Agronomic Strategies to Manipulate Kiwifruit Calcium Content to Understand Its Role in Fruit Physiology
Abstract
1. Introduction
2. Materials and Methods
2.1. Ca, K, and N Application—Experiment 1
2.1.1. Orchard Description and Treatments
2.1.2. Sampling and Analysis
2.1.3. Physiological Measurements
2.1.4. Statistical Analysis
2.2. Solar Radiation—Experiment 2
2.2.1. Orchard Description and Treatments
2.2.2. Sampling, Analysis, and Physiological Measurements
2.2.3. Statistical Analysis
2.3. Fruit Transpiration—Experiment 3
2.3.1. Experimental Conditions and Measurements
2.3.2. Statistical Analysis
3. Results
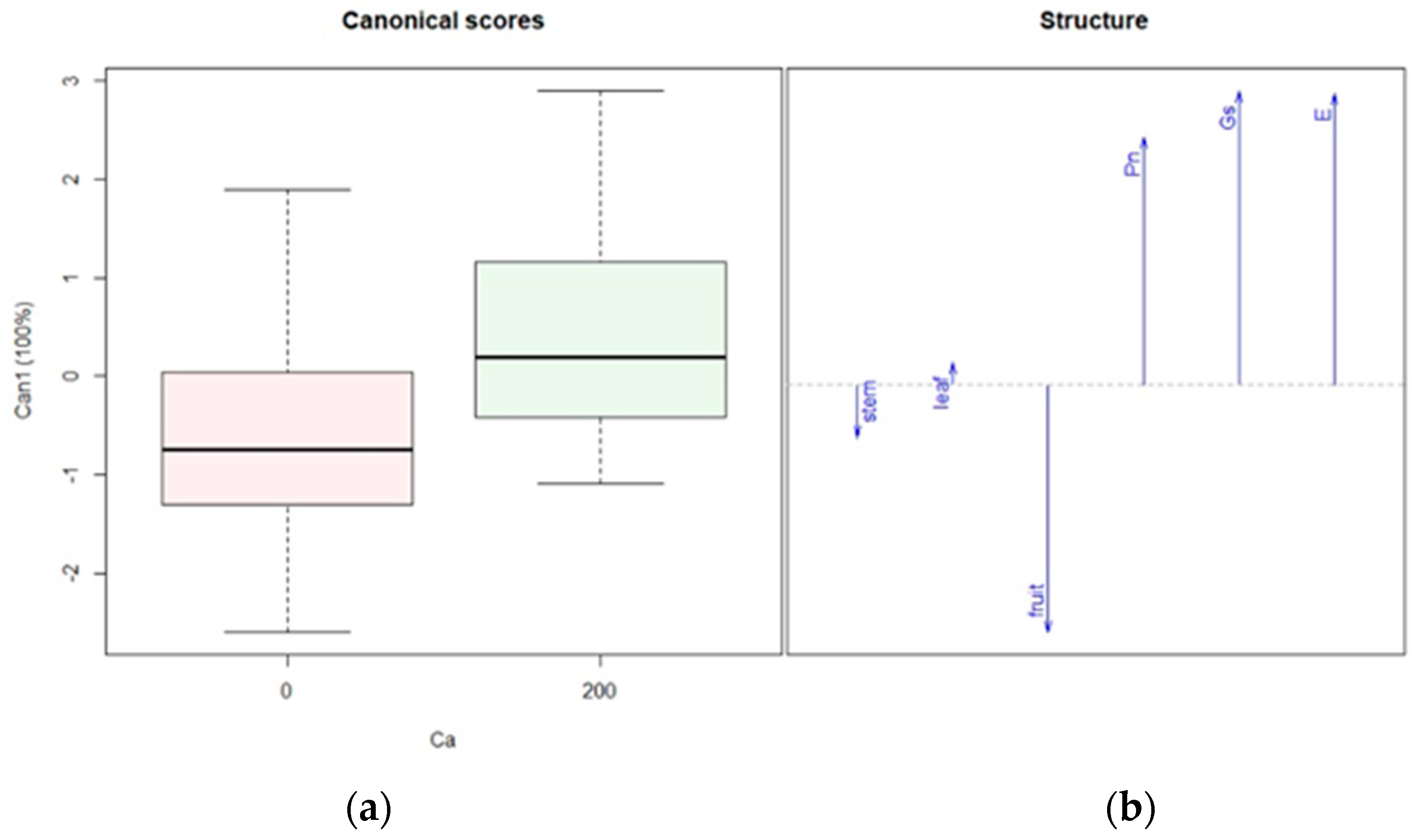
3.1. Ca, K, and N Application—Experiment 1
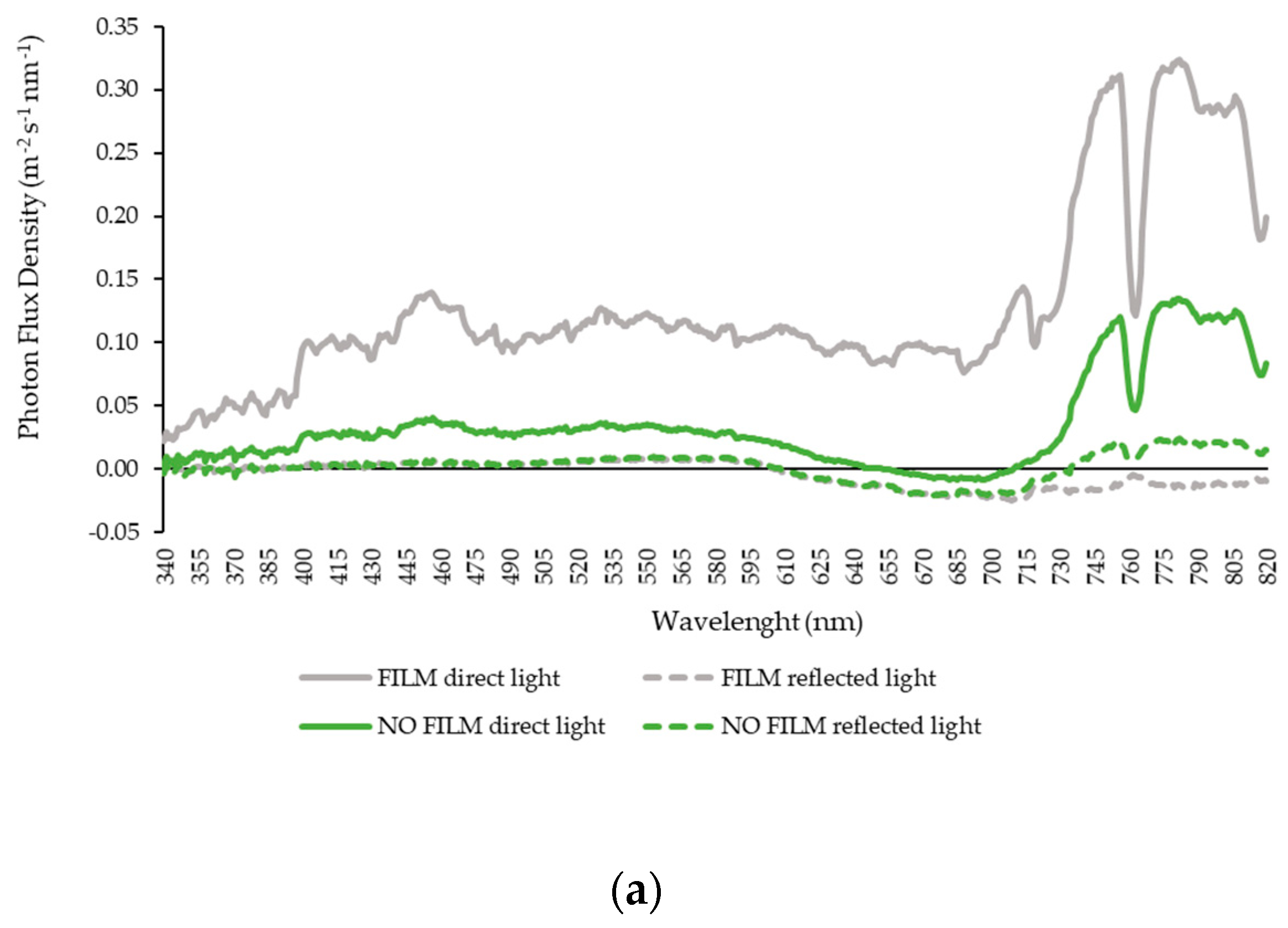
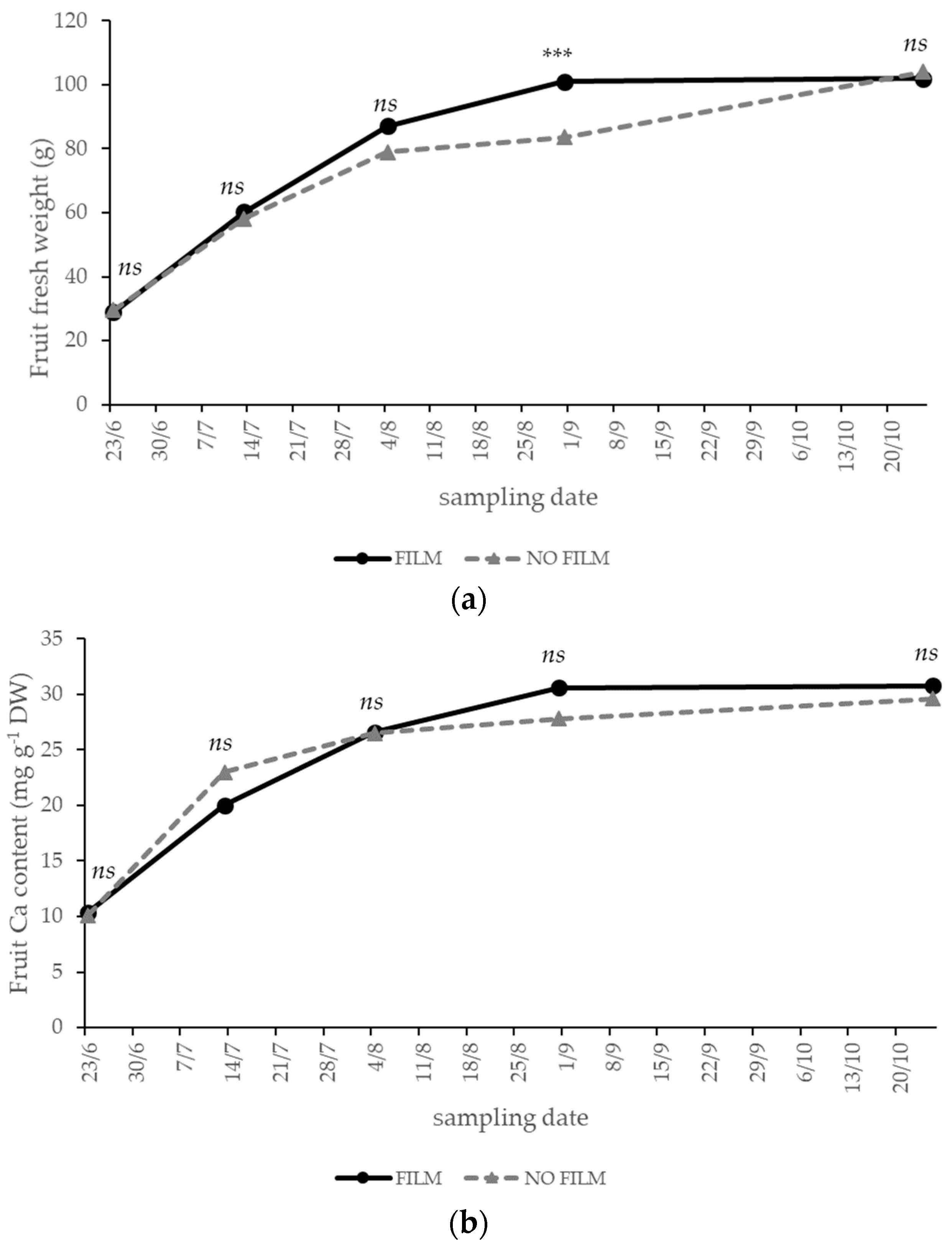
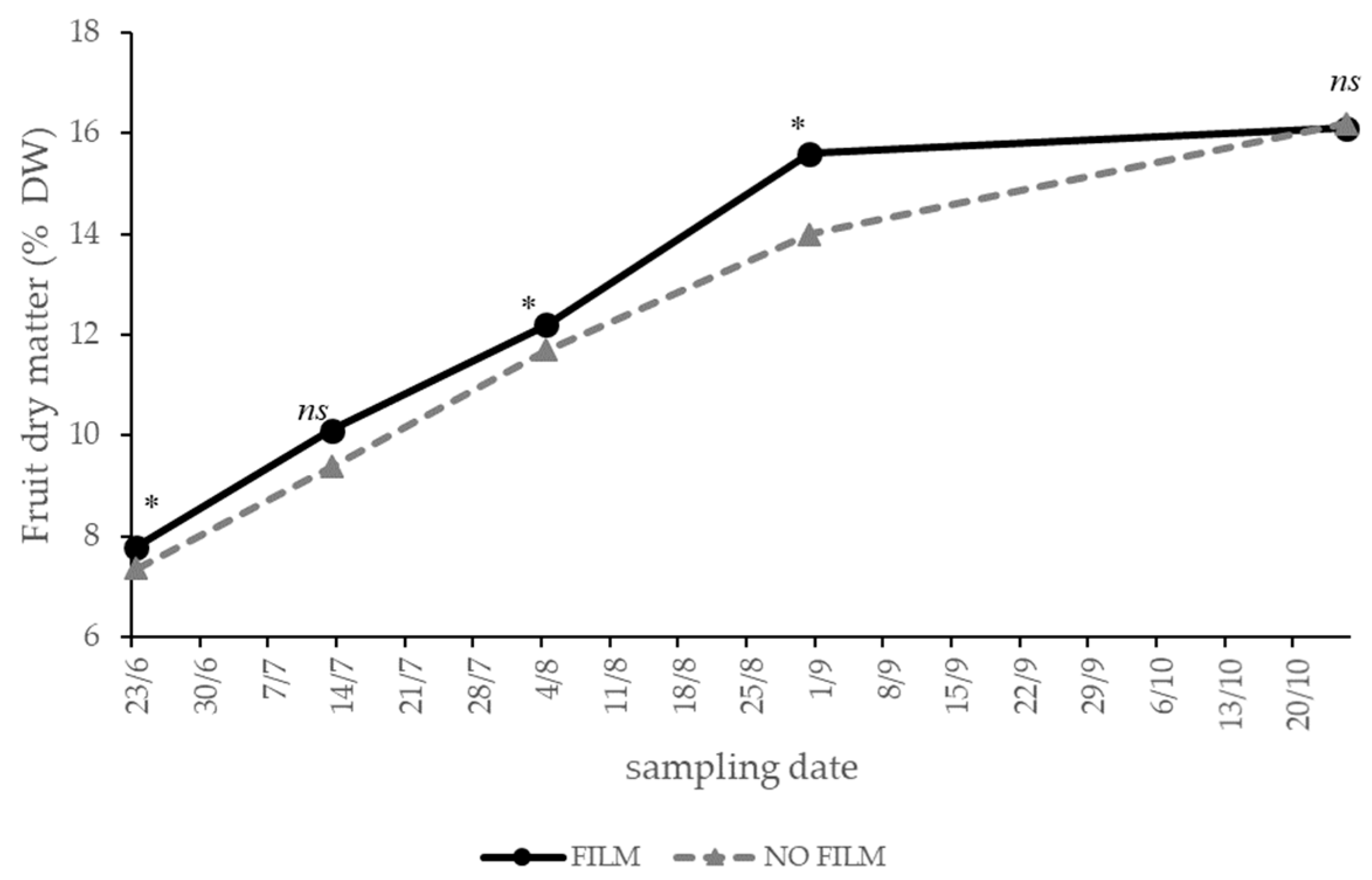
3.2. Solar Radiation—Experiment 2
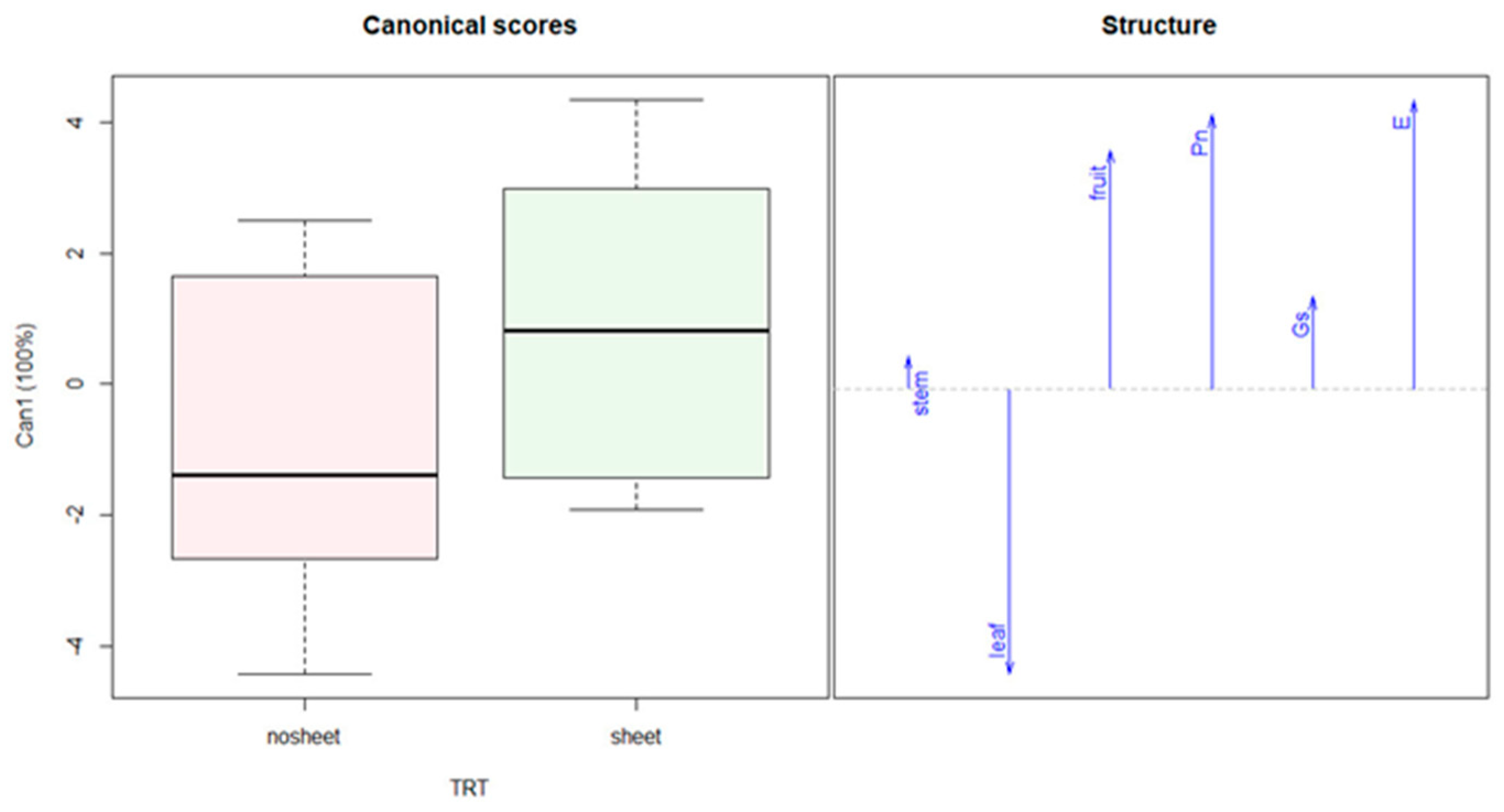
3.3. Fruit Transpiration—Experiment 3
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ca | Calcium |
| N | Nitrogen |
| K | Potassium |
| PAR | Photosynthetic active radiation |
| VPD | Vapor pressure deficit |
| SSC | Soluble solid content |
| DM | Dry matter |
| DCA | Discriminant canonical analysis |
| Ψω | Water potentials |
| ΨωS | Stem water potential |
| ΨwL | Leaf water potential |
| ΨwF | Fruit water potential |
| Pn | Total photosynthesis |
| Gs | Stomatal conductance |
| E | Transpiration |
References
- FAO. 2025. Available online: https://www.statista.com/statistics/812434/production-volume-of-leading-kiwi-producing-countries/ (accessed on 5 February 2025).
- Thor, K. Calcium—Nutrient and messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Negi, N.P.; Narwal, P.; Kumari, P.; Kisku, A.V.; Gahlot, P.; Mittal, N.; Kumar, D. Calcium signaling in coordinating plant development, circadian oscillations and environmental stress responses in plants. Environ. Exp. Bot. 2022, 201, 104935. [Google Scholar] [CrossRef]
- Wdowiak, A.; Podgórska, A.; Szal, B. Calcium in plants: An important element of cell physiology and structure, signaling, and stress responses. Acta Physiol. Plant 2024, 46, 108. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Hirschi, K.D. The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiol. 2004, 136, 2438–2442. [Google Scholar] [CrossRef] [PubMed]
- Saure, M.C. Reassessment of the role of calcium in development of bitter pit in apple. Funct. Plant Biol. 1996, 23, 237–243. [Google Scholar] [CrossRef]
- Ciccarese, A.; Stellacci, A.M.; Gentilesco, G.; Rubino, P. Effectiveness of pre-and post-veraison calcium applications to control decay and maintain table grape fruit quality during storage. Postharvest Biol. Tech. 2013, 75, 135–141. [Google Scholar] [CrossRef]
- Thorp, T.G.; Ferguson, I.B.; Boyd, L.M.; Bornett, A.M. Fruiting position, mineral concentration and incidence of physiological pitting in ‘Hayward’ kiwifruit. J. Hort. Sci. Biotech. 2003, 78, 512–517. [Google Scholar] [CrossRef]
- Montanaro, G.; Dichio, B.; Lang, A.; Mininni, A.N.; Nuzzo, V.; Clearwater, M.J.; Xiloyannis, C. Internal versus external control of calcium nutrition in kiwifruit. J. Plant Nutr. Soil. Sci. 2014, 177, 819–830. [Google Scholar] [CrossRef]
- Buwalda, J.G.; Smith, G.S. Accumulation and partitioning of dry matter and mineral nutrients in developing kiwifruit vines. Tree Physiol. 1987, 3, 295–307. [Google Scholar] [CrossRef]
- Xiloyannis, C.; Celano, G.; Montanaro, G.; Dichio, B.; Sebastiani, L.; Minnocci, A. Water relations, calcium and potassium concentration in fruits and leaves during annual growth in mature kiwifruit plants. Acta Hortic. 2000, 564, 129–134. [Google Scholar] [CrossRef]
- Clark, C.J.; Smith, G.S. Seasonal accumulation of mineral nutrients by kiwifruit 2. Fruit. New Phytol. 1988, 108, 399–409. [Google Scholar] [CrossRef]
- Quartieri, M.; Toselli, M.; Sorrenti, G.; Baldi, E.; Polidori, G.; Germani, M.A.; Xylogiannis, E. Dynamic of nutrient uptake and partitioning within yellow-fleshed kiwifruit (Actinidia chinensis var. chinensis) organs. Acta Hortic. 2021, 1333, 203–208. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Bangerth, F. Calcium-related physiological disorders of plants. Annu. Rev. Phytopathol. 1979, 17, 97–122. [Google Scholar] [CrossRef]
- Montanaro, G.; Dichio, B.; Xiloyannis, C.; Celano, G. Light influences transpiration and calcium accumulation in fruit of kiwifruit plants (Actinidia deliciosa var. deliciosa). Plant Sci. 2006, 170, 520–527. [Google Scholar] [CrossRef]
- Montanaro, G.; Dichio, B.; Xiloyannis, C.; Lang, A. Fruit transpiration in kiwifruit: Environmental drivers and predictive model. AoB Plants 2012, 2012, pls036. [Google Scholar] [CrossRef] [PubMed]
- Nobel, P.S. Physicochemical and Environmental Plant Physiology; Academic Press: Cambridge, MA, USA, 1999. [Google Scholar]
- Buchanan, B.; Gruissem, W.; Jones, R. Natural Products (Secondary Metabolites). In Biochemistry and Molecular Biology of Plants; Science Press: Beijing, China, 2002. [Google Scholar]
- Mohammad Sokri, S.; Babalar, M.; Barker, A.V.; Lesani, H.; Asgari, M.A. Fruit quality and nitrogen, potassium, and calcium content of apple as influenced by nitrate: Ammonium ratios in tree nutrition. J. Plant Nutr. 2015, 38, 1619–1627. [Google Scholar] [CrossRef]
- WRB, World Reference Base for Soil Resources 2014. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. World Soil Resources Reports 106. FAO, Rome. Available online: http://www.fao.org/soils-portal/soilsurvey/soil-classification/world-reference-base/en/ (accessed on 13 May 2024).
- ARPAE. 2024. Available online: https://www.arpae.it/sim/?osservazioni_e_dati/climatologia (accessed on 13 May 2024).
- Kingston, H.M. Microwave Assisted Acid Digestion of Siliceous and Organically-Based Matrices, Method 3052; U.S. Environmental Protection Agency IAG DWI-393254-01-0, Quarterly Report, 1 January–31 March; U.S. Environmental Protection Agency: Washington, DC, USA, 1988. [Google Scholar]
- Schumann, G.E.; Stanley, M.A.; Knudsen, D. Automated total nitrogen analysis of soil and plant samples. Soil Sci. Soc. Amer. J. 1973, 37, 480–481. [Google Scholar] [CrossRef]
- McCutchan, H.; Shackel, K.A. Stem water potential as a sensitive indicator of water stress in prune trees (Prunus domestica L. cv. French.). J. Am. Soc. Hort. Sci. 1992, 117, 607–611. [Google Scholar] [CrossRef]
- Emilia-Romagna. 2022. Available online: https://agricoltura.regione.emilia-romagna.it/produzioniagroalimentari/temi/bio-agro-climambiente/agricoltura-integrata/disciplinari-produzione-integratavegetale# (accessed on 13 May 2024).
- Ferguson, I.B. Calcium in plant senescence and fruit ripening. Plant Cell Environ. 1984, 7, 477–489. [Google Scholar] [CrossRef]
- Conway, W.S.; Sams, C.E. The effects of postharvest infiltration of calcium, magnesium, or strontium on decay, firmness, respiration, and ethylene production in apples. J. Am. Soc. Hort. Sci. 1987, 112, 300–303. [Google Scholar] [CrossRef]
- Toselli, M.; Marangoni, B.; Malaguti, D.; Sorrenti, G.; Bazzi, C.; Collina, M. Use of soil-applied calcium chloride to reduce fire blight and brown spot susceptibility of pear. Acta Hortic. 2002, 638, 301–305. [Google Scholar] [CrossRef]
- Quartieri, M.; Sorrenti, G.; Ciriani, A.; Baldi, E.; Collina, M.; Toselli, M. Combining quince (Cydonia oblonga) rootstock with soil-applied calcium chloride solution as a strategy to control brown spot (Stemphylium vesicarium) incidence in Abbé Fétel pear fruits. AIMS Agric. Food 2019, 4, 414–428. [Google Scholar] [CrossRef]
- Yang, H.M.; Zhang, X.Y.; Tang, Q.L.; Wang, G.X. Extracellular calcium is involved in stomatal movement through the regulation of water channels in broad bean. Plant Growth Regul. 2006, 50, 79–83. [Google Scholar] [CrossRef]
- Hocking, B.; Tyerman, S.D.; Burton, R.A.; Gilliham, M. Fruit calcium: Transport and physiology. Front. Plant Sci. 2016, 7, 569. [Google Scholar] [CrossRef] [PubMed]
- Knipfer, T.; Fei, J.; Gambetta, G.A.; McElrone, A.J.; Shackel, K.A.; Matthews, M.A. Water transport properties of the grape pedicel during fruit development: Insights into xylem anatomy and function using microtomography. Plant Physiol. 2015, 168, 1590–1602. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, M.; Dichio, B.; Clearwater, M.J.; Montanaro, G.; Xiloyannis, C. Hydraulic resistance of developing Actinidia fruit. Ann. Bot. 2013, 112, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Hochmal, A.K.; Schulze, S.; Trompelt, K.; Hippler, M. Calcium-dependent regulation of photosynthesis. Bioenergetics 2015, 1847, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Tarara, J.M. Microclimate modification with plastic mulch. HortScience 2000, 35, 169–180. [Google Scholar] [CrossRef]
- Abdelkader, A.B.; Tagliavini, M.; Zanotelli, D. Effects of hail nets and reflective ground covers on microclimate and evapotranspiration demand in an apple orchard. Acta Hortic. 2021, 1327, 647–654. [Google Scholar] [CrossRef]
- Green, S.R.; McNaughton, K.G.; Greer, D.H.; McLeod, D.J. Measurement of the increased PAR and net all-wave radiation absorption by an apple tree caused by applying a reflective ground covering. Agric. For. Meteorol. 1995, 76, 163–183. [Google Scholar] [CrossRef]
- Ju, Z.; Duan, Y.; Ju, Z. Effects of covering the orchard floor with reflecting films on pigment accumulation and fruit coloration in Fuji’ apples. Sci. Hort. 1999, 82, 47–56. [Google Scholar] [CrossRef]
- Tombesi, A.; Antognozzi, E.; Palliotti, A. Influence of light exposure on characteristics and storage life of kiwifruit. N. Z. J. Crop Hort. 1993, 21, 85–90. [Google Scholar] [CrossRef]
- Xiloyannis, C.; Celano, G.; Montanaro, G.; Dichio, B. Calcium absorption and distribution in mature kiwifruit plants. Acta Hortic. 2002, 610, 331–334. [Google Scholar] [CrossRef]
- Biasi, R.; Altamura, M.M. Light enhances differentiation of the vascular system in the fruit of Actinidia deliciosa. Physiol. Plantarum 1996, 98, 28–35. [Google Scholar] [CrossRef]
- Bukovac, M.J.; Wittwer, S.H. Absorption and mobility of foliar applied nutrients. Plant Physiol. 1957, 32, 428–435. [Google Scholar] [CrossRef]
- McLaughlin, S.B.; Wimmer, R. Tansley review No. 104. Calcium physiology and terrestrial ecosystem processes. New Phytol. 1999, 142, 373–417. [Google Scholar] [CrossRef]
- Zavalloni, C.; Marangoni, B.; Tagliavini, M.; Scudellari, D. Dynamics of uptake of calcium, potassium and magnesium into apple fruit in a high-density planting. Acta Hortic. 2000, 56, 113–121. [Google Scholar] [CrossRef]




| Parameter | Experiment 1 | Experiments 2 and 3 |
|---|---|---|
| pH | 7.56 | 8.16 |
| Total lime (% CaCO3) | 3 | 13 |
| Active carbonate (% CaCO3) | 12.7 | 11.6 |
| Organic matter (%) | 3.32 | 1.97 |
| Total N (%) | 2.04 | 1.30 |
| Available P (mg kg−1) | 98 | 29 |
| Exchangeable K (mg kg−1) | 322 | 324 |
| Exchangeable Ca (mg kg−1) | 5512 | 4141 |
| Exchangeable Mg (mg kg−1) | 183 | 408 |
| Salinity (mS cm−1) | 2.75 | - |
| CEC 1 (meq 100 g−1) | 30.6 | 25.0 |
| Sand (%) | 35 | 10 |
| Loam (%) | 49 | 49 |
| Clay (%) | 16 | 41 |
| Treatment | Fruit Average Weight (g) | Total Yield (kg pt−1) |
|---|---|---|
| N0 | 75.1 | 21.0 |
| N200 | 74.1 | 19.4 |
| Significance | ns | ns |
| K0 | 74.9 | 20.4 |
| K200 | 74.4 | 20.0 |
| Significance | ns | ns |
| Ca0 | 76.1 | 21.7 |
| Ca200 | 73.1 | 18.7 |
| Significance | ns 1 | * |
| N × K | ns | ns |
| N × Ca | ns | ns |
| K × Ca | ns | ns |
| N × K × Ca | ns | ns |
| Treatment | Harvest | 107 Days of Storage | 154 Days of Storage | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Dry Matter (%) | SSC (°Brix) | Firmness (kg) | Dry Matter (%) | SSC (°Brix) | Firmness (kg) | Dry Matter (%) | SSC (°Brix) | Firmness (kg) | |
| N0 | 19.3 | 8.0 | 7.63 | 19.8 | 16.2 | 1.92 | 19.3 | 16.0 | 1.04 |
| N200 | 19.2 | 8.1 | 7.53 | 19.7 | 16.9 | 1.67 | 19.2 | 16.2 | 0.99 |
| Significance | ns 1 | ns | ns | ns | ns | * | ns | ns | ns |
| K0 | 19.3 | 8.1 | 7.54 | 19.6 | 16.2 | 1.73 | 19.0 | 16.1 | 1.04 |
| K200 | 19.1 | 8.0 | 7.63 | 19.8 | 16.9 | 1.86 | 19.6 | 16.1 | 1.00 |
| Significance | ns | ns | ns | ns | ns | * | * | ns | ns |
| Ca0 | 19.1 | 7.9 | 7.72 | 19.6 | 16.6 | 1.86 | 19.1 | 16.0 | 1.05 |
| Ca200 | 19.4 | 8.2 | 7.45 | 19.9 | 16.5 | 1.73 | 19.5 | 16.2 | 0.99 |
| Significance | ns | ** | * | ns | ns | ns | ns | ns | ns |
| N × K | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| N × Ca | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| K × Ca | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| N × K × a | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Treatment | Firmness (kg) | SSC (%) | DM (%) |
|---|---|---|---|
| HARVEST | |||
| NO FILM | 6.97 | 6.20 | 16.2 |
| FILM | 7.53 | 6.92 | 16.1 |
| Significance | * 1 | *** | ns |
| 3-MONTH COLD STORAGE | |||
| NO FILM | 1.76 | 11.8 | 13.5 |
| FILM | 2.36 | 12.8 | 15.7 |
| Significance | *** | *** | *** |
| 5-MONTH COLD STORAGE | |||
| NO FILM | 1.18 | 12.2 | 14.7 |
| FILM | 1.25 | 14.5 | 17.1 |
| Significance | ns | *** | *** |
| Treatment | Firmness (kg) | SSC (%) | DM (%) |
|---|---|---|---|
| HARVEST | |||
| NO BAG | 6.97 | 6.20 | 16.2 |
| BAG | 5.82 | 6.03 | 16.2 |
| Significance | *** 1 | ns | ns |
| 3-MONTH COLD STORAGE | |||
| NO BAG | 1.76 | 11.8 | 13.5 |
| BAG | 1.74 | 12.2 | 14.7 |
| Significance | ns | ns | ** |
| 5-MONTH COLD STORAGE | |||
| NO BAG | 1.18 | 12.1 | 14.8 |
| BAG | 1.15 | 13.2 | 18.8 |
| Significance | ns | * | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldi, E.; Toselli, M.; Bonora, A.; Boini, A.; Quartieri, M.; Germani, M.; Polidori, G.; Corelli Grappadelli, L. Agronomic Strategies to Manipulate Kiwifruit Calcium Content to Understand Its Role in Fruit Physiology. Horticulturae 2025, 11, 237. https://doi.org/10.3390/horticulturae11030237
Baldi E, Toselli M, Bonora A, Boini A, Quartieri M, Germani M, Polidori G, Corelli Grappadelli L. Agronomic Strategies to Manipulate Kiwifruit Calcium Content to Understand Its Role in Fruit Physiology. Horticulturae. 2025; 11(3):237. https://doi.org/10.3390/horticulturae11030237
Chicago/Turabian StyleBaldi, Elena, Moreno Toselli, Alessandro Bonora, Alexandra Boini, Maurizio Quartieri, Margherita Germani, Greta Polidori, and Luca Corelli Grappadelli. 2025. "Agronomic Strategies to Manipulate Kiwifruit Calcium Content to Understand Its Role in Fruit Physiology" Horticulturae 11, no. 3: 237. https://doi.org/10.3390/horticulturae11030237
APA StyleBaldi, E., Toselli, M., Bonora, A., Boini, A., Quartieri, M., Germani, M., Polidori, G., & Corelli Grappadelli, L. (2025). Agronomic Strategies to Manipulate Kiwifruit Calcium Content to Understand Its Role in Fruit Physiology. Horticulturae, 11(3), 237. https://doi.org/10.3390/horticulturae11030237









