The Combined Effect of Lighting and Zinc on the Nutritional Quality of Lettuce (Lactuca sativa L.) Grown in Hydroponics
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. Biometric Measurements
2.3. Determination of Zinc in Lettuce
2.4. Antioxidant Activity and Total Phenolic Content
- ABTS scavenging activity
- DPPH scavenging activity
- FRAP
- Total phenolic content
2.5. Determination of Soluble Sugars by UFLC
2.6. Measurement of Soluble Protein Content
2.7. Antioxidant Enzymes Activities
2.8. Determination of Carotenoids
2.9. Statistical Analysis
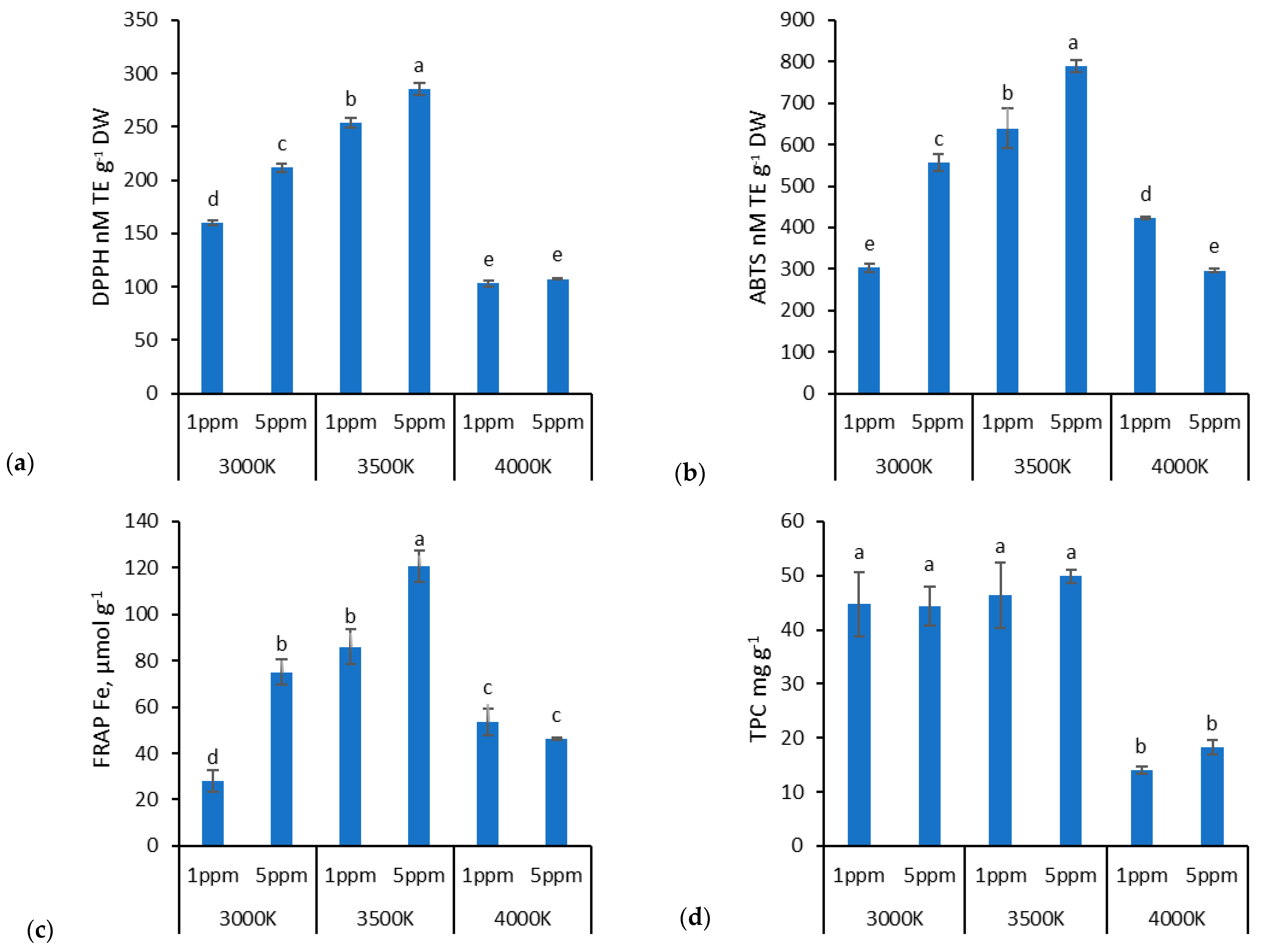
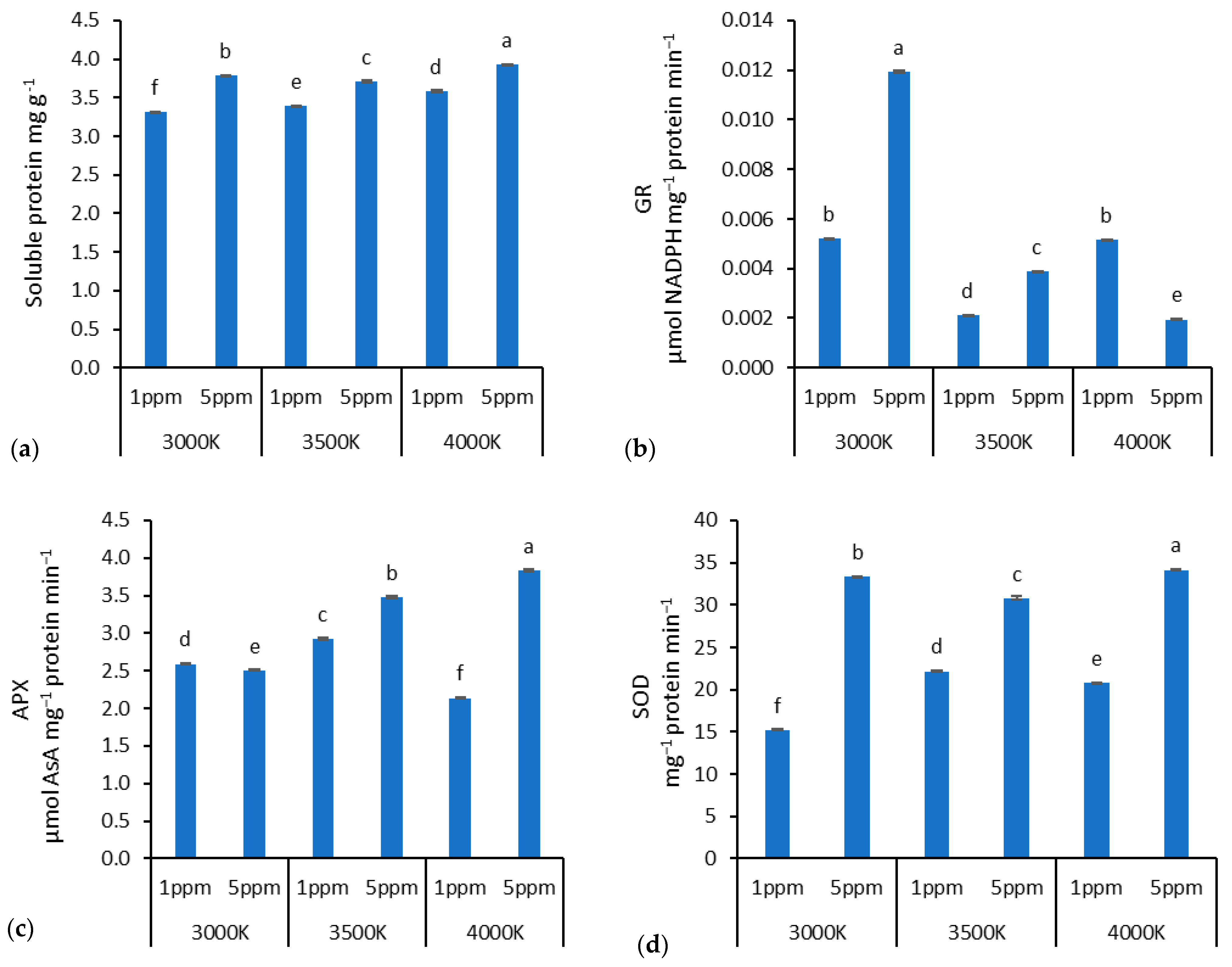
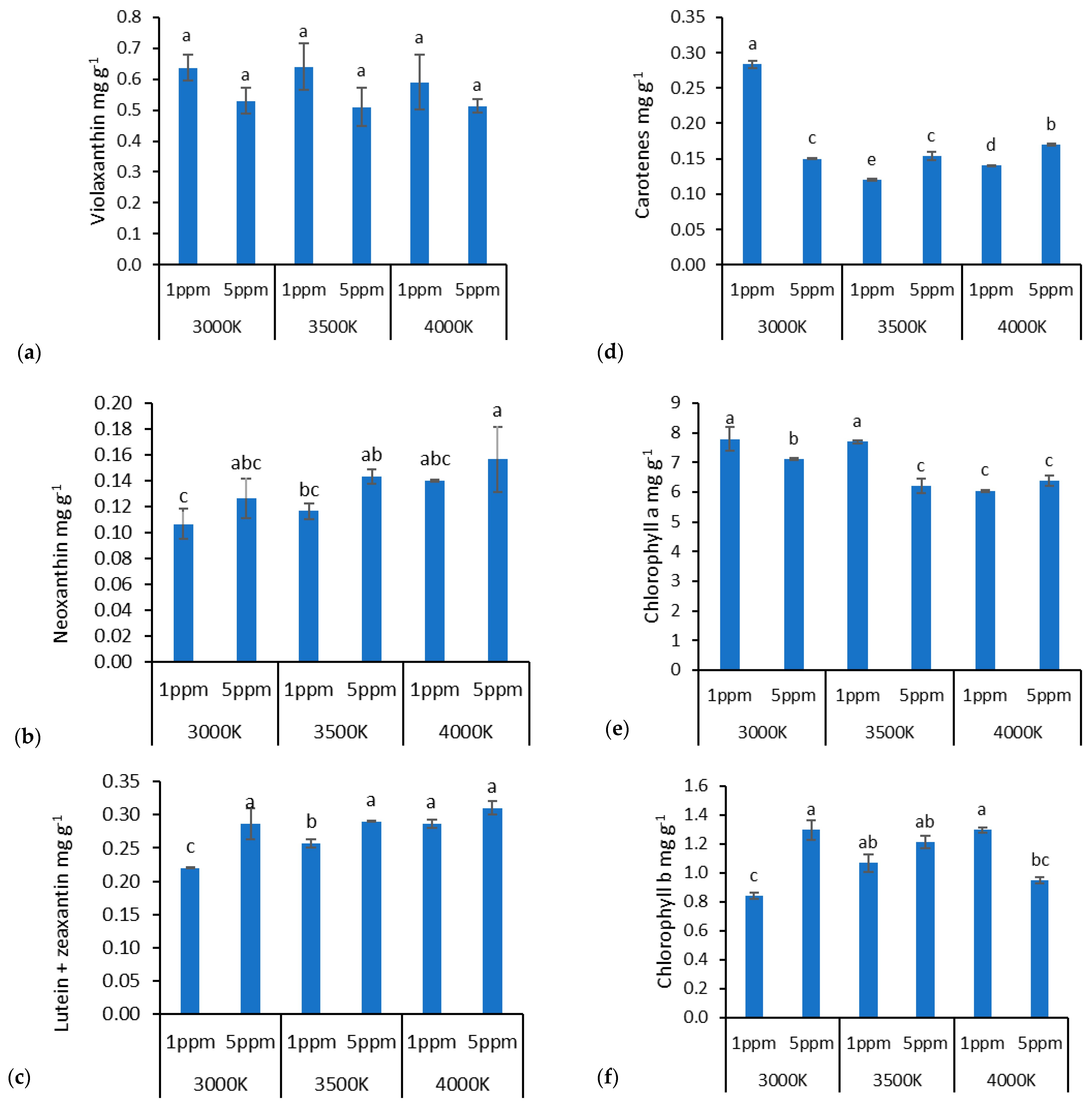
3. Results
4. Discussion
4.1. Zn Doses and Lighting Impact on Zn Accumulation in Lettuce Leaves
4.2. Zn Doses and Lighting Impact on Biochemical Properties in Lettuce
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| PPFD | Photosynthetic Photon Flux Density |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| FRAP | Ferric reducing antioxidant power |
| TPC | Total phenols content |
| LED | light-emitting diodes |
| SOD | superoxide dismutase |
| APX | ascorbate peroxidase |
| GR | glutathione reductase |
References
- Khan, S.T.; Malik, A.; Alwarthan, A.; Shaik, M.R. The Enormity of the Zinc Deficiency Problem and Available Solutions; an Overview. Arab. J. Chem. 2022, 15, 103668. [Google Scholar] [CrossRef]
- Gibson, R.S. Principles of Nutritional Assessment; Oxford University Press: Oxford, UK, 2005; ISBN 978-0-19-517169-3. [Google Scholar]
- Maares, M.; Haase, H. A Guide to Human Zinc Absorption: General Overview and Recent Advances of In Vitro Intestinal Models. Nutrients 2020, 12, 762. [Google Scholar] [CrossRef] [PubMed]
- Nandal, N.; Solanki, M. The Zn as a Vital Micronutrient in Plants. J. Microbiol. Biotechnol. Food Sci. 2021, 11, 4026. [Google Scholar] [CrossRef]
- Clemens, S. The Cell Biology of Zinc. J. Exp. Bot. 2022, 73, 1688–1698. [Google Scholar] [CrossRef]
- Hamzah Saleem, M.; Usman, K.; Rizwan, M.; Al Jabri, H.; Alsafran, M. Functions and Strategies for Enhancing Zinc Availability in Plants for Sustainable Agriculture. Front. Plant Sci. 2022, 13, 1033092. [Google Scholar] [CrossRef]
- Borrego, A.C.; Abreu, R.; Carreira, F.A.; Caetano, F.; Vasconcelos, A.L. Environmental Taxation on the Agri-Food Sector and the Farm to Fork Strategy: The Portuguese Case. Sustainability 2023, 15, 12124. [Google Scholar] [CrossRef]
- Manzoni, A. The Evolution of EU Agri-Food Policymaking: A Long Path Towards a Food System Approach. In The Food Commons Approach in the EU Agri-Food Law: Reframing the Common Agricultural Policy for More Ecological Food Systems in Europe; Manzoni, A., Ed.; Springer Nature: Cham, Switzerland, 2024; pp. 61–155. ISBN 978-3-031-64565-5. [Google Scholar]
- Fonseca, L.M.; Domingues, J.P.; Dima, A.M. Mapping the Sustainable Development Goals Relationships. Sustainability 2020, 12, 3359. [Google Scholar] [CrossRef]
- Szerement, J.; Szatanik-Kloc, A.; Mokrzycki, J.; Mierzwa-Hersztek, M. Agronomic Biofortification with Se, Zn, and Fe: An Effective Strategy to Enhance Crop Nutritional Quality and Stress Defense—A Review. J. Soil Sci. Plant Nutr. 2022, 22, 1129–1159. [Google Scholar] [CrossRef]
- Magwaza, S.T.; Magwaza, L.S.; Odindo, A.O.; Mditshwa, A. Hydroponic Technology as Decentralised System for Domestic Wastewater Treatment and Vegetable Production in Urban Agriculture: A Review. Sci. Total Environ. 2020, 698, 134154. [Google Scholar] [CrossRef]
- Shi, M.; Gu, J.; Wu, H.; Rauf, A.; Emran, T.B.; Khan, Z.; Mitra, S.; Aljohani, A.S.M.; Alhumaydhi, F.A.; Al-Awthan, Y.S.; et al. Phytochemicals, Nutrition, Metabolism, Bioavailability, and Health Benefits in Lettuce—A Comprehensive Review. Antioxidants 2022, 11, 1158. [Google Scholar] [CrossRef]
- Giordano, M.; El-Nakhel, C.; Pannico, A.; Kyriacou, M.C.; Stazi, S.R.; De Pascale, S.; Rouphael, Y. Iron Biofortification of Red and Green Pigmented Lettuce in Closed Soilless Cultivation Impacts Crop Performance and Modulates Mineral and Bioactive Composition. Agronomy 2019, 9, 290. [Google Scholar] [CrossRef]
- Lima, B.M.D.; Noboa, C.S.; Lima, F.M.D.; Mello, S.D.C.; Purquerio, L.F.V.; Sala, F.C. Agronomic Biofortification with Zinc in Hydroponically Cultivated Lettuce. Aust. J. Crop Sci. 2023, 17, 195–205. [Google Scholar] [CrossRef]
- Preciado-Rangel, P.; Campos-Ortiz, A.; Chávez, E.S.; Reyes-Gonzalez, A.; Ruiz-Espinoza, F.; Ojeda-Barrios, D.; Hernandez-Montiel, L. Zinc Biofortification Improves Yield, Nutraceutical Quality and Antioxidant Capacity in Lettuce. Trop. Subtrop. Agroecosystems 2021, 24, 1–10. [Google Scholar] [CrossRef]
- Behtash, F.; Fakhrghazi, H.; Hajizadeh, H.S.; Aghaee, A.; Pourmohammad, A. Effect of Zinc and Boron on Yield, Quality Attributes and Physiological Traits of Lettuce under Hydroponic Culture. Pak. J. Bot. 2023, 55, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Meneghelli, C.M.; Fontes, P.C.R.; Milagres, C.d.C.; da Silva, J.M.; Junior, E.G. Zinc-Biofortified Lettuce in Aeroponic System. J. Plant Nutr. 2021, 44, 2146–2156. [Google Scholar] [CrossRef]
- Xu, J.; Guo, Z.; Jiang, X.; Ahammed, G.J.; Zhou, Y. Light Regulation of Horticultural Crop Nutrient Uptake and Utilization. Hortic. Plant J. 2021, 7, 367–379. [Google Scholar] [CrossRef]
- Ramezani, S.; Yousefshahi, B.; Ramezan, D.; Zargar, M.; Pakina, E.; Bayat, M. Selenium, Iodine, and Supplementary Blue Light Enriched Fenugreek (Trigonella foenum-gracum L.) in Terms of Biochemical Quality, Mineral Uptake, and Trace Elements Accumulation in a Hydroponic System. Agriculture 2023, 13, 2009. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Miliauskienė, J.; Vaštakaitė-Kairienė, V.; Sutulienė, R.; Laužikė, K.; Stašytė, K.; Duchovskis, P.; Samuolienė, G. The Response of Baby Leaf Lettuce to Selenium Biofortification under Different Lighting Conditions. Biol. Life Sci. Forum 2021, 3, 10. [Google Scholar] [CrossRef]
- Hammock, H.A.; Kopsell, D.A.; Sams, C.E. Application Timing and Duration of LED and HPS Supplements Differentially Influence Yield, Nutrient Bioaccumulation, and Light Use Efficiency of Greenhouse Basil across Seasons. Front. Plant Sci. 2023, 14, 1174823. [Google Scholar] [CrossRef]
- Vaštakaitė-Kairienė, V.; Brazaitytė, A.; Miliauskienė, J.; Runkle, E.S. Red to Blue Light Ratio and Iron Nutrition Influence Growth, Metabolic Response, and Mineral Nutrients of Spinach Grown Indoors. Sustainability 2022, 14, 12564. [Google Scholar] [CrossRef]
- Li, X.; Liu, K.; He, X.; Chen, Y.; Tan, J.; Jiang, H.; He, R.; Li, Y.; Liu, H. Mutual Effects of Zinc Concentration and Ratio of Red-Blue Light on Growth and Nutritional Quality of Flowering Chinese Cabbage Sprouts. Horticulturae 2023, 9, 226. [Google Scholar] [CrossRef]
- Pinho, P.; Jokinen, K.; Halonen, L. The Influence of the LED Light Spectrum on the Growth and Nutrient Uptake of Hydroponically Grown Lettuce. Light. Res. Technol. 2017, 49, 866–881. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Spectral Effects of Light-Emitting Diodes on Plant Growth, Visual Color Quality, and Photosynthetic Photon Efficacy: White versus Blue plus Red Radiation. PLoS ONE 2018, 13, e0202386. [Google Scholar] [CrossRef] [PubMed]
- Tarakanov, I.G.; Tovstyko, D.A.; Lomakin, M.P.; Shmakov, A.S.; Sleptsov, N.N.; Shmarev, A.N.; Litvinskiy, V.A.; Ivlev, A.A. Effects of Light Spectral Quality on Photosynthetic Activity, Biomass Production, and Carbon Isotope Fractionation in Lettuce, Lactuca Sativa L., Plants. Plants 2022, 11, 441. [Google Scholar] [CrossRef] [PubMed]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green Light Drives Leaf Photosynthesis More Efficiently than Red Light in Strong White Light: Revisiting the Enigmatic Question of Why Leaves Are Green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef]
- Tewari, R.K.; Yadav, N.; Gupta, R.; Kumar, P. Oxidative Stress Under Macronutrient Deficiency in Plants. J. Soil Sci. Plant Nutr. 2021, 21, 832–859. [Google Scholar] [CrossRef]
- Korobova, A.; Ivanov, R.; Timergalina, L.; Vysotskaya, L.; Nuzhnaya, T.; Akhiyarova, G.; Kusnetsov, V.; Veselov, D.; Kudoyarova, G. Effect of Low Light Stress on Distribution of Auxin (Indole-3-Acetic Acid) Between Shoot and Roots and Development of Lateral Roots in Barley Plants. Biology 2023, 12, 787. [Google Scholar] [CrossRef]
- Metallo, R.M.; Kopsell, D.A.; Sams, C.E.; Bumgarner, N.R. Influence of Blue/Red vs. White LED Light Treatments on Biomass, Shoot Morphology, and Quality Parameters of Hydroponically Grown Kale. Sci. Hortic. 2018, 235, 189–197. [Google Scholar] [CrossRef]
- Tang, Y.; Mao, R.; Guo, S. Effects of LED Spectra on Growth, Gas Exchange, Antioxidant Activity and Nutritional Quality of Vegetable Species. Life Sci. Space Res. 2020, 26, 77–84. [Google Scholar] [CrossRef]
- Kang, C.H.; Yoon, E.K.; Muthusamy, M.; Kim, J.A.; Jeong, M.-J.; Lee, S.I. Blue LED Light Irradiation Enhances L-Ascorbic Acid Content While Reducing Reactive Oxygen Species Accumulation in Chinese Cabbage Seedlings. Sci. Hortic. 2020, 261, 108924. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH Antioxidant Assay Revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin–Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf Senescence: Correlated with Increased Levels of Membrane Permeability and Lipid Peroxidation, and Decreased Levels of Superoxide Dismutase and Catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Murshed, R.; Lopez-Lauri, F.; Sallanon, H. Microplate Quantification of Enzymes of the Plant Ascorbate–Glutathione Cycle. Anal. Biochem. 2008, 383, 320–322. [Google Scholar] [CrossRef]
- Smith, I.K.; Vierheller, T.L.; Thorne, C.A. Assay of Glutathione Reductase in Crude Tissue Homogenates Using 5,5′-Dithiobis(2-Nitrobenzoic Acid). Anal. Biochem. 1988, 175, 408–413. [Google Scholar] [CrossRef]
- Edelenbos, M.; Christensen, L.P.; Grevsen, K. HPLC Determination of Chlorophyll and Carotenoid Pigments in Processed Green Pea Cultivars (Pisum sativum L.). J. Agric. Food Chem. 2001, 49, 4768–4774. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zha, L.; Zhang, Y. Growth and Nutrient Element Content of Hydroponic Lettuce Are Modified by LED Continuous Lighting of Different Intensities and Spectral Qualities. Agronomy 2020, 10, 1678. [Google Scholar] [CrossRef]
- Lee, M.; Xu, J.; Wang, W.; Rajashekar, C.B. The Effect of Supplemental Blue, Red and Far-Red Light on the Growth and the Nutritional Quality of Red and Green Leaf Lettuce. Am. J. Plant Sci. 2019, 10, 2219–2235. [Google Scholar] [CrossRef]
- Soufi, H.R.; Roosta, H.R.; Fatehi, F.; Ghorbanpour, M. Spectral Composition of LED Light Differentially Affects Biomass, Photosynthesis, Nutrient Profile, and Foliar Nitrate Accumulation of Lettuce Grown under Various Replacement Methods of Nutrient Solution. Food Sci. Nutr. 2023, 11, 8143–8162. [Google Scholar] [CrossRef] [PubMed]
- Alrajhi, A.A.; Alsahli, A.S.; Alhelal, I.M.; Rihan, H.Z.; Fuller, M.P.; Alsadon, A.A.; Ibrahim, A.A. The Effect of LED Light Spectra on the Growth, Yield and Nutritional Value of Red and Green Lettuce (Lactuca sativa). Plants 2023, 12, 463. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Q.; Liu, W.; Li, B.; Shao, M.; Zhang, Y. Effects of Red/Blue versus White LED Light of Different Intensities on the Growth and Organic Carbon and Autotoxin Secretion of Hydroponic Lettuce. Hortic. Environ. Biotechnol. 2022, 63, 195–205. [Google Scholar] [CrossRef]
- Meng, Q.; Runkle, E.S. Blue Photons from Broad-Spectrum LEDs Control Growth, Morphology, and Coloration of Indoor Hydroponic Red-Leaf Lettuce. Plants 2023, 12, 1127. [Google Scholar] [CrossRef]
- Nguyen, T.K.L.; Cho, K.M.; Lee, H.Y.; Cho, D.Y.; Lee, G.O.; Jang, S.N.; Lee, Y.; Kim, D.; Son, K.-H. Effects of White LED Lighting with Specific Shorter Blue and/or Green Wavelength on the Growth and Quality of Two Lettuce Cultivars in a Vertical Farming System. Agronomy 2021, 11, 2111. [Google Scholar] [CrossRef]
- Smith, H.L.; McAusland, L.; Murchie, E.H. Don’t Ignore the Green Light: Exploring Diverse Roles in Plant Processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef]
- Tang, Y.K.; Guo, S.S.; Ai, W.D.; Qin, L.F. Effects of Red and Blue Light Emitting Diodes (LEDs) on the Growth and Development of Lettuce (Var. Youmaicai); SAE International: Warrendale, PA, USA, 2009. [Google Scholar]
- Qiao, J.; Hu, W.; Chen, S.; Cui, H.; Qi, J.; Yu, Y.; Liu, S.; Wang, J. Effect of LED Lights on Morphological Construction and Leaf Photosynthesis of Lettuce (Lactuca sativa L.). Horticulturae 2025, 11, 43. [Google Scholar] [CrossRef]
- Frutos-Totosa, A.; Hernández-Adasme, C.; Martínez, V.; Mestre, T.; Díaz-Mula, H.M.; Botella, M.A.; Flores, P.; Martínez-Moreno, A. Light Spectrum Effects on Rocket and Lamb’s Lettuce Cultivated in a Vertical Indoor Farming System. Sci. Hortic. 2023, 321, 112221. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Li, T.; Yang, Q.; Guo, W. Sugar Accumulation and Growth of Lettuce Exposed to Different Lighting Modes of Red and Blue LED Light. Sci. Rep. 2019, 9, 6926. [Google Scholar] [CrossRef] [PubMed]
- Darko, E.; Gondor, K.O.; Kovács, V.; Janda, T. Changes in the Light Environment: Short-Term Responses of Photosynthesis and Metabolism in Spinach. Physiol. Plant. 2023, 175, e13996. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, L.; Jiang, H.; He, R.; Song, S.; Su, W.; Liu, H. Supplementary Far-Red and Blue Lights Influence the Biomass and Phytochemical Profiles of Two Lettuce Cultivars in Plant Factory. Molecules 2021, 26, 7405. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Z.; Chen, X.; Zhu, B.; Liu, T.; Yang, J. Nutritional Composition and Antioxidant Activity of Gonostegia Hirta: An Underexploited, Potentially Edible, Wild Plant. Plants 2023, 12, 875. [Google Scholar] [CrossRef]
- Ciriello, M.; Formisano, L.; Kyriacou, M.; Soteriou, G.A.; Graziani, G.; De Pascale, S.; Rouphael, Y. Zinc Biofortification of Hydroponically Grown Basil: Stress Physiological Responses and Impact on Antioxidant Secondary Metabolites of Genotypic Variants. Front. Plant Sci. 2022, 13, 1049004. [Google Scholar] [CrossRef]




| Factor | Treatments | |||||
|---|---|---|---|---|---|---|
| Zinc concentration in hydroponic solution | 1 ppm | 5 ppm | 1 ppm | 5 ppm | 1 ppm | 5 ppm |
| Spectrum | 3000 K | 3500 K | 4000 K | |||
| Total Leaf Area | Dry and Fresh Weight | |
|---|---|---|
| Factor A (Light) | * | * |
| Factor B (Zn dose) | * | * |
| Interaction A × B | * | * |
| Zinc Content in Leaves | |
|---|---|
| Factor A (Light) | * |
| Factor B (Zn dose) | * |
| Interaction A × B | * |
| Fructose | Glucose | Sucrose | Maltose | Raffinose | |
|---|---|---|---|---|---|
| Factor A (Light) | * | * | * | ns | * |
| Factor B (Zn dose) | * | * | * | * | ns |
| Interaction A × B | * | * | * | * | * |
| DPPH | ABTS | FRAP | TPC | |
|---|---|---|---|---|
| Factor A (Light) | * | * | * | ns |
| Factor B (Zn dose) | * | * | * | * |
| Interaction A × B | * | * | * | * |
| Soluble Protein Content | Glutathione Reductase | Ascorbate Peroxidase | Superoxide Dismutase | |
|---|---|---|---|---|
| Factor A (Light) | * | * | * | * |
| Factor B (Zn dose) | * | * | * | * |
| Interaction A × B | * | * | * | * |
| Violaksantin | Neoxanthin | Liutein + Zeaksantin | Carotenes | Chlorophyll a | Chlorophyll b | |
|---|---|---|---|---|---|---|
| Factor A (Light) | ns | * | * | * | * | * |
| Factor B (Zn dose) | ns | * | * | * | * | * |
| Interaction A × B | ns | * | * | * | * | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laužikė, K.; Sutulienė, R.; Vaštakaitė-Kairienė, V.; Brazaitytė, A. The Combined Effect of Lighting and Zinc on the Nutritional Quality of Lettuce (Lactuca sativa L.) Grown in Hydroponics. Horticulturae 2025, 11, 284. https://doi.org/10.3390/horticulturae11030284
Laužikė K, Sutulienė R, Vaštakaitė-Kairienė V, Brazaitytė A. The Combined Effect of Lighting and Zinc on the Nutritional Quality of Lettuce (Lactuca sativa L.) Grown in Hydroponics. Horticulturae. 2025; 11(3):284. https://doi.org/10.3390/horticulturae11030284
Chicago/Turabian StyleLaužikė, Kristina, Rūta Sutulienė, Viktorija Vaštakaitė-Kairienė, and Aušra Brazaitytė. 2025. "The Combined Effect of Lighting and Zinc on the Nutritional Quality of Lettuce (Lactuca sativa L.) Grown in Hydroponics" Horticulturae 11, no. 3: 284. https://doi.org/10.3390/horticulturae11030284
APA StyleLaužikė, K., Sutulienė, R., Vaštakaitė-Kairienė, V., & Brazaitytė, A. (2025). The Combined Effect of Lighting and Zinc on the Nutritional Quality of Lettuce (Lactuca sativa L.) Grown in Hydroponics. Horticulturae, 11(3), 284. https://doi.org/10.3390/horticulturae11030284







