Droplet-Vitrification Protocol for Cryopreservation of Ginger (Zingiber officinale) Shoot Tips
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
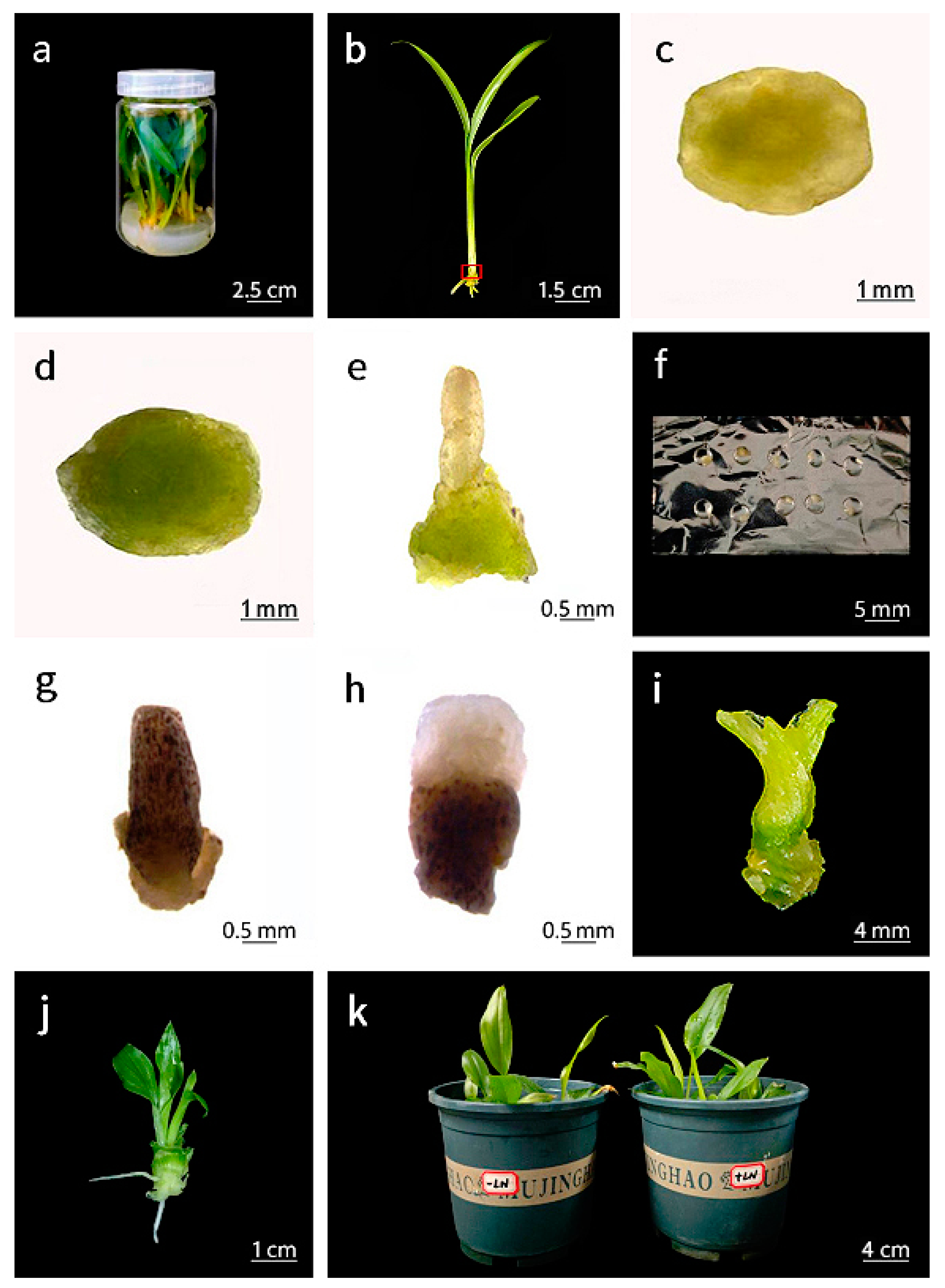
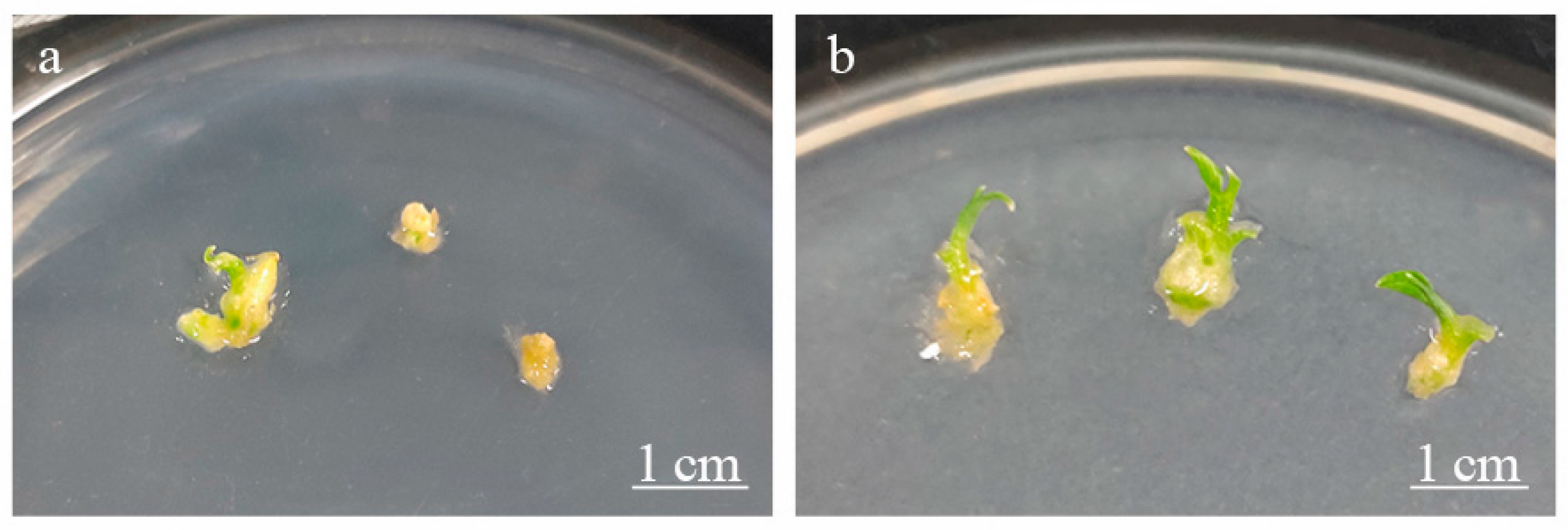
2.2. Cryopreservation Procedure
2.3. Addition of Exogenous Ascorbic Acid (AsA) and Glutathione (GSH)
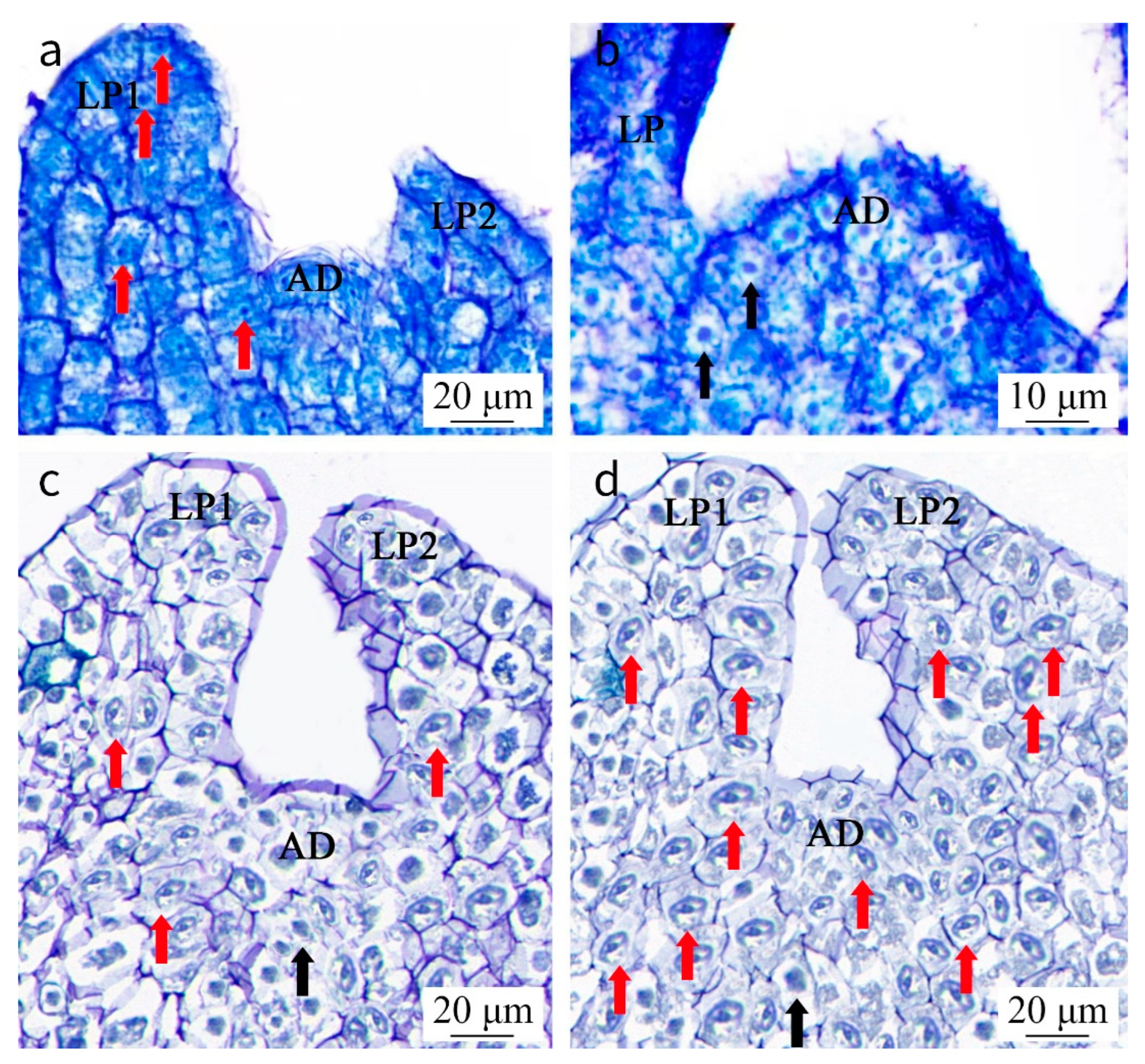
2.4. Analyses from a Histological and Ultrastructural Perspective of Shoot Tips
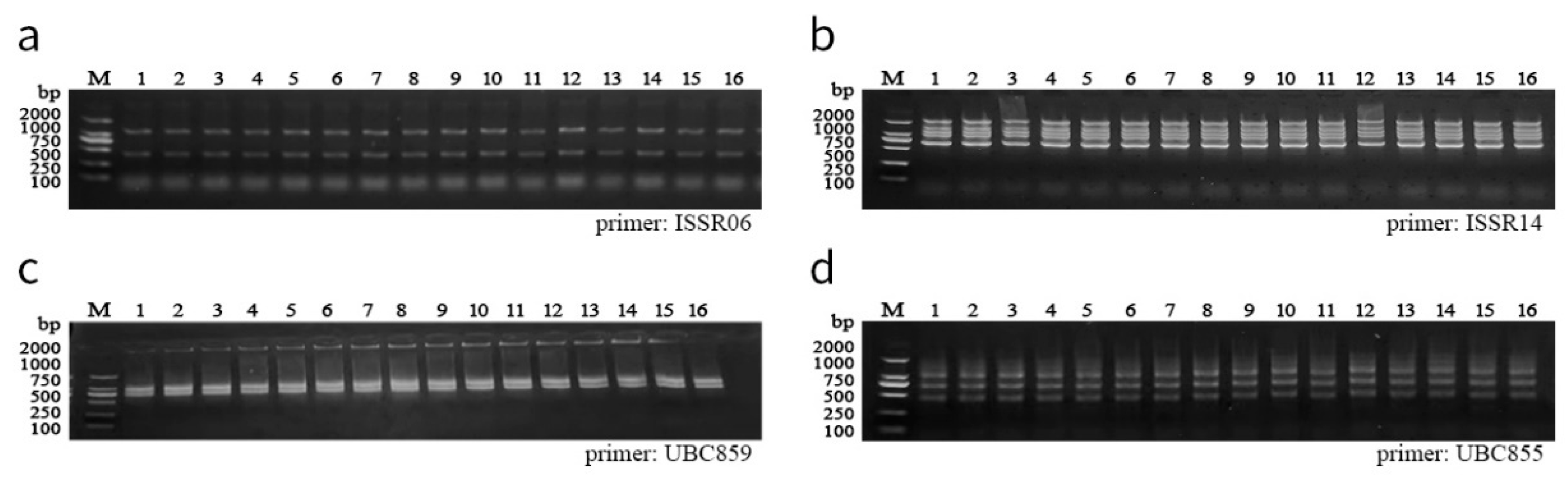
2.5. ISSR Analysis
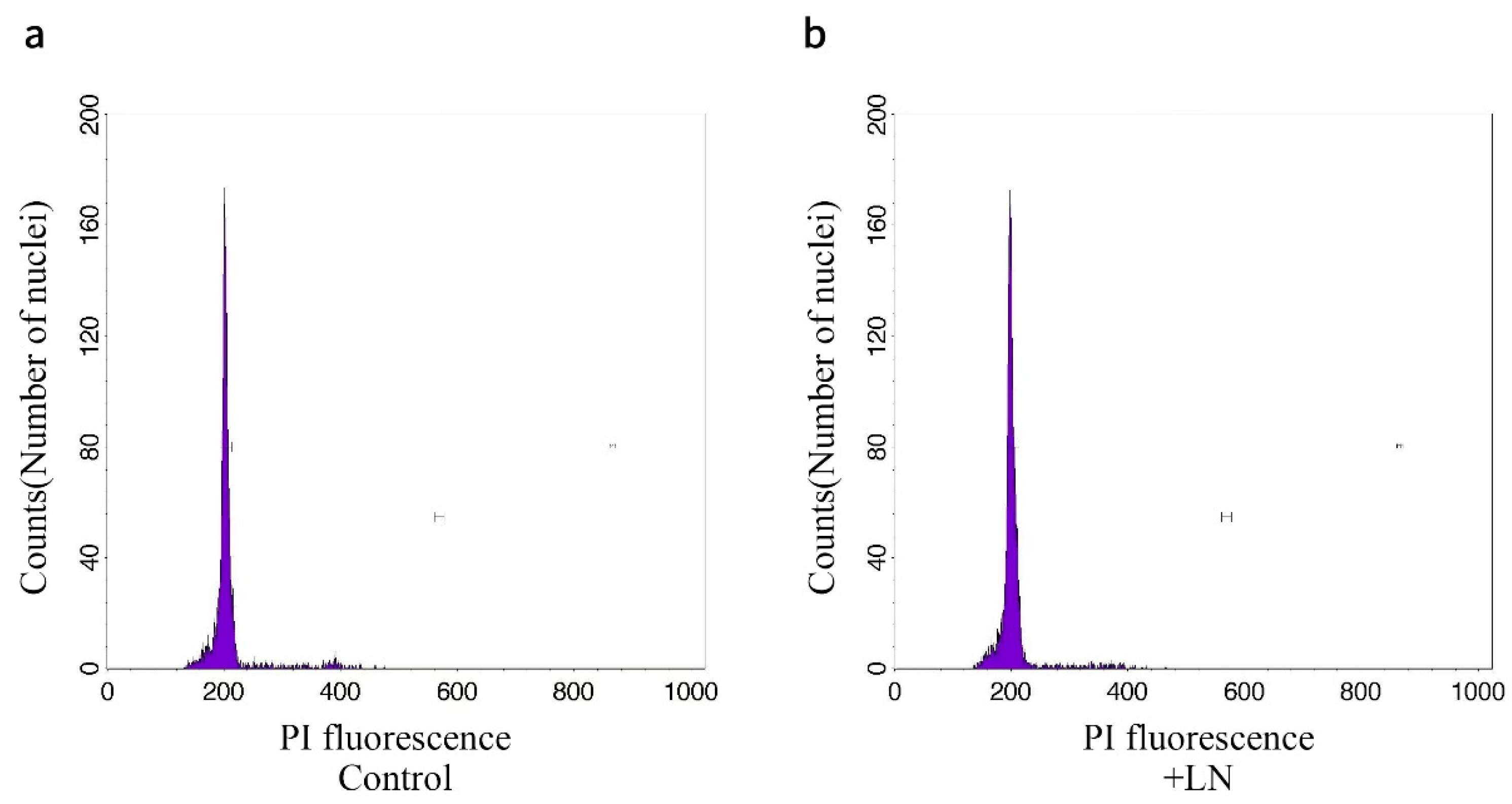
2.6. Flow Cytometry (FCM)
2.7. Acclimatization of Plants to Soil
2.8. Statistical Analysis
3. Results
3.1. Effect of Sucrose Preculture
3.2. Effect of the Exposure Time Duration to PVS2
3.3. Effect of SRM
3.4. Effect of AsA and GSH
3.5. Histological and Ultrastructural Observations
3.6. Analysis of Genetic Stability
3.6.1. ISSR
3.6.2. FCM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MS | Murashige and Skoog |
| PVS | Plant vitrification solutions |
| PVS2 | Plant vitrification solution 2 |
| AsA | Ascorbic acid |
| LN | Liquid nitrogen |
| SRM | Shoot recovery medium |
| GA3 | Gibberellic acid 3 |
| AD | Apical dome |
| LP | Leaf primordia |
| ISSR | Inter-simple sequence repeat |
| FCM | Flow cytometry |
| 6BAP | 6-benzyl-aminopurine |
| NAA | α-naphthaleneacetic acid |
| DMSO | Dimethylsulfoxide |
| TDZ | Thidiazuron |
| KT | 6-furfurylaminopurine |
| GSH | Glutathione |
| TB | Toluidine blue |
| WPM | Woody plant medium |
| TEM | Transmission electron microscope |
| RAPD | Random amplified polymorphic DNA |
| SSR | Simple sequence repeats |
| AFLP | Amplified fragment length polymorphism |
| rpm | Revolutions per minute |
| TE | Tris-EDTA |
| DNA | Deoxyribonucleic acid |
| PCR | Polymerase chain reaction |
| dNTP | Deoxy-ribonucleoside triphosphate |
| TBE | Tris-borate-EDTA |
| UV | ultraviolet |
References
- Yusuff, O.; Rafii, M.Y.; Fatai, A.; Murugesu, S.; Chukwu, S.; Salisu, M.A.; Fagbohun, I.; Kolawole, T.; Kadar, A.I. Genetic diversity and utilization of ginger (Zingiber officinale) for varietal improvement: A review. AIMS Agric. Food 2024, 9, 183–208. [Google Scholar]
- Moghaddasi, M.S. Ginger (Zingiber officinale): A review. J. Med. Plant Res. 2012, 6, 4255–4258. [Google Scholar]
- Priyanka, V.; Kumar, R.; Dhailiwal, I.; Kaushik, P. Germplasm conservation: Instrumental in agricultural biodiversity—A review. Sustainability 2021, 13, 6743. [Google Scholar] [CrossRef]
- Benelli, C. Plant cryopreservation: A look at the present and the future. Plants 2021, 10, 2744. [Google Scholar] [CrossRef]
- Wang, Q.C.; Panis, B.; Engelmann, F.; Lambardi, M.; Valkonen, J.P.T. Cryotherapy of shoot tips: A technique for pathogen eradication to produce healthy planting materials and prepare the healthy plant genetic resources for cryopreservation. Ann. Appl. Biol. 2009, 154, 351–363. [Google Scholar] [CrossRef]
- Reed, B.M. Plant Cryopreservation: A Practical Guide; Springer Science +Businuss Medium: New York, NY, USA, 2008; p. 7. [Google Scholar]
- Engelmann, F. Plant cryopreservation: Progress and prospects. Vitr. Cell. Dev. Biol.-Plant 2004, 40, 427–433. [Google Scholar] [CrossRef]
- Wilkinson, T.; Wetten, A.; Prychid, C.; Fay, M.F. Suitability of cryopreservation of the long-term storage of rare and endangered plant species: A case history for Cosmos atrosanguineus. Ann. Bot. 2003, 91, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Villalobos-Olivera, A.; Lorenzo-Feijoo, J.C.; Quintana-Bernabé, N.; Leiva-Mora, M.; Bettoni, J.C.; Maitínez-Montero, M.E. Morpho-anatomical and physiological assessments of cryo-derived pineapple plants (Ananas comosus var. comosus) after acclimatization. Horticulturae 2023, 9, 841. [Google Scholar] [CrossRef]
- Yamuna, G.; Sumathi, V.; Geetha, S.P.; Praveen, K.; Swapna, N.; Nirmal Babu, K. Cryopreservation of in vitro grown shoots of ginger (Zingiber officinale Rosc.). CryoLetters 2007, 28, 241–252. [Google Scholar]
- Du, L.S.; Feng, J.J.; Li, X.H.; Zhao, F.Z.; Wu, Y.Q.; Yu, H.T. Ginger vitrification cryopreservation technology. North. Horticult. 2014, 13, 123–125, (in Chinese with English abstract). [Google Scholar]
- Ozudogru, E.A.; Kirdok, E.; Kaya, E.; Capuana, M.; Benelli, C.; Engelmann, F. Cryopreservation of redwood (Sequoia sumpervirens) in vitro buds using vitrification-based techniques. CryoLetter 2011, 32, 99–110. [Google Scholar]
- Wang, B.; Wang, R.R.; Li, J.W.; Ma, Y.L.; Sheng, W.M.; Li, M.F.; Wang, Q.C. Development of three vitrification-based cryopreservation procedures of shoot tips for China’s potato. CryoLetter 2013, 34, 369–380. [Google Scholar]
- Kartha, K.K.; Leung, N.L.; Mroginski, L.A. In vitro growth responses and plant regeneration from cryopreserved meristems of cassava (Manihot esculenta Crantz). Z. Pflanzenphysiol. 1982, 107, 133–140. [Google Scholar] [CrossRef]
- Sakai, A.; Engelmann, F. Vitrification, encapsulation-vitrification and droplet-vitrification: A review. CryoLetters 2007, 28, 151–172. [Google Scholar]
- Kim, H.H.; Lee, Y.G.; Shin, D.J.; Ko, H.C.; Gwag, J.G.; Cho, E.G.; Engelmann, F. Development of alternative plant vitrification solutions in droplet-vitrification procedures. CryoLetters 2009, 30, 320–334. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.D.; Zhan, T.J.; Yang, H.Y.; Han, H.X.; Liu, L.F.; Yi, Y. Droplet generation, vitrification, and warming for cell cryopreservation: A review. ACS Biomater. Sci. Eng. 2023, 9, 1151–1163. [Google Scholar] [CrossRef]
- Wang, M.R.; Lambardi, M.; Engelmann, F.; Pathirana, R.; Panis, B.; Volk, G.M.; Wang, Q.C. Advances in cryopreservation of in vitro-derived propagules: Technologies and explant sources. Plant Cell Tiss. Org. 2021, 144, 7–20. [Google Scholar] [CrossRef]
- Wang, M.R.; Bi, W.L.; Bettoni, J.C.; Zhang, D.; Volk, G.M.; Wang, Q.C. Shoot tip cryotherapy for plant pathogen eradication. Plant Pathol. 2022, 71, 1241–1254. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Bonnart, R.; Volk, G.M. Challenges in implementing plant shoot tip cryopreservation technologies. Plant Cell Tiss. Org. 2021, 144, 21–34. [Google Scholar] [CrossRef]
- Yang, Q.S.; Wei, X.Y.; Yang, Q.; Wang, Y.M.; Tao, Y.H. Study on rapid propagation technology of ginger landraces in Yunnan. Chin. J. Trop. Agric. 2022, 42, 28–32, (in Chinese with English abstract). [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Wang, R.R.; Liu, C.C.; Wang, L.J.; Yuan, M.Y.; Li, J.; Tang, Z.K. An improved micropropagation via nodal segments of Zingiber officinale. Eur. J. Hortic. Sci. 2021, 86, 21–28. [Google Scholar] [CrossRef]
- Sakai, A.; Kobayashi, S.; Oiyama, I. Cryopreservation of nucellar cells of navelorange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep. 1990, 9, 30–33. [Google Scholar] [CrossRef]
- Wang, S.M.; Luo, L.S.; Hou, Y.H.; Leng, J.G.; Li, D.W. Establishment of virus-free and rapid propagation system for tissue culture of Guizhou ginger. Guizhou Agr. Sci. 2021, 49, 98–102, (in Chinese with English abstract). [Google Scholar]
- Wang, H.; Lin, G.Y.; Li, X.K. Tissue culture and rapid propagation of Curcuma wenyujin Y.H.Chen et C. Ling. Plant Physiol. J. 2007, 3, 509–510, (in Chinese with English abstract). [Google Scholar]
- Wang, Q.C.; Valkonen, J.P.T. Efficient elimination of sweetpotato little leaf phytoplasma from sweetpotato by cryotherapy of shoot tips. Plant Pathol. 2008, 57, 338–347. [Google Scholar] [CrossRef]
- Sakai, W. Simple method for different staining of paraffin embedded plant material using toluidine blue O. Stain Technol. 1973, 48, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.S. The use of lead at high xylem ray parenchyma cells of Red Osier dogwood (Cornus sericea L.) to freezing stress. Plant Physiol. 1963, 104, 737–746. [Google Scholar]
- Porebski, S.; Bailey, L.G.; Baum, B.R. Modification of a CTAB DNA extraction protocol for plant containing high polysaccharides and polyphenol component. Plant Mol. Biol. Rep. 1997, 15, 8–15. [Google Scholar] [CrossRef]
- Kizhakkayil, J.; Sasikumar, B. Genetic diversity analysis of ginger (Zingiber officinale Rosc.) germplasm based on RAPD and ISSR markers. Sci. Hortic. 2010, 125, 73–76. [Google Scholar] [CrossRef]
- Mohanty, S.; Panda, M.K.; Sahoo, S.; Nayak, S. Micropropagation of Zingiber rubens and assessment of genetic stability through RAPD and ISSR markers. Biol. Plant. 2011, 55, 16–20. [Google Scholar] [CrossRef]
- Ray, T.; Roy, S.C. Phylogenetic relationship between members of Amaranthaceae and Chenopodiaceae of lower gangetic plains using RAPD and ISSR markers. Bangl. J. Bot. 2007, 36, 21–28. [Google Scholar] [CrossRef]
- Huang, H.; Tong, Y.; Zhang, Q.J.; Gao, L.Z. Genome size variation among and within Camellia species by using flow cytometric analysis. PLoS ONE 2013, 8, e64981. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, D.W.; Harkins, K.R.; Maddox, J.M.; Ayres, N.M.; Sharma, D.P.; Firoozabady, E. Rapid flow cytometric analysis of the cell-cycle in intact plant-tissues. Science 1983, 220, 1049–1051. [Google Scholar] [CrossRef] [PubMed]
- Leunufna, S.; Keller, E.R.J. Investigating a new cryopreservation protocol for yam (Discorea spp.). Plant Cell Rep. 2003, 21, 1159–1166. [Google Scholar] [CrossRef]
- Kim, H. Optimizing the droplet-vitrification procedure by balancing the cryoprotection and cytotoxicity of alternative plant vitrification solutions based on the nature of donor plant vigor. Plants 2023, 12, 4040. [Google Scholar] [CrossRef]
- Lee, H.; Choi, B.; Oh, S.; Park, H.; Popova, E.; Paik, M.J.; Kim, H. Dynamics of organic acids during the droplet-vitrification cryopreservation procedure can be a signature of oxidative stress in Pogostemon yatabeanus. Plants 2023, 12, 3489. [Google Scholar] [CrossRef]
- Delgado-Aceves, L.; Corona, S.; Martin-Castro, U.R.; Rascó-Díaz, M.P.; Portillo, L.; Gutiérrez-Mora, A.; González-Arnao, M.T. Comparative studies for cryopreservation of Agave shoot tips by droplet-vitrification. Plants 2024, 13, 2609. [Google Scholar] [CrossRef]
- Panis, B. Sixty years of plant cryopreservation: From freezing hardy mulberry twigs to establishing reference crop collections for future generations. Acta Hortic. 2019, 1234, 1–7. [Google Scholar] [CrossRef]
- Matsumoto, T. Cryopreservation of plant genetic resources: Conventional and new methods. Rev. Agric. Sci. 2017, 5, 13–20. [Google Scholar] [CrossRef]
- Hirai, D.; Sakai, A. Cryopreservation of in vitro-grown meristems of potato (Solanum tuberosum L.) by encapsulation-vitrification. Potato Res. 1999, 42, 153–160. [Google Scholar] [CrossRef]
- Nair, D.S.; Reghunath, B.R. Cryoconservation and regeneration of axillary shoot meristems of Indigofera tinctoria (L.) by encapsulation-dehydration technique. Vitr. Cell. Dev. Biol.-Plant 2009, 45, 565–573. [Google Scholar] [CrossRef]
- Da Silva Cordeiro, L.; Simões-Gurgel, C.; Albarello, N.; Engelmann, F. Cryopreservation of in vitro-grown shoot tips of Cleome rosea Vahl (Cleopmaceae) using the V cryo-plate technique. Vitr. Cell. Dev. Biol.-Plant 2015, 51, 688–695. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Bonnart, R.; Shepherd, A.; Kretzschmar, A.A.; Volk, G.M. Cryopreservation of grapevine (Vitis spp.) shoot tips from growth chamber-sourced plants and histological observation. Vitis 2019, 58, 71–78. [Google Scholar]
- Wang, R.R.; Gao, X.X.; Chen, L.; Huo, L.Q.; Li, M.F.; Wang, Q.C. Shoot recovery and genetic integrity of Chrysanthemum morifolium shoot tips following cryopreservation by droplet-vitrification. Sci. Hortic. 2014, 176, 330–339. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Marković, Z.; Bi, W.L.; Volk, G.M. Grapevine shoot tip cryopreservation and cryotherapy: Secure storage of disease-free plants. Plants 2010, 10, 2190. [Google Scholar] [CrossRef]
- Benson, E.E. Cryopreservation of phytodiversity: A critical appraisal of theory & practice. Crit. Rev. Plant Sci. 2008, 27, 141–219. [Google Scholar]
- Wang, B.; Wang, R.R.; Cui, Z.H.; Li, J.W.; Bi, W.L.; Wang, Q.C. Potential applications of cryobiotechnology to plant genetic transformation and pathogen eradication. Biotechnol. Adv. 2014, 32, 583–595. [Google Scholar] [CrossRef]
- Li, J.W.; Ozudogru, E.A.; Li, J.; Wang, M.R.; Bi, W.L.; Lambardi, M.; Wang, Q.C. Cryobiotechnology of forest trees: Recent advances and future prospects. Biodivers. Conserv. 2018, 27, 795–814. [Google Scholar] [CrossRef]
- Leslie, S.B.; Israeli, E.; Lighthart, B.; Crowe, J.H.; Crowe, L.M. Trehalose and sucrose protect both membranes and protein in intact bacteria during drying. Appl. Environ. Microbiol. 1995, 61, 3592–3597. [Google Scholar] [CrossRef]
- Kim, H.H.; Lee, J.K.; Yoon, J.W.; Li, J.J.; Engelmann, F. Cryopreservation of garlic bulbil primordia by the droplet-vitrification procedure. CryoLetters 2006, 27, 143–153. [Google Scholar]
- Wang, L.Y.; Li, Y.D.; Sun, H.Y.; Liu, H.G.; Tang, X.D.; Wang, Q.C.; Zhang, Z.D. An efficient droplet-vitrification cryopreservation for valuable blueberry germplasm. Sci. Hortic. 2017, 219, 60–69. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Comb. Proc.-Int. Plant Propagators’ Soc. 1980, 30, 421–427. [Google Scholar]
- Park, S.U.; Kim, H.H. Cryopreservation of sweet potato shoot tips using a droplet-vitrification procedure. CryoLetter 2015, 36, 344–352. [Google Scholar]
- Teixeira, A.S.; Faltus, M.; Zámeámečník, J.; González-Benito, M.E.; Molina-García, A.D. Glass transition and heat capacity behaviors of plant vitrification solutions. Thermochim. Acta 2014, 593, 43–49. [Google Scholar] [CrossRef]
- Roque-Borda, C.A.; Kulus, D.; Vacaro de Souza, A.; Kaviani, B.; VaVicente, E.F. Cryopreservation of agronomic plant germplasm using vitrification-based methods: An overview of selected case studies. Int. J. Mol. Sci. 2021, 22, 6157. [Google Scholar] [CrossRef]
- Bi, W.L.; Hao, X.Y.; Cui, Z.H.; Volk, Z.H.; Wang, Q.C. Droplet-vitrification cryopreservation of in vitro-grown shoot tips of grapevine (Vitis spp.). Vitr. Cell. Dev. Biol.-Plant 2018, 54, 590–599. [Google Scholar] [CrossRef]
- Souza, F.V.D.; Kaya, E.; de Jesus Vieira, L.; de Souza, E.H.; de Oliveira Amorim, V.B.; Skogerboe, D.; Skogerboe, D.; Matsumoto, T.; Alves, A.A.C.; de Silva Ledo, C.A.; et al. Droplet-vitrification and morphohistological studies of cryopreserved shoot tips of cultivated and wild pineapple genotypes. Plant Cell Tiss. Org. 2016, 124, 351–360. [Google Scholar] [CrossRef]
- Zhang, J.M.; Han, L.; Lu, X.X.; Volk, G.M.; Xin, X.; Yin, G.K.; He, J.J.; Wang, L.; Chen, X.L. Cryopreservation of Jerusalem artichoke cultivars using an improved droplet-vitrification method. Plant Cell Tiss. Org. 2017, 128, 577–587. [Google Scholar] [CrossRef]
- Engelmann, F. Use of biotechnologies for the conservation of plant biodiversity. Vitr. Cell. Dev. Biol.-Plant 2011, 47, 5–16. [Google Scholar] [CrossRef]
- Martín, C.; Kremer, C.; González, I.; González-Benito, M.E. Influence of the cryopreservation technique, recovery medium and genotype on genetic stability of mint cryopreserved shoot tips. Plant Cell Tiss. Org. 2015, 122, 185–195. [Google Scholar] [CrossRef]
- Hedden, P.; Sponsel, V. A century of gibberellin research. J. Plant Growth Regul. 2015, 34, 740–760. [Google Scholar] [CrossRef] [PubMed]
- Avenido, R.A.; Endonela, L.E.; Patena, L.F.; Barba, R.C. Developing plant regeneration systems for in vitro conservation of mandarin (Citrus reticulata) and pummelo (Citrus maxima). Acta Hortic. 2005, 694, 133–136. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive species and antioxidants. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, D.; Shen, X.H.; Reed, B.M. Antioxidants and anti-stress compounds improve the survival of cryopreserved Arabidopsis seedlings. II Int. Symp. Plant Cryopreserv. 2013, 1039, 57–62. [Google Scholar] [CrossRef]
- Mathew, L.Y.; Burritt, D.J.; McLachalan, A.; Pathirana, R. Combined pre-treatments enhance antioxidant metabolism and improve survival of cryopreserved kiwifruit shoot tips. Plant Cell Tiss. Org. 2019, 138, 193–205. [Google Scholar] [CrossRef]
- Ren, R.F.; Li, Z.D.; Zhang, L.L.; Zhou, H.; Jiang, X.R.; Liu, Y. Enzymatic and nonenzymatic antioxidant systems impact the viability of cryopreserved Paeonia suffruticosa pollen. Plant Cell Tiss. Org. 2021, 144, 233–246. [Google Scholar] [CrossRef]
- Halder, T.; Upadhyaya, G.; Basak, C.; Das, A.; Chakraborty, C.; Ray, S. Dehydrins impact protection against oxidative stress in transgenic tobacco plants. Front. Plant Sci. 2018, 9, 136. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Bioch. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Huang, Y.; Bie, Z.; Ahmed, W.; Reiter, R.J.; Niu, M.; Hameed, S. Melatonin: Current status and future perspectives in plant science. Front. Plant Sci. 2016, 6, 1230. [Google Scholar] [CrossRef]
- Xing, X.D.; Liu, M.; Zhou, R.; Jiang, F.; Bai, Y.; Wei, H.Y.; Zhang, D.; Wei, J.J.; Wu, Z. Ascorbic acid addition during dehydration improves garlic shoot tips cryopreservation but does not affect viral load. Cryobiology 2022, 107, 64–73. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Deng, S.; Lin, H.F.; Chu, Y.X.; Huang, J.Y.; Li, S.G.; Lin, F.Z.; Zhang, S.M.; Jiang, W.L.; Ren, L.; et al. The development of a procedure for the cryopreservation of the callus of Anthurium andraeanum by vitrification. Plants 2024, 13, 3106. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhang, D.; Chen, G.Q.; Reed, B.M.; Shen, X.H.; Chen, H.Y. Transcriptomic profiling revealed the regulatory mechanism of Arabidopsis seedlings response to oxidative stress from cryopreservation. Plant Cell Tiss. Org. 2015, 34, 2161–2178. [Google Scholar] [CrossRef]
- Jaouad, B.; Torsten, B. Exogenous antioxidants-double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxidative Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar]
- Ozudogru, E.A.; Kaya, E.; Kirdok, E.; Issever-Ozturk, S. In vitro propagation from young and mature explants of thyme (Thymus vulgaris and T. loongicaulis) resulting in genetically stable shoots. Vitr. Cell. Dev. Biol.-Plant 2011, 47, 309–320. [Google Scholar] [CrossRef]
- Martín, C.; González-Benito, M.E. Survival and genetic stability of Dendranthema grandiflora Tzvelev shoot apices after cryopreservation by vitrification and encapsulation-dehydration. Cryobiology 2005, 51, 281–289. [Google Scholar] [CrossRef]
- Ai, P.F.; Lu, L.P.; Song, J.J. Cryopreservation of in vitro-grown shoot tips of Rabdosia rubescens by encapsulation-dehydration and evaluation of their genetic stability. Plant Cell Tiss. Org. 2012, 108, 381–387. [Google Scholar] [CrossRef]
- Vijayan, K. Inter simple sequence repeat (ISSR) polymorphism and its application in mulberry genome analysis. Int. J. Ind. Entomol. 2005, 10, 79–86. [Google Scholar]
- Kaya, E. ISSR analysis for determination of genetic diversity and relationship in some Turkish olive (Olea eruopaea L) cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 96–99. [Google Scholar] [CrossRef]
- Wang, R.R.; Mou, H.Q.; Gao, X.X.; Chen, L.; Li, M.F.; Wang, Q.C. Cryopreservation for eradication of Jujube witches’ broom phytoplasma from Chinese jujube (Ziziphus jujuba). Ann. Appl. Biol. 2015, 166, 128–288. [Google Scholar] [CrossRef]
- Krajňáková, J.; Sutela, S.; Aronen, T.; Gömöry, D.; Vianello, A.; Häggman, H.M. Long-term cryopreservation of Greek fir embryogenic cell line: Recovery, maturation and genetic fidelity. Cryobiology 2011, 63, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Li, H.H.; Wang, R.R.; Gao, X.X.; Wang, Q.C. Cryopreservation for retaining morphology, genetic integrity, and foreign genes in transgenic plants of Torenia fournieri. Acta Physiol. Plant. 2016, 38, 8. [Google Scholar] [CrossRef]



| Sucrose Concentration (M) | Survival Rate (%) | Regrowth Rate (%) | ||
|---|---|---|---|---|
| −LN | +LN | −LN | +LN | |
| 0.25 | 90.00 ± 0.31 x | 73.33 ± 0.45 a | 63.33 ± 0.49 x | 33.33 ± 0.48 a |
| 0.50 | 83.33 ± 0.38 xy | 63.33 ± 0.49 a | 43.33 ± 0.50 xy | 20.00 ± 0.41 ab |
| 0.75 | 60.00 ± 0.50 y | 40.00 ± 0.50 b | 23.33 ± 0.43 y | 3.33 ± 0.18 b |
| Preculture Duration (d) | Survival Rate (%) | Regrowth Rate (%) | ||
|---|---|---|---|---|
| −LN | +LN | −LN | +LN | |
| 1 | 90.00 ± 0.31 x | 73.33 ± 0.45 a | 63.33 ± 0.49 x | 33.33 ± 0.48 a |
| 2 | 80.00 ± 0.41 xy | 66.67 ± 0.48 ab | 40.00 ± 0.50 xy | 16.67 ± 0.38 ab |
| 3 | 66.67 ± 0.48 xy | 53.33 ± 0.51 ab | 23.33 ± 0.43 y | 6.67 ± 0.25 b |
| 4 | 56.67 ± 0.50 y | 40.00 ± 0.50 b | 16.67 ± 0.38 y | 6.67 ± 0.25 b |
| Exogenous Substances | Survival Rate (%) | Regrowth Rate (%) | ||
|---|---|---|---|---|
| −LN | +LN | −LN | +LN | |
| PVS2 | 90.00 ± 0.31 xy | 73.33 ± 0.45 bc | 63.33 ± 0.49 xy | 33.33 ± 0.48 bc |
| PVS2 + 0.1 mM AsA | 100.00 ± 0.00 x | 100.00 ± 0.00 a | 80.00 ± 0.41 x | 66.67 ± 0.48 a |
| PVS2 + 0.2 mM AsA | 100.00 ± 0.00 x | 96.67 ± 0.18 ab | 70.00 ± 0.47 y | 36.67 ± 0.49 b |
| PVS2 + 0.4 mM AsA | 93.33 ± 0.25 xy | 83.33 ± 0.38 abc | 63.33 ± 0.49 xy | 36.67 ± 0.49 b |
| PVS2 + 0.4 mM GSH | 80.00 ± 0.41 xy | 73.33 ± 0.45 bc | 33.33 ± 0.48 yz | 16.67 ± 6.92 b |
| PVS2 + 0.6 mM GSH | 76.67 ± 0.43 y | 63.33 ± 0.49 c | 30.00 ± 0.47 z | 6.67 ± 0.25 c |
| PVS2 + 0.8 mM GSH | 76.67 ± 0.43 y | 63.33 ± 0.49 c | 20.00 ± 0.41 z | 6.67 ± 0.25 c |
| Primer Name | Primer Sequence | Number of Amplified Bands | Number of Variation Bands | References |
|---|---|---|---|---|
| ISSR06 | (GA)8C | 2 | 0 | [31] |
| ISSR14 | (AGC)4GT | 5 | 0 | |
| SPS05 | (GA)9T | 5 | 0 | [32] |
| SPS 06 | T(GA)9T | 6 | 0 | |
| UBC816 | CACACACACACACACAT | 5 | 0 | [33] |
| UBC855 | ACACACACACACACACYT | 3 | 0 | |
| UBC851 | GTGTGTGTGTGTGTGTYG | 1 | 0 | |
| UBC859 | TGTGTGTGTGTGTGTGRC | 2 | 0 | |
| Total | 29 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.-R.; Li, X.; Song, R.-F.; Hou, J.-J.; Zhao, Y.; Song, X.-K.; Cai, X.-D.; Li, J. Droplet-Vitrification Protocol for Cryopreservation of Ginger (Zingiber officinale) Shoot Tips. Horticulturae 2025, 11, 283. https://doi.org/10.3390/horticulturae11030283
Wang R-R, Li X, Song R-F, Hou J-J, Zhao Y, Song X-K, Cai X-D, Li J. Droplet-Vitrification Protocol for Cryopreservation of Ginger (Zingiber officinale) Shoot Tips. Horticulturae. 2025; 11(3):283. https://doi.org/10.3390/horticulturae11030283
Chicago/Turabian StyleWang, Ren-Rui, Xin Li, Ren-Fan Song, Juan-Juan Hou, Yi Zhao, Xing-Kun Song, Xiao-Dong Cai, and Jie Li. 2025. "Droplet-Vitrification Protocol for Cryopreservation of Ginger (Zingiber officinale) Shoot Tips" Horticulturae 11, no. 3: 283. https://doi.org/10.3390/horticulturae11030283
APA StyleWang, R.-R., Li, X., Song, R.-F., Hou, J.-J., Zhao, Y., Song, X.-K., Cai, X.-D., & Li, J. (2025). Droplet-Vitrification Protocol for Cryopreservation of Ginger (Zingiber officinale) Shoot Tips. Horticulturae, 11(3), 283. https://doi.org/10.3390/horticulturae11030283






