Effects of Different Irrigation Rates and Microbial Fertilizers on Inter-Root Soil Environment and Yield and Quality of Brassica chinensis L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Experimental Area
2.2. Experimental Materials and Methods
2.3. Measurement Indicators and Methods
2.3.1. Yield
2.3.2. Quality of B. chinensis
2.3.3. Soil Microbial Communities
2.3.4. Soil Enzyme Activity
2.4. Data Analysis
3. Results
3.1. Effects of Different Treatments on the Yield of B. chinensis
Yield of B. chinensis
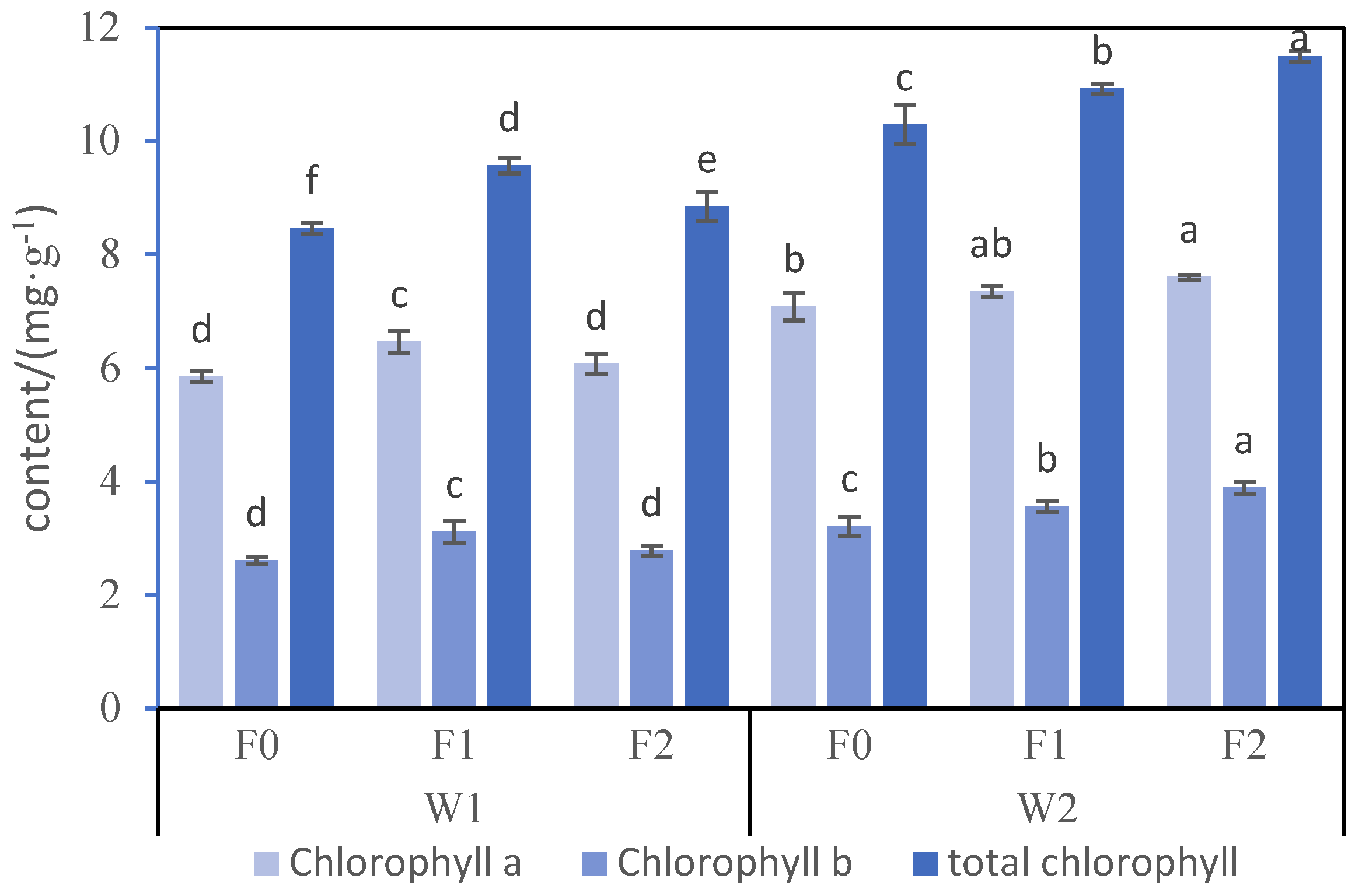
3.2. Chlorophyll in B. chinensis
3.3. Quality of B. chinensis
3.3.1. Vitamin C Content
3.3.2. Soluble Protein Content
3.3.3. Total Phenol Content
3.3.4. Nitrate Content
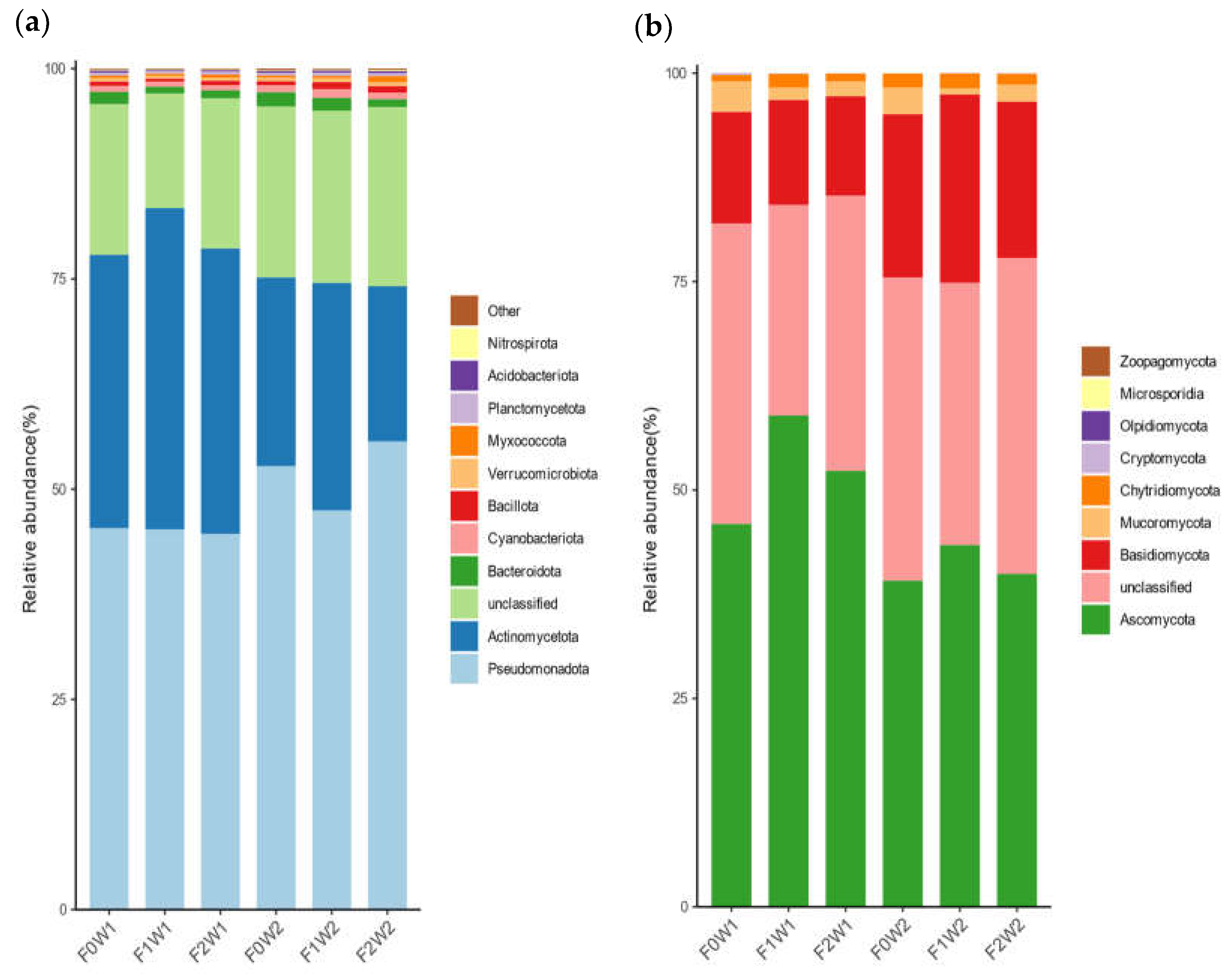
3.4. Soil Microbial Communities
3.5. Effect of Different Treatments on Soil Enzyme Activities
3.5.1. Sucrase Activity
3.5.2. Urease Activity
3.5.3. Catalase Activity
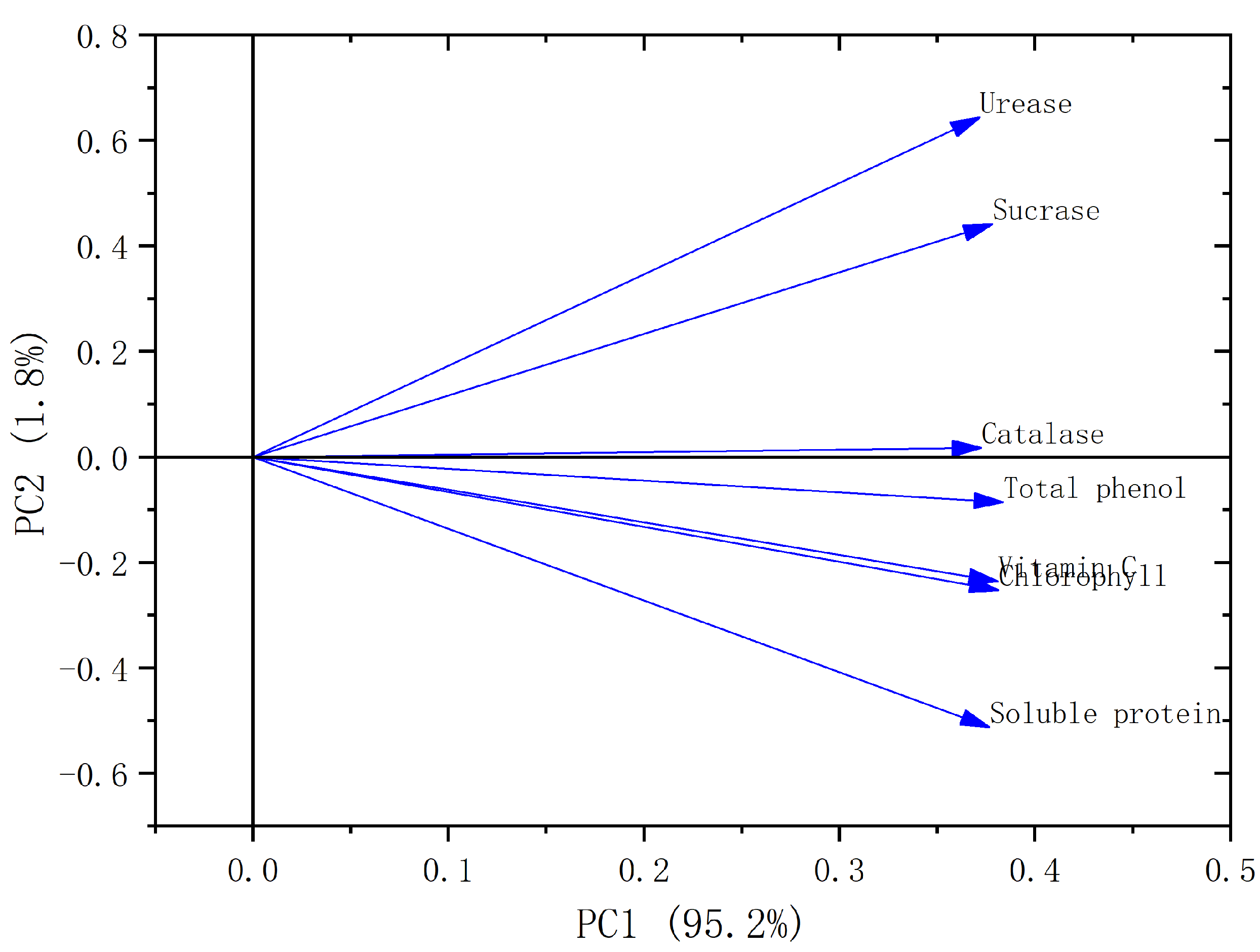
3.6. Principal Component Analysis
4. Discussion
4.1. Effects of Different Irrigation Rates and Microbial Fertilizers on Yield and Quality of B. chinensis
4.2. The Effect of Different Irrigation Rates and Microbial Fertilizer on Soil Microbial Activity
4.3. Impact of Varying Irrigation Rates and Microbial Fertilizers on Soil Enzyme Activities
4.4. Principal Component Analysis with Different Treatments
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mampholo, B.M.; Sivakumar, D.; Beukes, M.; van Rensburg, W.J. Effect of Modified Atmosphere Packaging on the Quality and Bioactive Compounds of Chinese Cabbage (Brasicca rapa L. ssp. chinensis). J. Sci. Food Agric. 2013, 93, 2008–2015. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Geng, S.; Wang, L.; Xing, J.; Fan, G.; Gao, Y.; Lu, X.; Zhang, Z. Effects of Modified Biochar on Growth, Yield, and Quality of Brassica chinensis L. in Cadmium Contaminated Soils. Plants 2025, 14, 524. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Chen, Y.; Dou, X.; Liao, D.; Li, K.; An, C.; Li, G.; Dong, Z. Microbial Fertilizers Improve Soil Quality and Crop Yield in Coastal Saline Soils by Regulating Soil Bacterial and Fungal Community Structure. Sci. Total Environ. 2024, 949, 175127. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.; Yuan, Z.; Feng, J.; Chen, S.; Sun, G.; Wei, Z.; Hu, T. Effects of Microbial Fertilizer and Irrigation Amount on Growth, Physiology and Water Use Efficiency of Tomato in Greenhouse. Sci. Hortic. 2024, 323, 112553. [Google Scholar] [CrossRef]
- Abdul-Halim, A.M.A.A.; Shivanand, P.; Krishnamoorthy, S.; Taha, H. A Review on the Biological Properties of Trichoderma spp. as a Prospective Biocontrol Agent and Biofertilizer. J. Appl. Biol. Biotechnol. 2023, 11, 34–46. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; He, P.; Chen, D.; Dai, X.; Jin, Q.; Su, X. The Optimal Irrigation Water Salinity and Salt Component for High-Yield and Good-Quality of Tomato in Ningxia. Agric. Water Manag. 2022, 274, 107940. [Google Scholar] [CrossRef]
- Yang, W.; Gong, T.; Wang, J.; Li, G.; Liu, Y.; Zhen, J.; Ning, M.; Yue, D.; Du, Z.; Chen, G. Effects of Compound Microbial Fertilizer on Soil Characteristics and Yield of Wheat (Triticum aestivum L). J. Soil. Sci. Plant Nutr. 2020, 20, 2740–2748. [Google Scholar] [CrossRef]
- Jin, N.; Jin, L.; Wang, S.; Li, J.; Liu, F.; Liu, Z.; Luo, S.; Wu, Y.; Lyu, J.; Yu, J. Reduced Chemical Fertilizer Combined with Bio-Organic Fertilizer Affects the Soil Microbial Community and Yield and Quality of Lettuce. Front. Microbiol. 2022, 13, 863325. [Google Scholar] [CrossRef]
- Ariza, M.T.; Miranda, L.; Gómez-Mora, J.A.; Medina, J.J.; Lozano, D.; Gavilán, P.; Soria, C.; Martínez-Ferri, E. Yield and Fruit Quality of Strawberry Cultivars Under Different Irrigation Regimes. Agronomy 2021, 11, 261. [Google Scholar] [CrossRef]
- Kuslu, Y.; Sahin, U.; Kiziloglu, F.M.; Memis, S. Fruit Yield and Quality, and Irrigation Water Use Efficiency of Summer Squash Drip-Irrigated with Different Irrigation Quantities in a Semi-Arid Agricultural Area. J. Integr. Agric. 2014, 13, 2518–2526. [Google Scholar] [CrossRef]
- Ma, Y.Z.; Pan, N.; Su, W.; Zhang, F.J.; Ye, G.J.; Pu, X.Q.; Zhou, Y.; Wang, J. Soil Water Stress Effects on Potato Tuber Starch Quality Formation. Potato Res. 2024, 67, 1829–1848. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, H.; Yang, C.; Li, H.; Wu, J. Effects of Water Stress on Nutrients and Enzyme Activity in Rhizosphere Soils of Greenhouse Grape. Front. Microbiol. 2024, 15, 1376849. [Google Scholar] [CrossRef]
- Khalaf, R.A.; Abdullah, K.M.; Khalaf, A.A.; Al-Fatlawi, M.M. Improving the Chemical Content and Mineral Elements of Drought Stressed (Moringa oleifera L.) by Application of Glutathione and Bio-Fertilizer. IOP Conf. Ser. Earth Environ. Sci. 2023, 1225, 012021. [Google Scholar] [CrossRef]
- Li, H.S. Modern Plant Physiology; Higher Education Press: Beijing, China, 2002; pp. 194–197. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Cintya, H.; Silalahi, J.; De Lux Putra, E.; Siburian, R. The Influence of Fertilizer on Nitrate, Nitrite and Vitamin C Contents in Vegetables. Orient. J. Chem. 2018, 34, 2614–2621. [Google Scholar] [CrossRef]
- Pang, L.; Wang, Y.; Ye, Y.; Zhou, Y.; Zhi, Q.; Lin, H. Metagenomic Analysis of Dental Plaque on Pit and Fissure Sites with and Without Caries Among Adolescents. Front. Cell Infect. Microbiol. 2021, 11, 740981. [Google Scholar] [CrossRef]
- Guan, S.Y.; Zhang, D.; Zhang, Z. Soil Enzyme and Its Research Methods; Chinese Agricultural Press: Beijing, China, 1986; pp. 274–297. [Google Scholar]
- Bai, X.; Liu, K.; Ning, T.; Deng, C.; Wang, L.; Li, D.; Wang, T.; Li, J. Effects of Multiple N, P, and K Fertilizer Combinations on Strawberry Growth and the Microbial Community. PLoS ONE 2023, 18, e0293088. [Google Scholar] [CrossRef]
- Sun, W.L.; Zhao, Y.G.; Yang, M. Microbial Fertilizer Improving the Soil Nutrients and Growth of Reed in Degraded Wetland. IOP Conf. Ser. Earth Environ. Sci. 2017, 69, 012062. [Google Scholar] [CrossRef]
- Nan, J.; Chao, L.; Ma, X.; Xu, D.; Mo, L.; Zhang, X.; Zhao, X.; Bao, Y. Microbial Diversity in the Rhizosphere Soils of Three Stipa Species from the Eastern Inner Mongolian Grasslands. Glob. Ecol. Conserv. 2020, 22, e00992. [Google Scholar] [CrossRef]
- Chang, E.-H.; Chung, R.-S.; Wang, F.-N. Effect of Different Types of Organic Fertilizers on the Chemical Properties and Enzymatic Activities of an Oxisol under Intensive Cultivation of Vegetables for 4 Years. Soil. Sci. Plant Nutr. 2008, 54, 587–599. [Google Scholar] [CrossRef]
- Al-Jiyashi, S.Z.S.; Noni, G.B. Effect of Scheduling Irrigation Water and Adding Organic Matter and Bio-Fertilizer on the Concentration of Some Nutrients in Wheat Plant (Triticum aestivum L.). IOP Conf. Ser. Earth Environ. Sci. 2023, 1225, 012001. [Google Scholar] [CrossRef]
- Nie, Z.; Wan, C.; Chen, C.; Chen, J. Comprehensive Evaluation of the Postharvest Antioxidant Capacity of Majiayou Pomelo Harvested at Different Maturities Based on PCA. Antioxidants 2019, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, X.; Gong, P.; Zhao, D.; Zhang, Y.; Wang, Z.; Zhang, J. Optimization of a Water-Saving and Fertilizer-Saving Model for Enhancing Xinjiang Korla Fragrant Pear Yield, Quality, and Net Profits under Water and Fertilizer Coupling. Sustainability 2022, 14, 8495. [Google Scholar] [CrossRef]
- Yao, L.; Kong, L.; Yang, Q.; Nian, H.; Liang, J. Analysis of Microbial Diversity in Rhizosphere Soil of Panax notoginseng under Different Water and Microbial Fertilizer Conditions. Agronomy 2024, 14, 922. [Google Scholar] [CrossRef]
- Li, X.; Lu, Q.; Li, D.; Wang, D.; Ren, X.; Yan, J.; Ahmed, T.; Li, B. Effects of Different Microbial Fertilizers on Growth and Rhizosphere Soil Properties of Corn in Newly Reclaimed Land. Plants 2022, 11, 1978. [Google Scholar] [CrossRef]
- Chen, C.; Lv, Q.; Tang, Q. Impact of Bio-Organic Fertilizer and Reduced Chemical Fertilizer Application on Physical and Hydraulic Properties of Cucumber Continuous Cropping Soil. Biomass Convers. Biorefin. 2022, 14, 921–930. [Google Scholar] [CrossRef]
- Fu, C.; Ma, W.; Qiang, B.; Jin, X.; Zhang, Y.; Wang, M. Effect of Chemical Fertilizer with Compound Microbial Fertilizer on Soil Physical Properties and Soybean Yield. Agronomy 2023, 13, 2488. [Google Scholar] [CrossRef]
- Kasprzyk, W.; Baranowska, M.; Korzeniewicz, R.; Behnke-Borowczyk, J.; Kowalkowski, W. Effect of Irrigation Dose on Powdery Mildew Incidence and Root Biomass of Sessile Oaks (Quercus petraea (Matt.) Liebl.). Plants 2022, 11, 1248. [Google Scholar] [CrossRef]
- Muñoz, V.; France, A.; Uribe, H.; Hirzel, J. Nitrogen and Irrigation Rates Affected Leaf Phosphorus and Potassium Concentrations in Different Cultivars of Pot-Grown Blueberry. J. Soil. Sci. Plant Nutr. 2023, 23, 965–973. [Google Scholar] [CrossRef]
- Nakielska, M.; Berbeć, A.K.; Madej, A.; Feledyn-Szewczyk, B. Microbial Fertilizing Products Impact on Productivity and Profitability of Organic Strawberry Cultivars. Horticulturae 2024, 10, 1112. [Google Scholar] [CrossRef]
- Sas-Paszt, L.; Sumorok, B.; Górnik, K.; Grzyb, Z.S.; Lisek, A.; Głuszek, S.; Trzciński, P.; Derkowska, E.; Frąc, M.; Treder, W.; et al. Influence of beneficial soil microorganisms and mineral fertilizers enriched with them on the flowering, fruiting, and physical and chemical parameters of the fruit of three-year-old strawberry plants in field cultivation. Hortic. Sci. 2023, 50, 112–126. [Google Scholar] [CrossRef]
- Caiyu, Z. Identification and Evaluation of the Characteristics of Local Varieties of Cabbage Without Balls; Zhejiang University: Hangzhou, China, 2018. [Google Scholar] [CrossRef]
- Teng, Z.; Yingying, X.; Kui, X. Effect of water and fertiliser regulation on potato tuber quality and water and fertiliser use efficiency. Northwest J. Agric. 2024, 33, 842–850. [Google Scholar]
- Tomar, V.; Topno, S.E.; Bahadur, V.; Kerketta, A. Effect of Organic, Inorganic and Biofertilizer on Growth, Yield and Quality of Bottle Gourd (Lagenaria siceraria Mol.). J. Adv. Biol. Biotechnol. 2024, 27, 683–691. [Google Scholar] [CrossRef]
- Jihadi, A.; Nikmatullah, A.; Sarjan, M. The Effect of Different Combinations of Biological Fertilizer and Different Doses of Chemical Fertilizer on the Severity of Late Blight on Potato Plants. IOP Conf. Ser. Earth Environ. Sci. 2023, 1220, 012023. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Chun, S.C.; Oh, J.W.; Paramasivan, M.; Saini, R.K.; Sahayarayan, J.J. Bacillus subtilis CBR05 for Tomato (Solanum lycopersicum) Fruits in South Korea as a Novel Plant Probiotic Bacterium (PPB): Implications from Total Phenolics, Flavonoids, and Carotenoids Content for Fruit Quality. Agronomy 2019, 9, 838. [Google Scholar] [CrossRef]
- Ali, D.F.I.; El-Nahrawy, S.; EL-Zawawy, H.A.H.; Omara, A.E.-D. Effective Applications of Bacillus subtilis and B. amyloliquefaciens as Biocontrol Agents of Damping-Off Disease and Biostimulation of Tomato Plants. Stresses 2025, 5, 9. [Google Scholar] [CrossRef]
- Sivojienė, D.; Masevičienė, A.; Žičkienė, L.; Ražukas, A.; Kačergius, A. Soil Microbial Community Structure and Carbon Stocks Following Fertilization with Organic Fertilizers and Biological Inputs. Biology 2024, 13, 534. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, D.; Ling, N.; Chen, F.; Fang, W.; Shen, Q. Bio-Organic Fertilizer Application Significantly Reduces the Fusarium oxysporum Population and Alters the Composition of Fungi Communities of Watermelon Fusarium Wilt Rhizosphere Soil. Biol. Fertil. Soils 2014, 50, 765–774. [Google Scholar] [CrossRef]
- Mozumder, A.B.; Chanda, K.; Chorei, R.; Prasad, H.K. An Evaluation of Aluminum Tolerant Pseudomonas aeruginosa A7 for In Vivo Suppression of Fusarium Wilt of Chickpea Caused by Fusarium oxysporum f. sp. ciceris and Growth Promotion of Chickpea. Microorganisms 2022, 10, 568. [Google Scholar] [CrossRef]
- Kumar, S.; Sindhu, S.S.; Kumar, R. Biofertilizers: An Ecofriendly Technology for Nutrient Recycling and Environmental Sustainability. Curr. Res. Microb. Sci. 2021, 3, 100094. [Google Scholar] [CrossRef]
- Shao, F.; Tao, W.; Yan, H.; Wang, Q. Effects of Microbial Organic Fertilizer (MOF) Application on Desert Soil Enzyme Activity and Jujube Yield and Quality. Agronomy 2023, 13, 2427. [Google Scholar] [CrossRef]
- Yang, C.Y.; Zhang, R.; Lin, B.J.; Dong, B.; Gao, Y.T. Effects of water stress on inter-root soil organic nitrogen and soil enzyme activities of delayed grape cultivation in facilities. Arid. Zone Res. 2021, 38, 1376–1384. [Google Scholar]
- Ali, A.; Liu, X.; Yang, W.; Li, W.; Chen, J.; Qiao, Y.; Gao, Z.; Yang, Z. Impact of Bio-Organic Fertilizer Incorporation on Soil Nutrients, Enzymatic Activity, and Microbial Community in Wheat–Maize Rotation System. Agronomy 2024, 14, 1942. [Google Scholar] [CrossRef]
- Caballero, P.; Macías-Benítez, S.; Moya, A.; Rodríguez-Morgado, B.; Martín, L.; Tejada, M.; Castaño, A.; Parrado Rubio, J. Biochemical and Microbiological Soil Effects of a Biostimulant Based on Bacillus licheniformis-Fermented Sludge. Agronomy 2022, 12, 1743. [Google Scholar] [CrossRef]
- Kumar, M.; Mishra, S.; Dixit, V.; Kumar, M.; Agarwal, L.; Chauhan, P.S.; Nautiyal, C.S. Synergistic effect of Pseudomonas putida and Bacillus amyloliquefaciens ameliorates drought stress in chickpea (Cicer arietinum L.). Plant Signal. Behav. 2016, 11, e1071004. [Google Scholar] [CrossRef]
- Brockett, B.F.T.; Prescott, C.E.; Grayston, S.J. Soil Moisture Is the Major Factor Influencing Microbial Community Structure and Enzyme Activities across Seven Biogeoclimatic Zones in Western Canada. Soil. Biol. Biochem. 2012, 44, 9–20. [Google Scholar] [CrossRef]
- Manici, L.M.; Caputo, F.; De Sabata, D.; Fornasier, F. The Enzyme Patterns of Ascomycota and Basidiomycota Fungi Reveal Their Different Functions in Soil. Appl. Soil. Ecol. 2024, 196, 105323. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, S.; Xue, M.; Wang, M.; Sun, H.; Li, H.; Han, Q.; Li, R. Effects of Different Irrigation Rates and Microbial Fertilizers on Inter-Root Soil Environment and Yield and Quality of Brassica chinensis L. Horticulturae 2025, 11, 321. https://doi.org/10.3390/horticulturae11030321
Guan S, Xue M, Wang M, Sun H, Li H, Han Q, Li R. Effects of Different Irrigation Rates and Microbial Fertilizers on Inter-Root Soil Environment and Yield and Quality of Brassica chinensis L. Horticulturae. 2025; 11(3):321. https://doi.org/10.3390/horticulturae11030321
Chicago/Turabian StyleGuan, Saisai, Mengyun Xue, Mengyang Wang, Hao Sun, Hui Li, Qibiao Han, and Rui Li. 2025. "Effects of Different Irrigation Rates and Microbial Fertilizers on Inter-Root Soil Environment and Yield and Quality of Brassica chinensis L." Horticulturae 11, no. 3: 321. https://doi.org/10.3390/horticulturae11030321
APA StyleGuan, S., Xue, M., Wang, M., Sun, H., Li, H., Han, Q., & Li, R. (2025). Effects of Different Irrigation Rates and Microbial Fertilizers on Inter-Root Soil Environment and Yield and Quality of Brassica chinensis L. Horticulturae, 11(3), 321. https://doi.org/10.3390/horticulturae11030321





