Abstract
The edible value of crabapple flowers remains unreported. In this study, the flower buds of three crabapple cultivars with different flower colors, Malus ‘Royalty’ (purple-red), ‘May’s Delight’ (pink), and ‘Snowdrift’ (white), were processed via hot-air drying at different temperatures and durations. The results showed that the sensory scores of ‘Royalty’ (4 h at 50 °C or 6 h at 40 °C), ‘Snowdrift’ (5 h at 50 °C), and ‘May’s Delight’ (4 h at 60 °C or 6 h at 50 °C) were higher (score > 90 points). The contents of phloridzin, total flavonoids (TFC), and total free amino acids (TFAC) in ‘May’s Delight’ under 50 or 60 °C were significantly higher than in other treatments. The differences in functional constituents among the different treatments of ‘Royalty’ were the smallest (C.V < 7%). The influences (C.Vk > 35%) of cultivar and drying temperature on the phlorizin content (PC) and TFAC were significantly higher than those of other treatments. The PC maintained the highest stability (C.Vk < 10%) under different temperatures and durations. The value of color parameter a* of dry flowers was significantly positively correlated with TFC, PC, and TFAC, and the total score of the sensory evaluation was positively correlated with the TFC. Based on functional constituents and sensory evaluation, ‘May’s Delight’ with air-drying at 50 °C for 6 h was the best option for crabapple flower tea.
1. Introduction
Crabapple trees (Malus spp.) are known for their stunning springtime blooms and decorative fruits that grace landscapes well into winter [1]. The genus Malus comprises around 30 species and about 1000 crabapple cultivars, with roughly 100 frequently chosen for planting due to their ornamental value and adaptability [2,3]. In addition to their ornamental value, crabapples also provide a rich source of bioactive substances, which can be utilized in pharmaceutical, chemical, and food fields, among others. The leaves and fruits of crabapple are reported to contain abundant polyphenols, terpenoids, vitamins, lipids, fibers, soluble sugars, microelements, organic acids, and amino acids. These compounds exhibit antioxidant, anticancer, lipid-lowering, anti-diabetic, and anti-inflammatory activities in vitro and/or in vivo [4,5,6,7]. The leaves of M. hupehensis and M. toringo have been consumed as herbal tea for over 400 years [8]. However, exploration of the edible potential of crabapple is currently limited to tea leaves and fruits [7,9], with no existing reports on the use of flower organs, such as crabapple floral tea.
The processing technology for flower tea is relatively advanced, with products such as rose tea [10], chrysanthemum tea [11], and jasmine tea [12] already enjoying popularity in the market. Scholars have also focused on the quality and efficacy of these scented teas, leading to studies on their volatile components, physical and chemical properties, antioxidant activity, and drying methods [13,14,15]. However, crabapple flower tea is not currently available on the market. Given the rich diversity of crabapple cultivars, these plants have significant potential as raw materials for flower tea. Nonetheless, their flavor profiles, what functional benefits they may offer, and whether these cultivars are suitable for flower tea production remain to be determined.
Therefore, in this study, the flower buds of three crabapple cultivars with different flower colors, Malus ‘Royalty’ (purple-red flowers), ‘May’s Delight’ (pink flowers), and ‘Snowdrift’ (white flowers), were processed via hot-air drying at different temperatures (40 °C, 50 °C, and 60 °C) and durations (4 h, 5 h, and 6 h). The differences in sensory quality, flower tea infusion color, and main functional constituents of different flower cultivars were explored to screen suitable cultivars with optimal processes and provide a reference for developing and applying crabapple flower resources.
2. Materials and Methods
2.1. Materials
Three cultivars with different colors were selected as materials, namely, Malus ‘Royalty’ (purple-red flowers), ‘May’s Delight’ (pink flowers), and ‘Snowdrift’ (white flowers). All cultivars were planted in the National Crabapple Germplasm Genetic Center (Yangzhou, lat. 32°42′ N, long. 119°55′ E). They were all 13-year-old grafted seedlings with stable flowering. In early April 2023, from 9:00 a.m. to 10:00 a.m. on a sunny day, flowers in the post-budding stage (when the female and stamen have not yet been revealed) were collected from the grating seedlings (Figure 1). The fresh samples were rinsed with tap water for 30 s to remove surface dust and then air-dried to a moisture content of approximately 60% for further processing [15].

Figure 1.
Flowers of three cultivars of crabapple in this study. A Colorchecker of X-rite was used for color correction of images.
2.2. Sample Drying
Our previous study showed that pretreatment by a microwave can inactivate the polyphenol oxidase (PPO) in crabapple flowers. After crabapple flowers were treated for 2 min at 300 W, the PPO activities declined to below 10%. So, the crabapple flowers were pretreated for 2 min by a microwave at 300 W, then dried in a hot-air dryer.
An L9 (33) orthogonal experimental design was adopted. Nine treatments, including three factors and three levels, cultivars, drying temperatures (40 °C, 50 °C, 60 °C), and drying durations (4 h, 5 h, 6 h), were set, namely, A1 (‘Royalty’, 40 °C, 6 h), A2 (‘Royalty’, 50 °C, 4 h), A3 (‘Royalty’, 60 °C, 5 h), B1 (‘May’s Delight’ 40 °C, 5 h), B2 (‘May’s Delight’, 50 °C, 6 h), B3 (‘May’s Delight’, 60 °C, 4 h), C1 (‘Snowdrift’, 40 °C, 4 h), C2 (‘Snowdrift’, 50 °C, 5 h), and C3 (‘Snowdrift’ 60 °C 6 h). Approximately 50 g samples for each treatment were spread on a stainless-steel plate in a hot-air dryer (Electrothermal Blowing Dry Box, DHG-9076A, Shanghai Jinghong Laboratory Instrument Co., Ltd., Shanghai, China). Each treatment included three replicates.
2.3. Sensory Evaluation
The sensory quality of the samples (nine treatments) was evaluated by a sensory panel consisting of one tea evaluation expert (officially qualified as a senior tea evaluator) and nine experienced evaluators, according to the methods of GB/T 23776-2018 [16] and ISO 13299:2016 [17], with some modifications. Samples of buds totaling 3 g were then covered with freshly boiled water in a 150 mL tea-tasting porcelain cup for 5 min. After steeping, the tea infusion and residue were transferred to white porcelain bowls and white enamel plates. The flower tea infusion was assessed in terms of the infusion color, aroma, taste, and infused flowers.
Based on GB/T 23776-2018 and GB/T 14487-2017, the final sensory score consisted of “appearance of dry flowers” (20%), “infusion color” (5%), “aroma” (35%), “taste” (30%), and “infused flowers” (10%) (Table 1) [16,18].

Table 1.
Rating scale for sensory evaluation of flower tea.
2.4. Parametrization of Sample Color
The preparation of the flower tea infusion and infusion flowers was consistent with the sensory evaluation method. The colorimetric values (L*, a*, and b*) of the dry samples, infusions, and infused flowers were measured by a color difference meter (IWAVE WF32, Shenzhen Wave Optoelectronics Technology Co., Ltd., Shenzhen, China). Each treatment was repeated with nine replications.
In the CIELab uniform color space, L* represents lightness, or how bright or dark a color is. The value ranges from 0 (black) to 100 (white). a* represents the red-green axis, with negative values representing green and positive values representing red. b* represents the blue-yellow axis, with negative values representing blue and positive values representing yellow. The values of a* and b* range from −128 to +128. Color difference (ΔE) is calculated by taking the square root of the sum of the squares of the differences between the L*, a*, and b* values of two states (dry flowers and infusion, infusion and infused flowers, and dry flowers and infused flowers), or between two treatments (A1 and A2, A2 and A3, B1 and B2, B2 and B3, C1 and C2, and C2 and C3). The formula is as follows [19]:
2.5. Measurement of the Content of Functional Constituents
Sample preparation: Functional constituents were extracted from dried crabapple flower tea using a method described by Liu et al. [6], with some modifications. A total of 1 g gram of ground sample (dry flower buds) was mixed with 50 mL of an 80% (v/v) methanol solution and sonicated for 20 min. The homogenate was centrifuged at 4025× g at 4 °C for 10 min. The extract was collected, and the residue underwent the same procedure two additional times to ensure maximum extraction. Supernatants from all extractions were collected, evaporated to dryness at 35 °C using a rotary vacuum evaporator, and then bulked to 25 mL with methanol. The supernatant was subsequently filtered through a medium-speed filter under vacuum at room temperature and stored at −4 °C until analysis.
The total free amino acid content (TFAC) was analyzed using ninhydrin [20]. A calibration curve was prepared, and the results are presented as glutamic acid equivalents per gram of dry weight (mg/g). All samples were analyzed in triplicates.
The total polyphenol content (TPC) was determined using the Folin–Ciocalteu colorimetric method, as outlined by Liu et al. [6]. Calibration and linear curves (based on gallic acid) were determined. The TPC was calculated and expressed as milligrams of gallic acid equivalent per gram of dry weight (mg/g). All samples were analyzed in triplicate.
The aluminum nitrate–sodium nitrite colorimetric determination method was adopted to measure the total flavonoid content (TFC), following the procedure described by Dong et al. [15]. Calibration and linear curves (based on rutin) were established. The TFC was calculated and expressed as milligrams of rutin equivalent per gram of dry weight (mg/g). All samples were analyzed using three replicates.
The phlorizin content (PC) was determined by HPLC (Alliance 2695, Water Co., Ltd., Milford, MA, USA) as previously described by Liu et al. [6]. After filtration through a Millipore membrane (0.22 μm), 10 μL of the solution was injected into the HPLC system. Chromatographic separation was performed using a reversed-phase column (C18, 250 mm × 4.6 mm i.d., 5 μm particle size, Phenomenex, Inc., Torrance, CA, USA) and a UV–vis detector. The mobile phase consisted of A (methanol) and B (H2O/acetic acid solution, 99.5: 0.5, v/v). The column temperature was maintained at 26 °C, and the flow rate was kept at 1.0 mL/min. Detection wavelengths were set at 280 nm. Quantitative determinations were conducted using an external phlorizin standard. All samples were analyzed using three replicates.
2.6. Statistical Analysis
Data were visualized using OriginPro 9.8.0.200 (OriginLab Corp., Northampton, MA, USA) and Adobe Illustrator CC 23.0.2 (Adobe Inc., San Jose, CA, USA) to process data and graphs. ANOVA was performed with SPSS 26.0 (IBM Corp., Armonk, NY, USA), and significant differences were assessed using Tukey’s HSD post hoc test (p < 0.05).
Kji value is defined as the sum of the evaluation indexes of all levels (i, i = 1, 2, 3) in each factor (j, j = cultivars, temperatures, durations), and (mean value of Kji) is used to determine the optimal level and combination of factors [21]. The optimal level for each factor can be obtained when is the largest. The formula for the variable coefficient of is as follows:
is the square deviation of , and is the square mean of .
3. Results
3.1. Sensory Evaluation of Crabapple Flower Tea
There were significant differences in the sensory scores of the nine treatments (p < 0.05), and the total scores from high to low were as follows: 95 points > A2 > C2 > A1 > B3 > B2 > 90 points > A3 = B1 > C3 > C1 > 75 points (Table 2, Figure 2).

Table 2.
Sensory scores on the quality of crabapple flower tea of different treatments.

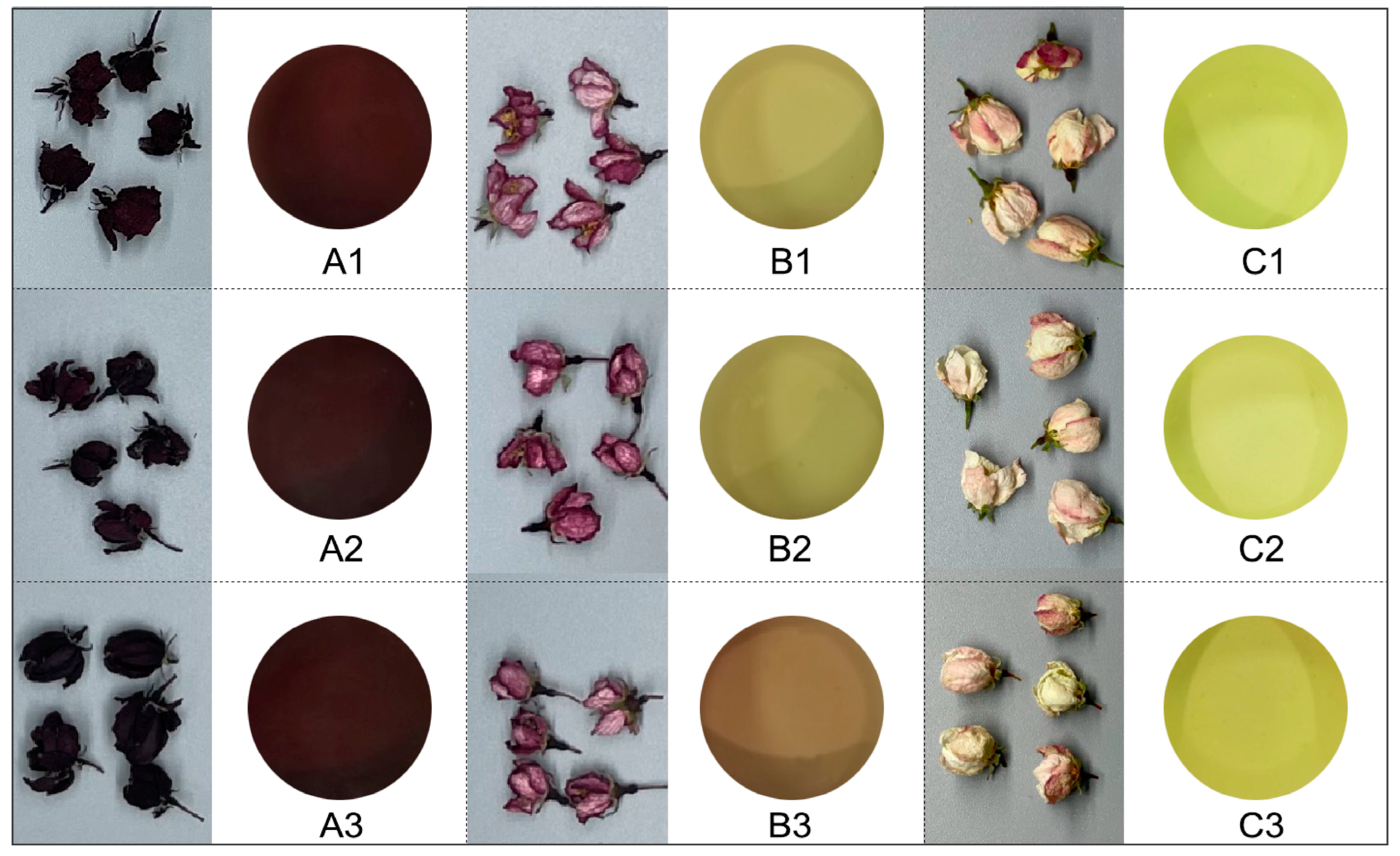
Figure 2.
The appearance of dry flowers and infusion of different treatments. (A1) ‘Royalty’, 40 °C, 6 h, (A2) ‘Royalty’, 50 °C, 4 h, (A3) ‘Royalty’, 60 °C, 5 h, (B1) ‘May’s Delight’ 40 °C, 5 h, (B2) ‘May’s Delight’, 50 °C, 6 h, (B3) ‘May’s Delight’, 60 °C, 4 h, (C1) ‘Snowdrift’, 40 °C, 4 h, (C2) ‘Snowdrift’, 50 °C, 5 h, (C3) ‘Snowdrift’ 60 °C, 6 h (the same as below).
As shown in Table 2, treatments A3, B3, and C2 exhibited significantly higher scores in the appearance of dried flowers than those of the other treatments (p < 0.05). The infusion color and aroma scores of ‘Royalty’ and ‘Snowdrift’ were significantly higher than those of ‘May’s Delight’ (p < 0.05). Nevertheless, ‘May’s Delight’ obtained a significantly higher score for taste (27.40–28.20 points). Treatment C1 achieved the lowest overall scores (78.18 points), suggesting that a low temperature with a short processing time was disadvantageous for enhancing the appearance, aroma, and taste quality of the flower tea.
3.2. Change in Color Parameters from Dry Flowers to Infusion and Infused Flowers
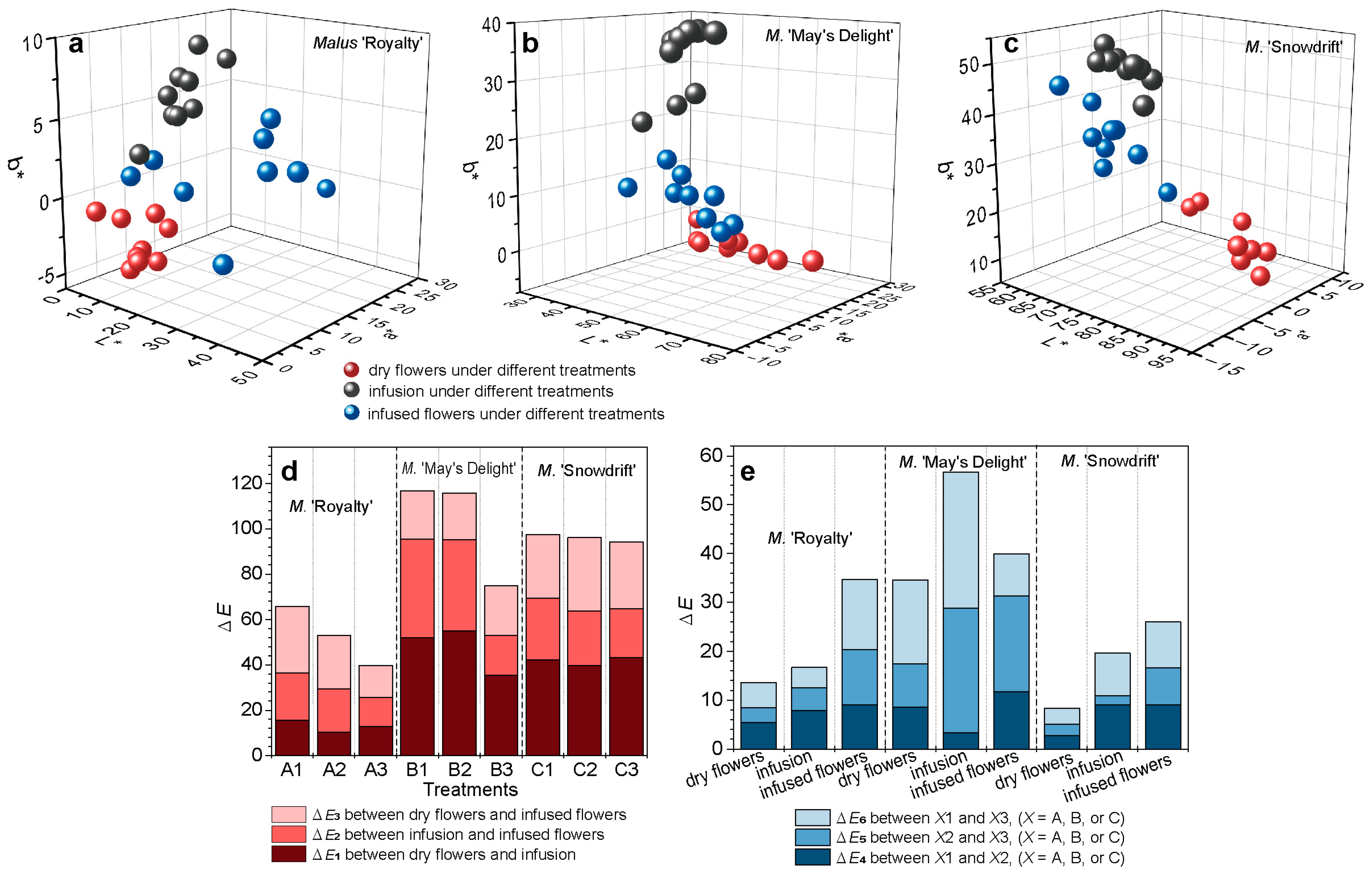
Figure 3a–c show that the three cultivars exhibited not completely consistent spatial distribution patterns and change trends across the L*, a*, and b* dimensions from dry flowers → infusion → infused flowers. The three cultivars exhibited an initial increase in the b* dimension followed by a decrease when evaluated based on dry flowers → infusion → infused flowers. The L* values of ‘May’s Delight’ and ‘Snowdrift’ demonstrated a downward trend, whereas those of ‘Royalty’ showed an opposite trend. The a* values of ‘May’s Delight’ and ‘Snowdrift’ initially decreased and then increased, while those of ‘Royalty’ consistently decreased. In addition, the lightness (L*) and yellow-blue (b*) values of ‘Snowdrift’ were significantly higher than those of the other two cultivars (p < 0.05) (Figure 3a–c).
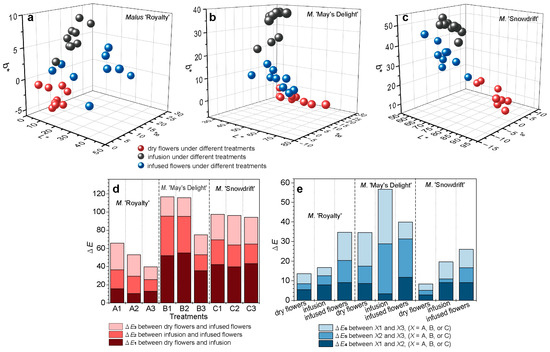
Figure 3.
Color parameter distribution in CIELab color space and color difference of crabapple flower teas with different treatments. (a) Malus ‘Royalty’; (b) M. ‘May’s Delight’; (c) M. ‘Snowdrift’. (d) The color difference (ΔE) among dry flowers, infusion, and infused flowers in each treatment. (e) The color difference (ΔE) among different treatments for dry, infusion, and infused flowers. L*: the degree of lightness, a*: the degree of redness (+) or greenness (−), b*: the degree of yellowness (+) or blueness (−). Each treatment comprised a total of nine replicates. The values from each set of three replicates were averaged and are represented as a single data point (sphere in the CIELab color space). Consequently, each treatment yields three spheres in the CIELab color space.
The color differences among the three stages (dry flower, infusion, and infused flowers) of ‘May’s Delight’ and ‘Snowdrift’ were significantly higher than those of ‘Royalty’ (Total ΔE = 39.67–65.82) (Figure 3d).
The color differences among different treatments in each stage (dry flower, infusion, or infused flowers) were also significantly different (p < 0.05) (Figure 3e). Within the same cultivar, the color differences among dry flowers processed with different parameters were more minor than those in infusions or infused flowers (Figure 3e). Among the cultivars, the processing treatments significantly influenced the color of the samples of ‘May’s Delight’ (Total ΔE = 34.59–56.68), which were higher than those of the two cultivars.
3.3. Differences in the Constituents of Dry Flower Tea of Different Crabapple Cultivars
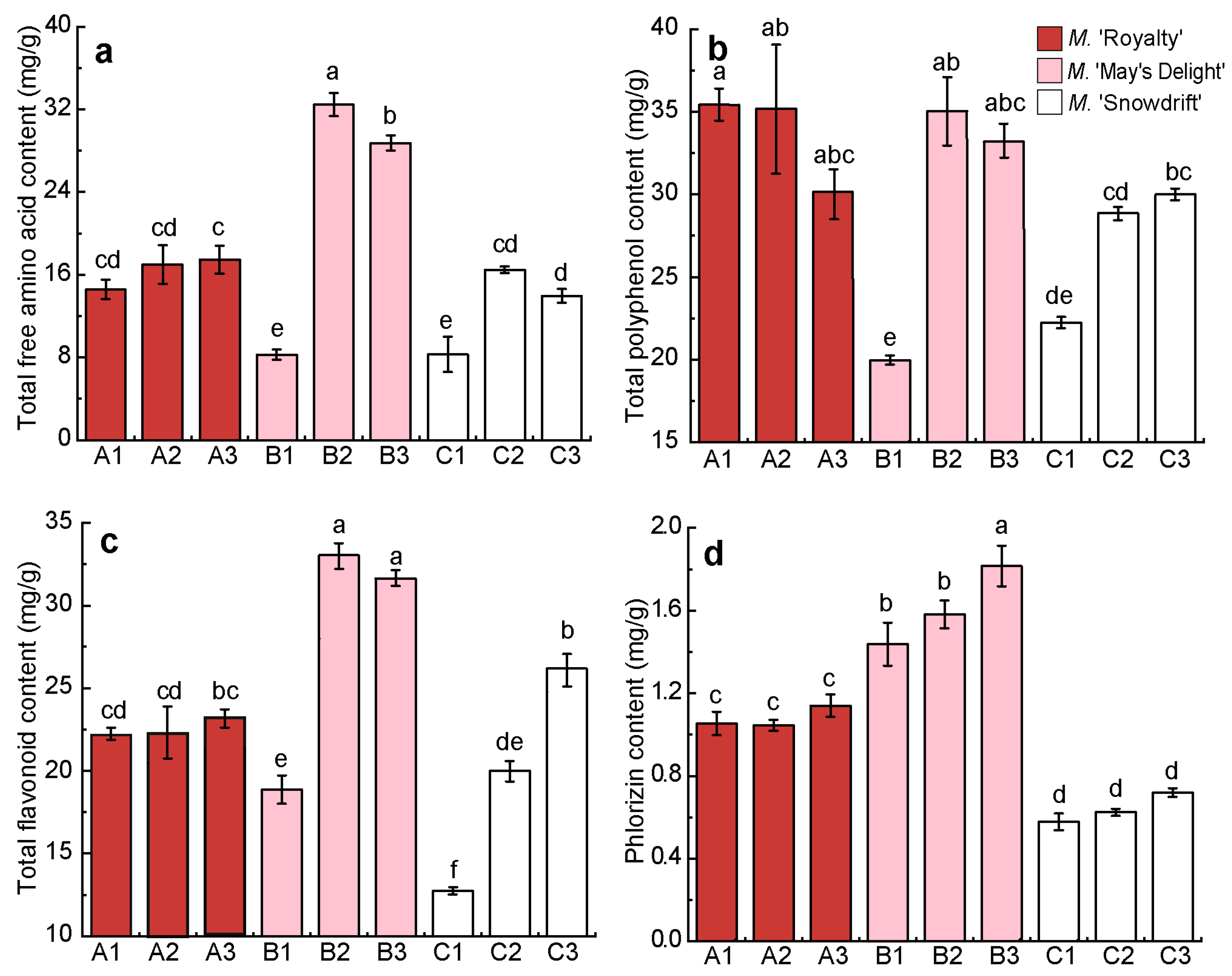
The contents of total free amino acids (TFAC), total polyphenols (TPC), total flavonoids (TFC), and phlorizin (PC) in the dry flowers of nine treatments are presented in Figure 4.
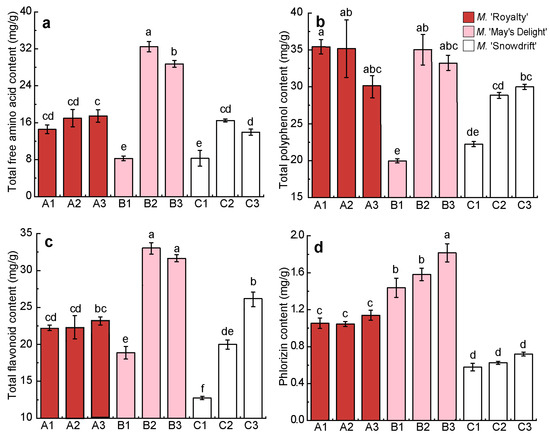
Figure 4.
Contents of the main functional constituents in the dry flower tea of different crabapple cultivars. (a) The content of total amino acid; (b) The content of total polyphenol; (c) The content of total flavonoid; (d) The content of phlorizin. Values are the means (±S.D.) of three replicates. The same letters above the columns mean there is no significant difference among the different treatments (p > 0.05), and different letters indicate a significant difference (p < 0.05), using Turkey’s multiple range test.
The TFAC, TFC, and PC of treatments B2 and B3 in ‘May’s Delight’ crabapple floral tea were significantly higher than those of the other two cultivars (p < 0.05) (Figure 4a,c,d). The TFAC, TPC, and TFC of treatments B1 (‘May’s Delight’, 40 °C, 5 h) and C1 (‘Snowdrift’, 40 °C, 4 h) were lower than those of the other treatments, including treatment A1 (‘Royalty’, 40 °C, 6 h). The differences in functional constituents between the different treatments (A1–A3) of ‘Royalty’ were the smallest (C.V < 7%). The TFC, TPC, and PC of ‘Snowdrift’ flower tea continued to increase with increasing processing temperature and duration (40 °C → 60 °C, 4 h → 6 h) (Figure 4).
3.4. Comparison of the Influence of Different Factors on the Functional Constituent Content of Crabapple Flower Tea
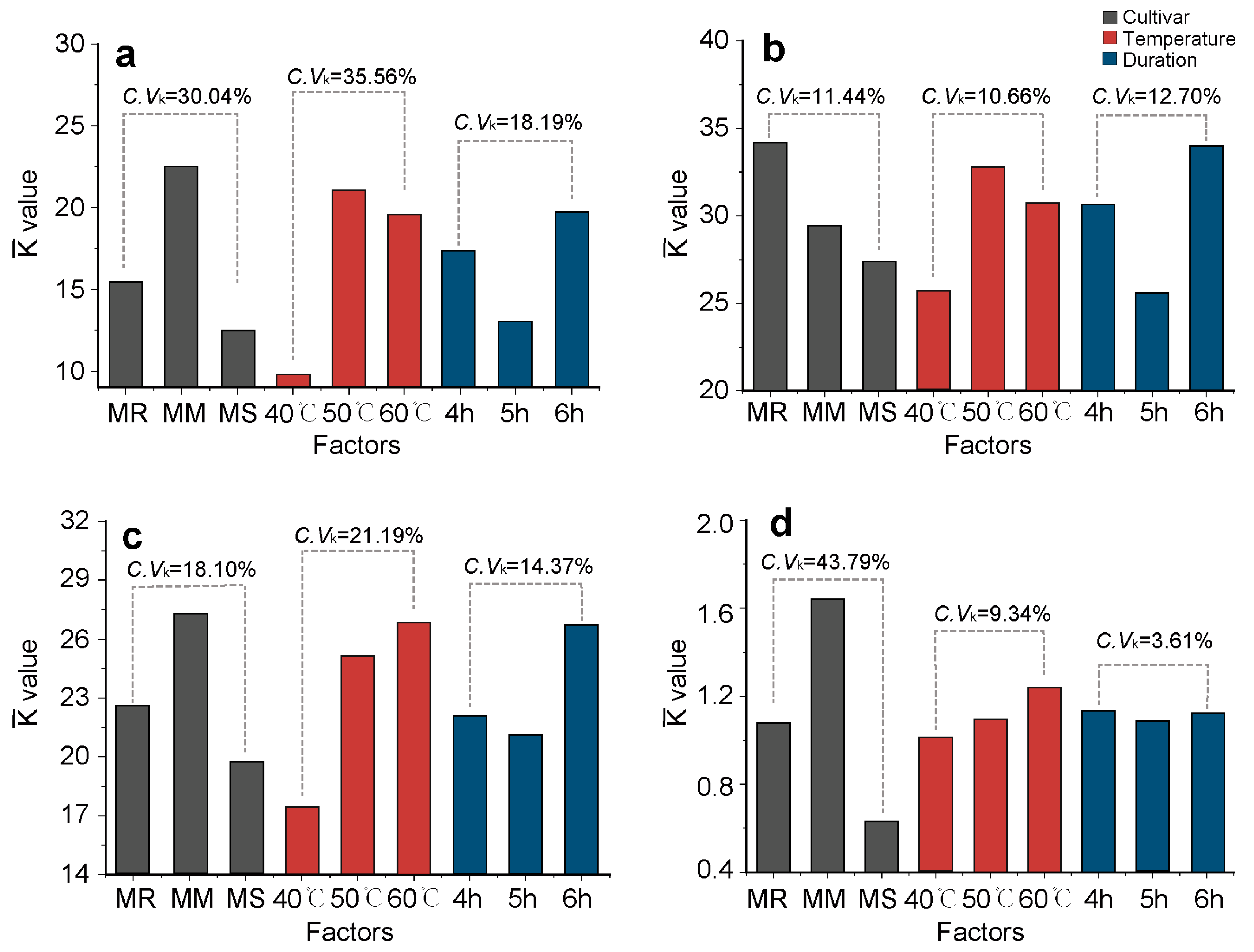
As shown in Figure 5, the degree of influence ( values) of the different factors (cultivar, temperature, and duration) on the functional constituent content of crabapple flower tea varied significantly (p < 0.05).
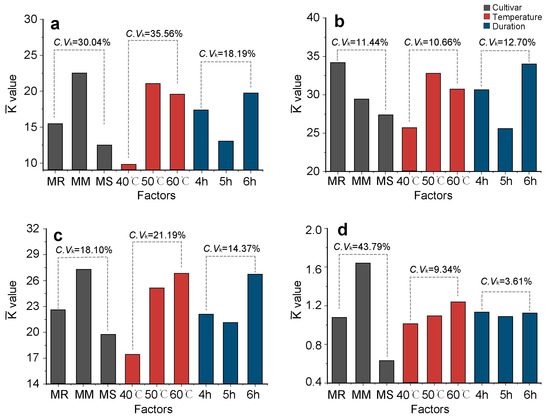
Figure 5.
The effects ( values) of different treatment factors (cultivar, temperature, duration) on the content of functional constituents of dry crabapple flower tea. (a) Total free amino acids; (b) total polyphenols; (c) total flavonoids; (d) phlorizin. The optimal level for each factor could be obtained when values were the largest. C.Vk was the variable coefficient of the values of three levels in each factor. MR, Malus ‘Royalty’; MM, M. ‘May’s Delight’; MS, M. ‘Snowdrift’.
The influence of different cultivars on the TFAC and PC (C.Vk > 30%) was significantly higher than those on the TPC and TFC. The influence of different drying temperatures on the TFAC and TFC (C.Vk > 21%) was significantly higher than those on the other functional constituents. The differences in the impact of varying drying temperatures and durations on the PC were minimal (C.Vk < 10%).
The level combination of each factor with the largest values for TFAC, TPC, TFC and PC was ‘May’s Delight’ with 6 h at 50 °C, ‘Royalty’ with 6 h at 50 °C, ‘May’s Delight’ with 6 h at 50 °C, and ‘May’s Delight’ with 4 or 6 h at 50 °C, respectively.
3.5. Correlation Analysis Between Sensory Attributes and Functional Constituents
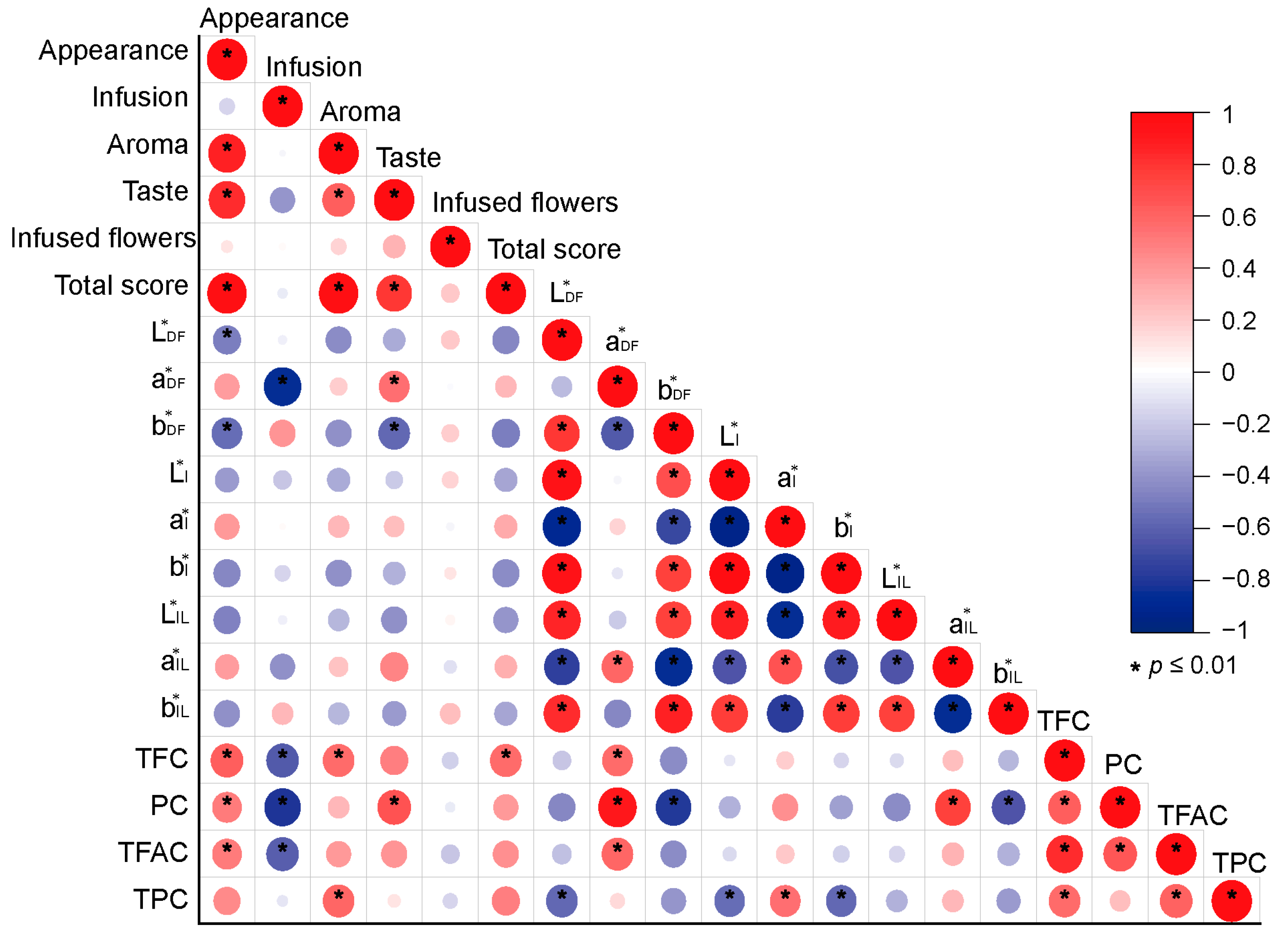
Correlation analysis showed that the total sensory evaluation score was significantly positively correlated with the TFC (R = 0.59) (Figure 6). The appearance score of dry flowers was positively correlated with the TFC, PC, and TFAC (R = 0.52–0.66, p < 0.01). However, the infusion color score was significantly negatively correlated with the TFC, PC, and TFAC (R = −0.62–−0.81, p < 0.01). The aroma score was significantly positively correlated with the TFC (R = 0.57) and TPC (R = 0.59). A significant positive correlation existed between taste and PC (R = 0.68, p < 0.01). The a* value of dry flowers was significantly positively correlated with PC (R = 0.91), TFC (R = 0.61), and TFAC (R = 0.60).
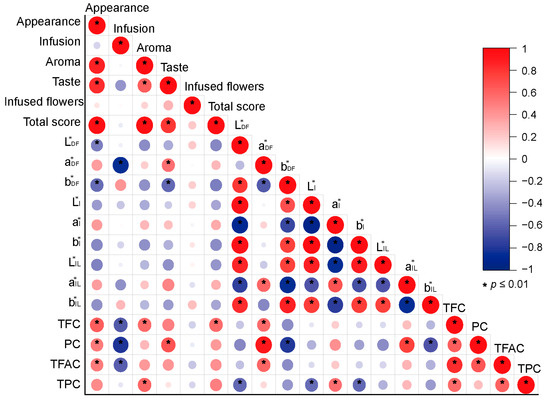
Figure 6.
Correlation analysis between sensory evaluation, color parameters, and functional constituent contents. L*DF, a*DF, and b*DF are the color parameters of the dry flowers; L*I, a*I, and b*I are the color parameters of the infusions; and L*IL, a*IL, and b*IL are the color parameters of the infused flowers. TFC: total flavonoid content; PC: phlorizin content; TFAC: total free amino acid content; TPC: total polyphenol content.
4. Discussion
4.1. The Cultivar and Drying Temperature Are Important Factors Influencing the Flower Tea Quality of Crabapple
Processing technology significantly impacts the quality of flower tea [22,23,24]. Factors such as the drying method, temperature, and storage conditions all influence the final quality of flower tea. Various processing technologies, including pan-baking, microwave, freeze-drying, hybrid drying, and far-infrared technology, have enhanced flower tea quality [14,15,24,25]. In the production of functional tea, bioactive substances are particularly susceptible to decomposition when subjected to the pan-baking drying method [15]. Conversely, a brief duration of microwave drying typically deactivates enzymes and enhances the aroma of the products without causing mechanical damage, resulting in satisfactory sensory quality and functional constituents [26,27]. However, our preliminary experiments indicated that microwave drying can cause the browning of crabapple petals (unpublished), adversely affecting their appearance quality. Consequently, this study processed crabapple flower tea using hot-air drying with varying parameters.
Our results indicated that the factors influencing the quality were ranked from highest to lowest () as follows: cultivars, drying temperature, and duration. The quality of the aroma and the color of the infusion of crabapples with purple-red and white flowers were better than those of pink flowers, while pink flowers had a better taste. This could be due to the higher TFAC in pink flowers (treatments B2 and B3, Figure 3a), which contribute to a more pronounced fresh flavor [28]. The sensory quality and functional constituent content of the purple-red flowers were less affected by temperature and duration (C.V < 7%) than those of the pink and white flowers. However, for cultivars with white or pink flowers, as processing temperature increased in a certain range (40–60 °C), the content of most functional constituents tended to rise, which aligns with the findings of Dong et al. [15]. This increase may be attributed to the transforming of certain polyphenols and flavonoids from a bound state to a free state at elevated temperatures [29]. Low temperature with a short processing time was disadvantageous for enhancing the sensory quality and functional constituent contents of flower tea. The optimal level for each factor could be obtained when values were the largest for the orthogonal test [21]. The level combination of each factor with the largest values for most functional constituents was ‘May’s Delight’ treated at 50 °C for six hours, which was also evaluated to have a high sensory score (90.55 points).
In summary, the selection of crabapple flower cultivars is crucial in the development of flower tea products. The pink- and white-flowered cultivars have stricter processing temperature and time requirements than the purple-flowered cultivars, as their sensory quality and functional constituents are more sensitive to fluctuations in the processing parameters.
4.2. Functional Constituents in Crabapple Flower Tea
Free amino acids have been confirmed as key chemical compounds that influence the taste and bioactive properties of tea infusions [30]. Polyphenols, particularly flavonoids, are important chemical compounds responsible for the health benefits of tea and serve as critical indicators of tea quality [31]. In studies of flower teas such as Chrysanthemum, Camellia, and Rosa, significant differences in functional constituents were observed among different cultivars [13,32,33]. In this study, we found that most functional constituents of pink and red flower cultivars were significantly higher than those of white flower cultivars, which was also observed in peony flowers [34]. Compared with other plant flowers, the total phenolic content (TPC) and total flavonoid content (TFC) in crabapple flower tea were approximately 0.25 to 1 times higher than in loquat flower tea [14]. However, the TPC in crabapple flower tea was about 5 to 10 times higher than in French rose buds, Jasmine, or Osmanthus flowers [35]. The TFAC was approximately one to eight times higher than in loquat flower tea [14], suggesting that crabapple flower tea may possess superior sensory qualities.
Additionally, we observed that the phloridzin content maintained relative stability (C.Vk < 10%) under different temperatures and durations. Phloridzin (phloretin 2′-O-glucoside) is a prominent member of the chemical class of dihydrochalcones, which are phenylpropanoids [36]. In contrast to other flavonoids, dihydrochalcones are rare and classified as “minor flavonoids” [37]. Phloridzin is a characteristic compound of Malus species that accounts for about 10% of the dry weight of Malus [38] and has attracted much attention due to its beneficial effects in the prevention of obesity, hepatic steatosis, inflammation, and insulin resistance [39,40]. Currently, 14 kinds of dihydrochalcones have been reported in Malus, of which phloridzin is the main form, including phloridtin, trihydroxyphloridzin, and phloridzin. In addition, trilobin and trihydroxytrilobin are commonly found in wild apple cultivars and ornamental crabapple [41,42].
Phloretin accumulates in large quantities in roots, leaves, fruits, and seeds, among which the content of phloretin in leaves is the highest, and the content in young leaves is significantly higher than in old leaves [43]. Recent studies have shown that it is also present in the fresh flowers and leaves of crabapple [6]. Compared to fresh crabapple flowers, the TPC and TFC in dried flowers in this study significantly increased, while the PC was slightly reduced [6]. This further substantiates that drying can promote the increase in the TPC and TFC.
Interestingly, we also found that the color parameter a* value of dry flowers was positively correlated with phloridzin content (R = 0.91, p < 0.01). The red-green value (a*) has been demonstrated to positively correlate with anthocyanin content, which may significantly contribute to the red hue [44]. Both anthocyanins and phloridzin were synthesized through the common pathway from phenylalanine to cinnamic acid, coumaric acid, and chalcone [45,46], which may have resulted in a positive correlation. The relationship model between the color parameters and phloridzin content will be further established and demonstrated, and it will be used to screen high-phloridzin cultivars rapidly.
5. Conclusions
In this study, the flower buds of three crabapple cultivars with different flower colors were processed for hot-air drying at different temperatures and durations. Under various processing conditions, the indices of sensory evaluation score and functional component content exhibited significant differences. Cultivar factors exhibited significant impacts on the content of functional constituents. Most index scores or contents of the pink flower cultivar (M. ‘May’s Delight’) were significantly higher than those of other cultivars or treatments. The value of the color parameter a* of dry flowers was positively correlated with the TFC, PC, and TFAC, and the total score of the sensory evaluation was positively correlated with the TFC. Considering functional constituents and sensory evaluation, ‘May’s Delight’ air-drying at 50 °C for six hours was best for crabapple flower tea.
Author Contributions
Conceptualization, H.L. and J.F.; methodology, H.L. and R.D.; software, J.M. and Y.C.; formal analysis, J.M. and Y.C.; investigation, Z.Y. and H.Z.; resources, J.F.; data curation, J.F.; writing—original draft preparation, H.L., Z.Y. and H.Z.; writing—review and editing, J.F.; visualization, J.F.; supervision, R.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (Grant No. 32201622), the Qing Lan Project of Jiangsu, the National Germplasm Center of Crabapple in China (Grant No. 164010065), and the High-Level Talent Foundation of Jinling Institute of Technology (Grant No. jit-b-202037).
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, Y.N. Researches of Germplasm Resources of Malus Mill; China Agriculture Press: Beijing, China, 2001; pp. 181–183+315–335. [Google Scholar]
- Rehder, A. Manual of Cultivated Trees and Shrubs, 2nd ed.; Environmental Science, Biology: New York, NY, USA, 1940; pp. 389–399. [Google Scholar]
- Fiala, J.L. Flowering Crabapples: The Genus Malus; Timber Press: Portland, OR, USA, 1994; pp. 106–273. [Google Scholar]
- Han, M.; Li, G.; Liu, X.; Li, A.; Mao, P.; Liu, P.; Li, H. Phenolic profile, antioxidant activity and anti-proliferative activity of crabapple fruits. Hortic. Plant J. 2019, 5, 155–163. [Google Scholar] [CrossRef]
- Li, N.; Shi, J.; Wang, K. Profile and antioxidant activity of phenolic extracts from 10 crabapples (Malus wild species). J. Agric. Food Chem. 2014, 62, 574–581. [Google Scholar] [CrossRef]
- Liu, F.; Wang, M.; Wang, M. Phenolic compounds and antioxidant activities of flowers, leaves and fruits of five crabapple cultivars (Malus Mill. species). Sci. Hortic. 2018, 235, 460–467. [Google Scholar] [CrossRef]
- Zeng, X.; Li, H.; Jiang, W.; Li, Q.; Xi, Y.; Wang, X.; Li, J. Phytochemical compositions, health-promoting properties and food applications of crabapples: A review. Food Chem. 2022, 386, 132789. [Google Scholar] [CrossRef]
- Yun, J.; Jiang, H.; Han, W. Analysis and evaluation of free amino acid in different cultivars of crabapple leaf tea. Food Ferment. Ind. 2020, 46, 237–243. [Google Scholar]
- Liu, B.; Zhang, C.; Zhang, J.; Zhao, X. Wu Shan Shen Cha (Malus asiatica Nakai. Leaves)-derived flavonoids alleviate alcohol-induced gastric injury in mice via an anti-oxidative mechanism. Biomolecules 2019, 9, 169. [Google Scholar] [CrossRef]
- Qin, H.; Deng, X.Q.; Li, B.C.; Dai, W.F.; Jiao, S.Y.; Qin, Y.; Zhang, M. Volatiles, polysaccharides and total polyphenols in Chinese rose tea infusions and their antioxidant activities. J. Food Process. Preserv. 2018, 42, e13323.1–e13323.7. [Google Scholar] [CrossRef]
- Li, Y.F.; Yang, P.Y.; Luo, Y.H.; Gao, B.Y.; Sun, J.H.; Lu, W.Y.; Liu, J.; Chen, P.; Zhang, Y.Q.; Yu, L.L. Chemical compositions of chrysanthemum teas and their anti-inflammatory and antioxidant properties. Food Chem. 2019, 286, 8–16. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, C.; Tian, C.; Xu, K.; Lai, Z.; Lin, Y.; Guo, Y. Volatilomics analysis of jasmine tea during multiple rounds of scenting processes. Foods 2023, 12, 812. [Google Scholar] [CrossRef]
- Han, A.R.; Nam, B.; Kim, B.R.; Lee, K.C.; Song, B.S.; Kim, S.H.; Kim, J.B.; Jin, C.H. Phytochemical Composition and Antioxidant Activities of Two Different Color Chrysanthemum Flower Teas. Molecules 2019, 24, 329. [Google Scholar] [CrossRef]
- Zheng, M.; Xia, Q.; Lu, S. Study on drying methods and their influences on effective components of loquat flower tea. LWT Food Sci. Technol. 2015, 63, 14–20. [Google Scholar] [CrossRef]
- Dong, J.; Ma, X.; Fu, Z.; Guo, Y. Effects of microwave drying on the contents of functional constituents of Eucommia ulmoides flower tea. Ind. Crops Prod. 2011, 34, 1102–1110. [Google Scholar] [CrossRef]
- GB/T 23776-2018; Methodology for Sensory Evaluation of Tea. Standard of the People’s Republic of China: Beijing, China, 2018.
- ISO 13299; 2016 Sensory Analysis-Methodology-General Guidance for Establishing a Sensory Profile. International Organization for Standardization (ISO): Geneva, Switzerland, 2016.
- GB/T 14487-2017; Tea Vocabulary for Sensory Evaluation. Standard of the People’s Republic of China: Beijing, China, 2018.
- Zhu, J.; Wang, J.; Yuan, H.; Ouyang, W.; Li, J.; Hua, J.; Jiang, Y. Effects of fermentation temperature and time on the color attributes and tea pigments of Yunnan Congou black tea. Foods 2022, 11, 1845. [Google Scholar] [CrossRef]
- Wang, H.F.; Tsai, Y.S.; Lin, M.L.; Ou, A.S.M. Comparison of bioactive components in GABA tea and green tea produced in Taiwan. Food Chem. 2006, 96, 648–653. [Google Scholar] [CrossRef]
- Wu, X.; Leung, D.Y. Optimization of biodiesel production from camelina oil using orthogonal experiment. Appl. Energy 2011, 88, 3615–3624. [Google Scholar] [CrossRef]
- Huang, Y.; Shang, H.; Zhu, J.W.; Liu, P.; Sun, W.J. Effects of processing treatments on quality and antioxidant activity of tea plant flower. Food Sci. 2020, 41, 165–170. [Google Scholar]
- Shi, L.; Gu, Y.; Wu, D.; Wu, X.; Grierson, D.; Tu, Y.; Wu, Y. Hot air drying of tea flowers: Effect of experimental temperatures on drying kinetics, bioactive compounds and quality attributes. Int. J. Food Sci. Technol. 2019, 54, 526–535. [Google Scholar] [CrossRef]
- Zhao, L.; Fan, H.; Zhang, M.; Chitrakar, B.; Bhandari, B.; Wang, B. Edible flowers: Review of flower processing and extraction of bioactive compounds by novel technologies. Food Res. Int. 2019, 126, 108660. [Google Scholar] [CrossRef]
- Valadez-Carmona, L.; Plazola-Jacinto, C.P.; Hernández-Ortega, M.; Hernández-Navarro, M.D.; Villarreal, F.; Necoechea-Mondragón, H.; Ortiz-Moreno, A.; Ceballos-Reyes, G. Effects of microwaves, hot air and freeze-drying on the phenolic compounds, antioxidant capacity, enzyme activity and microstructure of cacao pod husks (Theobroma cacao L.). Innov. Food Sci. Emerg. Technol. 2017, 41, 378–386. [Google Scholar] [CrossRef]
- Shi, L.; Kim, E.; Yang, L.; Huang, Y.; Ren, N.; Li, B.; He, P.; Tu, Y.; Wu, Y. Effect of a combined microwave-assisted drying and air drying on improving active nutraceutical compounds, flavor quality, and antioxidant properties of Camellia sinensis L. (cv. Longing 43) flowers. Food Qual. Saf. 2021, 5, 1–7. [Google Scholar] [CrossRef]
- Qu, F.; Zhu, X.; Ai, Z.; Ai, Y.; Qiu, F.; Ni, D. Effect of different drying methods on the sensory quality and chemical components of black tea. LWT 2019, 99, 112–118. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Q.Q.; Granato, D.; Xu, Y.Q.; Ho, C.T. Association between chemistry and taste of tea: A review. Trends Food Sci. Technol. 2020, 101, 139–149. [Google Scholar] [CrossRef]
- Irina, I.; Mohamed, G. Biological Activities and Effects of Food Processing on Flavonoids as Phenolic Antioxidants. In Advances in Applied Biotechnology; Petre, M., Ed.; Intech: Rijeka, Croatia, 2012; pp. 101–124. [Google Scholar]
- Horanni, R.; Engelhardt, U.H. Determination of amino acids in white, green, black, oolong, pu-erh teas and tea products. J. Food Compos. Anal. 2013, 31, 94–100. [Google Scholar] [CrossRef]
- Yang, J.; Liu, R.H. The Phenolic Profiles and Antioxidant Activity in Different Types of Tea. Int. J. Food Sci. Technol. 2013, 48, 163–171. [Google Scholar] [CrossRef]
- Shen, X.; Shi, L.; Pan, H.; Li, B.; Wu, Y.; Tu, Y. Identification of triterpenoid saponins in flowers of four Camellia sinensis cultivars from Zhejiang province: Differences between cultivars, developmental stages, and tissues. Ind. Crops Prod. 2017, 95, 140–147. [Google Scholar] [CrossRef]
- Vinokur, Y.; Rodov, V.; Reznick, N.; Goldman, G.; Horev, B.; Umiel, N.; Friedman, H. Rose petal tea as an antioxidant-rich beverage: Cultivar effects. J. Food Sci. 2006, 71, S42–S47. [Google Scholar] [CrossRef]
- Feng, Z.W.; Yang, X.G.; Pan, J.; Ren, G.H.; Zhang, E.X.; Fan, B.Y.; Shi, G.A. Antioxidation analisis of extracts from petals of 6 tree peonies in vitro. J. Northwest A F Univ. Nat. Sci. Ed. 2009, 37, 205–210. [Google Scholar]
- Hussain, N.; Ishak, I.; Harith, N.M.; Kuan, G.L.P. Comparison of bioactive compounds and sensory evaluation on edible flowers tea infusion. Ital. J. Food Sci. 2019, 31, 264–273. [Google Scholar]
- Baldisserotto, A.; Malisardi, G.; Scalambra, E.; Andreotti, E.; Romagnoli, C.; Vicentini, C.B.; Manfredini, S.; Vertuani, S. Synthesis, antioxidant and antimicrobial activity of a new phloridzin derivative for dermo-cosmetic applications. Molecules 2012, 17, 13275–13289. [Google Scholar] [CrossRef] [PubMed]
- Riviere, C. Dihydrochalcones: Occurrence in the plant kingdom, chemistry and biological activities. Stud. Nat. Prod. Chem. 2016, 51, 253–381. [Google Scholar]
- Ben-Othman, S.; Kaldmäe, H.; Rätsep, R.; Bleive, U.; Aluvee, A.; Rinken, T. Optimization of ultrasound-assisted extraction of phloretin and other phenolic compounds from apple tree leaves (Malus domestica Borkh.) and Comparison of Different Cultivars from Estonia. Antioxidants 2021, 10, 189. [Google Scholar] [CrossRef]
- Puel, C.; Quintin, A.; Mathey, J.; Obled, C.; Davicco, M.J.; Lebecque, P.; Kati-Coulibaly, S.; Horcajada, M.N.; Coxam, V. Prevention of bone loss by phloridzin, an apple polyphenol, in ovariectomized rats under inflammation conditions. Calcif. Tissue Int. 2005, 77, 311–318. [Google Scholar] [CrossRef]
- Anastasiadi, M.; Mohareb, F.; Redfern, S.P.; Berry, M.; Simmonds, M.S.; Terry, L.A. Biochemical profile of heritage and modern apple cultivars and application of machine learning methods to predict usage, age, and harvest season. J. Agric. Food. Chem. 2017, 65, 5339–5356. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, B.L.; Zhong, G.Y.; Brown, S.K. Genetic diversity of dihydrochalcone content in Malus germplasm. Genet. Resour. Crop Evol. 2018, 65, 1485–1502. [Google Scholar] [CrossRef]
- Guyot, S.; Marnet, N.; Sanoner, P.; Drilleau, J.F. Variability of the polyphenolic composition of cider apple (Malus domestica) fruits and juices. J. Agric. Food. Chem. 2003, 51, 6240–6247. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Hu, L.; Li, P.; Gong, X.; Ma, F. Genome-wide identification of glycosyltransferases converting phloretin to phloridzin in Malus species. Plant Sci. 2017, 265, 131–145. [Google Scholar] [CrossRef]
- Chen, H.; Wang, M.; Zhang, L.; Ren, F.; Li, Y.; Chen, Y.; Liu, Y.; Zhang, Z.; Zeng, Q. Anthocyanin profiles and color parameters of fourteen grapes and wines from the eastern foot of Helan Mountain in Ningxia. Food Chem. 2024, 24, 102034. [Google Scholar] [CrossRef]
- Gosch, C.; Halbwirth, H.; Stich, K. Phloridzin: Biosynthesis, distribution and physiological relevance in plants. Phytochemistry 2010, 71, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, Z.; Wu, Y.; Zheng, L.; Zhang, G. Regulatory mechanisms of anthocyanin biosynthesis in apple and pear. Int. J. Mol. Sci. 2021, 22, 8441. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).