Effects of Glyoxylic Acid on Metabolism and Ripening of ‘Rocha’ Pears Treated with 1-MCP
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Material and Experimental Design
2.2. Fruit Quality Evaluation
2.3. Ethylene Production, and Related Metabolites and Biosynthetic Enzymes
2.4. Respiration Measurement
2.5. Volatile Aromatic Compounds Profiling by SPME-GC-MS
2.6. Determination of Sugars and Organic Acids
2.7. Statistical Analysis
3. Results
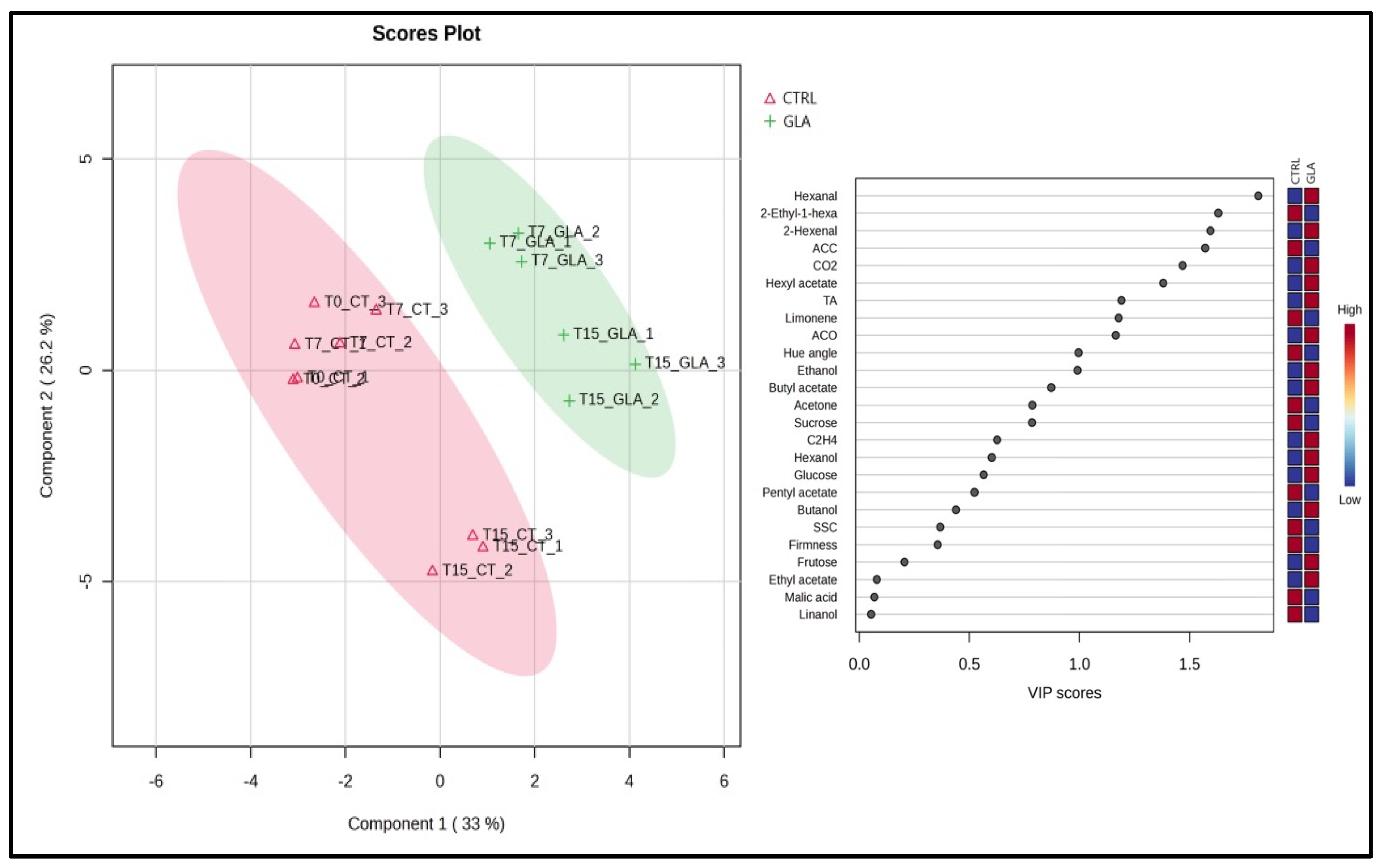
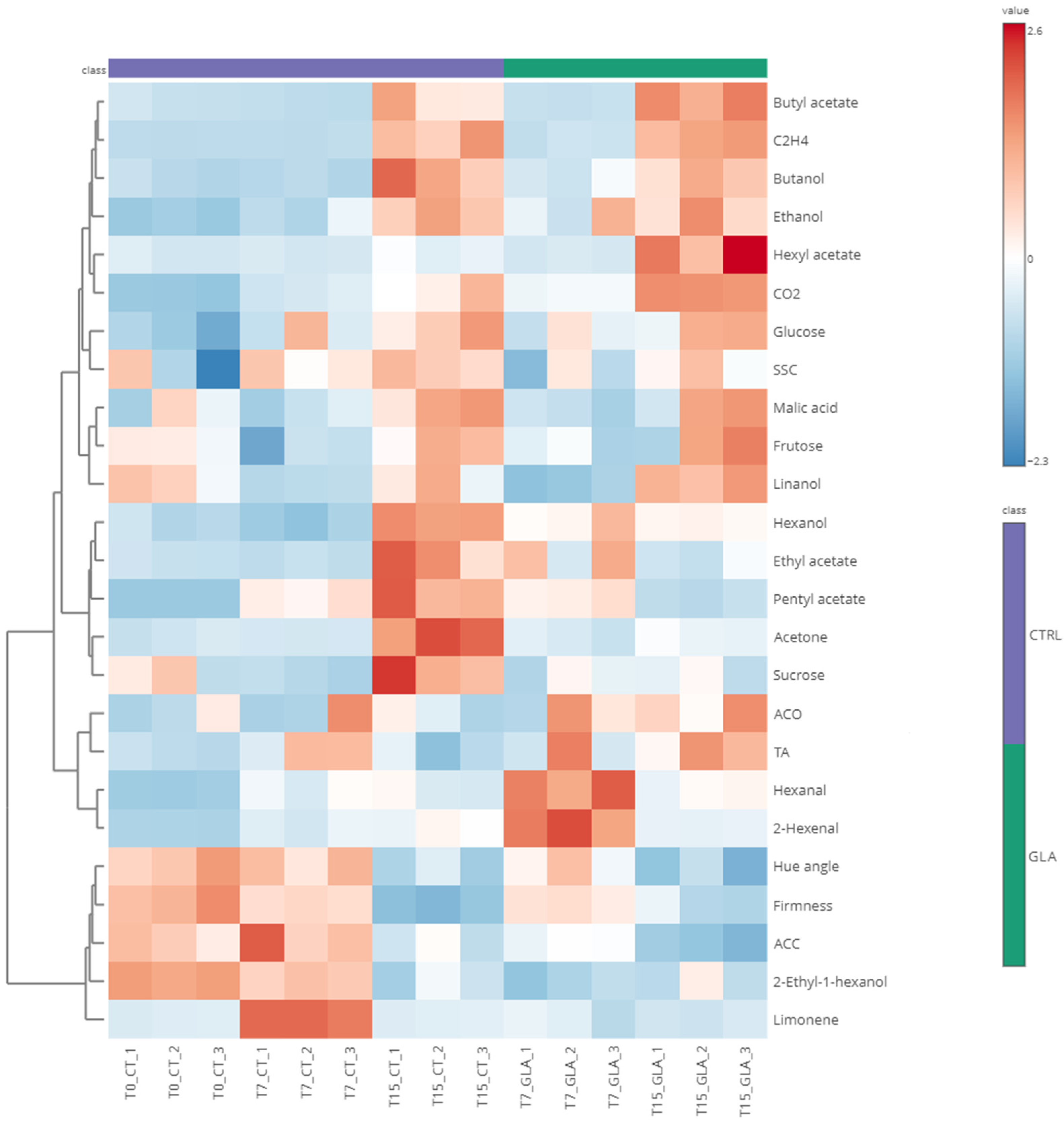
3.1. Multivariate Analysis Reveals Distinct Clustering of GLA-Treated and CTRL Samples
3.2. Multivariate Analysis Corroborates GLA-Induced Physiological Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
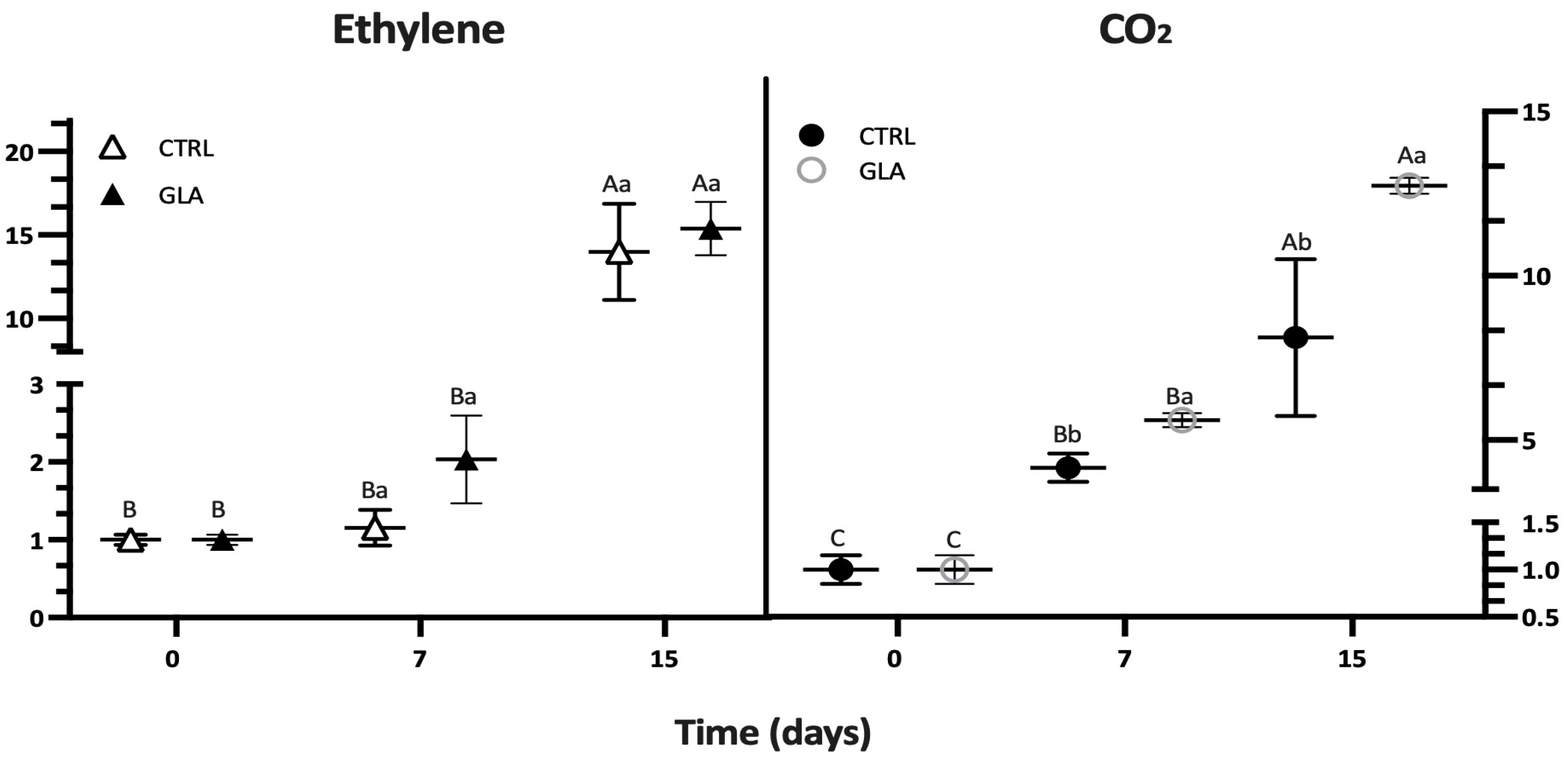
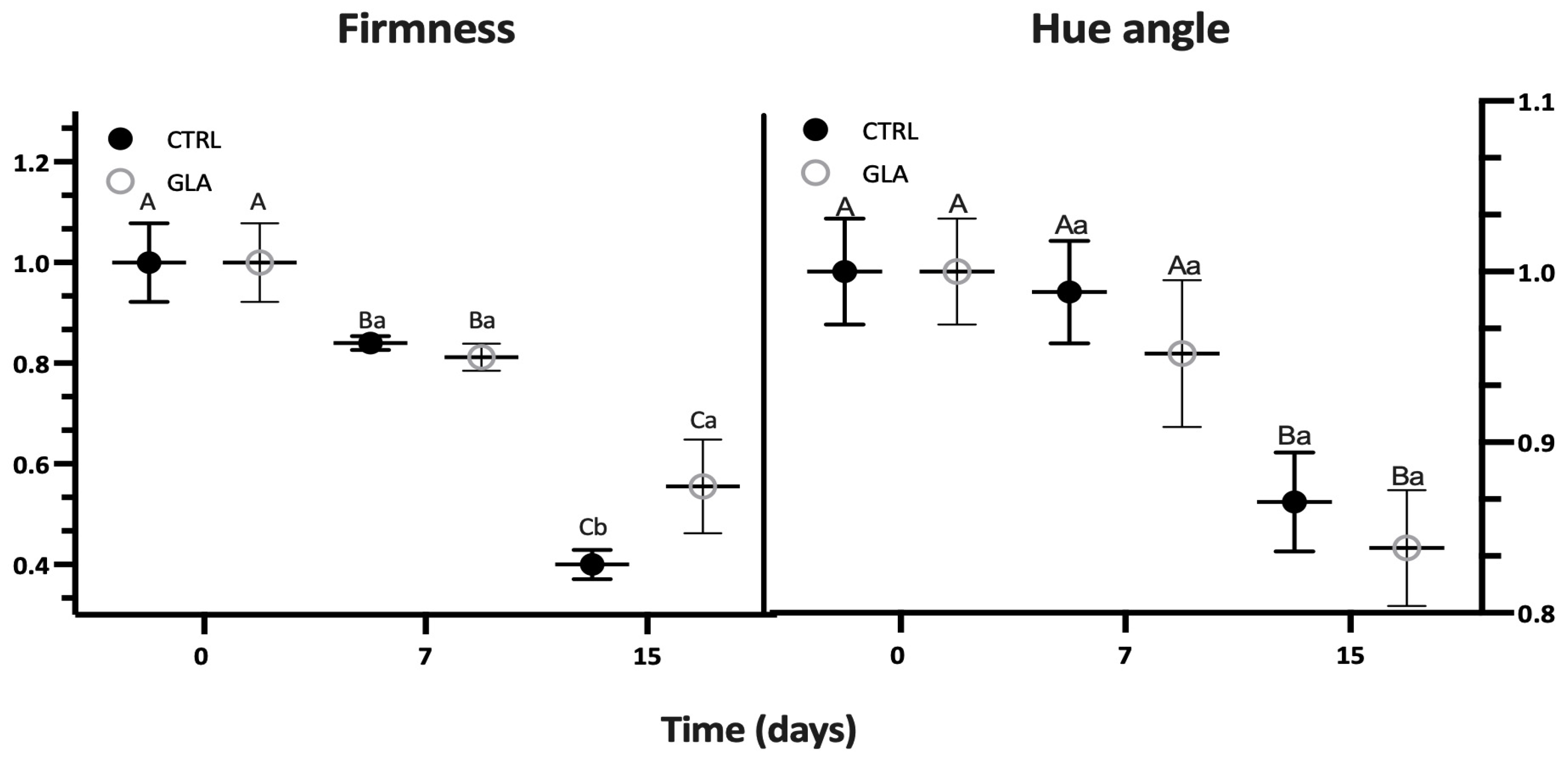
| Time (d) | SSC (%) | TA (g kg−1) | Firmness (N) | Hue Angle (°) | CO2 (mg (kg.h)−1) | C2H4 (μg (kg.h)−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTRL | GLA | CTRL | GLA | CTRL | GLA | CTRL | GLA | CTRL | GLA | CTRL | GLA | |
| 0 | 12.32 ± 0.80 | 12.32 ± 0.80 | 11.74 ± 0.18 | 11.74 ± 0.18 | 43.93 ± 3.43 | 43.93 ± 3.43 | 95.91 ± 2.99 | 95.91 ± 2.99 | 1.98 ± 0.30 | 1.98 ± 0.30 | 0.74 ± 0.05 | 0.74 ± 0.05 |
| 7 | 12.93 ± 0.20 | 12.40 ± 0.46 | 13.80 ± 1.30 | 13.29 ± 2.08 | 36.88 ± 0.61 | 35.68 ± 1.18 | 94.81 ± 2.83 | 91.27 ± 4.08 | 8.22 ± 0.86 | 11.07 ± 1.42 | 0.86 ± 0.17 | 1.51 ± 0.42 |
| 15 | 13.12 ± 0.13 | 12.90 ± 0.27 | 11.65 ± 0.84 | 14.36 ± 1.11 | 17.56 ± 1.27 | 24.4 ± 4.10 | 83.00 ± 2.82 | 83.40 ± 3.25 | 16.04 ± 4.72 | 25.17 ± 0.48 | 10.39 ± 2.14 | 11.41 ± 1.18 |
| Sucrose (mg g−1) | Sorbitol (mg g−1) | Glucose (mg g−1) | Fructose (mg g−1) | Malic acid (mg g−1) | ACC (nmol g−1) | |||||||
| 0 | 5.07 ± 1.12 | 5.07 ± 1.12 | 22.11 ± 1.50 | 22.11 ± 1.50 | 6.47 ± 0.80 | 6.47 ± 0.80 | 46.48 ± 1.28 | 46.48 ± 1.28 | 4.24 ± 0.73 | 4.24 ± 0.73 | 0.70 ± 0.07 | 0.70 ± 0.07 |
| 7 | 3.71 ± 0.20 | 4.41 ± 0.74 | 20.61 ± 4.98 | 17.03 ± 2.16 | 9.41 ± 0.70 | 8.77 ± 1.52 | 40.66 ± 3.09 | 43.44 ± 2.98 | 3.82 ± 0.32 | 3.76 ± 0.20 | 0.81 ± 0.14 | 0.56 ± 0.03 |
| 15 | 6.94 ± 1.05 | 4.46 ± 0.61 | 15.59 ± 1.80 | 14.78 ± 1.57 | 14.98 ± 3.46 | 10.39 ± 2.13 | 49.58 ± 2.98 | 49.23 ± 7.21 | 5.32 ± 0.50 | 5.07 ± 0.96 | 0.49 ± 0.08 | 0.33 ± 0.04 |
| ACO (nmol (g h) −1) | MACC (nmol g−1) | ACS (nmol (g h) −1) | Hexanal (ng g−1) | Hexanol (ng g−1) | Butyl acetate (ng g−1) | |||||||
| 0 | 2.04 ± 0.20 | 2.04 ± 0.20 | 36.84 ± 4.34 | 36.84 ± 4.34 | Not detected | 9.99 ± 2.35 | 9.99 ± 2.35 | 79.30 ± 11.76 | 79.30 ± 11.76 | 14.38 ± 4.33 | 14.38 ± 4.33 | |
| 7 | 2.14 ± 0.41 | 2.27 ± 0.33 | 42.43 ± 4.10 | 41.29 ± 2.45 | 135.2 ± 36.61 | 386.68 ± 55.2 | 55.92 ± 9.88 | 153.36 ± 33.6 | 7.23 ± 1.94 | 12.09 ± 1.31 | ||
| 15 | 2.08 ± 0.17 | 2.39 ± 0.21 | 43.86 ± 3.84 | 41.44 ± 4.36 | 124.59 ± 46.1 | 160.76 ± 31.1 | 216.83 ± 8.01 | 137.57 ± 3.34 | 78.64 ± 28.96 | 119.62 ± 15.1 | ||
| Hexyl acetate (ng g−1) | 2-ethyl-hexanol (ng g−1) | Butanol (ng g−1) | Ethanol (mg g−1) | Acetone (ng g−1) | (E)-2-Hexenal (ng g−1) | |||||||
| 0 | 2.07 ± 0.94 | 2.07 ± 0.94 | 5.25 ± 0.11 | 5.25 ± 0.11 | 59.35 ± 20.25 | 59.35 ± 20.25 | 3.90 ± 2.32 | 3.90 ± 2.32 | 90.66 ± 25.46 | 90.66 ± 25.46 | 3.12 ± 0.60 | 3.12 ± 0.60 |
| 7 | 1.99 ± 0.55 | 1.90 ± 0.49 | 4.44 ± 0.19 | 1.94 ± 0.38 | 51.19 ± 8.25 | 113.54 ± 38.5 | 5.82 ± 1.42 | 8.49 ± 3.48 | 103.02 ± 0.66 | 81.00 ± 25.00 | 25.65 ± 5.59 | 106.26 ± 17.1 |
| 15 | 4.89 ± 1.88 | 19.64 ± 14.63 | 2.49 ± 0.67 | 2.72 ± 0.86 | 347.24 ± 85.1 | 281.09 ± 52.9 | 11.77 ± 1.29 | 11.37 ± 2.33 | 593.47 ± 88.4 | 125.74 ± 19.6 | 38.82 ± 7.62 | 29.36 ± 0.51 |
| Limonene (ng g−1) | Hexane (ng g−1) | Linanol (ng g−1) | Ethyl acetate (ng g−1) | Pentyl acetate (ng g−1) | ||||||||
| 0 | 10.38 ± 0.16 | 10.38 ± 0.16 | Not detected | 0.153 ± 0.02 | 0.153 ± 0.02 | 67.11 ± 4.57 | 67.11 ± 4.57 | 0.05 ± 0.008 | 0.05 ± 0.008 | |||
| 7 | 10.96 ± 0.26 | 9.99 ± 1.52 | 0.11 ± 0.03 | 0.10 ± 0.01 | 60.54 ± 5.43 | 157.99 ± 69.1 | 1.06 ± 0.13 | 1.08 ± 0.11 | ||||
| 15 | 10.57 ± 0.17 | 9.67 ± 0.36 | 0.152 ± 0.02 | 0.175 ± 0.01 | 218.08 ± 62.1 | 81.52 ± 25.53 | 1.86 ± 0.41 | 0.31 ± 0.06 | ||||
References
- Gwanpua, S.G.; Verlinden, B.E.; Hertog, M.L.; Nicolai, B.M.; Geeraerd, A.H. A Conceptual Model of the Inhibitory Effect of 1-methylcyclopropene on Ripening of Apples. Acta Hortic. 2017, 1154, 41–46. [Google Scholar] [CrossRef]
- Hu, B.; Sun, D.-W.; Pu, H.; Wei, Q. Recent Advances in Detecting and Regulating Ethylene Concentrations for Shelf-Life Extension and Maturity Control of Fruit: A Review. Trends Food Sci. Technol. 2019, 91, 66–82. [Google Scholar] [CrossRef]
- Vanoli, M.; Grassi, M.; Buccheri, M.; Cortellino, G.; Lovati, F.; Caramanico, R.; Levoni, P.; Dalla Mora, A.; Spinelli, L.; Torricelli, A. Quality Characteristics, Sensory Profiles and Ethylene Production of Stored ‘Abate Fetel’ Pears Sorted at Harvest by Time-Resolved Reflectance Spectroscopy. Plants 2023, 12, 4013. [Google Scholar] [CrossRef] [PubMed]
- Watkins, C.B. The Use of 1-Methylcyclopropene (1-MCP) on Fruits and Vegetables. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef]
- Gago, C.M.; Miguel, M.G.; Cavaco, A.M.; Almeida, D.P.; Antunes, M.D. Combined Effect of Temperature and Controlled Atmosphere on Storage and Shelf-Life of ‘Rocha’ Pear Treated with 1-Methylcyclopropene. Food Sci. Technol. Int. 2015, 21, 94–103. [Google Scholar] [CrossRef]
- Zhi, H.; Dong, Y. Effect of 1-Methylcyclopropene on Superficial Scald Associated with Ethylene Production, α-Farnesene Catabolism, and Antioxidant System of Over-Mature ‘d’Anjou’ Pears After Long-Term Storage. Food Bioprocess Tech. 2018, 11, 1775–1786. [Google Scholar] [CrossRef]
- Hewitt, S.L.; Ghogare, R.; Dhingra, A. Glyoxylic Acid Overcomes 1-MCP-Induced Blockage of Fruit Ripening in Pyrus communis L. Var. ‘D’Anjou’. Sci. Rep. 2020, 10, 7084. [Google Scholar] [CrossRef]
- Dias, C.; Ribeiro, T.; Rodrigues, A.C.; Ferrante, A.; Vasconcelos, M.W.; Pintado, M. Improving the Ripening Process after 1-MCP Application: Implications and Strategies. Trends Food Sci. Technol. 2021, 113, 382–396. [Google Scholar] [CrossRef]
- Isidoro, N.; Almeida, D.P.F. α-Farnesene, Conjugated Trienols, and Superficial Scald in ‘Rocha’ Pear as Affected by 1-Methylcyclopropene and Diphenylamine. Postharvest Biol. Technol. 2006, 42, 49–56. [Google Scholar] [CrossRef]
- Minas, I.S.; Tanou, G.; Krokida, A.; Karagiannis, E.; Belghazi, M.; Vasilakakis, M.; Papadopoulou, K.K.; Molassiotis, A. Ozone-Induced Inhibition of Kiwifruit Ripening Is Amplified by 1-Methylcyclopropene and Reversed by Exogenous Ethylene. BMC Plant Biol. 2018, 18, 358. [Google Scholar] [CrossRef]
- Pongprasert, N.; Srilaong, V.; Sugaya, S. An Alternative Technique Using Ethylene Micro-Bubble Technology to Accelerate the Ripening of Banana Fruit. Sci. Hortic. 2020, 272, 109566. [Google Scholar] [CrossRef]
- Rizzolo, A.; Grassi, M.; Vanoli, M. Storage Protocol Modulates Ripening Behavior and Physiological Disorders of 1-MCP Treated ‘Abate Fetel’ Pears. Acta Hortic. 2018, 1194, 701–708. [Google Scholar] [CrossRef]
- Zhou, X.; Dong, L.; Zhou, Q.; Wang, J.; Chang, N.; Liu, Z.; Ji, S. Effects of Intermittent Warming on Aroma-Related Esters of 1-Methylcyclopropene-Treated ‘Nanguo’ Pears during Ripening at Room Temperature. Sci. Hortic. 2015, 185, 82–89. [Google Scholar] [CrossRef]
- Brumos, J. Gene Regulation in Climacteric Fruit Ripening. Curr. Opin. Plant Biol. 2021, 63, 102042. [Google Scholar] [CrossRef]
- Dias, C.; Rodrigues, A.C.; Vasconcelos, M.W.; Ferrante, A.; Pintado, M. Preliminary Results on the Effect of 1-Naphthaleneacetic Acid on Restoring ‘Rocha’ Pear Ripening Treated with 1-MCP. Acta Hortic. 2024, 1396, 61–66. [Google Scholar] [CrossRef]
- Kondo, S.; Seto, H. Changes of Jasmonic Acid During Ripening in Pear Fruit and Interactions Between Jasmonic Acid and Abscisic Acid. Acta Hortic. 2004, 636, 537–543. [Google Scholar] [CrossRef]
- Siebeneichler, T.J.; Crizel, R.L.; Camozatto, G.H.; Paim, B.T.; da Silva Messias, R.; Rombaldi, C.V.; Galli, V. The Postharvest Ripening of Strawberry Fruits Induced by Abscisic Acid and Sucrose Differs from Their In Vivo Ripening. Food Chem. 2020, 317, 126407. [Google Scholar] [CrossRef]
- Dhingra, A.; Hendrickson, C. Control of Ripening and Senescence in Pre-Harvest and Post-Harvest Plants and Plant Materials by Manipulating Alternative Oxidase Activity. U.S. Patent 9,123,456, 17 July 2017. [Google Scholar]
- Nikiforova, V.J.; Giesbertz, P.; Wiemer, J.; Bethan, B.; Looser, R.; Liebenberg, V.; Ruiz Noppinger, P.; Daniel, H.; Rein, D. Glyoxylate, a New Marker Metabolite of Type 2 Diabetes. J. Diabetes Res. 2014, 2014, 685204. [Google Scholar] [CrossRef]
- Norikura, T.; Sasaki, Y.; Kojima-Yuasa, A.; Kon, A. Glyoxylic Acid, an α-Keto Acid Metabolite Derived from Glycine, Promotes Myogenesis in C2C12 Cells. Nutrients 2023, 15, 1763. [Google Scholar] [CrossRef]
- Dias, C.; Ribeiro, T.; Rodrigues, A.C.; Ferrante, A.; Vasconcelos, M.W.; Pintado, M. Cold Storage Demand for “Rocha” Pear Ripening: A Comparison between a Shorter and Longer Cold Period. Sci. Hortic. 2022, 299, 111033. [Google Scholar] [CrossRef]
- Fernández-Cancelo, P.; Muñoz, P.; Echeverría, G.; Larrigaudière, C.; Teixidó, N.; Munné-Bosch, S.; Giné-Bordonaba, J. Ethylene and Abscisic Acid Play a Key Role in Modulating Apple Ripening after Harvest and after Cold-Storage. Postharvest Biol. Technol. 2022, 188, 111902. [Google Scholar] [CrossRef]
- De Martin, M.S.; Steffens, C.A.; Do Amarante, C.V.T.; Brackmann, A.; Rodrigues, M.F.; Soethe, C. Pears Stored Under Controlled Atmosphere with Ultra-Low and Low O2 Associated with Different CO2 Levels. Rev. Bras. Frutic. 2017, 39, e143. [Google Scholar] [CrossRef]
- Saquet, A.; Almeida, D. Internal Disorders of ‘Rocha’ Pear Affected by Oxygen Partial Pressure and Inhibition of Ethylene Action. Postharvest Biol. Technol. 2017, 128, 54–62. [Google Scholar] [CrossRef]
- Bulens, I.; Van de Poel, B.; Hertog, M.L.; De Proft, M.P.; Geeraerd, A.H.; Nicolaï, B.M. Protocol: An Updated Integrated Methodology for Analysis of Metabolites and Enzyme Activities of Ethylene Biosynthesis. Plant Methods 2011, 7, 17. [Google Scholar] [CrossRef]
- Giné-Bordonaba, J.; Echeverria, G.; Ubach, D.; Aguiló-Aguayo, I.; López, M.L.; Larrigaudière, C. Biochemical and Physiological Changes during Fruit Development and Ripening of Two Sweet Cherry Varieties with Different Levels of Cracking Tolerance. Plant Physiol. Biochem. 2017, 111, 216–225. [Google Scholar] [CrossRef]
- Lindo-García, V.; Larrigaudière, C.; Echeverría, G.; Murayama, H.; Soria, Y.; Giné-Bordonaba, J. New Insights on the Ripening Pattern of ‘Blanquilla’ Pears: A Comparison between on- and off-Tree Ripened Fruit. Postharvest Biol. Technol. 2019, 150, 112–121. [Google Scholar] [CrossRef]
- Alsberg, B.K.; Kell, D.B.; Goodacre, R. Variable Selection in Discriminant Partial Least-Squares Analysis. Anal. Chem. 1998, 70, 4126–4133. [Google Scholar] [CrossRef]
- Gao, Q.; Tian, Y.; Zhang, J.; Zhang, P.; Zhang, M.; Bi, J.; Li, J.; Xue, Y. Discriminant Analysis of ‘Fuji’ and ‘Hanfu’ Apples under 1-Methylcyclopropene (1-MCP) and Cold Storage Conditions Based on Their Postharvest Quality and Aroma Properties. J. Food Meas. Charact. 2024, 18, 9492–9507. [Google Scholar] [CrossRef]
- Lee, H.Y.; Yoon, G.M. Kinase Assay for CONSTITUTIVE TRIPLE RESPONSE 1 (CTR1) in Arabidopsis Thaliana. In Ethylene Signaling; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2017; Volume 1573, pp. 133–140. ISBN 9781493968541. [Google Scholar]
- Wu, Q.; Tao, X.; Ai, X.; Luo, Z.; Mao, L.; Ying, T.; Li, L. Contribution of Abscisic Acid to Aromatic Volatiles in Cherry Tomato (Solanum lycopersicum L.) Fruit during Postharvest Ripening. Plant Physiol. Biochem. 2018, 130, 205–214. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, G.; Tian, L.; Cao, Y.; Dong, X.; Huo, H.; Qi, D.; Zhang, Y.; Xu, J.; Liu, C. Changes of Volatile Organic Compounds of Different Flesh Texture Pears during Shelf Life Based on Headspace Solid-Phase Microextraction with Gas Chromatography–Mass Spectrometry. Foods 2023, 12, 4224. [Google Scholar] [CrossRef]
- Barbosa, R. Avaliação dos Compostos Orgânicos Voláteis Associados à Maturação da Pera Rocha; Instituto Técnico de Lisboa: Lisbon, Portugal, 2020. [Google Scholar]
- Kou, X.; He, Y.; Li, Y.; Chen, X.; Feng, Y.; Xue, Z. Effect of Abscisic Acid (ABA) and Chitosan/Nano-Silica/Sodium Alginate Composite Film on the Color Development and Quality of Postharvest Chinese Winter Jujube (Zizyphus jujuba Mill. cv. Dongzao). Food Chem. 2019, 270, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Farcuh, M.; Rivero, R.M.; Sadka, A.; Blumwald, E. Ethylene Regulation of Sugar Metabolism in Climacteric and Non-Climacteric Plums. Postharvest Biol. Technol. 2018, 139, 20–30. [Google Scholar] [CrossRef]
- Tipu, M.M.H.; Sherif, S.M. Ethylene and Its Crosstalk with Hormonal Pathways in Fruit Ripening: Mechanisms, Modulation, and Commercial Exploitation. Front. Plant Sci. 2024, 15, 1475496. [Google Scholar] [CrossRef] [PubMed]
- Ashitha, G.N.; Sunny, A.C.; Nisha, R. Effect of Pre-Harvest and Post-Harvest Hexanal Treatments on Fruits and Vegetables: A Review. Agric. Rev. 2020, 41, 124–131. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, H.; Zhong, T.; Chen, D.; Wu, Y.; Xie, Z. Molecular Regulatory Mechanisms Affecting Fruit Aroma. Foods 2024, 13, 1870. [Google Scholar] [CrossRef]
- Torregrosa, L.; Echeverria, G.; Illa, J.; Giné-Bordonaba, J. Emission of VOCs and Quality Evolution in Response to Repeated Oxygen Pull Downs on ‘Conference’ Pears during Long-Term Cold Storage. Postharvest Biol. Technol. 2020, 170, 111322. [Google Scholar] [CrossRef]
- Khan, S.; Alvi, A.F.; Saify, S.; Iqbal, N.; Khan, N.A. The Ethylene Biosynthetic Enzymes, 1-Aminocyclopropane-1-Carboxylate (ACC) Synthase (ACS) and ACC Oxidase (ACO): The Less Explored Players in Abiotic Stress Tolerance. Biomolecules 2024, 14, 90. [Google Scholar] [CrossRef]
- Polko, J.K.; Kieber, J.J. 1-Aminocyclopropane 1-Carboxylic Acid and Its Emerging Role as an Ethylene-Independent Growth Regulator. Front. Plant Sci. 2019, 10, 1602. [Google Scholar] [CrossRef]
- Tucker, G.; Yin, X.; Zhang, A.; Wang, M.; Zhu, Q.; Liu, X.; Xie, X.; Chen, K.; Grierson, D. Ethylene and Fruit Softening. Food Qual. Saf. 2017, 1, 253–267. [Google Scholar] [CrossRef]
- Zhu, X.; Song, Z.; Li, Q.; Li, J.; Chen, W.; Li, X. Physiological and Transcriptomic Analysis Reveals the Roles of 1-MCP in the Ripening and Fruit Aroma Quality of Banana Fruit (Fenjiao). Food Res. Int. 2020, 130, 108968. [Google Scholar] [CrossRef]
- Roy Choudhury, S.; Roy, S.; Das, R.; Sengupta, D.N. Differential Transcriptional Regulation of Banana Sucrose Phosphate Synthase Gene in Response to Ethylene, Auxin, Wounding, Low Temperature and Different Photoperiods during Fruit Ripening and Functional Analysis of Banana SPS Gene Promoter. Planta 2008, 229, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, L.; Su, M.; Zhang, X.; Du, J.; Li, X.; Zhang, M.; Hu, Y.; Zheng, X.; Ye, Z.; et al. Comparative Network Analysis Reveals the Regulatory Mechanism of 1-Methylcyclopropene on Sugar and Acid Metabolisms in Yellow Peach Stored at Non-Chilling Temperatures. Plant Physiol. Biochem. 2024, 216, 109100. [Google Scholar] [CrossRef] [PubMed]
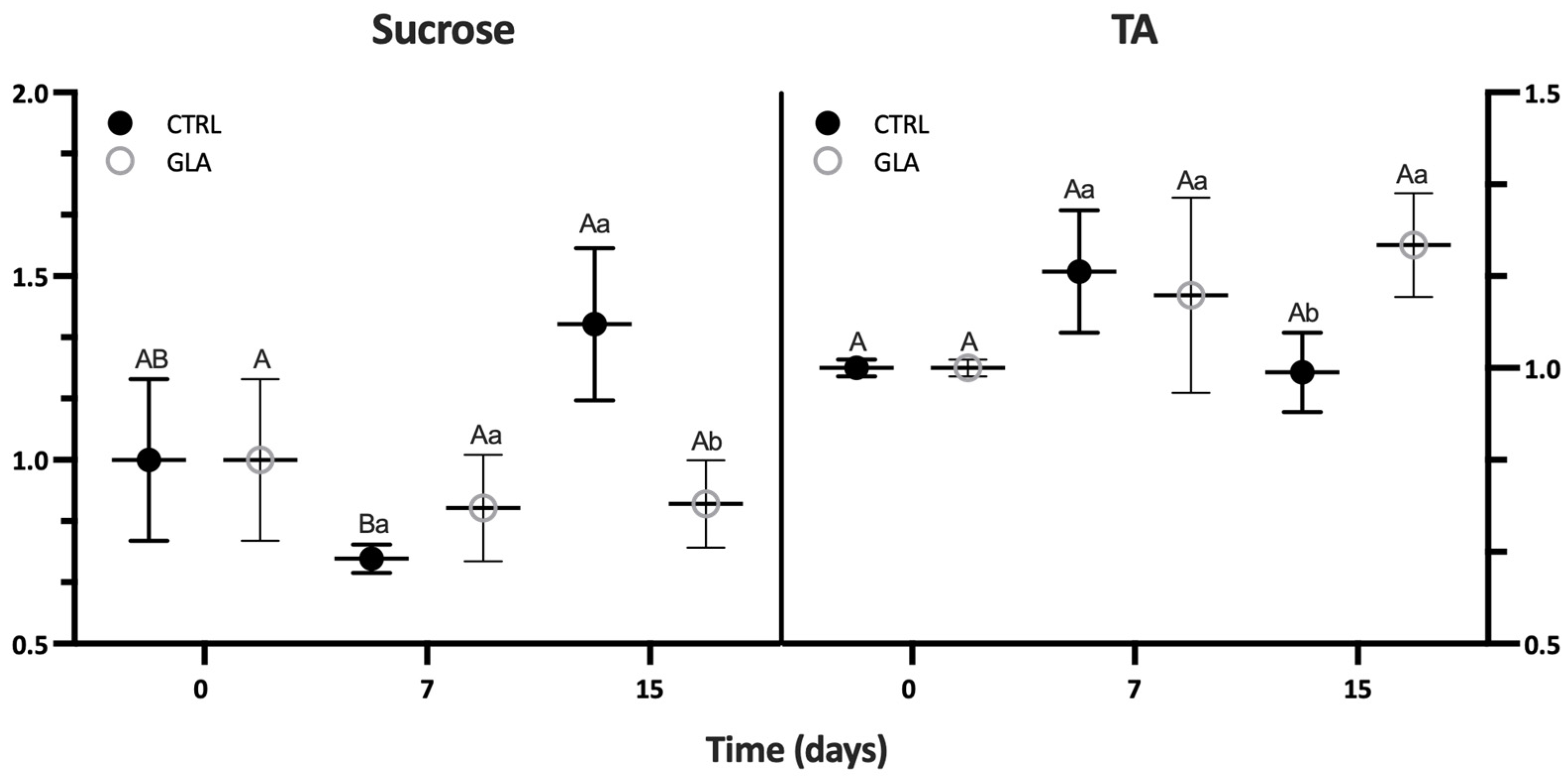
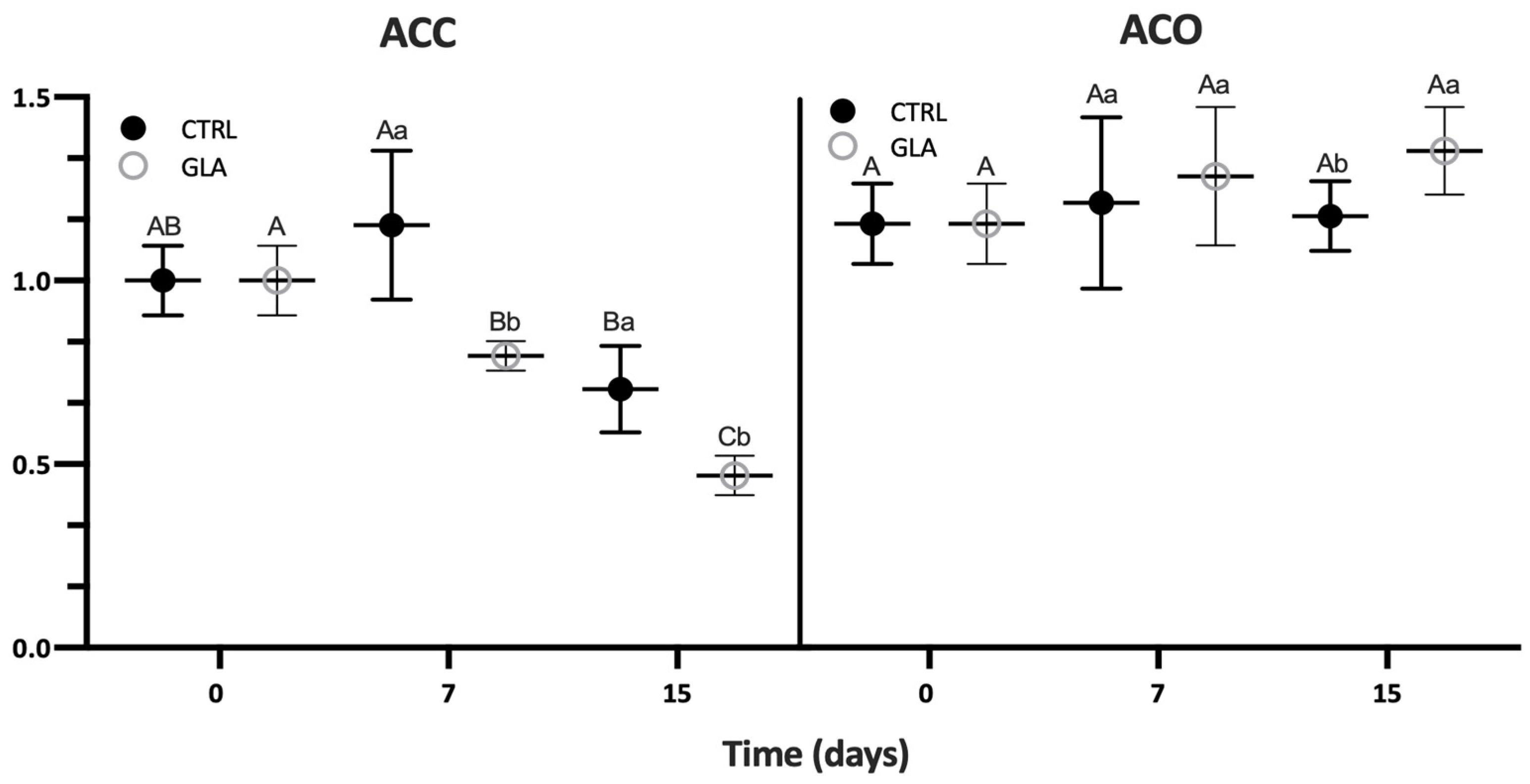
| Aroma Volatile | 0 D | 7 D | 15 D | |
|---|---|---|---|---|
| Hexanal | CTRL | 1 ± 0.22 B | 12.94 ± 3.50 Ab | 11.92 ± 4.41 Aa |
| GLA | 1 ± 0.22 C | 37.00 ± 5.28 Aa | 15.38 ± 2.98 Ba | |
| Hexyl acetate | CTRL | 1 ± 0.45 A | 0.96 ± 0.26 Aa | 2.36 ± 0.91 Aa |
| GLA | 1 ± 0.45 B | 0.92 ± 0.24 Ba | 12.70 ± 4.34 Ab | |
| Butyl acetate | CTRL | 1 ± 0.28 B | 0.46 ± 0.13 Bb | 5.04 ± 1.86 Aa |
| GLA | 1 ± 0.28 B | 0.77 ± 0.08 Ba | 7.66 ± 0.96 Aa | |
| (E)-2-Hexenal | CTRL | 1 ± 0.19 B | 8.24 ± 1.79 Ab | 12.46 ± 2.45 Aa |
| GLA | 1 ± 0.19 C | 34.11 ± 5.48 Aa | 9.43 ± 0.16 Ba | |
| Limonene | CTRL | 1 ± 0.02 B | 2.08 ± 0.05 Aa | 1.02 ± 0.02 Ba |
| GLA | 1 ± 0.02 A | 0.96 ± 0.15 Ab | 0.93 ± 0.03 Aa | |
| 2-Ethyl-hexanol | CTRL | 1 ± 0.02 B | 0.85 ± 0.04 Aa | 0.48 ± 0.13 Aa |
| GLA | 1 ± 0.02 A | 0.37 ± 0.07 Bb | 0.52 ± 0.17 Ba | |
| Ethanol | CTRL | 1 ± 0.06 B | 1.49 ± 0.36 Ba | 3.02 ± 0.33 Aa |
| GLA | 1 ± 0.06 B | 2.18 ± 0.9 ABa | 2.92 ± 0.60 Aa | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, C.; Sousa, C.; Vasconcelos, M.W.; Ferrante, A.; Pintado, M. Effects of Glyoxylic Acid on Metabolism and Ripening of ‘Rocha’ Pears Treated with 1-MCP. Horticulturae 2025, 11, 314. https://doi.org/10.3390/horticulturae11030314
Dias C, Sousa C, Vasconcelos MW, Ferrante A, Pintado M. Effects of Glyoxylic Acid on Metabolism and Ripening of ‘Rocha’ Pears Treated with 1-MCP. Horticulturae. 2025; 11(3):314. https://doi.org/10.3390/horticulturae11030314
Chicago/Turabian StyleDias, Cindy, Clara Sousa, Marta W. Vasconcelos, António Ferrante, and Manuela Pintado. 2025. "Effects of Glyoxylic Acid on Metabolism and Ripening of ‘Rocha’ Pears Treated with 1-MCP" Horticulturae 11, no. 3: 314. https://doi.org/10.3390/horticulturae11030314
APA StyleDias, C., Sousa, C., Vasconcelos, M. W., Ferrante, A., & Pintado, M. (2025). Effects of Glyoxylic Acid on Metabolism and Ripening of ‘Rocha’ Pears Treated with 1-MCP. Horticulturae, 11(3), 314. https://doi.org/10.3390/horticulturae11030314











