11.74 | 11.32 | 13.54 | 12.13 | 1.03 | 1.08 | 0.20 | 9.14 | 6.74 | 5.46 | 44.11 |
| G17 | 1.56 | 13.61 | 12.14 | 12.47 | 12.69 | 0.93 | 1.41 | 0.15 | 7.68 | 6.72 | 5.37 | 46.81 |
| G18 | 1.53 | 12.52 | 12.27 | 12.74 | 12.48 | 1.00 | 1.34 | 0.19 | 8.67 | 7.37 | 5.92 | 42.22 |
| G19 | 1.21 | 11.93 | 10.83 | 12.67 | 11.76 | 0.99 | 1.01 | 0.20 | 9.10 | 6.82 | 5.52 | 53.42 |
| G20 | 2.48 | 15.88 | 14.10 | 15.08 | 14.96 | 0.94 | 2.26 | 0.22 | 9.40 | 7.86 | 5.77 | 42.67 |
| G21 | 0.92 | 11.76 | 8.76 | 11.64 | 10.60 | 0.90 | 0.75 | 0.17 | 9.30 | 7.48 | 6.09 | 47.39 |
| G22 | 0.70 | 10.56 | 9.58 | 10.71 | 10.25 | 0.97 | 0.54 | 0.16 | 8.24 | 6.81 | 5.96 | 46.36 |
| G23 | 1.19 | 12.56 | 11.50 | 11.71 | 11.89 | 0.95 | 1.02 | 0.17 | 7.92 | 7.80 | 6.24 | 53.72 |
| G24 | 0.78 | 10.80 | 9.54 | 9.95 | 10.06 | 0.93 | 0.68 | 0.10 | 7.30 | 6.44 | 5.17 | 42.72 |
| G25 | 0.77 | 9.47 | 9.13 | 10.62 | 9.70 | 1.02 | 0.68 | 0.09 | 8.41 | 5.99 | 4.83 | 41.36 |
| G26 | 0.96 | 10.51 | 10.05 | 12.10 | 10.83 | 1.03 | 0.71 | 0.25 | 9.48 | 7.48 | 6.03 | 63.83 |
| G27 | 1.14 | 12.11 | 10.69 | 11.64 | 11.44 | 0.94 | 0.96 | 0.18 | 8.52 | 8.48 | 5.93 | 56.56 |
| G28 | 1.44 | 12.98 | 11.90 | 12.70 | 12.49 | 0.96 | 1.17 | 0.27 | 8.83 | 7.82 | 6.76 | 44.75 |
| G29 | 1.13 | 11.59 | 10.86 | 11.61 | 11.32 | 0.98 | 0.90 | 0.23 | 8.21 | 7.95 | 6.48 | 44.83 |
| G30 | 1.73 | 15.12 | 12.82 | 12.85 | 13.52 | 0.89 | 1.52 | 0.21 | 8.19 | 7.38 | 6.69 | 56.08 |
| G31 | 1.10 | 12.10 | 11.40 | 11.40 | 11.60 | 0.96 | 0.92 | 0.18 | 8.17 | 6.11 | 6.01 | 46.72 |
| G32 | 0.61 | 9.96 | 8.66 | 9.02 | 9.18 | 0.92 | 0.51 | 0.10 | 6.88 | 6.90 | 5.19 | 51.94 |
| G33 | 0.96 | 11.10 | 10.27 | 11.88 | 11.04 | 0.99 | 0.79 | 0.17 | 8.87 | 6.62 | 5.22 | 50.58 |
| G34 | 0.99 | 12.10 | 10.15 | 11.61 | 11.23 | 0.93 | 0.84 | 0.15 | 7.93 | 6.80 | 5.12 | 55.00 |
| G35 | 1.26 | 12.19 | 11.40 | 12.26 | 11.91 | 0.98 | 1.12 | 0.14 | 8.50 | 6.91 | 5.48 | 44.92 |
| G36 | 1.48 | 13.51 | 12.61 | 12.68 | 12.89 | 0.95 | 1.35 | 0.13 | 7.88 | 6.30 | 5.59 | 36.33 |
| G37 | 1.00 | 11.59 | 9.81 | 10.77 | 10.67 | 0.92 | 0.87 | 0.13 | 7.31 | 8.83 | 4.89 | 40.47 |
| G38 | 2.82 | 16.70 | 15.38 | 16.52 | 16.14 | 0.97 | 2.50 | 0.32 | 9.84 | 7.75 | 7.10 | 52.47 |
| G39 | 2.65 | 21.17 | 18.49 | 21.48 | 20.27 | 0.96 | 2.36 | 0.29 | 10.49 | 7.75 | 6.32 | 46.33 |
| Average | 1.67 | 13.85 | 12.41 | 13.23 | 13.10 | 0.95 | 1.47 | 0.20 | 8.68 | 7.43 | 5.91 | 46.35 |
| Min | 0.61 | 9.47 | 8.66 | 9.02 | 9.18 | 0.81 | 0.51 | 0.09 | 6.88 | 5.99 | 4.83 | 36.28 |
| Max | 3.20 | 21.17 | 18.49 | 21.48 | 20.27 | 1.11 | 2.94 | 0.32 | 10.49 | 8.83 | 7.10 | 63.83 |
| STDEV | 0.76 | 2.86 | 2.38 | 2.31 | 2.42 | 0.05 | 0.72 | 0.06 | 0.77 | 0.71 | 0.58 | 5.71 |
| COVAR | 45.44 | 20.62 | 19.15 | 17.45 | 18.44 | 5.53 | 48.72 | 28.96 | 8.85 | 9.58 | 9.84 | 12.32 |
| Genotype | TSS | TP | TF | AA |
|---|---|---|---|---|
| (°Brix) | (mg GAE·g−1 f.w.) | (mg EC·g−1 f.w.) | (%) | |
| G1 | 17.02 | 2.55 | 0.37 | 83.20 |
| G2 | 14.00 | 0.87 | 0.18 | 83.29 |
| G3 | 21.00 | 2.49 | 0.45 | 89.95 |
| G4 | 16.80 | 2.12 | 0.41 | 78.01 |
| G5 | 26.00 | 3.85 | 0.87 | 55.06 |
| G6 | 20.40 | 1.14 | 0.48 | 72.26 |
| G7 | 11.20 | 1.61 | 0.34 | 74.11 |
| G8 | 21.00 | 3.97 | 0.79 | 54.11 |
| G9 | 16.60 | 2.70 | 0.46 | 51.10 |
| G10 | 15.00 | 3.06 | 0.63 | 81.43 |
| G11 | 22.00 | 2.72 | 0.60 | 73.89 |
| G12 | 16.80 | 0.24 | 0.24 | 82.02 |
| G13 | 24.60 | 0.79 | 0.96 | 71.73 |
| G14 | 22.50 | 0.82 | 0.67 | 42.84 |
| G15 | 20.40 | 0.57 | 0.52 | 75.00 |
| G16 | 22.40 | 0.55 | 0.44 | 76.32 |
| G17 | 22.00 | 0.98 | 0.81 | 46.28 |
| G18 | 22.40 | 0.57 | 0.57 | 62.88 |
| G19 | 28.20 | 0.61 | 0.55 | 44.79 |
| G20 | 21.40 | 0.35 | 0.26 | 70.74 |
| G21 | 21.20 | 0.24 | 0.14 | 97.52 |
| G22 | 23.00 | 0.30 | 0.22 | 84.76 |
| G23 | 20.00 | 0.40 | 0.25 | 73.19 |
| G24 | 26.00 | 0.81 | 0.56 | 54.39 |
| G25 | 24.00 | 0.42 | 0.28 | 91.47 |
| G26 | 16.00 | 0.28 | 0.16 | 96.20 |
| G27 | 14.30 | 0.27 | 0.17 | 90.79 |
| G28 | 22.60 | 0.28 | 0.23 | 79.91 |
| G29 | 18.20 | 0.67 | 0.58 | 73.39 |
| G30 | 16.33 | 0.15 | 0.08 | 98.22 |
| G31 | 12.50 | 0.17 | 0.13 | 96.71 |
| G32 | 22.70 | 0.88 | 0.60 | 70.40 |
| G33 | 22.00 | 0.51 | 0.37 | 79.21 |
| G34 | 11.40 | 0.18 | 0.15 | 87.35 |
| G35 | 21.40 | 0.16 | 0.10 | 93.06 |
| G36 | 22.20 | 0.63 | 0.38 | 92.63 |
| G37 | 18.60 | 0.47 | 0.26 | 85.28 |
| G38 | 23.10 | 0.50 | 0.38 | 75.41 |
| G39 | 19.00 | 0.64 | 0.45 | 51.45 |
| Average | 19.90 | 1.04 | 0.41 | 75.39 |
| Min | 11.20 | 0.15 | 0.08 | 42.84 |
| Max | 28.20 | 3.97 | 0.96 | 98.22 |
| STDEV | 4.07 | 1.06 | 0.22 | 15.68 |
| COVAR | 20.44 | 101.58 | 54.27 | 20.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sîrbu, S.; Oprică, L.; Popovici, L.-F.; Sîrbu, C.; Mineață, I.; Ungureanu, I.V.; Golache, I.E. Fruit Characteristics of In Situ Collected Sweet Cherry (Prunus avium L.) Genotypes. Horticulturae 2025, 11, 340. https://doi.org/10.3390/horticulturae11030340
Sîrbu S, Oprică L, Popovici L-F, Sîrbu C, Mineață I, Ungureanu IV, Golache IE. Fruit Characteristics of In Situ Collected Sweet Cherry (Prunus avium L.) Genotypes. Horticulturae. 2025; 11(3):340. https://doi.org/10.3390/horticulturae11030340
Chicago/Turabian StyleSîrbu, Sorina, Lăcrămioara Oprică, Lucia-Florina Popovici, Culiţă Sîrbu, Iulia Mineață, Ionuț Vasile Ungureanu, and Iuliana Elena Golache. 2025. "Fruit Characteristics of In Situ Collected Sweet Cherry (Prunus avium L.) Genotypes" Horticulturae 11, no. 3: 340. https://doi.org/10.3390/horticulturae11030340
APA StyleSîrbu, S., Oprică, L., Popovici, L.-F., Sîrbu, C., Mineață, I., Ungureanu, I. V., & Golache, I. E. (2025). Fruit Characteristics of In Situ Collected Sweet Cherry (Prunus avium L.) Genotypes. Horticulturae, 11(3), 340. https://doi.org/10.3390/horticulturae11030340







