Magnetic-Water-Enhanced Tomato Yield and Antioxidant Response Under Arid Conditions of South Tunisia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area, Experimental Design, and Treatments
2.2. Magnetic Treatment and Device
2.3. Vegetative Growth and Yield Traits
2.4. Physicochemical Quality Parameters
2.5. Hydrogen Peroxide and Antioxidant Enzymes
2.6. Malondialdehyde and Osmoticum Determination
2.7. RNA Isolation and Quantitative RT-qPCR Analysis
2.8. Statistical Analysis
3. Results
3.1. Yield Characteristics
3.2. Physicochemical Quality Parameters
3.3. Hydrogen Peroxide and Antioxidant Enzyme Activity
3.4. Malondialdehyde and Osmoticum Accumulation
3.5. Correlation Analysis
3.6. Different Expressed Gene Patterns
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Norbert, S.; Kaka, Y.; Gona, A. Analysis of Tomato (Solanum lycopersicum L) Value Chain in Kebbi State, Nigeria. Direct Res. J. Agri. Food Sci. 2023, 11, 217–223. [Google Scholar] [CrossRef]
- Hong, Y.X.X.; Jiang, X.L.; Xu, H.; Yu, C. The impacts of China’s dual carbon policy on green innovation: Evidence from Chinese heavy-polluting enterprises. J. Environ. Manag. 2023, 350, 119620. [Google Scholar] [CrossRef] [PubMed]
- CheikhRouhou, M.; Souhir, A.; Hager, A.; Anne, L.; Guadalupe, V.M.; Christine, R.; Dorra, G. Pilot scale production of dietary fibers from Tunisian tomato and red pepper by-products. Sust. Chem. Pharm. 2024, 39, 101521. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#search/Pistachios%2C%20in%20shell (accessed on 23 January 2025).
- Yacoubi, S.; Slatnia, A.; Azzib, R.; Oueslati, T. Impact of deficit irrigation strategies on water use and productivity of vegetable crops in a semi-arid context of Tunisia. Desal. Water Treat. 2022, 263, 109–115. [Google Scholar] [CrossRef]
- Zhai, Y.; Yang, Q.; Wu, Y. Soil Salt Distribution and Tomato Response to Saline Water Irrigation under Straw Mulching. PLoS ONE 2016, 11, e0165985. [Google Scholar] [CrossRef]
- Grancharova, N.E.; Elenov, B.; Janevska, E. Quantitative prediction of tomato irrigation needs using Cropwat model. J. Agri. Food Environ. Sci. 2022, 76, 46–51. [Google Scholar] [CrossRef]
- Kanzari, S.; Šimůnek, J.; Daghari, I.; Younes, A.; Ali, K.B.; Mariem, S.B.; Ghannem, S. Modeling Irrigation of Tomatoes with Saline Water in Semi-Arid Conditions Using Hydrus-1D. Land 2024, 13, 739. [Google Scholar] [CrossRef]
- Dominguez, G.; Wilkins, G.; Thiemens, M.H. Isotope fractionation in silicate melts. Nature 2011, 473, 70–73.e1. [Google Scholar] [CrossRef]
- Corbari, C.; Ben Charfi, I.; Mancini, M. Optimizing Irrigation Water Use Efficiency for Tomato and Maize Fields across Italy Combining Remote Sensing Data and the AquaCrop Model. Hydrology 2021, 8, 39. [Google Scholar] [CrossRef]
- Ullah, I.; Mao, H.; Rasool, G.; Gao, H.; Javed, Q.; Sarwar, A.; Khan, M.I. Effect of Deficit Irrigation and Reduced N Fertilization on Plant Growth, Root Morphology, and Water Use Efficiency of Tomato Grown in Soilless Culture. Agronomy 2021, 11, 228. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Liao, S.; Zou, G.; Zhao, T.; Chen, Y.; Yang, J.; Zhang, L. Effects of two slow-release nitrogen fertilizers and irrigation on yield, quality, and water-fertilizer productivity of greenhouse tomato. Agric. Water Manag. 2017, 186, 139–146. [Google Scholar] [CrossRef]
- Colimba-Limaico, J.E.; Zubelzu-Minguez, S.; Rodríguez-Sinobas, L. Optimal Irrigation Scheduling for Greenhouse Tomato Crop (Solanum lycopersicum L.) in Ecuador. Agronomy 2022, 12, 1020. [Google Scholar] [CrossRef]
- Wang, X.; Xing, Y. Evaluation of the effect of irrigation and fertilization by drip fertigation on tomato yield and water use efficiency in greenhouse. Int. J. Agron. 2016, 2016, 3961903. [Google Scholar] [CrossRef]
- Kuscu, H.; Turhan, A.; Ozmen, N.; Aydinol, P.; Demir, A.O. Optimizing levels of water and nitrogen applied through drip irrigation for yield, quality, and water productivity of processing tomato (Lycopersicon esculentum Mill.). Hortic. Environ. Biotechnol. 2014, 55, 103–114. [Google Scholar] [CrossRef]
- Halimi, A.H.; Tefera, A.H. Application of Cropwat Model for Estimation of Irrigation Scheduling of Tomato in Changing Climate of Eastern Europe: The Case Study of Godollo, Hungary. Intern. J. Agri. Environ. Sci. 2019, 6, 1–11. [Google Scholar]
- Adamtie, T.F.; Mitku, D.T.; Hassen, A. Validations of CROPWAT Based Irrigation Practice for Tomato Productivity in Lowland Hot Humid Area of Ethiopia. Am. J. Life Sci. Innov. 2022, 1, 27–35. [Google Scholar] [CrossRef]
- Oke, O.S.; Oyaniyi, T.; Adewumi, O.T.; Bamigboye, O.T.; Lawah, M.O.; Jatto, K.A.; Adara, C.T.; Marizu, J.T.; Ogunbela, A.A. Economic, nutritional and medicinal values of african walnut (Tetracarpidium conophorum) in Nigeria (Hutch. & Dalziel): A Review. J. Res. For. Wildl. Environ. 2020, 12. [Google Scholar]
- Xiang, W.; Tan, M.; Yang, X.; Li, H. The impact of cropland spatial shift on irrigation water use in China. Environ. Impact Assess. Rev. 2022, 97, 106904. [Google Scholar] [CrossRef]
- Smith, M. CROPWAT A computer program for irrigation planning and management. In Rome FAO Irrigation and Drainage Paper; FAO: Rome, Italy, 1992; p. 46. [Google Scholar]
- Solangi, G.S.; Shah, S.A.; Alharbi, R.S.; Panhwar, S.; Keerio, H.A.; Kim, T.-W.; Memon, J.A.; Bughio, A.D. Investigation of Irrigation Water Requirements for Major Crops Using CROPWAT Model Based on Climate Data. Water 2022, 14, 2578. [Google Scholar] [CrossRef]
- Aydın, Y. Quantification of water requirement of some major crops under semi-arid climate in Turkey. Peer J. 2022, 10, e13696. [Google Scholar] [CrossRef]
- Akrimi, R.; Hajlaoui, H.; Batelli, G.; Ruggiero, A.; Badri, M.; Grillo, S.; Mhamdi, M. Changes in potato metabolism and agro-physiological attributes in response to saline irrigation. J. Agron. Crop Sci. 2021, 207, 44–58. [Google Scholar] [CrossRef]
- Kishorea, G.; Singha, R.K.; Saxenaa, C.K.; Al-Ansari, N.; Vishwakarma, D.K.; Heddamd, S. Magnetic treatment of irrigation water and its effect on Frenchbean (Phaseolus vulgaris). Water Reuse 2023, 13, 545. [Google Scholar] [CrossRef]
- Colic, M.; Morse, D. The elusive mechanism of the magnetic ‘memory’ of water. Colloids Surf. Physicoch. Eng. Aspects 1999, 154, 167–174. [Google Scholar] [CrossRef]
- Samarah, N.H.; Bany Hani, M.M.I.; Makhadmeh, I.M. Effect of Magnetic Treatment of Water or Seeds on Germination and Productivity of Tomato Plants under Salinity Stress. Horticulturae 2021, 7, 220. [Google Scholar] [CrossRef]
- Khaskhoussy, K.; Mohamed, B.; Mariem, D.; Mohamed, H. Performance of different magnetic and electromagnetic water treatment devices on soil and two tomato cultivars. Sci. Hort. 2023, 322, 112437. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Dobránszki, J. Impact of magnetic water on plant growth. Environ. Exper. Biol. 2014, 12, 137–142. [Google Scholar]
- Akrimi, R.; Hajlaoui, H.; Mhamdi, M. Effects of electromagnetic treated saline water on potatoes (Solanum tuberosum L.) physiological and nutritional characteristics. J. Hortic. Postharvest Res. 2023, 6, 247–260. [Google Scholar] [CrossRef]
- Zaidi, N.S.; Sohaili, J.; Muda, K.; Sillanpää, M. Magnetic field application and its potential in water and wastewater treatment systems. Separ. Purif. Rev. 2014, 43, 206–240. [Google Scholar] [CrossRef]
- Abedinpour, M.; Rohani, E. Effects of magnetized water application on soil and maize growth indices under different amounts of salt in the water. J. Water Reuse Desal. 2017, 7, 319–325. [Google Scholar] [CrossRef]
- Zhao, G.; Mu, Y.; Wang, Y.; Wang, L. Magnetization and oxidation of irrigation water to improve winter wheat (Triticumaestivum L.) production and water-use efficiency. Agri. Water Manag. 2022, 259, 107254. [Google Scholar] [CrossRef]
- Maheshwari, B.L.; Grewal, H.S. Magnetic treatment of irrigation water: Its effects on vegetable crop yield and water productivity. Agric. Water Manag. 2009, 96, 1229–1236. [Google Scholar] [CrossRef]
- Hozayn, M.; Abdul Qados, A.M. Irrigation with magnetized water enhances growth, chemical constituent and yield of chick pea (Cicerarietinum L.). Agric. Biol. J. North Am. 2010, 1, 671–676. [Google Scholar]
- Al-Khazan, M.; Abdullatif, B.M.; Al-Assaf, N. Effects of magnetically treated water on water status, chlorophyll pigments and some elements content of jojoba (Simmondsia chinensis L.) at different growth stages. Afr. J. Environ. Sci. Technol. 2011, 5, 722–731. [Google Scholar]
- Chang, K.T.; Weng, C.I. The effect of an external magnetic field on the structure of liquid water using molecular dynamics simulation. J. Appl. Phys. 2006, 100, 043917. [Google Scholar] [CrossRef]
- Selim, A.F.; El-Nady, M. Physio-anatomical responses of drought stressed tomato plants to magnetic field. Acta Astronaut. 2011, 69, 387–396. [Google Scholar] [CrossRef]
- Belyavskaya, N.A. Biological effects due to weak magnetic field on plants. Adv. Space Res. 2004, 34, 1566–1574. [Google Scholar] [CrossRef]
- Turker, M.; Temirci, C.; Battal, P.; Erez, M.E. The Effects of an Artificial and Static Magnetic Field on Plant Growth, Chlorophyll and Phytohormone Levels in Maize and Sunflower Plants. Phyton Ann. Rei Bot 2007, 46, 271–284. [Google Scholar]
- Gabrielli, C.; Jaouhari, R.; Maurin, G.; Keddam, M. Magnetic water treatment for scale prevention. Water Res. 2001, 35, 3249–3259. [Google Scholar] [CrossRef]
- Surendran, U.; Sandeep, O.; George, M.; Joseph, E.J. A Novel technique of magnetic treatment of saline and hard water for irrigation and its impact on cow pea growth and water properties. Int. J. Agric. Environ. Biotech. 2013, 6, 85–92. [Google Scholar]
- Kahn, B.A.; Damicone, J.P. Kaolin particle film product applications before harvest begins may not improve marketable yields of fresh tomatoes. Hort. Technol. 2008, 42, 1010. [Google Scholar] [CrossRef]
- Nangare, D.D.; Singh, Y.; Kumar, P.S.; Minhas, P.S. Growth, fruit yield and quality of tomato (Lycopersicun esculentum Mill) as affected by deficit irrigation regulated on phonological basis. Agri. Water Manag. 2016, 171, 73–79. [Google Scholar] [CrossRef]
- Dashti, N.H.; Montasser, M.S.; Ali, N.Y.; Cherian, V.M. Influence of plant growth promoting rhizobacteria on fruit yield, pomological characteristics and chemical contents in cucumber mosaic virus-infected tomato plants. Kuwait J. Sci. 2014, 41. [Google Scholar]
- Murshed, R.; Lopez-Lauri, F.; Keller, C.; Monnet, F.; Sallanon, H. Acclimation to drought stress enhances oxidative stress tolerance in Solanum lycopersicum L. Fruits Plant Stress 2008, 2, 145–151. [Google Scholar]
- Azevedo, R.A.; Alas, R.M.; Smith, R.J.; Lea, P.J. Response of antioxidant enzymes to transfer from elevated carbon dioxide to air and ozone fumigation, in the leaves and roots of wild-type and a catalase deficient mutant of barley. Physiol. Plant. 1998, 104, 280–292. [Google Scholar] [CrossRef]
- Gomes-Junior, R.A.; Gratão, P.L.; Gaziola, S.A.; Mazzafera, P.; Lea, P.J.; Azevedo, R.A. Selenium-induced oxidative stress in coffee cell suspension cultures. Funct. Plant Biol. 2007, 34, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Zhang, M.; Zhang, H.; Zhang, P. Improved tolerance to various abiotic stresses in transgenic sweet potato (Ipomoea batatas) expressing spanichbetaine aldehyde dehydrogenase. PLoS ONE 2012, 7, e37344. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spanich chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Hodges, D.M.; De Long, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive substances assay for estimating lipid peroxidtion in plant tissues containing anthocynin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Man, P.; Griffiths, P.G.; Chinnery, P.F. Mitochondrial optic neuropathies—Disease mechanisms and therapeutic strategies. Prog. Retin Eye Res. 2011, 30, 81–114. [Google Scholar] [CrossRef]
- Robyt, J.F.; White, B.J. Biochemical Techniques-Theory and Practice; Books/Cole Publishing Company: Monterey, CA, USA, 1987; Volume XVI, pp. 267–275. [Google Scholar]
- Chang, S.; Puryear, J.; Cairney, J. A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 1993, 11, 113–116. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kalleli, F.; Abid, G.; Ben Salem, I.; Boughalleb, N.; M’hamdi, M. Essential oil from fen nel seeds (Foeniculum vulgare) reduces Fusarium wilt of tomato (Solanum lycopersicon). Phytopathol. Medi Terranea 2020, 59, 63–76. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Surendran, U.; Sandeep, O.; Joseph, E.J. The impacts of magnetic treatment of irrigation water on plant, water and soil characteristics. Agri. Water Manag. 2016, 178, 21–29. [Google Scholar] [CrossRef]
- Ogunlela, A.O.; Yusuf, K.O. Effect of magnetic treatment of water treatment of water on chemical properties of water and sodium adsorption ration. J. Resear Forest Wildl. Environ. 2016, 8, 73–79. [Google Scholar]
- Vashisth, A.; Nagarajan, S. Effect on germination and early growth characteristics in sunflower (Helianthus annuus) seeds exposed to static magnetic field. J. Plant Physiol. 2010, 167, 149–156. [Google Scholar] [CrossRef]
- Yue, K.; Li, L.; Xie, J.; Fudjoe, S.K.; Zhang, R.; Luo, Z.; Anwar, S. Nitrogen Supply Affects Grain Yield by Regulating Antioxidant Enzyme Activity and Photosynthetic Capacity of Maize Plant in the Loess Plateau. Agronomy 2021, 11, 1094. [Google Scholar] [CrossRef]
- Li, Y.L.; Stanghellini, C.; Challa, H. Response of tomato plants to a step-change in root-zone salinity under two different transpiration regimes. Sci. Hort. 2002, 93, 26779. [Google Scholar] [CrossRef]
- Patanè, C.; Cosentino, S.L.; Romano, D.; Toscano, S. Relative Water Content, Proline, and Antioxidant Enzymes in Leaves of Long Shelf-Life Tomatoes under Drought Stress and Rewatering. Plants 2022, 11, 3045. [Google Scholar] [CrossRef]
- Raziq, A.; Wang, Y.; MohiUd Din, A.; Sun, J.; Shu, S.; Guo, S.A. Comprehensive evaluation of salt tolerance in tomato (var. Ailsa Craig): Responses of physiological and transcriptional changes in RBOH’s and ABA biosynthesis and signalling genes. Int. J. Mol. Sci. 2022, 23, 1603. [Google Scholar] [CrossRef]
- Jensen, C.R.; Battilani, A.; Plauborg, F.; Psarras, G.; Chartzoulakis, K.; Janowiak, F. Deficit irrigation based on drought tolerance and root signalling in potatoes and tomatoes. Agri. Water Manag. 2010, 98, 403–413. [Google Scholar] [CrossRef]
- Alian, A.; Altman, A.; Heuer, B. Genotypic difference in salinity and water stress tolerance of fresh market tomato genotypes. Plant Sci. 2000, 152, 59–65. [Google Scholar] [CrossRef]
- Lu, S. Effects of salt stress on sugar content and sucrose metabolism in tomato fruit. China Veg. 2012, 20, 56–61. [Google Scholar]
- Blum, A. Drought resistance water-use efficiency, and yield potential-are they compatible, dissonant, or mutually exclusive? Aust. J. Agric. Res 2005, 56, 1159–1168. [Google Scholar] [CrossRef]
- Samira, B.; Chandrappa, K.G.; Sharifah, B.A.H. Generation of Hematite Nanoparticles via Sol-Gel Method. Res. J. Chem. Sci. 2013, 3, 62–68. [Google Scholar]
- Parasad, B.; Dimir, D.C.; Bora, L. Effect of pre-harvest foliar spray of calcium and potassium on fruit quality of pear cv. Pathernakh. Sci. Resear. Essays 2015, 10, 376–380. [Google Scholar] [CrossRef]
- Akrimi, R.; Hajlaoui, H.; Rizzo, V.; Muratore, G.; Mhamdi, M. Agronomical traits, phenolic compounds and antioxidant activity in raw and cooked potato tubers growing under saline conditions. J. Sci. Food Agri 2020, 100, 3719–3728. [Google Scholar] [CrossRef]
- Pnueli, L.; Liang, H.; Rozenberg, M.; Mittler, R. Growth suppression, altered stomatal responses, and augmented induction of heat shock proteins in cytosolic ascorbate peroxidase (Apx1)-deficient Arabidopsis plants. Plant J. 2003, 34, 187–203. [Google Scholar] [CrossRef]
- Do, J.H.; Park, S.Y.; Park, S.H.; Kim, H.M.; Ma, S.H.; Mai, T.D.; Shim, J.S.; Joung, Y.H. Development of a Genome-Edited Tomato with High Ascorbate Content During Later Stage of Fruit Ripening Through Mutation of SlAPX4. Front. Plant Sci. 2022, 13, 836916. [Google Scholar] [CrossRef]
- Locato, V.; Cimini, S.; Gara, L.D. Strategies to increase vitamin C in plants: From plant defense perspective to food biofortification. Front. Plant Sci. 2013, 4, 152. [Google Scholar] [CrossRef]
- Guo, K.; Li, Z.; Tian, H.; Du, X.; Liu, Z.; Huang, H. Cytosolic ascorbate peroxidases plays a critical role in photosynthesis by modulating reactive oxygen species level in stomatal guard cell. Front. Plant Sci. 2020, 11, 446. [Google Scholar] [CrossRef] [PubMed]
- Pardo, J.M.; Rubio, F. Na+ and K+ transporters in plant signalling. In Transporters and Pumps in Plant Signaling; Geisler, M., Venema, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 65–99. [Google Scholar]
- Zuo, K.J.; Qin, J.; Zhao, J.Y.; Ling, H.; Zhang, L.D.; Tang, K.X. Over-expression GbERF2 transcription factor in tobacco enhances brown spots disease resistance by activating expression of downstream genes. Gene 2007, 391, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Backues, S.K.; Baba, M.; Heo, J.M.; Harper, J.W.; Klionsky, D.J. Phosphorylation of Atg9 regulates movement to the phagophore assembly site and the rate of autophagosome formation. Autophagy 2016, 12, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Asins, M.J.; Irene, V.; Mohamed, M.A.; Raquel, O.; Paz Álvarez, D.M.R.S.; Jun, L.; Noelia, J.P.; Rosario, H.; Veronica, R.; Emiliona, C.; et al. Two closely linked tomato HKT coding genes are positional candidates for the major tomato QTL involved in Na+/K+ homeostasis. Plant Cell Environ. 2013, 36, 1171–1191. [Google Scholar] [CrossRef]
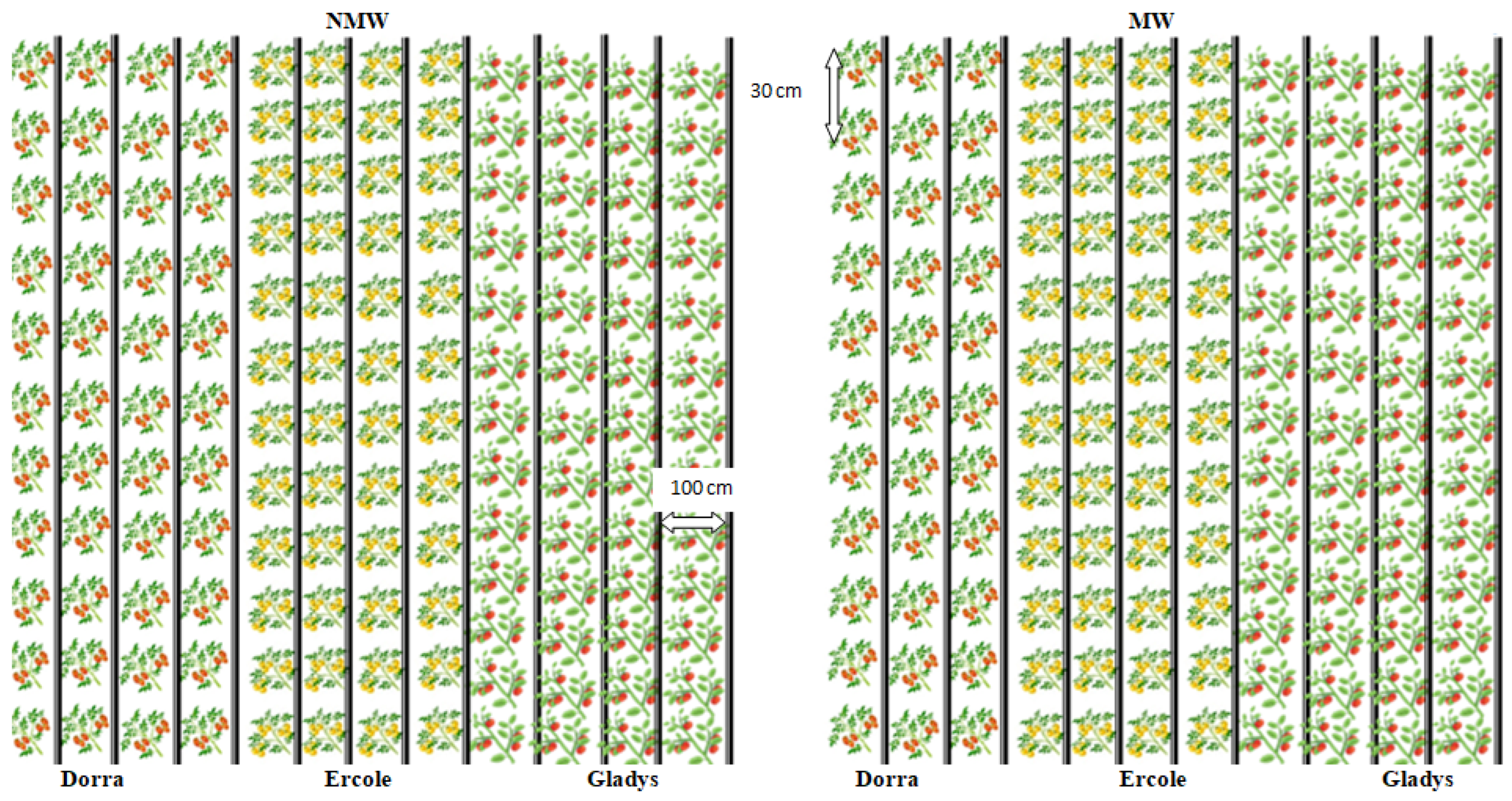
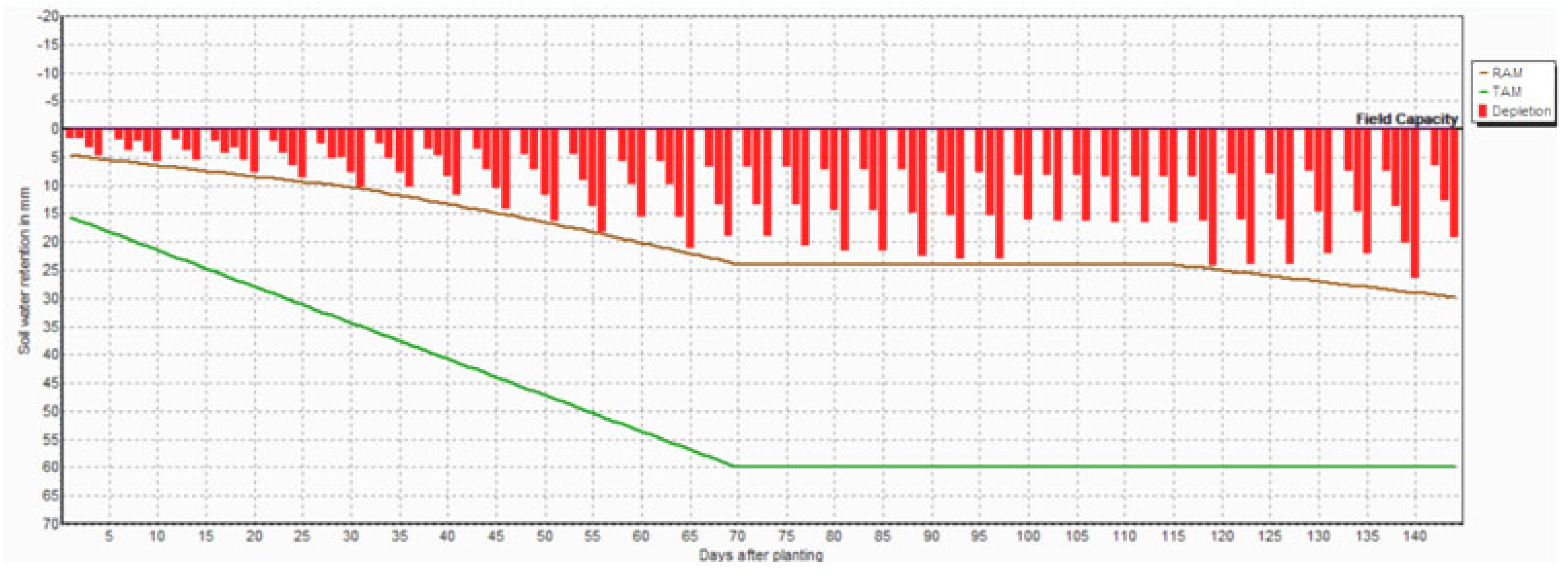
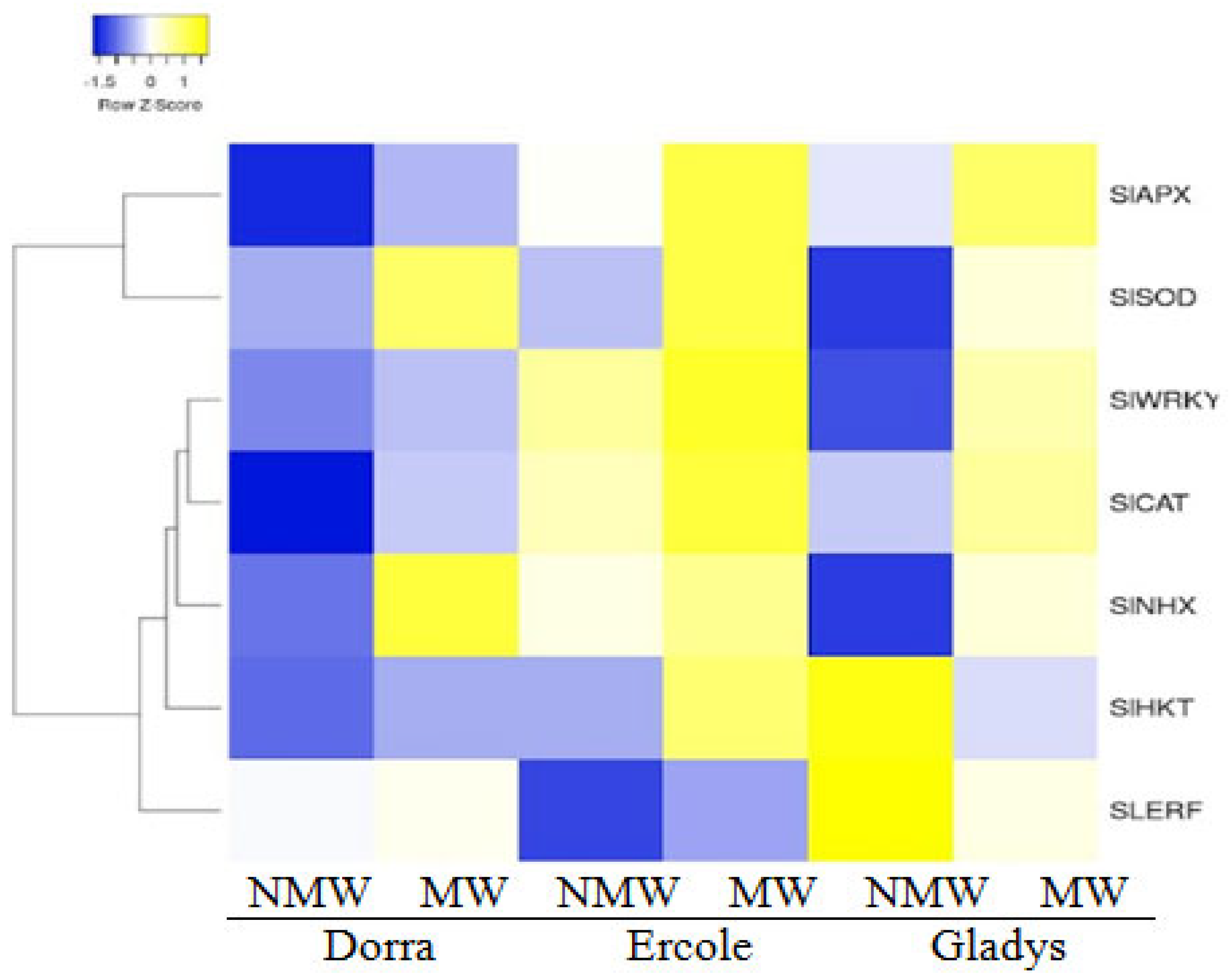

| Month | Min Temp (°C) | Max Temp (°C) | Humidity (%) | Wind (km/Day) | Sun Hours | Rad MJ/m2/Day | ET0 mm/Day | Rain (mm) | Eff rain (mm) | ETc (mm) | Irr. Req (mm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| March | 10.0 | 21.6 | 73 | 285 | 7.1 | 16.6 | 3.11 | 20.0 | 19.4 | 50.50 | 34.6 |
| April | 13.8 | 25.5 | 63 | 311 | 7.6 | 19.8 | 4.62 | 14.0 | 13.7 | 106.90 | 93.4 |
| May | 16.6 | 30.0 | 63 | 285 | 9.7 | 24.1 | 5.75 | 7.0 | 6.9 | 204.0 | 197.0 |
| June | 20.5 | 4.3 | 58 | 277 | 9.9 | 24.8 | 6.88 | 1.0 | 1.0 | 241.4 | 240.4 |
| July | 21.6 | 37.7 | 54 | 225 | 11.3 | 26.5 | 7.47 | 0.0 | 0.0 | 205.1 | 205.1 |
| pH | EC (mS cm−1) | TDR (mg L−1) | Ca2+ (meq L−1) | Mg2+ (meq L−1) | Na+ (meq L−1) | HCO3− (meq L−1) | Cl− (meq L−1) | SO42− (meq L−1) | P (mg L−1) | K+ (mg L−1) | NO3− (mg L−1) | SAR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7.20 | 7.99 | 5647.00 | 22.03 | 18.79 | 35.19 | 2.60 | 55.90 | 35.95 | 204 | 32.90 | 0.80 | 7.79 |
| Gene Names | Accession Number | Primers |
|---|---|---|
| SlCAT | KU933832.1 | F:GGCTCCCAAGTGTGGTCATCA |
| R:GGATCAAACCTCGAGGGCAA | ||
| SlSOD | X14040.1 | F:AGGGAGGACATGAGCTCAGT |
| R: ACAGACACCACCGCTAGTTG | ||
| SlAPX | DQ096286.1 | F:GTTTCCCACCCTCTCCCATG |
| R: CTCTCTGCCAGGGTGAAAGG | ||
| SlWRKY | XM_026029062.1 | F: CCGGAAAGCCTCCATTGTCT |
| R: CCGGAAGATCCCACTGCACTT | ||
| SlERF | XM_004251654.4 | F: ATCGTCGTCGTTGTATCGGG |
| R: TAGCAGTTTTTCCGGCGTA | ||
| SlNHX | NM_001246987.1 | F: CCCTTCCCAGGCACTTAGTG |
| R: AATCTCGCCACAGCATCCAA | ||
| SlHKT | HE962483.1 | F: TGTTTTGTGCTTTGGAGTGGA |
| R: TGGGGGTGAAAGAGTGGAGA |
| TY (t ha−1) | APFW (g Plant−1) | FFW (g Plant−1) | MF (g Plant−1) | NMF (g Plant−1) | IWUE (kg ha mm−1) | IWP (kg ha mm−1) | ||
|---|---|---|---|---|---|---|---|---|
| Dorra | NMW | 50.70 ± 1.41 bA | 434.00 ± 48.38 bA | 1696.6 ± 0.03 aA | 1570 ± 0.03 bA | 126.6 ± 0.00 aA | 3.38 ± 0.33 bA | 0.10 ± 0.00 bA |
| MW | 67.20 ± 3.15 aA | 700.66 ± 65.27 aA | 2240.0 ± 0.07 bB | 2200 ± 0.04 aA | 40.0 ± 0.00 bB | 4.48 ± 0.10 aA | 0.15 ± 0.00 aA | |
| Ercole | NMW | 39.60 ± 0.90 bB | 382.00 ± 36.38 aA | 1320.0 ± 0.02 aA | 1180.66 ± 0.0 bB | 138.33 ± 0.00 aA | 2.64 ± 0.09 bB | 0.08 ± 0.00 bB |
| MW | 60.90 ± 1.73 aA | 538.33 ± 126.50 aA | 2233.3 ± 0.04 aA | 2100 ± 0.07 aA | 133.33 ± 0.00 aB | 4.66 ± 0.73 aA | 0.14 ± 0.00 aA | |
| Gladys | NMW | 27.20 ± 0.51 bC | 423.66 ± 76.32 bA | 740.0 ± 0.12 aB | 673.33 ± 0.1 bC | 66.60 ± 0.01 aB | 1.48 ± 0.05 bC | 0.04 ± 0.00 bC |
| MW | 42.90 ± 2.16 aB | 638.00 ± 31.82 aA | 1430.0 ± 0.05 aB | 1370 ± 0.03 aB | 60 ± 0.00 aB | 2.86 ± 0.20 aB | 0.09 ± 0.00 aB | |
| ANOVA | V | * | ns | ** | * | * | * | * |
| T | ** | * | * | * | * | * | * | |
| V × T | * | ns | * | * | * | * | * |
| Firmness | TSSs (°Brix) | TA (mg AC 100 g−1 FW) | ||
|---|---|---|---|---|
| Dorra | NMW | 0.60 ± 0.01 aA | 6.26 ± 0.04 aB | 0.55 ± 0.00 Aa |
| MW | 0.86 ± 0.04 aA | 6.16 ± 0.07 aB | 0.58 ± 0.06 aA | |
| Ercole | NMW | 0.60 ± 0.01 aA | 6.40 ± 0.28 aB | 0.29 ± 0.01 bC |
| MW | 0.87 ± 0.03 aA | 6.30 ± 0.18 aB | 0.60 ± 0.00 aA | |
| Gladys | NMW | 0.45 ± 0.03 aB | 6.30 ± 0.07 bB | 0.42 ± 0.01 aB |
| MW | 0.86 ± 0.02 aA | 6.93 ± 0.10 aA | 0.41 ± 0.01 aB | |
| ANOVA | T | * | ns | ns |
| V | ns | ns | ns | |
| T × V | ns | ns | ns |
| H2O2 (µmol g−1 FW) | CAT (µmol mg−1 Prot) | APX (µmol mg−1 Prot) | SOD (U mg−1 Prot) | GPOX (µmol mg−1 Prot) | ||
|---|---|---|---|---|---|---|
| Dorra | NMW | 6.30 ± 0.01 aB | 19.68 ± 0.87 aC | 46.91 ± 1.00 aB | 52.53 ± 1.93 aB | 45.22 ± 1.41 aB |
| MW | 4.40 ± 0.03 bB | 10.16 ± 1.14 bC | 17.53 ± 0.45 bB | 43.27 ± 3.19 aB | 36.28 ± 1.41 bB | |
| Ercole | NMW | 5.47 ± 0.03 aC | 28.21 ± 0.69 aB | 60.96 ± 1.40 aA | 46.38 ± 2.27 aB | 75.61 ± 0.68 aA |
| MW | 3.44 ± 0.00 bC | 12.41 ± 1.06 bB | 22.93 ± 1.13 bA | 28.93 ± 3.63 bC | 65.04 ± 1.01 bA | |
| Gladys | NMW | 6.82 ± 0.03 aA | 32.69 ± 0.70 aA | 36.30 ± 0.74 aC | 106.89 ± 4.57 aA | 74.53 ± 1.76 aA |
| MW | 5.89 ± 0.01 bA | 17.05 ± 1.06 bA | 23.25 ± 1.34 bA | 53.66 ± 1.92 bA | 22.11 ± 1.90 bB | |
| ANOVA | T | ** | * | * | * | ** |
| V | ns | * | * | * | * | |
| T × V | ns | * | * | * | * |
| MDA (µmol g−1 FW) | Proline (µmol mg−1 Prot) | SSC (µmol mg−1 Prot) | ||
|---|---|---|---|---|
| Dorra | NMW | 9.31 ± 0.20 aB | 11.06 ± 0.10 aA | 29.97 ± 0.37 aC |
| MW | 6.88 ± 0.46 bB | 6.33 ± 0.14 bA | 23.50 ± 0.78 bC | |
| Ercole | NMW | 8.96 ± 0.18 aC | 8.24 ± 0.08 aB | 46.65 ± 0.92 aA |
| MW | 6.92 ± 0.02 bB | 5.18 ± 0.07 bC | 39.26 ± 0.53 bA | |
| Gladys | NMW | 10.90 ± 0.16 aA | 10.71 ± 0.10 aA | 41.46 ± 0.67 aB |
| MW | 7.95 ± 0.06 bA | 7.28 ± 0.07 bB | 30.21 ± 0.62 bB | |
| ANOVA | T | * | * | ** |
| V | * | * | * | |
| T × V | ns | * | * |
| APFW | FFW | MF | NMF | IWUE | IWP | F | TSSs | TA | H2O2 | CAT | APX | SOD | GPOX | MDA | Proline | SSC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TY | 0.08 ns | −0.99 ** | 0.42 ns | −1.0 ** | −1.0 ** | 0.15 ns | 0.18 ns | 0.46 ns | 0.75 ns | 0.97 ** | 0.99 ** | −0.21 ns | 0.94 ** | −0.04 ns | 0.09 ** | 0.97 ** | |
| APFW | 1 | 0.04 ns | 0.10 ns | 0.04 ns | 0.1 ns | 0.04 ns | 0.32 ns | 0.08 ns | 0.1 ns | 0.05 ns | 0.09 ns | 0.20 ns | 0.01 ns | 0.07 ns | −0.10 ns | 0.12 ns | 0.80 ns |
| FFW | 1 | −0.17 ns | 0.48 ns | 1.00 ** | 1.00 ** | 0.66 ns | 0.42 ns | −0.32 ns | −0.31 ns | 0.12 ns | 0.06 ns | −0.12 ns | 0.31 ns | 0.04 ns | 0.07 ns | 0.07 ns | |
| MF | 1 | −475 | 0.02 ns | 1.00 ns | 0.17 ns | 0.2 ns | 0.12 ns | 0.16 ns | 0.84 ns | 0.09 ns | 0.52 ns | 0.06 ns | 0.32 ns | 0.41 ns | |||
| NMF | 1 | −1.0 ** | −1.0 ** | 0.38 ns | −0.27 ns | 0.48 ns | −0.14 ns | 0.40 ns | 0.43 ns | 0.42 ns | 0.48 ns | −0.23 ns | 0.39 ns | 0.53 ns | |||
| IWUE | 1 | 1.0 ** | 1.0 ** | −1.0 ** | −1.0 ** | −0.78 * | −1.0 ** | −1.0 ** | −1.0 ** | −1.0 ** | −1.0 ** | −1.0 ** | −1.0 ** | ||||
| IWP | 1 | 1.0 ** | −1.0 ** | −1.0 ** | −1.0 ** | −1.0 ** | −1.0 ** | −1.0 ** | −1.0 ** | −1.0 ** | −1.0 ** | −1.0 ** | |||||
| F | 1 | −0.12 ns | −0.03 ns | −0.69 ns | −0.34 ns | −0.29 ns | 0.07 ns | −0.12 ns | −0.44 ns | −0.26 ns | −0.21 ns | ||||||
| TSSs | 1 | −0.78 ns | 0.46 ns | 0.34 ns | 0.18 ns | −0.23 ns | 0.20 ns | 0.12 ns | 0.22 ns | 0.00 ns | |||||||
| TA | 1 | 0.05 ns | 0.29 ns | 0.46 ns | 0.01 ns | 0.42 ns | −0.12 ns | 0.43 ns | 0.61 ns | ||||||||
| H2O2 | 1 | 0.81 * | 0.76 ns | −0.15 ns | 0.57 ns | 0.01 ns | 0.77 ns | 0.63 ns | |||||||||
| CAT | 1 | 0.97 ** | −0.12 ns | 0.90 * | −0.01 ns | 0.97 ** | 0.92 ** | ||||||||||
| APX | 1 | −0.18 ns | 0.93 ** | −0.02 ns | 0.99 ** | 0.97 ** | |||||||||||
| SOD | 1 | −0.38 ns | −0.58 ns | −0.20 ns | −0.19 ns | ||||||||||||
| GPOX | 1 | 0.17 ns | 0.92 ** | 0.94 ** | |||||||||||||
| MDA | 1 | −0.07 ns | 0.21 ns | ||||||||||||||
| Proline | 1 | 0.96 ** |
| APFW | FFW | MF | NMF | IWUE | IWP | F | TSSs | TA | H2O2 | CAT | APX | SOD | GPOX | MDA | Proline | SSC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TY | 0.12 ns | −0.99 ** | −0.68 ns | −0.93 ** | −1.0 ** | −1.00 ** | 0.20 ns | 0.47 ns | −0.01 ns | 0.11 ns | −0.99 ** | −0.99 ** | 0.92 ** | −0.98 ** | −0.98 ** | −0.99 ** | −0.97 ** |
| APFW | 1 | 0.81 ns | 0.42 ns | 0.02 ns | 0.51 ns | 0.22 ns | 0.03 ns | 0.07 ns | 0.32 ns | 0.04 ns | 0.11 ns | 0.96 ns | 0.05 ns | 0.80 ns | −0.21 * | 0.22 ns | 0.17 ns |
| FFW | 1 | 0.70 ns | 0.93 ** | −1.0 ** | −1.00 ** | 0.40 ns | −0.47 ns | 0.04 ns | −0.13 ns | 0.99 ** | 0.99 ** | −0.91 * | 0.98 ** | 0.99 ** | 0.99 ** | 0.97 ** | |
| MF | 1 | 0.86 * | −1.0 ** | −1.00 ** | 0.22 ns | −0.64 ns | 0.61 ns | −0.60 ns | 0.67 ns | 0.63 ns | −0.44 ns | 0.72 ns | 0.69 ns | 0.68 ns | 0.65 ns | ||
| NMF | 1 | −1.0 ** | −1.00 ** | 0.39 ns | −0.70 ns | 0.21 ns | −0.41 ns | 0.93 ** | 0.90 * | −0.83 * | 0.94 ** | 0.90 * | 0.92 ** | 0.87 * | |||
| IWUE | 1 | 1.00 ** | 1.0 ** | 1.0 ** | 0.30 ns | 0.64 ns | −1.0 ** | −1.0 ** | 1.0 ** | −1.0 ** | −1.0 ** | −1.0 ** | −1.0 ns | ||||
| IWP | 1 | 1.0 ** | 1.0 ** | 0.02 ns | 1.0 ** | −1.0 ** | −1.0 ** | 1.0 ** | −1.0 ** | −1.0 ** | −1.0 ** | −1.0 ** | |||||
| F | 1 | −0.18 ns | 0.29 ns | 0.29 ns | 0.42 ns | 0.43 ns | −0.51 ns | 0.37 ns | 0.38 ns | 0.42 ns | 0.33 ns | ||||||
| TSSs | 1 | 0.31 ns | 0.46 ns | 0.52 ns | 0.43 ns | 0.50 ns | −0.47 ns | −0.36 ns | −0.43 ns | −0.29 ns | |||||||
| TA | 1 | −0.23 ns | −0.03 ns | −0.05 ns | 0.27 ns | 0.0 ns | 0.03 ns | −0.01 ns | 0.01 ns | ||||||||
| H2 O2 | 1 | −0.15 ns | −0.08 ns | 0.01 ns | −0.25 ns | −0.12 ns | −0.13 ns | 0.10 ns | |||||||||
| CAT | 1 | 0.99 ** | −0.95 ** | 0.98 ** | 0.97 ** | 0.99 ** | 0.957 ** | ||||||||||
| APX | 1 | −0.94 ** | 0.97 ** | 0.98 ** | 0.99 ** | 0.97 ** | |||||||||||
| SOD | 1 | −0.89 * | −0.87 * | −0.92 ** | −0.86 * | ||||||||||||
| GPOX | 1 | 0.98 ** | 0.98 ** | 0.97 ** | |||||||||||||
| MDA | 1 | 0.99 ** | 0.99 ** | ||||||||||||||
| Proline | 1 | 0.98 ** |
| FFW | MF | NMF | IWUE | IWP | F | TSSs | TA | H2O2 | CAT | APX | SOD | GPOX | MDA | Proline | SSC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TY | 0.99 ** | 0.32 ns | 0.00 ns | −1.00 ** | −1.00 ** | −0.14 ns | 0.67 ns | 0.94 ** | −0.05 ns | −0.99 ** | −0.98 ** | 0.98 ** | −0.99 ** | 0.34 ns | −0.99 ** | −0.99 ** |
| APFW | 0.12 ns | 0.61 ns | 0.10 ns | 0.32 ns | 0.55 ns | −0.36 ns | 0.11 ns | 0.25 ns | −0.07 ns | −0.33 ns | 0.22 ns | 0.17 ns | −0.12 ns | −0.02 * | 0.05 ns | −0.10 ns |
| FFW | 1 | 0.34 | 0.11 | 1.00 ** | 1.00 ** | −0.02 ns | 0.67 ns | 0.92 ** | 0.03 ns | −0.99 ** | −0.95 ** | 0.97 ** | −0.98 ** | 0.24 ns | −0.99 ** | −0.99 ** |
| MF | 1 | 0.37 | 1.00 ** | 1.00 ** | −0.39 ns | 0.27 ns | 0.07 | 0.27 ns | −0.28 ns | −0.31 ns | 0.26 ns | −0.32 ns | 0.52 ns | −0.34 ns | −0.38 | |
| NMF | 1 | −1.00 ** | −1.00 ** | 0.32 ns | 0.00 ns | −0.17 | 0.65 ns | −0.06 ns | 0.13 ns | −0.02 ns | 0.05 ns | −0.56 | −0.04 ns | −0.08 | ||
| IWUE | 1 | 1.00 ** | −1.00 ** | 1.00 ** | 1.00 ** | 1.00 ** | −1.00 ** | −1.00 ** | 1.00 ** | −1.00 ** | 1.00 ** | −1.00 ** | −1.00 ** | |||
| IWP | 1 | −1.00 ** | 1.00 ** | 1.00 ** | 1.00 ** | −1.00 ** | −1.00 ** | 1.00 ** | −1.00 ** | 1.00 ** | −1.00 ** | −1.00 ** | ||||
| F | 1 | −0.63 ns | 0.08 ns | −0.34 ns | −0.03 ns | 0.11 ns | −0.11 ns | 0.14 ns | −0.65 ns | 0.05 ns | −0.05 | |||||
| TSSs | 1 | 0.52 ns | 0.51 ns | −0.62 ns | −0.61 ns | 0.70 ns | −0.71 ns | 0.28 ns | −0.66 ns | −0.60 | ||||||
| TA | 1 | −0.29 ns | −0.94 ** | −0.96 ** | 0.95 ** | −0.94 ** | 0.29 ns | −0.94 ** | −0.93 ** | |||||||
| H2O2 | 1 | 0.03 ns | 0.20 ns | −0.04 ns | 0.04 ns | −0.37 ns | 0.03 ns | 0.06 | ||||||||
| CAT | 1 | 0.95 ** | −0.96 ** | 0.97 ** | −0.23 ns | 0.98 ** | 0.98 ** | |||||||||
| APX | 1 | −0.97 ** | 0.98 ** | −0.47 ns | 0.97 ** | 0.97 ** | ||||||||||
| SOD | 1 | −0.99 ** | 0.34 ns | −0.98 ** | −0.97 ** | |||||||||||
| GPOX | 1 | −0.38 ns | 0.99 ** | 0.97 ** | ||||||||||||
| MDA | 1 | −0.32 ns | 0.72 ns | |||||||||||||
| Proline | 1 | 0.99 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akrimi, R.; Mhamdi, M.; Abid, G.; Ezzeddine, H.; Nouiri, I. Magnetic-Water-Enhanced Tomato Yield and Antioxidant Response Under Arid Conditions of South Tunisia. Horticulturae 2025, 11, 301. https://doi.org/10.3390/horticulturae11030301
Akrimi R, Mhamdi M, Abid G, Ezzeddine H, Nouiri I. Magnetic-Water-Enhanced Tomato Yield and Antioxidant Response Under Arid Conditions of South Tunisia. Horticulturae. 2025; 11(3):301. https://doi.org/10.3390/horticulturae11030301
Chicago/Turabian StyleAkrimi, Rawaa, Mahmoud Mhamdi, Ghassen Abid, Hedyea Ezzeddine, and Issam Nouiri. 2025. "Magnetic-Water-Enhanced Tomato Yield and Antioxidant Response Under Arid Conditions of South Tunisia" Horticulturae 11, no. 3: 301. https://doi.org/10.3390/horticulturae11030301
APA StyleAkrimi, R., Mhamdi, M., Abid, G., Ezzeddine, H., & Nouiri, I. (2025). Magnetic-Water-Enhanced Tomato Yield and Antioxidant Response Under Arid Conditions of South Tunisia. Horticulturae, 11(3), 301. https://doi.org/10.3390/horticulturae11030301






