Abstract
Specklinia digitale is an epiphytic orchid endemic to Mexico. The destruction of its habitat has resulted in it being regarded as an endangered species, and, to our knowledge, no effort has been made to establish ex situ conservation programs. Here, we describe an in situ assessment of flower and fruit phenology and subsequent in vitro germination. We also established a successful protocol for asymbiotic in vitro germination and acclimatization using Murashige–Skoog (MS) medium at full (full-MS) and half (1/2 MS) strength as well as activated charcoal (AC). All-year flower and fruit production was confirmed. Nevertheless, we observed a low pollination occurrence. No significant difference in germination rate was found for the four treatments tested; however, after protocorm evaluation, full-MS plus AC emerged as the best treatment for S. digitale in vitro propagation. Germination of up to 90.53% was achieved in full-MS. The acclimatization survival was 95%. These observations and our effective germination protocol can be used in a comprehensive approach for conservation efforts of this species.
1. Introduction
Among the Orchidaceae family, the subtribe Pleurothallidinae, a term previously used as synonym of the genus term Pleurothalis, includes the majority of the orchid species [1]. This subtribe has been subjected to recent reassessments based on molecular phylogenetic studies [2], establishing the genus Specklinia, encompassed in this subtribe, with 86 species, although this is a debatable assertion, since DNA tests are constantly challenging the actual number [2].
Within this genus, Specklinia digitale (Luer) Pridgeon & M.W. Chase is a Mexican endemic orchid, reported by Hagsater et al. [3] as a species that thrives in semiarid and warm environments along with a xerophile shrub vegetation that includes succulent plants and cacti; according to these authors, the orchid is found 250 m above sea level at the top of hills, specifically in Nizanda, Oaxaca. Further observations made through the online portal inaturalist.org [4] place this species in the Mexican states of Chiapas, Oaxaca [5], Tabasco and Veracruz [6]. Specifically in Veracruz, where the most observations have been made, S. digitale is present in the municipalities of Córdoba, Amatlán De Los Reyes, Fortín de las Flores, Atoyac and Xalapa. Additionally, Soto-Arenas and Solano-Gomez [7] describe the climate in which S. digitale grows as semi-warm with average temperatures ranging from 18 °C to 27 °C, and precipitation ranging from 1000 mm to 2500 mm.
According to the Mexican Official Norm NOM-059-SEMARNAT-2010 updated in 2019 [8], S. digitale is regarded as a threatened species, adaptable to disturbed environments and therefore found in coffee and fruit plantations that have taken over its original habitat. S. digitale was first described by Leur (1976) [9] as a small, 10 cm long epiphytic plant, with thick leaves that are purple-spotted under the surface and inflorescence of 4–15 cm long with successive, pale green flowers.
The understanding of species phenology is important for conservation efforts, since reproductive success relies on flower and fruit production and synchronicity with the ideal abiotic and biotic agents that allow plants to achieve pollination, germination and, subsequently, growth [10]. Eleven species of the Specklinia genus are reported in Mexico [3], and nine of them are reported in Veracruz [11]; of them, only Specklinia endotrachys, S. marginata, S. tribuloides and S. digitale phenology has been previously reported [12,13]. As for the previously presented phenology of S. digitale, Soto-Arenas et al. [7] reported a flowering period from July to April, although flowering may extend throughout the year.
Ex situ conservation has not yet been implemented for S. digitale, but individuals of this species are successfully maintained in private and scientific collections [14,15]. Therefore, in vitro germination, a well-known and widely implemented technique, can be used as a strategy for conservation of threatened or commercially important species. The efficiency of this method relies on the proper selection of nutrients present in the culture medium [16]. The restriction of access to S. digitale seeds enhances the urgency of highly efficient germination protocols. The commonly used Murashige–Skoog medium (MS) has proven effective for seed germination [17,18] of a broad diversity of orchid species. Although this medium has not being thoroughly tested for the Specklinia genus, it has been used for the closely related genus Pleurothallis, leading to 21.39% of germination in P. pulchela [19]. Additionally, the miniature orchids Ericyna pusilla as well as Gastrochilus matsuran (Makino) Schltr. successfully germinate in MS medium [20,21]. Meanwhile, activated charcoal (AC) is being increasingly used in tissue culture due to its ability to absorb toxic metabolites and to decrease phenolic exudation and brown exudate accumulation [22]. In this research, our aim was to study the reproductive phenology of S. digital to expand our understanding of this endemic species and to establish a successful asymbiotic in vitro germination protocol that could be used in future efforts for the conservation of this threatened species, since the development of a successful tissue culture system is a prerequisite for the long-term conservation of threatened plant species [23].
2. Materials and Methods
2.1. Plant Material
A wild Specklinia digitale population (15 plants) established on two Pouteria zapota trees, located in Amatlán de los Reyes, Veracruz, Mexico, was used for observations and as a source of plant material (Figure 1). This site has an altitude of 647 m above sea level, and its climate is warm humid with abundant summer rainfall, an average annual temperature of 20 °C and an average total annual precipitation of 1900 mm [13]. During the study, the annual temperature averaged 25.8° and 25.6° in 2023 and 2024, respectively [24]. That population was used to register floral and fruit phenology and to collect fruits for seed counting and in vitro germination.

Figure 1.
Wild plant of S. digital located in Amatlán de los Reyes, Veracruz, during the flowering peak.
2.2. Reproductive Phenology
Since November 2022, observations were conducted of the previously mentioned outdoor population to record its reproductive phenology and the following: inflorescence onset, inflorescence buds, flowers and fruits. In 2024, all observations were put together (Figure 2).

Figure 2.
Fifty-two-week-long phenology of S. digitale showing the presence of flower organs in each week. As observed, three peaks in flowering were noted at week 6, 10 and 48.
2.3. Capsule Development and Total Seed Number
Three capsules were monitored between October and February. Photographs were taken daily, and measures were performed using Image J 1.54 software. The first capsule was left to achieve natural dehiscence at day 30, the second was harvested on day 27 for in vitro cultivation, and the third was also harvested on day 27 and used for total seed count. Once harvested, the capsule used for seed counting was cut longitudinally, and all the seeds were extracted and placed on a paper sheet divided into quadrants of 1 cm2 each, under a Zeiss Stemi 200C microscope (Carl Zeiss AG, Oberkochen, Germany), for counting.
2.4. In Vitro Germination and Protocorm Development
Previous attempts to conduct in vitro germination failed to produce protocorms when using 20- and 24-day-old fruits; therefore, a mature, 27-day-old, naturally pollinated fruit was harvested for the in vitro germination experiments reported. The capsule was transferred to the Plant Tissue Culture Laboratory at Colegio de Postgraduados, Córdoba Campus, Mexico, petal remnants were carefully cut, and a disinfection protocol was immediately implemented.
The capsule was washed in a 3 g·L−1 commercial detergent solution (Roma®) for 10 min and then submerged in a 1.8% sodium hypochlorite solution for 10 min with constant stirring. That was followed by three washes using sterile distilled water. The capsule was then submerged in a 0.5 g·L−1 solution of Captan® and maintained in a sterile laminar flow hood. A wash with sterile distilled water was repeated three times before the capsule was sprayed with 70% alcohol and flamed. The capsule was placed in a Petri dish and, after waiting for it to cool down, a longitudinal cut was made using a scalpel. With the help of a clean scalpel blade, approximately 60 seeds were dispersed in each culture medium.
2.5. Culture Medium for In Vitro Germination and Protocorm Growth
Four treatments were implemented for in vitro germination: two concentrations of MS medium (Murashige and Skoog, 1962) [25] without plant growth regulators were tested (full and 1/2) in the presence or absence of AC (0 and 0.15% w/v) [17], resulting in a 2 × 2 factorial arrangement. Regardless of the treatment, the culture medium was prepared using 2.5 g·L−1 Phytagel™, and the pH was adjusted to 5.7 ± 0.1 using HCl before Phytagel was added. Twenty milliliters of germination medium was poured inside 100 mL glass flasks and sterilized at 120 °C for 15 min.
2.6. In Vitro Germination and Protocorm Growth
The cultures were incubated at 24 ± 1 °C under a 16 h photoperiod at a light intensity of 57 µmol m−2 s−1. The earliest germination onset was observed after 4 weeks. At week 14, protocorm development had reached stage 3 or further according to staging by Gao et al. [26], who classified protocorm development in six stages, stage 1 being seed imbibition, and stage 6 defined as the appearance of true leaves and roots.
2.7. Experimental Design and Statistical Analysis
A completely random design with ten repetitions was used in both experiments. The data obtained were statistically processed with Prims Software version 8.0.2. Analysis of variance (ANOVA) followed by Tukey’s test (p ≤ 0.05) was performed to determine the significance of the differences between treatments.
2.8. Acclimatization
One-year-old plantlets with more than 100 leaves and 1 cm long roots on average, which were obtained in full-MS + AC and subcultured three times every four months using the same medium, were washed to eliminate any traces of culture medium and submerged for 10 min in a commercial fungicide Captan® solution. The plantlets were placed in closed plastic trays containing red volcanic rock (tezontle) and transferred to a greenhouse with temperature ranging 20 to 36 °C and a 70% relative humidity [27]. The substrate was sterilized for 20 min at 121 °C. Twenty-five plantlets were placed in closed trays that were gradually opened in the following two weeks; survival percentage was measured after 60 days.
3. Results
3.1. Specklinia Digitale Reproductive Phenology
The phenology data are condensed in Figure 2, presented per week, where each colored box shows the start and duration of each inflorescence onset, the presence of inflorescence buds and flowers, the flower peak and fruit formation.
According to the observations made, we confirmed an all-year-long flowering cycle divided into three main periods. At the start of the year, each plant showed at least one flower bud that kept growing for 9 days and developed secondary floral buds at its tip. As new primary floral buds emerged, the previous ones began to show well-formed blooms that later opened into fully developed flowers, as shown in Figure 3E. Since the opening of fresh flowers occurred progressively, the flowering period extended for five to seven weeks, depending on the number of flowers per inflorescence.

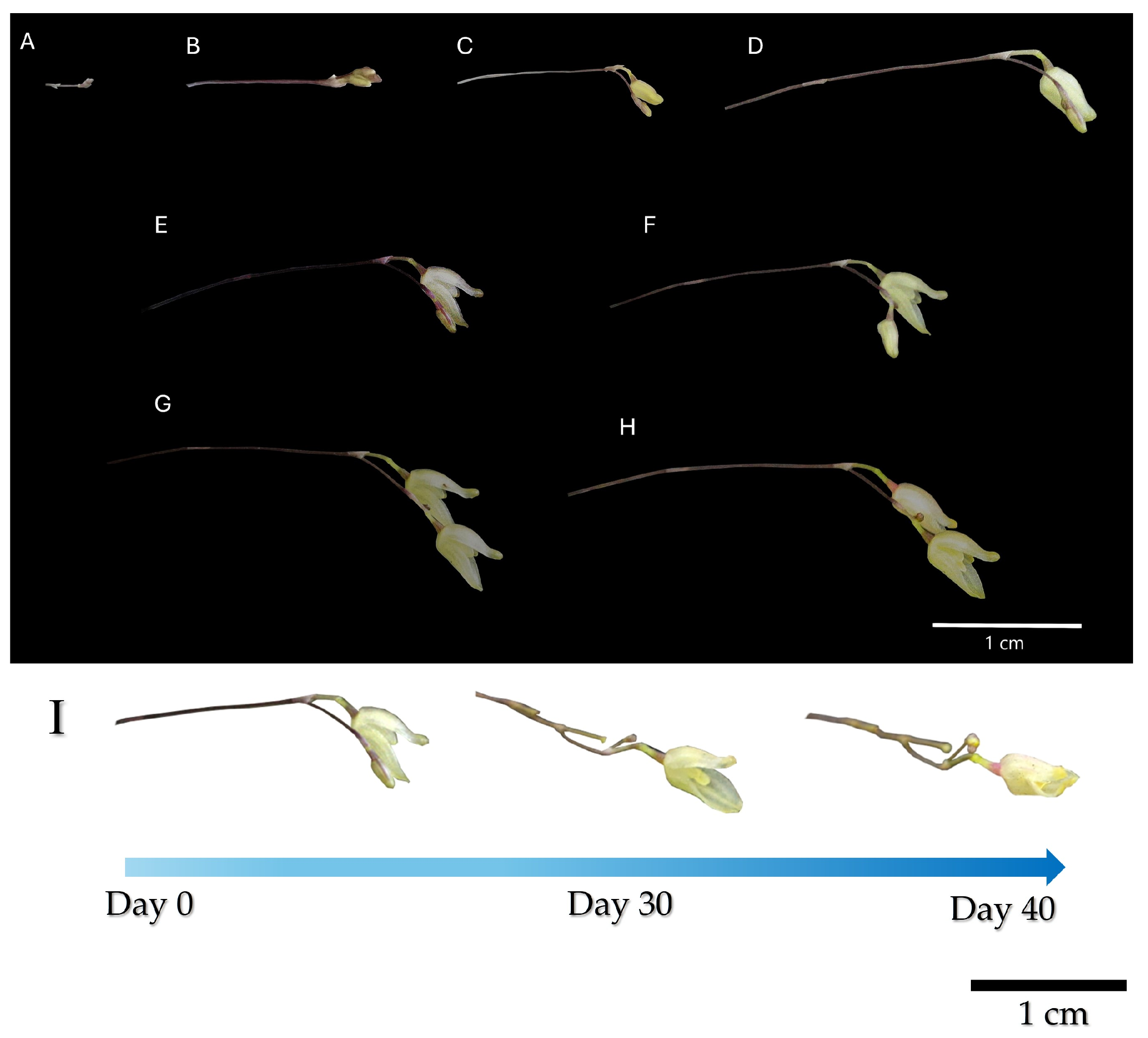
Figure 3.
Bloom progression of S. digitale from flower bud appearance to the closure of the first opened flower. (A) First flower bud, day 3; (B) day 9; (C) day 12; (D) day 14; (E) day 15; (F) day 17; (G) day 18; (H) day 24, the flower that first opened on day 15 is close; (I) inflorescence time lapse. Depending on the number of secondary flower buds, the inflorescence will last up to 30 more days.
During the study, three main stages of floral development were observed: once a S. digitale plant achieved a 2–3 cm width, it reached the end of its juvenile stage, approximately three to four years after germination. The flowering period started with the appearance of floral buds (day 1–3) that appeared as slim, purple sticks that lasted about a week (Figure 3A,B). During this week, the floral buds developed secondary floral buds at their tip, that would later become flowers (Figure 3C,D). These secondary floral buds opened one by one in successive order, the older flower remaining at the back of the inflorescence and lasting up to 8 days (Figure 3E–G). The inflorescences’ blooming period lasted up to 40 days since the first flower completely opened.
3.2. Capsule Development and Number of Seeds per Capsule
Pollination was a rare occurrence, since throughout the duration of the study, only ten capsules in total were observed among the population, one per flowering period. If pollination occurred, the flower began to close and acquired a purple tone. Over the course of 28 days, the fruits grew to a size of 0.56 × 0.3 cm and a weight of 0.0154 g, as determined by observing six fruits. The fruits turned pale green and finally developed brown spots upon achieving maturity (Figure 1). The fruits opened on day 30 after pollination.
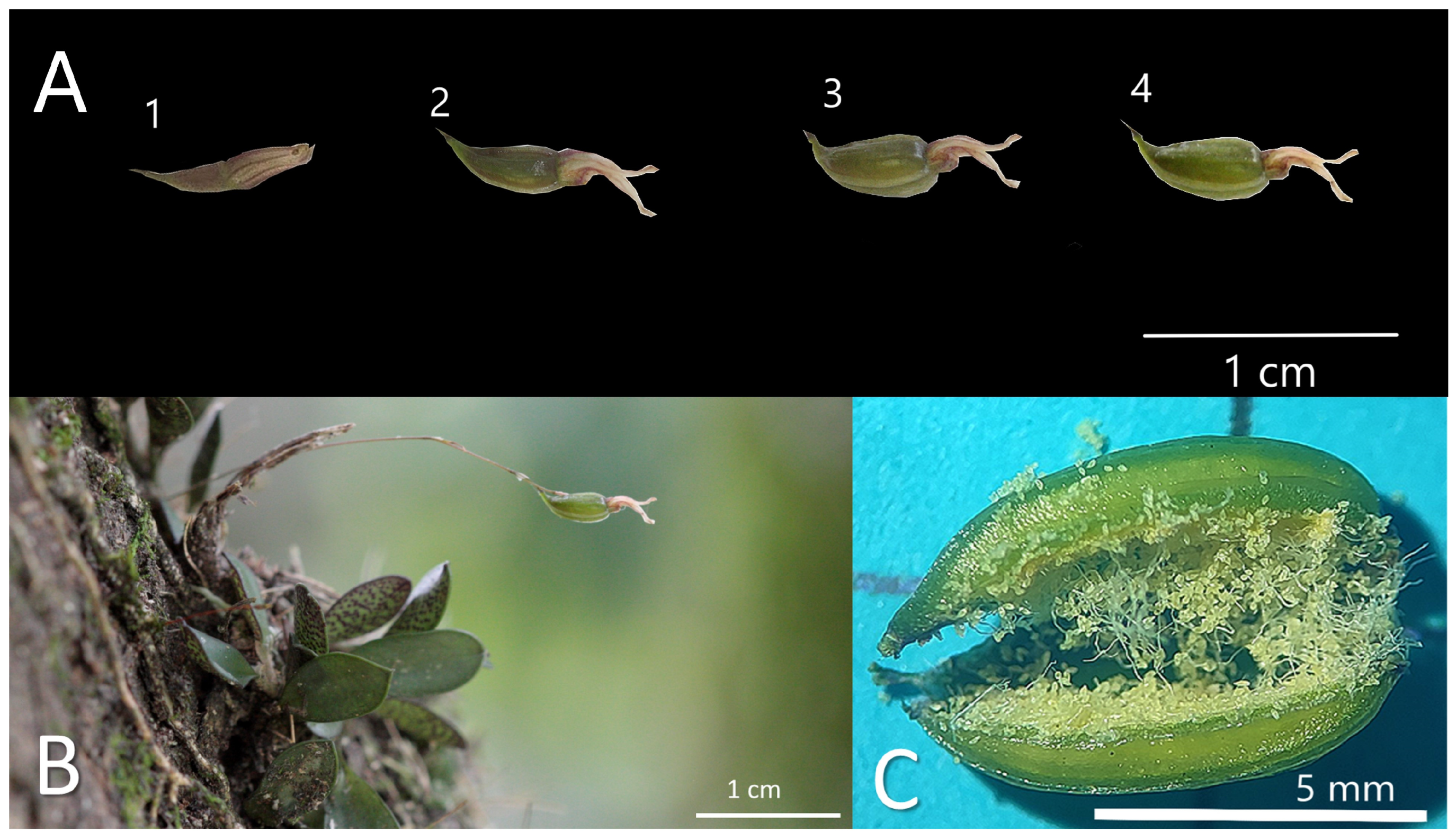
Figure 4 shows the developmental progression of a capsule from day 2 post pollination to day 27, when the capsule could be harvested for in vitro asymbiotic germination (Figure 4B). Finally, we found that a single capsule of S. digitale contained 5144 seeds (Figure 4C).

Figure 4.
S. digitale capsule phenology. (A) 1: Starting on day 2 post pollination, capsule formation began; 2: day 10; 3: day 24; 4: day 27; (B) a mature capsule on day 27, suitable for in vitro germination.
3.3. In Vitro Germination and Protocorm Development
The flasks containing S. digitale seeds presented no contamination, and seed germination was observed six weeks after sowing. However, very few seeds presented a change in color, signaling the start of the germination process; therefore, the evaluation of the germination percentage was performed in week 10 (Figure 5), and the measurement of protocorm size in week 14 (Figure 6A).

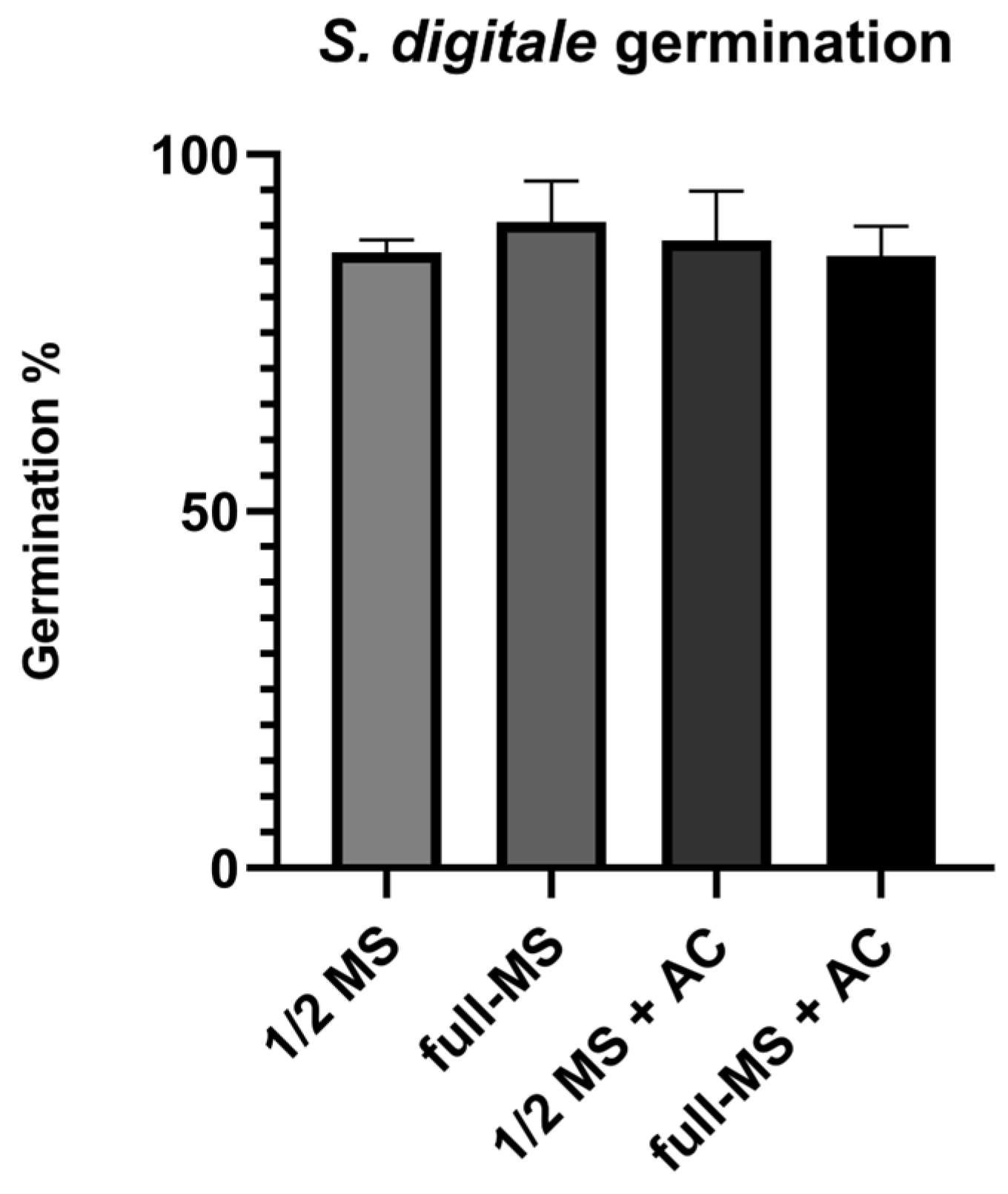
Figure 5.
Germination percentage ten weeks after sowing. No significant difference was found between treatments. Full-strength Murashige–Skoog (full-MS), Murashige–Skoog at half-strength (1/2 MS), activated charcoal (AC).

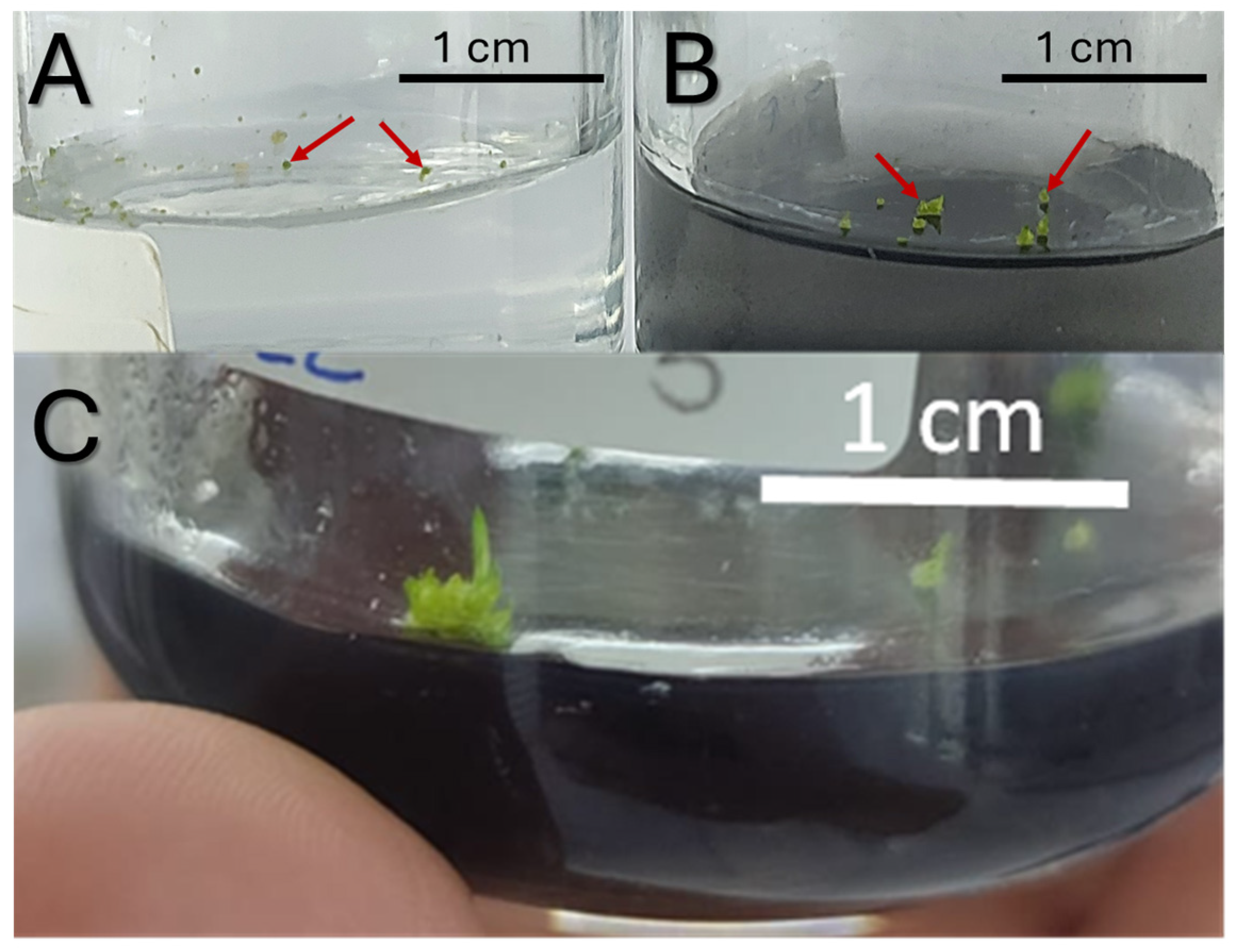
Figure 6.
S. digitale in vitro germination (A) S. digitale protocorm eight weeks after sowing full-MS (B) full-MS + AC and (C) Fourteen-week-old S. digitale protocorm during germination full-MS + AC; full-MS + AC. Red arrows indicate protocorms.
The highest germination percentage was observed in full-MS, with a value of 90.53, then in 1/2 MS + AC with a value of 87.99%, followed by 1/2 MS with a value of 86.26 and finally by full-MS + AC with a value of 85.78%. Even though no significant differences were observed in germination, the morphological characteristics were evidently superior when AC was used (Figure 6B). Ten-week-old protocorms were bigger in the presence of AC, achieving stage 4 regardless of MS concentration [26], while the protocorms growing in the absence of AC seemed to remain in stage 2 (Figure 6B).
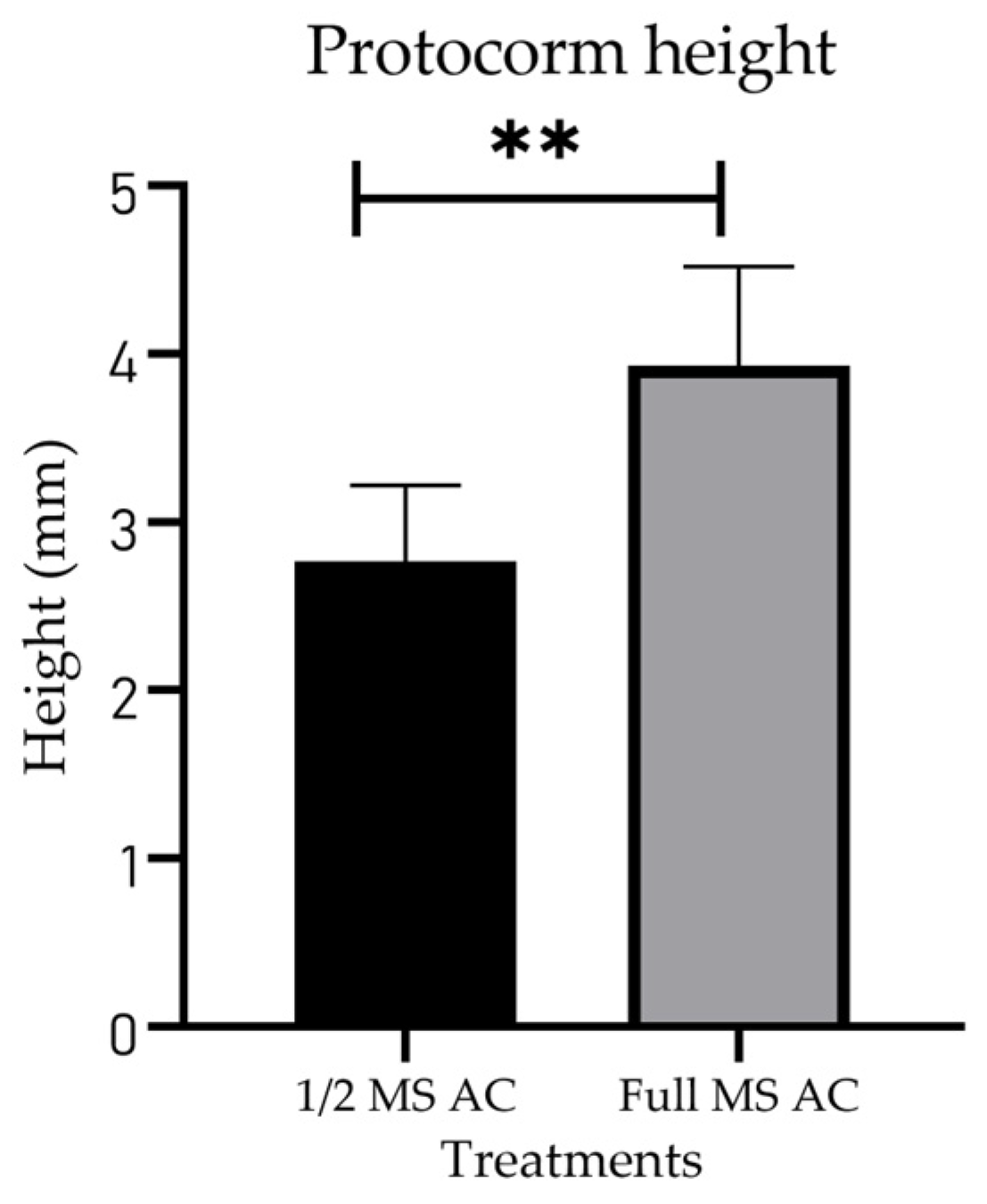
Due to the differences in stage development, protocorm size was measured only for plants subjected to the AC treatment, since the plants cultured in full-MS and 1/2 MS were two stages behind those treated with AC. Significant differences were found between the plants grown in 1/2 MS + AC and those cultured in full-MS + AC: the protocorms grown in full-MS + AC were, on average, 3.83 mm tall, while those grown in 1/2 MS + AC had an average height of 2.76 mm (Figure 7).

Figure 7.
Mean height of S. digitale protocorms 14 weeks after sowing. ** Significant difference according to an unpair t test at p < 0.05 was found. p = 0.0077. Full strength Murashige–Skoog (full-MS), Murashige–Skoog at half-strength (1/2 MS), activated charcoal (AC).
3.4. Acclimatization
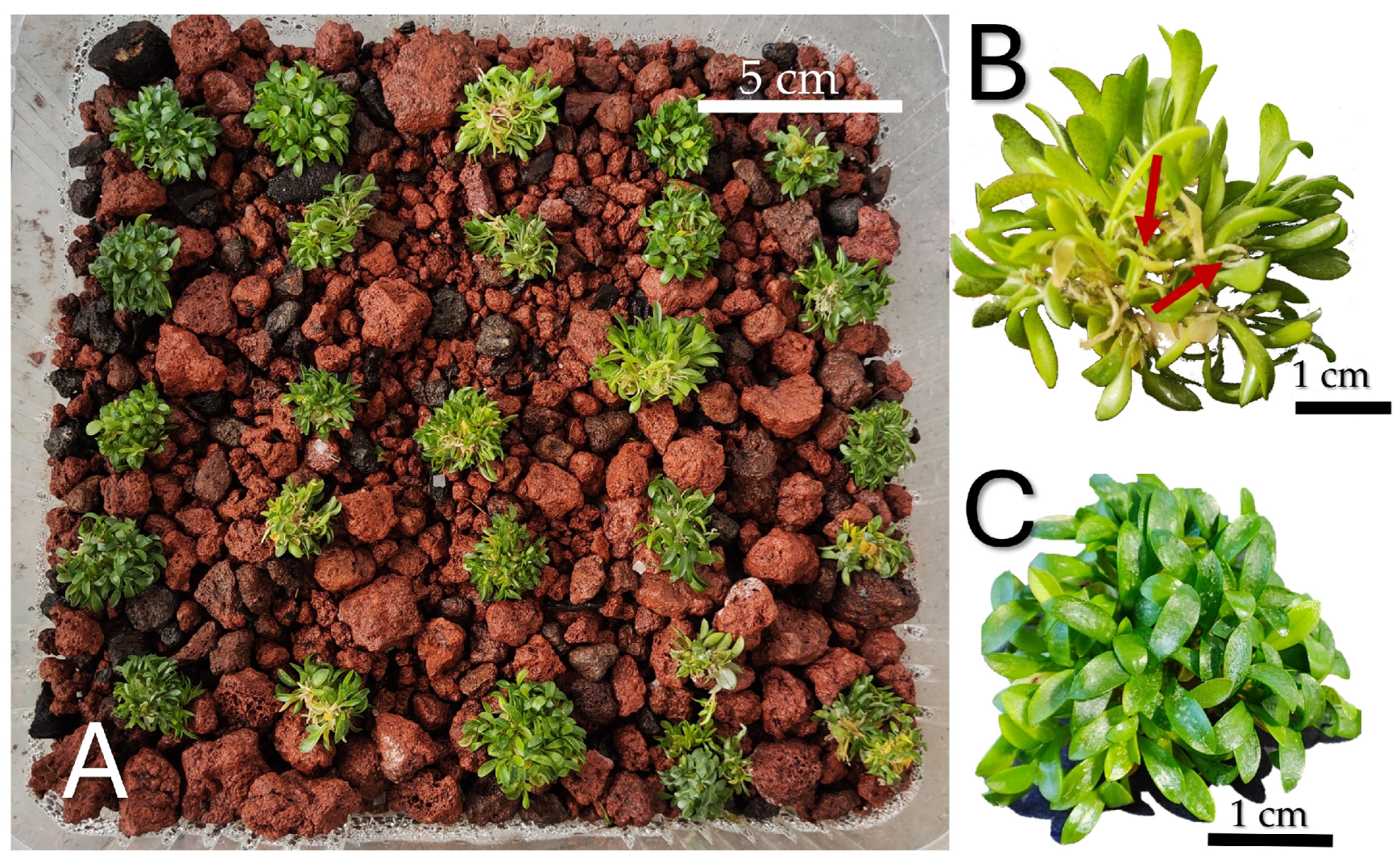
The survival rate was 95% after 60 days in greenhouse conditions (Figure 8A). The average height of the acclimatized plants was 2.44 cm (Figure 8B), a mean of 123 leaves was counted, and newly formed roots were observed (Figure 8C).

Figure 8.
Acclimatization of S. digitale. (A) Plantlets; (B) roots formed; (C) aerial parts after 60 days.
4. Discussion
S. digitale presents flowers all year round, similar to Specklinia endotrachys, S. pfavii, S. remotiflora and S. spectabilis [28], which are species from Costa Rica. S. digitale also presents flowering peaks at the start and at the end of the year; these are followed by a resting period that is interrupted by a short flowering period with less flower production and a second resting period. It is worth noting that the resting periods matched with the drought periods, which are irregular in the region. On the first year of this study, a moderate drought affected the region, starting in July and ending in October, whereas on the second year, a severe drought started in May and ended in June, followed by an abnormally dry half month [29]. These conditions might explain the resting periods and the slow flowering onset between them.
Part of the reason for the all-year flowering of this plant is the presence of long-lasting inflorescences. This plant produces flowers for at least a month, with overlapping flowering cycles, (Figure 2). Besides being in accordance with the flowering periods reported by Baltazar-Bernal et al. [13], the obtained results allow for a deeper understanding of the reproductive patterns of S. digitale. We observed that, despite its low occurrence, pollination was possible throughout the year regardless of the flowering peaks, and the number of produced fruits was unaffected. Although we could not determine a pollinator, Cecidomydae and Phoridae flies have been reported to transport the pollinium of Specklinia marginata in Sonocusco, Oaxaca [12]. It is also worth noting that the low fruit production aligns with the data reported for S. endotrachys [28], suggesting that the long-lasting flowers are essential for attracting pollinators.
The naturally pollinated fruit (0.56 × 0.3 cm) of S. digitale has >5000 seeds; Sonkoly et al. [30] studied 48 orchid species’ fruits whose seed number ranged from 910 to 19,726, which places S. digitale at the lower end of this range. Additionally, the small orchid Erycina crista-galli, whose fruit is 1.4 × 0.4 cm in size [31], is reported to have between 17,000 and 12,000 seeds [32].
The S. digitale fruit reaches maturity 27 days post-pollination (dpp), weighing on average 0.0154 g, but there is no evident morphological difference between a 24 dpp immature capsule and a 27 dpp mature capsule. Therefore, a strict surveillance of capsule development is important for successful in vitro germination.
During the in vitro germination, the first protocorms appeared 6 weeks after sowing, and at week 8, morphological differences in size between protocorms grown in MS and MS + AC were evident. The size difference, however, was not reflected in the germination rate. We observed that the full-MS and 1/2 MS media without AC slowed protocorm growth, while protocorms in full-MS and 1/2 MS + AC achieved a mean height of 3.933 and 2.767 mm, respectively. Ten-week-old protocorms were bigger in AC, achieving stage 4 [26] regardless of MS concentration, while protocorms growing in the absence of AC seemed to remain in stage 2 (Figure 6B). In the current research, seeds in MS medium failed to overcome stage 3 at week 14 after sowing, while MS + AC protocorms were already in stage 5 (Figure 6A). Thus, activated charcoal proved to be necessary for protocorm development.
Activated charcoal has been used for in vitro germination with various species, with different results. When tested in 18 European orchid species [33], the germination percentage decreased for all the species including Dactylorhiza maculata, whose germination rate was reduced from 89.6% in basal medium to 53.6% when 0.1% w/v AC was added. Conversely, AC helped to reduce the mortality rate 4 months after sowing, from 85.4% in basal medium to 10.2% in basal medium + 0.1% w/v AC. Meanwhile, Valencia-Glushchenko et al. [19] reported favorable results for Pleurothallis pulchella germination using 1/2 MS + AC, showing a significantly better germination rate compared to that achieved in 1/2 MS and reporting an increase from 21.39 to 33.15%. Our results fall between those two outcomes, and no significant differences were found between the treatments for germination.
While AC allowed for protocorm development, MS strength played an important role in protocorm size. MS was associated with a height mean of 3.933 mm, whereas 1/2 MS was associated with a height mean of 2.767 mm, which means that a higher MS concentration was required for protocorm development. So, while AC is essential for seedling development in S. digitale, a greater availability of nutrients provided by a higher MS strength also plays an important role. Besides having an effect on the germination rate, AC also has different effects on seedling development, depending on the species. Sorgato et al. concluded that AC delayed the growth and development of protocorms and seedlings of D. nobile and D. phalaenopsis [16], while Pacek-Bieniek et al. obtained results similar to ours for Zygostates grandiflora, showing that a greater AC concentration (3 g·L−1) resulted in larger primary leaves 18 months after sowing [34]
The discrepancy between the results obtained for different species seems to confirm that each species has distinct energy requirements, and AC can either promote or inhibit seedling growth. Our results suggest that the environment provided by AC, characterized by low light intensity and the adsorption of inhibitory compounds as well as naturally produced growth regulators [22], is essential for protocorm development, but so are compounds present in MS; therefore, a balance between compound availability and adsorbed compounds is required.
This efficient method for the in vitro germination and propagation of S. digitale allows for obtaining plant material for subsequent studies in this species such as those on its reintroduction into its natural habitat or on early in vitro flowering for ornamental purposes.
The substrate used for the acclimatization of S. digitale proved to be successful, showing high survival rates. Tezontle led to a greater survival rate than that obtained for L. speciosa [35]. Plantlets obtained following the in vitro germination protocol were able to overcome the environmental stress during acclimatization and were even able to develop new roots.
5. Conclusions
S. digitale formed inflorescences that lasted more than 30 days and were present almost all year. The only times that flowers were not observed among the population were drought seasons, and three peaks in flowering were observed. Although fruit formation was not common in the population studied, the in vitro germination rates were high.
A successful protocol for in vitro propagation and acclimatization was developed. Activate charcoal proved to be essential for seedling development, and full-strength MS enhanced their growth. After acclimatization, S. digitale plants can be reintroduced into their natural environment in order to contribute to the conservation of this endangered species.
Author Contributions
Conceptualization, research suggestions and manuscript finalization, O.B.-B.; fieldwork, statistical analysis, result interpretation and manuscript draft, E.G.M.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no competing interests.
References
- Rojas-Alvarado, G.; Karremans, A. A Typological and Morphological Analysis of the Pleurothallidinae (Orchidaceae) Inflorescences. Bot. Rev. 2024, 90, 221–250. [Google Scholar] [CrossRef]
- Karremans, A.P.; Albertazzi, F.J.; Bakker, F.T.; Bogarín, D.; Eurlings, M.C.M.; Pridgeon, A.; Pupulin, F.; Gravendeel, B. Phylogenetic Reassessment of Specklinia and Its Allied Genera in the Pleurothallidinae (Orchidaceae). Phytotaxa 2016, 272, 1. [Google Scholar] [CrossRef]
- Hágsater, E.; Soto Arenas, M.A.; Salazar Chávez, G.A.; Jiménez Machorro, R.; López Rosas, M.A.; Dressler, R.L. Las Orquídeas de México; Primera Edición; Productos Farmacéuticos, S.A. de C.V.: México, Mexico, 2005; ISBN 978-968-7889-07-8. [Google Scholar]
- Specklinia Digitale. Available online: https://www.inaturalist.org/taxa/206347-Specklinia-digitale (accessed on 30 January 2025).
- Vista de Vegetación y Flora de La Región de Nizanda, Istmo de Tehuantepec, Oaxaca, México. Available online: https://abm.ojs.inecol.mx/index.php/abm/article/view/879/1045 (accessed on 6 January 2025).
- Villaseñor, J.L. Checklist of the Native Vascular Plants of Mexico. Rev. Mex. Biodivers. 2016, 87, 559–902. [Google Scholar] [CrossRef]
- Soto-Arenas, M.A.; Solano-Gómez, A.R. Ficha Técnica de Specklinia Digitale. Información Actualizada Sobre las Especies de Orquídeas del PROY-NOM-059 ECOL-2000; Bases de datos SNIB-CONABIO; Proyecto No. W029; Instituto Chinoin A.C., Herbario de la Asociación Mexicana de Orquideología A.C.: México, Mexico, 2007. [Google Scholar]
- DOF-Diario Oficial de La Federación. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5578808&fecha=14/11/2019#gsc.tab=0 (accessed on 6 January 2025).
- Luer, C.A. Una Nueva Especie Del Golfo de México: Pleurothallis digitale. Orquidea 1976, 6, 3–7. [Google Scholar]
- Sakai, S. Phenological Diversity in Tropical Forests. Popul. Ecol. 2001, 43, 77–86. [Google Scholar] [CrossRef]
- Viccon Esquivel, J.; Castañeda Zárate, M.; Castro-Cortés, R.; Cetzal Ix, W. (Eds.) Las Orquídeas de Veracruz; Primera Edición; Universidad Veracruzana Dirección Editorial: Xalapa, Mexico, 2021; ISBN 978-607-502-903-0. [Google Scholar]
- Damon, A.; Salas-Roblero, P. A Survey of Pollination in Remnant Orchid Populations in Soconusco, Chiapas, Mexico. Trop. Ecol. 2007, 48, 1–14. [Google Scholar]
- Baltazar-Bernal, O.; De La Cruz-Martínez, V.M.; Hernández-García, A.; Zavala-Ruiz, J. An Exploratory Study of Orchidaceae Species Fruits in the Central Zone of Veracruz State, Mexico. Agrociencia 2023. [Google Scholar] [CrossRef]
- Baltazar-Bernal, O.; De La Cruz-Martínez, V.M.; Zavala-Ruiz, J. Las Orquídeas Del Campus Córdoba, Su Estudio y Preservación; Colegio de Postgradruados: Texcoco, Mexico, 2020. [Google Scholar]
- Baltazar-Bernal, O.; Zavala-Ruiz, J.; Hernández-García, A. Orchid Diversity (Orchidaceae) in Two Urban Sites in the State of Veracruz, Mexico. Agrociencia 2024, 58, 571–583. [Google Scholar] [CrossRef]
- Sorgato, J.C.; Soares, J.S.; Damiani, C.R.; Ribeiro, L.M. Effects of Light, Agar, Activated Charcoal, and Culture Medium on the Germination and Early Development of Dendrobium Seedlings. Aust. J. Crop Sci. 2020, 14, 557–564. [Google Scholar] [CrossRef]
- Baltazar-Bernal, O.; De la Cruz-Martínez, V.M.; Ramírez-Mosqueda, M.A.; Zavala-Ruiz, J. In Vitro Seed Germination and Acclimatization of Encyclia Cordigera (Kunth) Dressler. S. Afr. J. Bot. 2022, 151, 578–582. [Google Scholar] [CrossRef]
- Manokari, M.; Latha, R.; Priyadharshini, S.; Shekhawat, M.S. Effect of Activated Charcoal and Phytohormones to Improve in Vitro Regeneration in Vanda tessellata (Roxb.) Hook. Ex G. Don. Vegetos 2021, 34, 383–389. [Google Scholar] [CrossRef]
- Valencia-Glushchenko, N.; Oña-Arias, C.G.; Orellana, M.; Ortega, M.; Montero-Oleas, A.; De Lourdes Torres, M. In Vitro Asymbiotic Seed Germination and Seedling Development of Four Endangered Ecuadorian Orchids: Epidendrum jamiesonis, Pleurothallis pulchella, Oncidium pentadactylon, and Elleanthus capitatus. Plant Cell Tissue Organ Cult. 2024, 158, 60. [Google Scholar] [CrossRef]
- Chiu, Y.-T.; Chang, C. In Vitro Flowering and Breeding of Erycina Pusilla. In Orchid Propagation: From Laboratories to Greenhouses—Methods and Protocols; Lee, Y.-I., Yeung, E.C.-T., Eds.; Springer Protocols Handbooks; Springer: New York, NY, USA, 2018; pp. 257–265. ISBN 978-1-4939-7770-3. [Google Scholar]
- Kang, H.; Kang, K.W.; Kim, D.H.; Sivanesan, I. In Vitro Propagation of Gastrochilus matsuran (Makino) Schltr., an Endangered Epiphytic Orchid. Plants 2020, 9, 524. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.D. The Role of Activated Charcoal in Plant Tissue Culture. Biotechnol. Adv. 2008, 26, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Bettoni, J.C.; Bonnart, R.; Volk, G.M. Challenges in Implementing Plant Shoot Tip Cryopreservation Technologies. Plant Cell Tissue Organ Cult. 2021, 144, 21–34. [Google Scholar] [CrossRef]
- Comisión Nacional del Agua; Coordinación General Del Servicio Meteorológico Nacional Base de Datos Climatológica Nacional. Available online: https://smn.conagua.gob.mx/tools/RESOURCES/Normales_Climatologicas/Mensuales/ver/mes30340.txt (accessed on 22 February 2025).
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Y.; Deng, D.; Luo, Y.; Shao, S.; Luo, Y. Morphogenesis Changes in Protocorm Development during Symbiotic Seed Germination of Dendrobium chrysotoxum (Orchidaceae) with Its Mycobiont, Tulasnella Sp. Horticulturae 2023, 9, 531. [Google Scholar] [CrossRef]
- Solís-Zanotelli, F.Y.; Hidalgo-Contreras, J.V.; Baltazar-Bernal, O. Crecimiento Ex Vitro de Plántulas de Lycaste Aromatica (Graham) Lindl. RevFitotecMex 2022, 45, 341. [Google Scholar] [CrossRef]
- Karremans, A.P.; Pupulin, F.; Grimaldi, D.; Beentjes, K.K.; Butôt, R.; Fazzi, G.E.; Kaspers, K.; Kruizinga, J.; Roessingh, P.; Smets, E.F.; et al. Pollination of Specklinia by Nectar-Feeding Drosophila: The First Reported Case of a Deceptive Syndrome Employing Aggregation Pheromones in Orchidaceae. Ann. Bot. 2015, 116, 437–455. [Google Scholar] [CrossRef]
- CONAGUA Monitor de Sequía En México. Available online: https://smn.conagua.gob.mx/es/climatologia/monitor-de-sequia/monitor-de-sequia-en-mexico (accessed on 23 January 2025).
- Sonkoly, J.; Vojtkó, A.E.; Tökölyi, J.; Török, P.; Sramkó, G.; Illyés, Z.; Molnár, V.A. Higher Seed Number Compensates for Lower Fruit Set in Deceptive Orchids. J. Ecol. 2016, 104, 343–351. [Google Scholar] [CrossRef]
- Hagsater, E.; Salazar, G.A. Icones Orchidacearum Fascicle I, Orchid of Mexico Part 1; Asociación Mexicana de Orquideología A. C.: Mexico City, Mexico, 1990. [Google Scholar]
- Pérez-Hérnandez, H.; Damon, A.; Valle-Mora, J.; Sánchez-Guillen, D. Orchid Pollination: Specialization in Chance? Bot. J. Linn. Soc. 2011, 165, 251–266. [Google Scholar] [CrossRef]
- Van Waes, J. Effect of Activated Charcoal on in Vitro Propagation of Western European Orchids. Acta Hortic. 1987, 212, 131–138. [Google Scholar] [CrossRef]
- Pacek-Bieniek, A.; Dyduch-Siemińska, M.; Rudaś, M. Influence of Activated Charcoal on Seed Germination and Seedling Development by the Asymbiotic Method in Zygostates Grandiflora (Lindl.) Mansf. (Orchidaceae). Folia Hortic. 2010, 22, 45–50. [Google Scholar] [CrossRef][Green Version]
- Ávila-Díaz, I.; Oyama, K.; Gómez-Alonso, C.; Salgado-Garciglia, R. In Vitro Propagation of the Endangered Orchid Laelia speciosa. Plant Cell Tissue Organ Cult. 2009, 99, 335–343. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).