Abstract
Biostimulants such as seaweed extracts are emerging as crop management products that can enhance crop productivity and nutritional quality under abiotic stress conditions. Therefore, this study aimed to assess the effectiveness of a seaweed-derived biostimulant (Kelpak®) in alleviating salinity stress in spinach. A greenhouse experiment which consisted of five treatments (T1 = Control plants (no NaCl or seaweed extract (SWE), T2 = plants subjected to 300 mM NaCl without SWE, T3 = 300 mM NaCl + 1% dilution of SWE, T4 = 300 mM NaCl + 2.5% dilution of SWE, and T5 = 300 mM NaCl + 5% dilution of SWE) was conducted. The results showed that salinity without the addition of SWE reduced crop growth, relative water content, chlorophyll, and nutritional quality. Similarly, salinity induced severe oxidative stress, indicated by excessive amounts of superoxide radicals, malondialdehyde and the upregulation of catalase, peroxidase, polyphenols, and flavonoids. Interestingly, plants treated with 5% SWE displayed a substantial enhancement in crop performance, reduction in oxidative stress, and improved nutritional quality, characterised by considerable amounts of minerals, proximate constituents, and vitamins. These results support the use of seaweed extract (Kelpak®) as a biostimulant in enhancing growth and nutritional quality of spinach under saline cultivation.
1. Introduction
Salinity is one of the major factors limiting the growth and yield of vegetable crops worldwide [1,2]. While exact measures remain elusive, the prevalence of salinity-affected soil is on the rise, particularly in irrigated areas [3]. This has been attributed to the overuse of low-quality water and extensive irrigation coupled with intensive farming and inadequate drainage systems, resulting in the build-up of soluble salt ions in the soil [4]. These salt ions impede the ability of the plant roots to absorb water, causing osmotic stress and nutritional deficiencies that disrupt essential physiological processes such as photosynthesis and tissue hydration, and may also trigger oxidative stress and membrane damage, leading to diminished plant biomass [5].
To curb the devastating effects of salinity on food crops, farmers have been using synthetic chemical amendments for the amelioration of saline and sodic soils [6]. However, this process has become expensive, especially for small-scale farmers in developing countries, due to the competing demand from industry [7,8]. Thus, it is crucial to identify cost-effective and environmentally friendly techniques such as the use of naturally derived plant biostimulants to favourably modify physiological processes and enhance plant productivity under saline soils [9]. Recent studies have shown that the exogenous application of biostimulants from seaweed extracts could improve water retention, nutrient availability, and plant defence systems under abiotic stress [10,11,12]. Additionally, the positive effect of seaweed extract under saline conditions was reported in maize (Zea mays), tomato (Solanum lycopersicum L.), and in bell pepper (Capsicum annuum L.), where its application optimised photosynthetic performance, antioxidant defence systems, leaf relative water content, leaf greenness, and the accumulation of potassium and phosphorous, resulting in higher yield [13,14,15]. Hence, seaweed extracts have gained popularity as commercial biostimulants that positively influence agronomic and physio-biochemical responses in plants under saline conditions.
Spinacia oleracea (Fordhook giant Swiss chard), a widely consumed vegetable in South Africa, is recognised for its nutritional value due to the abundance of antioxidant compounds, minerals, vitamins, and dietary fibres [16]. However, its production has been negatively impacted by increasing soil salinity in South African vegetable farms, especially in semi-arid regions [17], which necessitates the application of cost-effective and environmentally friendly farming techniques to alleviate salinity. Despite the findings of El-Nakhel et al. [18], no comprehensive studies have been conducted on the use of seaweed-derived biostimulants to assess salt tolerance and increase the yield and nutritional profile of spinach under saline conditions. Thus, this study was conducted to assess the effectiveness of a South African seaweed-derived biostimulant (Kelpak®) in alleviating salinity stress in spinach. The results from this study are hoped to provide a sustainable and cost-effective technique to small-scale vegetable farmers in semi-arid regions affected by soil salinity.
2. Material and Methods
2.1. Greenhouse Cultivation
2.1.1. Growth Conditions
This study was conducted in the research greenhouse facility of the Cape Peninsula University of Technology during October and November 2023. The temperature within the greenhouse was configured to be 21–26 °C during the daytime and 12–17 °C at night, with 60% relative humidity. Under natural light conditions, the daily average photosynthetic photon flux density (PPFD) was 420 µmol/m−2s−1, with the intensity peaking at 1020 µmol/m−2s−1. The photoperiod corresponded to the prevalent conditions of early spring to summer.
2.1.2. Preparation of Plant Material
Seeds of Spinacia oleracea were acquired from a commercial garden store (StodelsTM Eversdal Rd, Bellville, Cape Town, South Africa) and germinated in seedling trays following the procedure outlined by Yavuz et al. [19]. Evenly established S. oleracea seedlings were transplanted individually in black plastic pots filled with a combination of peat moss and silica sand (1:1) and stationed in the hardening off area to acclimatise. The plants were then watered daily with a full Nutrifeed TM solution produced by STARKE AYRES Pty. Ltd., Hartebeesfontein Farm, Bredell Rd, Kaalfontein, Kempton Park, Gauteng, South Africa. After sixteen days of plant development, plants were irrigated with distilled water for six days to eliminate any salt residue before being subjected to salinity and seaweed extract treatments.
2.1.3. Seaweed Extract Preparation
Seaweed extract (Kelpak®) was sourced from Kelp Products (Pty) Ltd., Capricorn Business Park, 2 Link, Capricorn, Simon’s Town, South Africa and the treatments were prepared by diluting the liquid concentrate with distilled water to obtain three dilutions at 1%, 2.5%, and 5% (v/v) as described by Aremu et al. [20]. The seaweed extract was then applied weekly via soil drenching using 50 mL per potted plant. The chemical composition of Kelpak® is shown in Supplementary Tables S1 and S2.
2.1.4. Seaweed Extract and Saline Treatment
Fifty healthy plants were arranged into five treatments each containing ten replicates: T1—plants irrigated with nutrient solution (control); T2—plants irrigated with nutrient solution spiked with 300 mM of NaCl; T3—plants irrigated with nutrient solution spiked with 300 mM and treated with 1% seaweed extract; T4—plants irrigated with nutrient solution spiked with 300 mM and treated with 2.5% seaweed extract; and T5—plants irrigated with nutrient solution spiked with 300 mM and treated with 5% seaweed extract. To avoid osmotic shock, NaCl was gradually elevated by 50 mM daily until the maximum concentration (300 mM) was attained. Thereafter, the plants were irrigated every two days with 300 mL of nutrient solution with or without NaCl.
2.2. Biomass Evaluation
After 60 days of plant development under the tested treatments, plants were thoroughly watered with distilled water to loosen up the soil and were harvested cautiously to prevent root and plant damage. They were then rinsed multiple times with distilled water and dried with tissue paper. Thereafter, the shoots and roots were split, and the fresh samples were weighed using a precision weighing scale, followed by oven-drying at 30 °C to complete dryness, and the dry samples were also weighed.
2.3. Physiological Attributes
Chlorophyll a and b content was estimated spectrophotometrically as demonstrated by Wang et al. [21]. Fresh leaf material (100 mg) was extracted with 5 mL of 99.5% dimethyl sulfoxide (DMSO; Sigma-Aldrich, Gauteng, South Africa). The samples were then incubated at 35 °C for three hours in the dark before being centrifuged at 13,300 rpm for 10 min at 4 °C. The absorbance of the supernatants was measured at 649 and 665 nm. The contents of Chl a and Chl b were subsequently determined using the formula outlined by Lichtenthaler and Wellburn [22].
The variability in leaf relative water content (RWC) was evaluated using the procedure previously described by Bistgani et al. [23]. Briefly, three leaves were excised from three distinct plants in each treatment and weighed (FW). Subsequently, they were immersed in distilled water and kept in darkness for 4 h, following which the turgid weight (TW) was determined. Finally, the leaves were air-dried in an oven at 56 °C for 24 h to determine the dry weight (DW). The RWC percentage was calculated using the equation below:
2.4. Oxidative Stress Markers
The superoxide radical content was measured following the technique reported by Gokul et al. [24]. Three 1 cm2 leaf sections were excised from three distinct plants in each treatment. These leaf sections were then immersed in 800 µL of potassium phosphate buffer (pH 7.0) [50 mM potassium phosphate buffer (pH 7.0) containing 10 mM potassium cyanide, 10 mM hydrogen peroxide, 2% sodium dodecyl sulphate and 80 µM NBT]. Leaf materials were then incubated in this buffer for 20 min at 21 °C before being crushed within the solution with a small pestle. The samples were centrifuged at 13,000 rpm for 5 min, and the supernatant was transferred to a clean 2 mL Eppendorf tube. A spectrophotometer was used to read the sample at 600 nm after loading 200 µL of it into a microtiter plate. The extinction coefficient of 12.8 mM cm−1 was used to calculate the superoxide radical content, expressed as nanomole (nmol) g−1 FW.
To quantify the malondialdehyde content within the tested samples, the procedure detailed by González-Orenga et al. [25] was followed. Leaf extracts were mixed with 0.5% thiobarbituric acid (TBA) prepared in 20% trichloroacetic acid (TCA) and with 20% TCA without TBA for the control and then incubated at 95 °C for 20 min before being cooled on ice and centrifuged at 13,300 rpm for 10 min at 4 °C. The absorbance of the supernatants was measured at 532 nm. The non-specific absorbance at 600 and 440 nm was subtracted, and the MDA concentration was calculated and expressed as nanomole (nmol) g−1 FW.
2.5. Antioxidative Enzymes
The method outlined by Ali and Ludidi [26] was utilised to extract protein from the leaf samples. Briefly, 200 mg of plant tissue from each treatment was homogenised in 400 µL of protein extraction solution, consisting of 40 mM phosphate buffer at pH 7.4, 1 mM ethylenediaminetetraacetic acid (EDTA), and 5% (w/v) polyvinylpolypyrrolidone (PVPP). The homogenate was centrifuged at 13,000 rpm for 20 min at 4 °C, and the supernatant was then used for various enzymatic assays. The protein concentration in the extracts was measured using the Bio-Rad reagent and bovine serum albumin (BSA) as standard.
The activity of superoxide dismutase (SOD) was measured at 560 nm spectrophotometrically by measuring the suppression and photoreduction of nitro blue tetrazolium (NBT); the reaction solution contained riboflavin as a determinant of superoxide radicals. Briefly, 1 mL of the reaction solution was prepared in 50 mM potassium phosphate buffer using 2 μM riboflavin, 75 μM Nitrotetrazolium blue (NBT), 100 μM EDTA, 13 mM DL-methionine, and 50 μL of enzyme extract. A unit SOD was labelled as the quantity of enzyme required to block NBT photoreduction by 50% in experimental conditions as outlined in the methodology of Kaur et al. [27] and expressed as U g−1 FW.
Catalase (CAT) activity was measured by observing a decrease in absorbance at 240 nm following the consumption of H2O2 in a 1 mL reactive mixture consisting of 10 mM H2O2 and 20 μL of enzyme extract in 50 mM of potassium phosphate buffer (pH = 7) [28]. One CAT unit (U) was defined as the quantity of enzyme degrading one millimole (mmol) of H2O2 at 25 °C per minute and expressed as U g−1 FW. Meanwhile, the enzyme activity of peroxidase (POD) was determined using the techniques and principles described by Omran [29] with slight modifications. A 1 mL reactive mixture consisting of 50 mM of potassium phosphate buffer (pH = 7), 3.5 guaiacol, 10 mM H2O2, and 20 μL of enzyme extract. The rate change in absorbance at 436 nm was measured and POD was expressed as U g−1 FW.
2.6. Metabolites and Antioxidant Activity
Crude extracts were obtained by mixing approximately 100 mg of finely pulverised dry plant materials into 25 mL of 70% (v/v) ethanol (EtOH) (Merck, Modderfontein, South Africa) for 1 h. The mixture was centrifuged at 4000 rpm for 5 min, and the supernatants were utilised for phytochemical and antioxidant assays.
The Folin–Ciocalteu method, as elucidated by Jasson et al. [30] was employed to assess the total phenolic content of the analysed samples. A 7.5% solution of sodium carbonate (Sigma-Aldrich, Gauteng, South Africa) and diluted Folin and Ciocalteu’s phenol reagent (2 N, Sigma, Gauteng, South Africa) were made. A 25 μL volume of the crude extract, 125 μL of Folin and Ciocalteu’s phenol reagent, and 100 μL of Na2CO3 were mixed in a 96-well plate at room temperature for 30 min. A Multiskan Spectrum plate reader was then used to measure the absorbance at 765 nm. The values were calculated using a gallic acid (Sigma-Aldrich, Gauteng, South Africa) standard curve with concentration varying between 0 and 500 mg/L and the results were expressed as mg gallic acid equivalents (GAE) per g dry weight (mg GAE/g DW).
The total flavonol content of the extracts was estimated from the quercetin standard curve established from concentration ranges of 0, 5, 10, 20, 40, and 80 mg/L of quercetin dissolved in 95% ethanol. A volume of 12.5 µL crude extracts was combined with 12.5 µL of 0.1% HCl (Merck, Modderfontein, South Africa) in 95% ethanol and 225 µL of 2% HCl for each sample. The mixture was then incubated at room temperature for 30 min before obtaining absorbance at 360 nm. The results were represented as milligrams of quercetin equivalent (mg QE/g DW) per gram of dry weight [31].
The radical scavenging activity was determined using the DPPH free radical scavenging assay following the methods described by Ngxabi et al. [32]. A solution of 0.135 mM DPPH was used to produce DPPH radicals About 300 µL of the DPPH solution was mixed with 25 µL of the crude extract and graded concentrations (0 and 500 µM) of Trolox standard (6-hydroxy-2,5,7,8-tetramethylchroman-2-20 carboxylic acid). After incubating for 30 min, the absorbance at 517 nm was measured and expressed as µM/Trolox equivalent per gram of dry weight (µM TE/g DW).
2.7. Nutritional Constituents
The mineral constituents (macro- and micronutrients) of the tested samples were analysed using the Inductively Coupled Plasma-Optical Emission Spectrometer (ICP-OES; Varian 710–ES series, SMM Instruments, Cape Town, South Africa) as previously explained by Bulawa et al. [33]. The proximate composition (moisture, ash, crude fat, crude protein, non-fibre carbohydrate and neutral detergent fibre) was determined using the accredited in-house standard Association of Official Analytical Chemists (AOAC) procedures reported by Tshayingwe et al. [34]. The composition of vitamin C (ascorbic acid) and vitamin E (α-tocopherol) in the leaf samples was also evaluated using the AOAC methods described by Nemzer et al. [35].
2.8. Statistical Analysis
The experimental data were analysed with Minitab 17 statistical software and a multivariate analysis of variance was employed to discern significant differences across treatments followed by Tukey’s (LSD) test at p ≤ 0.05. The data were subsequently presented as mean ± standard error.
3. Results
3.1. Biomass Evaluation
Salinity and the application of seaweed extracts (SWE) had a considerable impact on the biomass yield of spinach, as seen in Table 1. Plants irrigated with salinity without seaweed extract had the lowest biomass yield when compared to other treatments. The highest yield in shoot fresh weight was noted in control plants, and this was comparable to the yield obtained in plants treated with 5% SWE under saline irrigation. The same trend was also noted for shoot dry weight, root fresh weight, and total fresh weight. On the contrary, both the root dry weight and total dry weight were substantially higher in control plants as compared to plants subjected to either salinity or its combination with various SWE dilutions.

Table 1.
Effects of salinity and seaweed extracts (SWE) on biomass yield of spinach.
3.2. Physiological Attributes
Salinity and seaweed extracts induced physiological changes in spinach leaves, as shown in Table 2. The highest photosynthetic pigments (Chl a and b) were noted in control plants, but these values were comparable to those recorded in plants subjected to salinity and treated with 5% SWE. On the contrary, salinity without SWE caused a substantial reduction in photosynthetic pigments as anticipated. When assessing the relative water content within treatments, the same trend was noted where the control had the highest relative water content, but this value was again comparable to plants treated with 5% SWE.

Table 2.
Effects of salinity and seaweed extracts (SWE) on the photosynthetic pigments and relative water content (RWC) of spinach leaves.
3.3. Oxidative Stress Markers
Salinity and seaweed extracts had an influence on the accumulation of superoxide radicals and malondialdehyde (MDA) within the leaves of spinach, as displayed in Figure 1. Salinity without the addition of SWE upregulated the accumulation of oxidative stress markers, which are depicted by excessive amounts of superoxide and MDA. Interestingly, plants treated with 5% SWE had lower contents of superoxide radicals and MDA, which were comparable to the control plants.
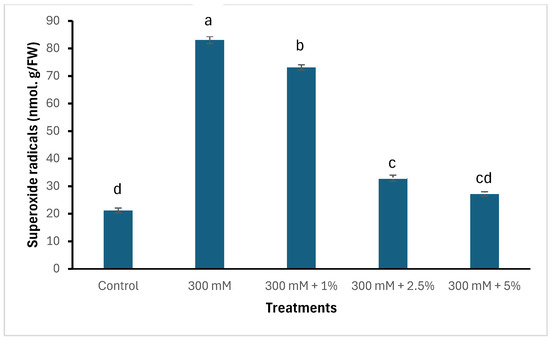
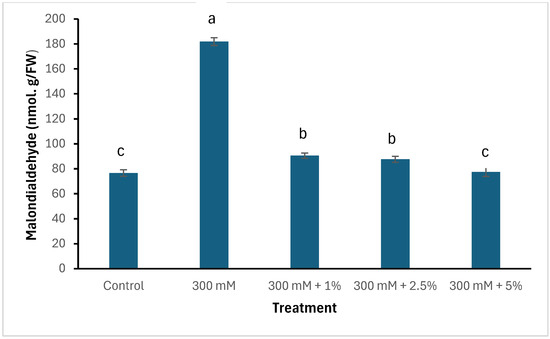
Figure 1.
Effects of salinity and seaweed extracts on oxidative stress markers in spinach. Means with different letters are significantly different (Tukey LSD, p ≤ 0.05).
3.4. Antioxidant Defence Mechanisms
Salinity and seaweed extract stimulated the activation of antioxidative defence system in spinach, as seen in Table 3. The contents of antioxidative enzymes (CAT and POD) were all significantly enhanced in plants subjected to salinity without SWE. Superoxide dismutase (SOD), on the other hand, was substantially upregulated in plants treated with 5% SWE. When assessing the phytochemicals and antioxidant activity of the leaf samples, the highest polyphenol, flavonol, and DPPH contents were all noted in plants irrigated with salinity with no SWE treatment.

Table 3.
Effects of salinity and seaweed extracts (SWE) on the antioxidant defence attributes of spinach.
3.5. Nutritional Evaluation
3.5.1. Minerals and Vitamin Constituents
Salinity and the application of SWE significantly influenced the accumulation of minerals and vitamins in spinach leaves (Table 4 and Table 5). Macronutrients such as nitrogen, potassium, magnesium, and calcium were all noted in enhanced quantities in the control plants. However, the contents of magnesium and calcium were comparable to the values recorded in plants treated with 5% SWE, while a high amount of sodium was recorded in plants subjected to saline irrigation only. When assessing the accumulation of trace elements and vitamins, we observed that plants treated with 5% SWE had an enhanced quantity of zinc, manganese, and iron as compared to other treatments, including the control. On the contrary, vitamin C and E contents were more pronounced in control plants, but these values were comparable to plants treated with 5% SWE.

Table 4.
Effects of salinity and seaweed extracts (SWE) on the macronutrient contents of spinach leaves.

Table 5.
Effects of salinity and seaweed extracts (SWE) on the micronutrient and vitamin contents of spinach leaves.
3.5.2. Proximate Composition
The concentration of proximate constituents within the leaves of spinach was significantly affected by saline irrigation and the application of seaweed extracts (Table 6). The ash and neutral detergent fibre were found in abundance in plants subjected to salinity without SWE addition. These values were substantially higher than all treatments, including the control. On the contrary, crude fat was found in higher quantities in plants treated with 5% SWE, while protein, moisture, and non-fibre carbohydrate contents were enhanced in control plants. Nevertheless, it must be noted that the protein content recorded in control plants was comparable to the value attained in plants treated with 5% SWE.

Table 6.
Effects of salinity and seaweed extracts (SWE) on the proximate constituents of spinach leaves.
4. Discussion
Salinity is one of the key factors limiting the development and production of vegetable crops [36]. This is primarily due to the high concentration of sodium ions either in the soil or in irrigation water [37]. These ions disrupt essential physiological activities such as stomatal conductance, photosynthesis, and tissue hydration and trigger oxidative stress and membrane damage, leading to diminished plant biomass [5]. To curb the devasting effects of salinity on food crops, biostimulants such as seaweed extracts are used as crop management products to enhance crop productivity under abiotic stress conditions [10].
In the present study, the effectiveness of a South African seaweed-derived biostimulant (Kelpak®) in alleviating salinity stress in spinach was assessed. The results revealed a substantial improvement in crop development and yield in plants treated with seaweed extracts (SWE) under saline irrigation. Notably, the 5% SWE concentration displayed the greatest improvement in crop yield, whereas the 2.5% concentration also showed good benefits, albeit to a lesser extent when compared to the control. Moreover, the application of SWE also enhanced root development in salt-stressed spinach. These findings suggest that SWE upregulated enzymes’ activity and increased the level of endogenous hormones, which accelerated cell division and elongation processes to promote root growth, which enabled efficient water and mineral nutrient uptake, thus leading to improved plant biomass [38]. Radwan et al. [39] and Rouphael et al. [40] also reported similar findings on seed watermelon (Citrullus lanatus) and zucchini squash (Cucurbita pepo) under saline conditions, where SWE enhanced the root hydraulic conductivity and root activity, enabling efficient water and mineral absorption from the rhizosphere, in turn leading to a higher plant biomass. Additionally, the enhancement of growth parameters in salt-stressed spinach treated with SWE could be attributed to macro and micronutrients as well as growth regulators such as amino acids, auxins, abscisic acid, and gibberellins that have been reported in the SWE (Kelpak®) used in this study [41].
Salinity has also been reported to cause severe impact on various physiological traits in crops such as photosynthetic pigments and relative water content (RWC) [42]. Thus, the assessment of chlorophyll content is frequently used as an index for salt tolerance in many plant species, whilst relative water content is regarded as an appropriate indicator of plant hydration levels [43]. In this study, salt-stressed spinach without the application of SWE exhibited reduced chlorophyll content and RWC. This is mostly due to the impairment of the photosynthetic machinery caused by the excessive build-up of sodium ions in the chloroplasts which disturbs the carbon metabolism and photophosphorylation, resulting in reduced photosynthesis [44]. Interestingly, salt-stressed spinach treated with 5% SWE exhibited an improved chlorophyll content and an RWC which was comparable to the control plants. The improvement in these two physiological traits could be attributed to growth regulators found in the SWE, which are known to promote the absorption of water and nutrients and enhance the endogenous synthesis of phytohormones, particularly cytokinin, which plays a crucial role in protecting chlorophyll from photodamage, resulting in enhanced chlorophyll content [11,12]. Krid et al. [45] also reported similar findings for tomato grown under saline conditions, where an increase in chlorophyll was noted in plants treated with SWE. This was attributed to the presence of various organic substances within the SWE that played a role in sustaining the functions of photosynthetic enzymes, chlorophyll composition, photosynthetic apparatus, and the ultrastructure of thylakoid membranes.
Moreover, salinity triggers oxidative stress in crops by producing superoxide radicals through the Mehler reaction [46,47,48]. The overproduction of these free radicals interferes with normal metabolic functions in the cytoplasm, mitochondria, and peroxisomes by inducing oxidative damage to proteins and lipids [49,50,51]. This leads to cellular malfunction, which further causes cell death. Several biochemical indicators, such as cell death viability, superoxide radicals, and malondialdehyde, are routinely utilised to determine the extent of oxidative stress in plants exposed to harsh conditions [4,52]. Therefore, this study found that plants subjected to salinity without SWE treatment had higher amounts of superoxide radicals and malondialdehyde than those treated with SWE. This has been reported in numerous studies conducted on salt sensitive species such as spinach [19,53,54,55]. Intriguingly, salt-stressed spinach treated with 5% SWE had reduced oxidative stress and the amounts were comparable to the control plants. This was followed by the upregulation of superoxide dismutase, an antioxidative enzyme known to be the first line of defence against the excessive production of superoxide radicals [56,57]. This suggests that the SWE triggered this enzyme to dismutate these superoxide radicals into H2O2 and O2 and mitigated salinity stress in spinach.
Nutritional imbalances in vegetable crops due to salinity have been documented in the literature, typically resulting from the disruption of ion transport and accumulation induced by competing Na+ and Cl− ions, which restrict the absorption of vital nutrients necessary for optimal growth [58]. This was the case in this study, where the accumulation of Na+ in spinach without SWE resulted in a substantial reduction in the content of essential nutrients, such as phosphorus, potassium, calcium, magnesium, and vitamins C and E. Nonetheless, spinach plants treated with 5% SWE had an improved magnesium and calcium content which was comparable to the control plants. Sufficient potassium (K), calcium (Ca), and magnesium (Mg) contents are required to meet the basic metabolic processes such as intracellular K homeostasis, which is essential for the optimal functioning of the photosynthetic machinery and the maintenance of stomatal opening [55]. These results imply that the application of SWE upregulated the absorption of K, Ca, and Mg by new shoots under saline irrigation and maintained a suitable ratio needed for normal metabolism, thus the improvement in plant growth. These results are in line with those reported by Yarşı [59] on pepper (Capsicum annuum L.) seedlings subjected to saline irrigation, where the application of SWE improved the contents of K, Ca, and Mg. Furthermore, salt-stressed spinach treated with 5% SWE exhibited an increase in Zn, Mn, and Fe content. This enhancement in micronutrient contents might have also influenced nutrient homeostat in salt-stressed spinach, which is demonstrated by enhanced crop productivity. This is supported by numerous studies which indicated that the supplementation of zinc (Zn) and manganese (Mn) improves plant performance under saline conditions by facilitating plant growth through the protection of photosynthesis and the mitigation of oxidative stress [58,60,61].
When assessing the proximate constituents of the tested samples, ash and neutral detergent fibre (NDF) were found in abundance in plants subjected to salinity without SWE. It is commonly asserted that, with increasing salinity, plants absorb significant amounts of sodium ions and subsequently translocate them to the shoots, resulting in elevated ash content [62]. Furthermore, elevated salinity augments polysaccharide contents in the cell walls while diminishing soluble carbohydrate contents, resulting in an increase in the content of insoluble fibres such as NDF [63]. Interestingly, salt-stressed spinach treated with 2.5 and 5% SWE had lower contents of ash and NDF, whereas the non-fibre carbohydrate content was enhanced. This shows that the addition of SWE inhibited the formation of polysaccharides in the cell wall, therefore increasing the soluble carbohydrate contents in spinach. Protein content was also increased in salt-stressed spinach treated with 5% SWE, and this could be attributed to the increase in proteolysis as well as amino acid synthesis found in this SWE and the renaturation of enzymes involved in protein and amino acid synthesis [64].
5. Conclusions
This study reveals that the addition of seaweed extract (Kelpak®) by soil drenching at 5% dilution alleviated salinity stress in spinach by enhancing the morphophysiological, biochemical, and nutritional quality of these plants. Therefore, these findings support the use of seaweed extract (Kelpak®) as a biostimulant in vegetable farming systems in regions affected by salinity. Nonetheless, field studies in salinity-affected soils are needed to broaden the mechanism of action and to ascertain the salt alleviation potential of this South African seaweed extract.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10121340/s1, Table S1: The chemical composition of Kelpak seaweed extract. Adapted from Lötze and Hoffman. Table S2: Amino acid composition of kelpak expressed in mg.100 g-1.
Author Contributions
Conceptualization, A.S. and M.O.J.; Methodology, M.O.J.; Validation, B.L.N. and C.P.L.; Formal analysis, A.S. and L.K.; Resources, B.L.N., L.K. and C.P.L.; Writing—original draft, A.S.; Writing—review & editing, A.S., B.L.N., M.O.J. and C.P.L.; Supervision, M.O.J. and L.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Directorate, Consolidated Research Fund (CRF), and the National Research Foundation (NRF) of South Africa (Grant no: 140847).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank the Cape Peninsula University of Technology and Durban University of Technology for providing the necessary resources required for this study.
Conflicts of Interest
The authors declare no conflicts of interest in this study.
References
- Jameel, J.; Anwar, T.; Siddiqi, E.H.; Alomrani, S.O. Alleviation of NaCl Stress in Tomato Varieties by Promoting Morpho-Physiological Attributes and Biochemical Characters. Sci. Hortic. 2024, 323, 112496. [Google Scholar] [CrossRef]
- Mndi, O.; Sogoni, A.; Jimoh, M.O.; Wilmot, C.M.; Rautenbach, F.; Laubscher, C.P. Interactive Effects of Salinity Stress and Irrigation Intervals on Plant Growth, Nutritional Value, and Phytochemical Content in Mesembryanthemum crystallinum L. Agriculture 2023, 13, 1026. [Google Scholar] [CrossRef]
- Amerian, M.; Palangi, A.; Gohari, G.; Ntatsi, G. Enhancing Salinity Tolerance in Cucumber through Selenium Biofortification and Grafting. BMC Plant Biol. 2024, 24, 24. [Google Scholar] [CrossRef] [PubMed]
- Sogoni, A.; Jimoh, M.O.; Barker, A.M.; Keyster, M.; Kambizi, L.; Laubscher, C.P. Salinity Modulates Morpho-Physiology, Biochemical and Antioxidant Defence System in Tetragonia decumbens Mill.: A Neglected Wild Leafy Vegetable in South Africa. Plant Physiol. Rep. 2024, 1–14. [Google Scholar] [CrossRef]
- Kanwal, R.; Maqsood, M.F.; Shahbaz, M.; Naz, N.; Zulfiqar, U.; Ali, M.F.; Jamil, M.; Khalid, F.; Ali, Q.; Sabir, M.A.; et al. Exogenous Ascorbic Acid as a Potent Regulator of Antioxidants, Osmo-Protectants, and Lipid Peroxidation in Pea under Salt Stress. BMC Plant Biol. 2024, 24, 247. [Google Scholar] [CrossRef] [PubMed]
- Rani, J.; Paul, B. Challenges in Arid Region Reclamation with Special Reference to Indian Thar Desert—Its Conservation and Remediation Techniques. Int. J. Environ. Sci. Technol. 2023, 20, 12753–12774. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Bhowmik, P.C.; Hossain, M.A.; Rahman, M.M.; Prasad, M.N.V.; Ozturk, M.; Fujita, M. Potential Use of Halophytes to Remediate Saline Soils. Biomed Res. Int. 2014, 2014, 589341. [Google Scholar] [CrossRef]
- Qadir, M.; Oster, J.D.; Schubert, S.; Noble, A.D.; Sahrawat, K.L. Phytoremediation of Sodic and Saline-Sodic Soils. Adv. Agron. 2007, 96, 197–247. [Google Scholar]
- Mughunth, R.J.; Velmurugan, S.; Mohanalakshmi, M.; Vanitha, K. A Review of Seaweed Extract’s Potential as a Biostimulant to Enhance Growth and Mitigate Stress in Horticulture Crops. Sci. Hortic. 2024, 334, 113312. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant Properties of Seaweed Extracts in Plants: Implications towards Sustainable Crop Production. Plants 2021, 10, 531. [Google Scholar] [CrossRef] [PubMed]
- Deolu-Ajayi, A.O.; van der Meer, I.M.; van der Werf, A.; Karlova, R. The Power of Seaweeds as Plant Biostimulants to Boost Crop Production under Abiotic Stress. Plant Cell Environ. 2022, 45, 2537–2553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yin, J.; Ma, Y.; Peng, Y.; Fenton, O.; Wang, W.; Zhang, W.; Chen, Q. Unlocking the Potential of Biostimulants Derived from Organic Waste and By-Product Sources: Improving Plant Growth and Tolerance to Abiotic Stresses in Agriculture. Environ. Technol. Innov. 2024, 34, 103571. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Sánchez-Hernández, C.V.; Palmeros-Suárez, P.A.; Ocampo-Alvarez, H.; Santacruz-Ruvalcaba, F.; Meza-Canales, I.D.; Becerril-Espinosa, A. Seaweed Extract Improves Growth and Productivity of Tomato Plants under Salinity Stress. Agronomy 2022, 12, 2495. [Google Scholar] [CrossRef]
- Pal, S.C.; Hossain, M.B.; Mallick, D.; Bushra, F.; Abdullah, S.R.; Dash, P.K.; Das, D. Combined Use of Seaweed Extract and Arbuscular Mycorrhizal Fungi for Alleviating Salt Stress in Bell Pepper (Capsicum annuum L.). Sci. Hortic. 2024, 325, 112597. [Google Scholar] [CrossRef]
- Gul, S.; Nawaz, M.F.; Bin Yousaf, M.T.; Haroon U Rashid, M.; Adnan, M.Y.; Tausif, S.; Javed, A.; Abideen, Z.; El Keblawy, A. Brown Macro-Seaweeds Derived Agro-Biostimulant for Zea mays Farming in Saline Conditions: Growth Enhancement and Optimum Biochemical and Ion Feedback. Biocatal. Agric. Biotechnol. 2024, 57, 103105. [Google Scholar] [CrossRef]
- Salehi, B.; Tumer, T.B.; Ozleyen, A.; Peron, G.; Dall’Acqua, S.; Rajkovic, J.; Naz, R.; Nosheen, A.; Mudau, F.N.; Labanca, F.; et al. Plants of the Genus Spinacia: From Bioactive Molecules to Food and Phytopharmacological Applications. Trends Food Sci. Technol. 2019, 88, 260–273. [Google Scholar] [CrossRef]
- Shokri, N.; Hassani, A.; Sahimi, M. Multi-Scale Soil Salinization Dynamics from Global to Pore Scale: A Review. Rev. Geophys. 2024, 62, e2023RG000804. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Cozzolino, E.; Ottaiano, L.; Petropoulos, S.A.; Nocerino, S.; Pelosi, M.E.; Rouphael, Y.; Mori, M.; Di Mola, I. Effect of Biostimulant Application on Plant Growth, Chlorophylls and Hydrophilic Antioxidant Activity of Spinach (Spinacia oleracea L.) Grown under Saline Stress. Horticulturae 2022, 8, 971. [Google Scholar] [CrossRef]
- Yavuz, D.; Kılıç, E.; Seymen, M.; Dal, Y.; Kayak, N.; Kal, Ü.; Yavuz, N. The Effect of Irrigation Water Salinity on the Morph-Physiological and Biochemical Properties of Spinach under Deficit Irrigation Conditions. Sci. Hortic. 2022, 304, 111272. [Google Scholar] [CrossRef]
- Aremu, A.O.; Plačková, L.; Gruz, J.; Bíba, O.; Novák, O.; Stirk, W.A.; Doležal, K.; Van Staden, J. Seaweed-Derived Biostimulant (Kelpak®) Influences Endogenous Cytokinins and Bioactive Compounds in Hydroponically Grown Eucomis autumnalis. J. Plant Growth Regul. 2016, 35, 151–162. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Altman, A. Plant Responses to Drought, Salinity and Extreme Temperatures: Towards Genetic Engineering for Stress Tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Hashemi, M.; DaCosta, M.; Craker, L.; Maggi, F.; Morshedloo, M.R. Effect of Salinity Stress on the Physiological Characteristics, Phenolic Compounds and Antioxidant Activity of Thymus vulgaris L. and Thymus Daenensis Celak. Ind. Crops Prod. 2019, 135, 311–320. [Google Scholar] [CrossRef]
- Gokul, A.; Fahiem Carelse, M.; Niekerk, L.A.; Klein, A.; Ludidi, N.; Mendoza-Cozatl, D.; Keyster, M. Exogenous 3,3′-Diindolylmethane Improves Vanadium Stress Tolerance in Brassica napus Seedling Shoots by Modulating Antioxidant Enzyme Activities. Biomolecules 2021, 11, 436. [Google Scholar] [CrossRef]
- González-Orenga, S.; Leandro, M.E.D.A.; Tortajada, L.; Grigore, M.N.; Llorens, J.A.; Ferrer-Gallego, P.P.; Laguna, E.; Boscaiu, M.; Vicente, O. Comparative Studies on the Stress Responses of Two Bupleurum (Apiaceae) Species in Support of Conservation Programmes. Environ. Exp. Bot. 2021, 191, 104616. [Google Scholar] [CrossRef]
- Ali, A.E.E.; Ludidi, N. Antioxidant Responses Are Associated with Differences in Drought Tolerance between Maize and Sorghum. J. Oasis Agric. Sustain. Dev. 2021, 3, 1–12. [Google Scholar] [CrossRef]
- Kaur, N.; Dhawan, M.; Sharma, I.; Pati, P.K. Interdependency of Reactive Oxygen Species Generating and Scavenging System in Salt Sensitive and Salt Tolerant Cultivars of Rice. BMC Plant Biol. 2016, 16, 131. [Google Scholar] [CrossRef]
- Din Muhammad, H.M.; Anjum, M.A.; Naz, S. Silicon-Mediated Alleviation of Salinity Stress in Petunia (Petunia hybrida) by Modulation of Morphological, Physiological and Biochemical Indices. J. Soil Sci. Plant Nutr. 2024, 24, 2221–2231. [Google Scholar] [CrossRef]
- Omran, R.G. Peroxide Levels and the Activities of Catalase, Peroxidase, and Indoleacetic Acid Oxidase during and after Chilling Cucumber Seedlings. Plant Physiol. 1980, 65, 407–408. [Google Scholar] [CrossRef]
- Jasson, T.I.; Jimoh, M.O.; Daniels, C.W.; Nchu, F.; Laubscher, C.P. Enhancement of Antioxidant Potential, Phytochemicals, Nutritional Properties, and Growth of Siphonochilus aethiopicus (Schweinf.) B.L.Burtt with Different Dosages of Compost Tea. Horticulturae 2023, 9, 274. [Google Scholar] [CrossRef]
- Zantanta, N.; Kambizi, L.; Etsassala, N.G.E.R.; Nchu, F. Comparing Crop Yield, Secondary Metabolite Contents, and Antifungal Activity of Extracts of Helichrysum odoratissimum Cultivated in Aquaponic, Hydroponic, and Field Systems. Plants 2022, 11, 2696. [Google Scholar] [CrossRef] [PubMed]
- Ngxabi, S.; Jimoh, M.O.; Kambizi, L.; Laubscher, C.P. Growth Characteristics, Phytochemical Contents, and Antioxidant Capacity of Trachyandra ciliata (L.f) Kunth Grown in Hydroponics under Varying Degrees of Salinity. Horticulturae 2021, 7, 244. [Google Scholar] [CrossRef]
- Bulawa, B.; Sogoni, A.; Jimoh, M.O.; Laubscher, C.P. Potassium Application Enhanced Plant Growth, Mineral Composition, Proximate and Phytochemical Content in Trachyandra divaricata Kunth (Sandkool). Plants 2022, 11, 3183. [Google Scholar] [CrossRef]
- Tshayingwe, A.; Jimoh, M.O.; Sogoni, A.; Wilmot, C.M.; Laubscher, C.P. Light Intensity and Growth Media Influence Growth, Nutrition, and Phytochemical Content in Trachyandra divaricata Kunth. Agronomy 2023, 13, 247. [Google Scholar] [CrossRef]
- Nemzer, B.; Al-Taher, F.; Abshiru, N. Extraction and Natural Bioactive Molecules Characterization in Spinach, Kale and Purslane: A Comparative Study. Molecules 2021, 26, 2515. [Google Scholar] [CrossRef]
- Verma, O.; Sharma, S.; Kumar, V.; Singh, T.; Kumar, R.; Auji, R. Salinity Stress Effect on Staple Food Crops and Novel Mitigation Strategies. Biologia 2024, 79, 2359–2374. [Google Scholar] [CrossRef]
- Azeem, M.; Pirjan, K.; Qasim, M.; Mahmood, A.; Javed, T.; Muhammad, H.; Yang, S.; Dong, R.; Ali, B.; Rahimi, M. Salinity Stress Improves Antioxidant Potential by Modulating Physio-Biochemical Responses in Moringa oleifera Lam. Sci. Rep. 2023, 13, 2895. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.E.-S.; Shalaby, O.A. Effect of magnetized water and seaweed extract on growth and yield of squash (Cucurbita pepo L.) plants grown under saline conditions. Egypt. J. Desert Res. 2024, 74, 197–215. [Google Scholar] [CrossRef]
- Radwan, A.M.; Ahmed, E.A.; Donia, A.M.; Mustafa, A.E.; Balah, M.A. Priming of Citrullus lanatus var. colocynthoides Seeds in Seaweed Extract Improved Seed Germination, Plant Growth and Performance under Salinity Conditions. Sci. Rep. 2023, 13, 11884. [Google Scholar] [CrossRef]
- Rouphael, Y.; De Micco, V.; Arena, C.; Raimondi, G.; Colla, G.; De Pascale, S. Effect of Ecklonia Maxima Seaweed Extract on Yield, Mineral Composition, Gas Exchange, and Leaf Anatomy of Zucchini Squash Grown under Saline Conditions. J. Appl. Phycol. 2017, 29, 459–470. [Google Scholar] [CrossRef]
- Wilmot, C.M.; Jimoh, M.; Laubscher, C. Stimulatory Effects of an Exogenously Applied Seaweed Extract on the Morphological and Physiological Growth and Yield in Juvenile Amaryllis Belladonna L. Bulbs. Egypt. J. Bot. 2024, 64, 52–68. [Google Scholar] [CrossRef]
- Boussora, F.; Triki, T.; Bennani, L.; Bagues, M.; Ben Ali, S.; Ferchichi, A.; Ngaz, K.; Guasmi, F. Mineral Accumulation, Relative Water Content and Gas Exchange Are the Main Physiological Regulating Mechanisms to Cope with Salt Stress in Barley. Sci. Rep. 2024, 14, 14931. [Google Scholar] [CrossRef] [PubMed]
- Banakar, M.H.; Amiri, H.; Sarafraz Ardakani, M.R.; Ranjbar, G.H. Susceptibility and Tolerance of Fenugreek (Trigonella Foenum-Graceum L.) to Salt Stress: Physiological and Biochemical Inspections. Environ. Exp. Bot. 2022, 194, 104748. [Google Scholar] [CrossRef]
- Khatri, K.; Rathore, M.S. Salt and Osmotic Stress-Induced Changes in Physio-Chemical Responses, PSII Photochemistry and Chlorophyll a Fluorescence in Peanut. Plant Stress 2022, 3, 100063. [Google Scholar] [CrossRef]
- Krid, A.; El Hallabi, M.; Ennoury, A.; Nhhala, N.; Aberkani, K.; Nhiri, M.; Zerrouk, M.H. The Potential of Seaweed Extracts as a Biostimulant for Improving Salt Stress Tolerance of Solanum Lycopersicum L. S. Afr. J. Bot. 2023, 161, 305–316. [Google Scholar] [CrossRef]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to Drought and Salt Stress in Plants: Unraveling the Signaling Networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef] [PubMed]
- González-Orenga, S.; Grigore, M.-N.; Boscaiu, M.; Vicente, O. Constitutive and Induced Salt Tolerance Mechanisms and Potential Uses of Limonium Mill. Species. Agronomy 2021, 11, 413. [Google Scholar] [CrossRef]
- Rodrigues de Queiroz, A.; Hines, C.; Brown, J.; Sahay, S.; Vijayan, J.; Stone, J.M.; Bickford, N.; Wuellner, M.; Glowacka, K.; Buan, N.R.; et al. The Effects of Exogenously Applied Antioxidants on Plant Growth and Resilience. Phytochem. Rev. 2023, 22, 407–447. [Google Scholar] [CrossRef]
- Ozgur, R.; Uzilday, B.; Sekmen, A.H.; Turkan, I.; Ozgur, R.; Uzilday, B.; Sekmen, A.H.; Turkan, I. Reactive Oxygen Species Regulation and Antioxidant Defence in Halophytes. Funct. Plant Biol. 2013, 40, 832–847. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J.; Colmer, T.D. Plant Salt Tolerance: Adaptations in Halophytes. Ann. Bot. 2015, 115, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A.; Zulfiqar, A.; Ali, B.; Naseeb, M.A.; Almasaudi, A.S.; Harakeh, S. Iron Sulfate (FeSO4) Improved Physiological Attributes and Antioxidant Capacity by Reducing Oxidative Stress of Oryza Sativa L. Cultivars in Alkaline Soil. Sustainability 2022, 14, 16845. [Google Scholar] [CrossRef]
- Shahid, M.; Singh, U.B.; Farah, M.A.; Al-Anazi, K.M. Phyto-Toxicological Effect of Fipronil to Plant Seedlings: Assessing Germination Attributes, Root-Tip Morphology, Oxidative Stress, and Cellular Respiration Indices. Pestic Biochem. Physiol. 2024, 205, 106135. [Google Scholar] [CrossRef]
- Saddique, M.; Kausar, A.; Iqra, I.; Akhter, N.; Mujahid, N.; Parveen, A.; Zaman, Q.; Hussain, S. Amino Acids Application Alleviated Salinity Stress in Spinach (Spinacia Oleracea L.) by Improving Oxidative Defense, Osmolyte Accumulation, and Nutrient Balance. Turk. J. Agric. For. 2022, 46, 875–887. [Google Scholar] [CrossRef]
- Ors, S.; Suarez, D.L. Spinach Biomass Yield and Physiological Response to Interactive Salinity and Water Stress. Agric. Water Manag. 2017, 190, 31–41. [Google Scholar] [CrossRef]
- Naz, T.; Mazhar Iqbal, M.; Tahir, M.; Hassan, M.M.; Rehmani, M.I.A.; Zafar, M.I.; Ghafoor, U.; Qazi, M.A.; El Sabagh, A.; Sakran, M.I. Foliar Application of Potassium Mitigates Salinity Stress Conditions in Spinach (Spinacia Oleracea L.) through Reducing NaCl Toxicity and Enhancing the Activity of Antioxidant Enzymes. Horticulturae 2021, 7, 566. [Google Scholar] [CrossRef]
- Gill, S.S.; Anjum, N.A.; Gill, R.; Yadav, S.; Hasanuzzaman, M.; Fujita, M.; Mishra, P.; Sabat, S.C.; Tuteja, N. Superoxide Dismutase—Mentor of Abiotic Stress Tolerance in Crop Plants. Environ. Sci. Pollut. Res. 2015, 22, 10375–10394. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Ashraf, M. Antioxidants as Modulators of Arsenic-Induced Oxidative Stress Tolerance in Plants: An Overview. J. Hazard. Mater. 2022, 427, 127891. [Google Scholar] [CrossRef]
- Umair Hassan, M.; Chattha, M.U.; Khan, I.; Khan, T.A.; Nawaz, M.; Tang, H.; Noor, M.A.; Asseri, T.A.Y.; Hashem, M.; Guoqin, H. Zinc Seed Priming Alleviates Salinity Stress and Enhances Sorghum Growth by Regulating Antioxidant Activities, Nutrient Homeostasis, and Osmolyte Synthesis. Agronomy 2024, 14, 1815. [Google Scholar] [CrossRef]
- Yarşı, G. Effects of Mycorrhiza, Seaweed and Bionutrient Applied to Reduce the Salt Stress on Nutrient Content, Plant Growth, Malondialdehyde (MDA) and Proline in Pepper. J. Elem. 2023, 28, 533–545. [Google Scholar] [CrossRef]
- Alsherif, E.A.; Yaghoubi Khanghahi, M.; Crecchio, C.; Korany, S.M.; Sobrinho, R.L.; AbdElgawad, H. Understanding the Active Mechanisms of Plant (Sesuvium portulacastrum L.) against Heavy Metal Toxicity. Plants 2023, 12, 676. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Azhar, W.; Fan, X.; Ulhassan, Z.; Salam, A.; Ashraf, M.; Liu, Y.; Gan, Y. Efficacy of Zinc-Based Nanoparticles in Alleviating the Abiotic Stress in Plants: Current Knowledge and Future Perspectives. Environ. Sci. Pollut. Res. 2023, 30, 110047–110068. [Google Scholar] [CrossRef]
- Nabati, J.; Kafi, M.; Nezami, A.; Rezvani Moghaddam, P.; Masoumi, A.; Zare Mehrjerdi, M. Evaluation of Quantitative and Qualitative Characteristic of Forage Kochia (Kochia scoparia) in Different Salinity Levels and Time. Iran. J. Field Crops Res. 2014, 12, 613–620. [Google Scholar] [CrossRef]
- Hedayati-Firoozabadi, A.; Kazemeini, S.A.; Pirasteh-Anosheh, H.; Ghadiri, H.; Pessarakli, M. Forage Yield and Quality as Affected by Salt Stress in Different Ratios of Sorghum bicolor-Bassia indica Intercropping. J. Plant Nutr. 2020, 43, 2579–2589. [Google Scholar] [CrossRef]
- Athar, H.U.R.; Zulfiqar, F.; Moosa, A.; Ashraf, M.; Zafar, Z.U.; Zhang, L.; Ahmed, N.; Kalaji, H.M.; Nafees, M.; Hossain, M.A.; et al. Salt Stress Proteins in Plants: An Overview. Front. Plant Sci. 2022, 13, 999058. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).