Transcriptomic Analysis Reveals the Biosynthesis Mechanism of Coixol Under Salicylic Acid Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. SA Treatments
2.2. Determination of Coixol Content
2.3. RNA-Seq Library Preparation and Sequencing
2.4. De Novo Assembly, Functional Annotation, and Enrichment Analysis of DEGs
2.5. qRT-PCR Analysis
2.6. Statistical Analysis
3. Results
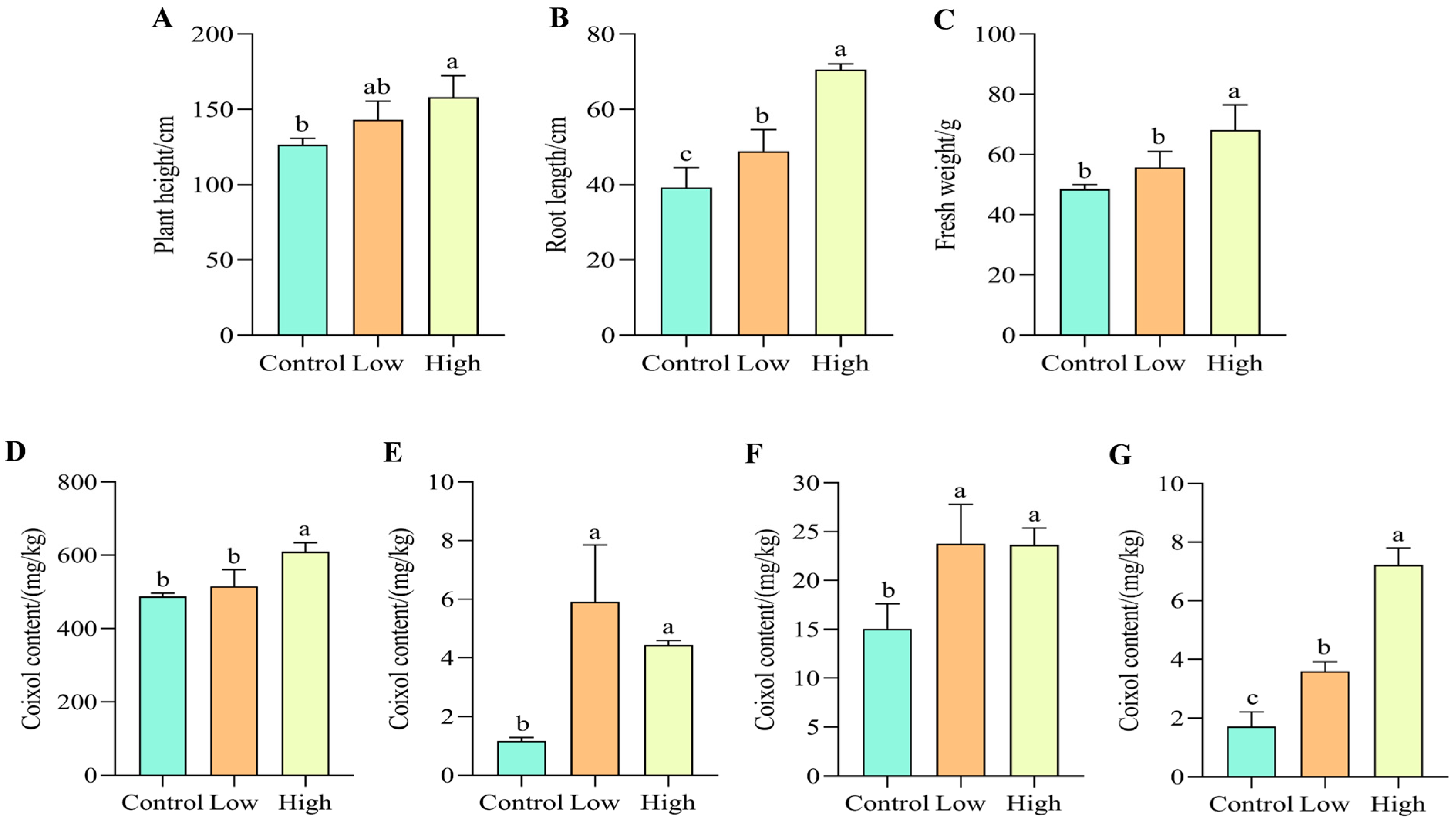
3.1. Effect of SA on Growth Indicators of Coix
3.2. The Effect of SA on the Content of Coixol in Different Parts of Coix
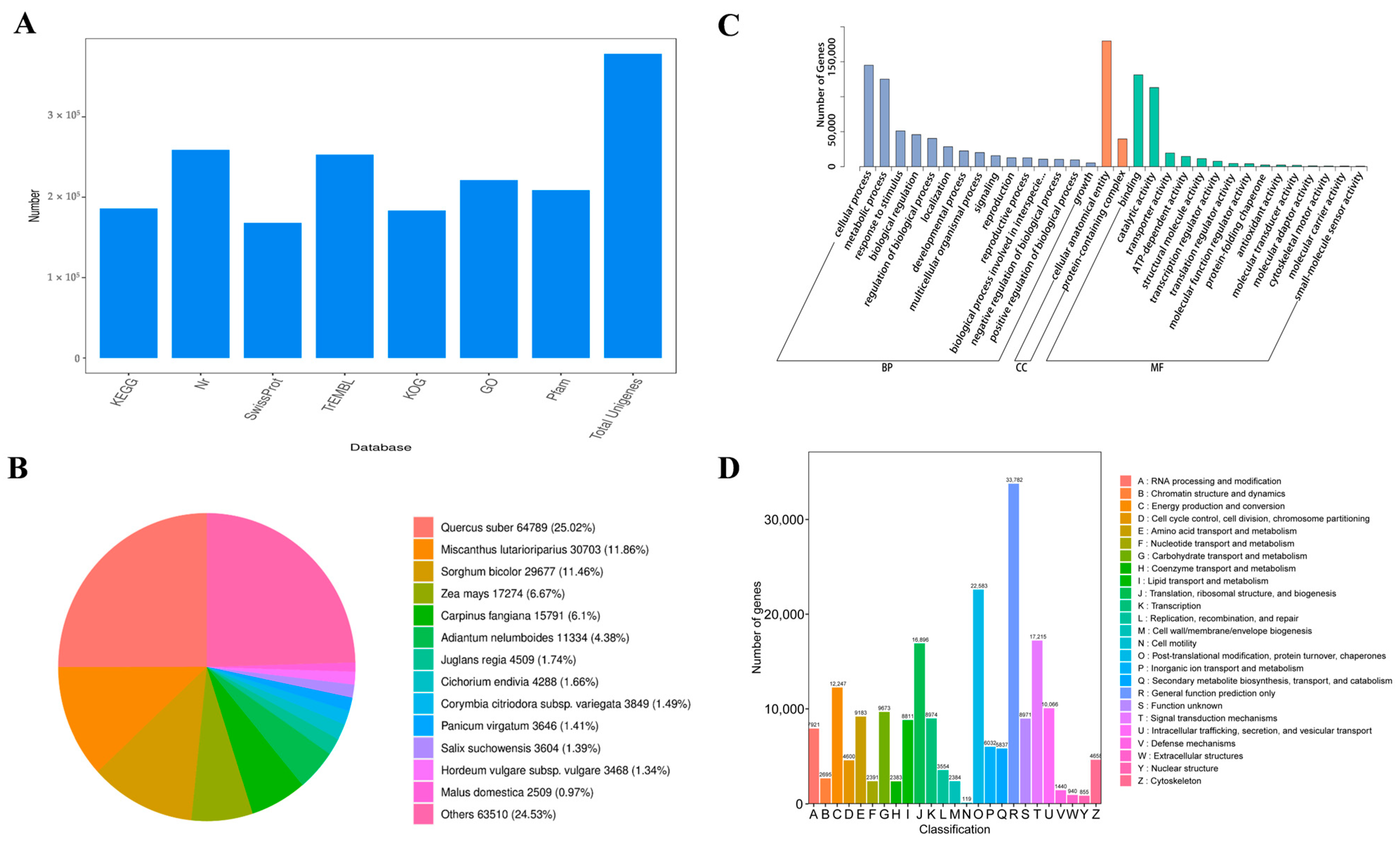
3.3. Transcriptome Analysis
3.4. Gene Function Annotation
3.5. Differential Gene Expression Screening
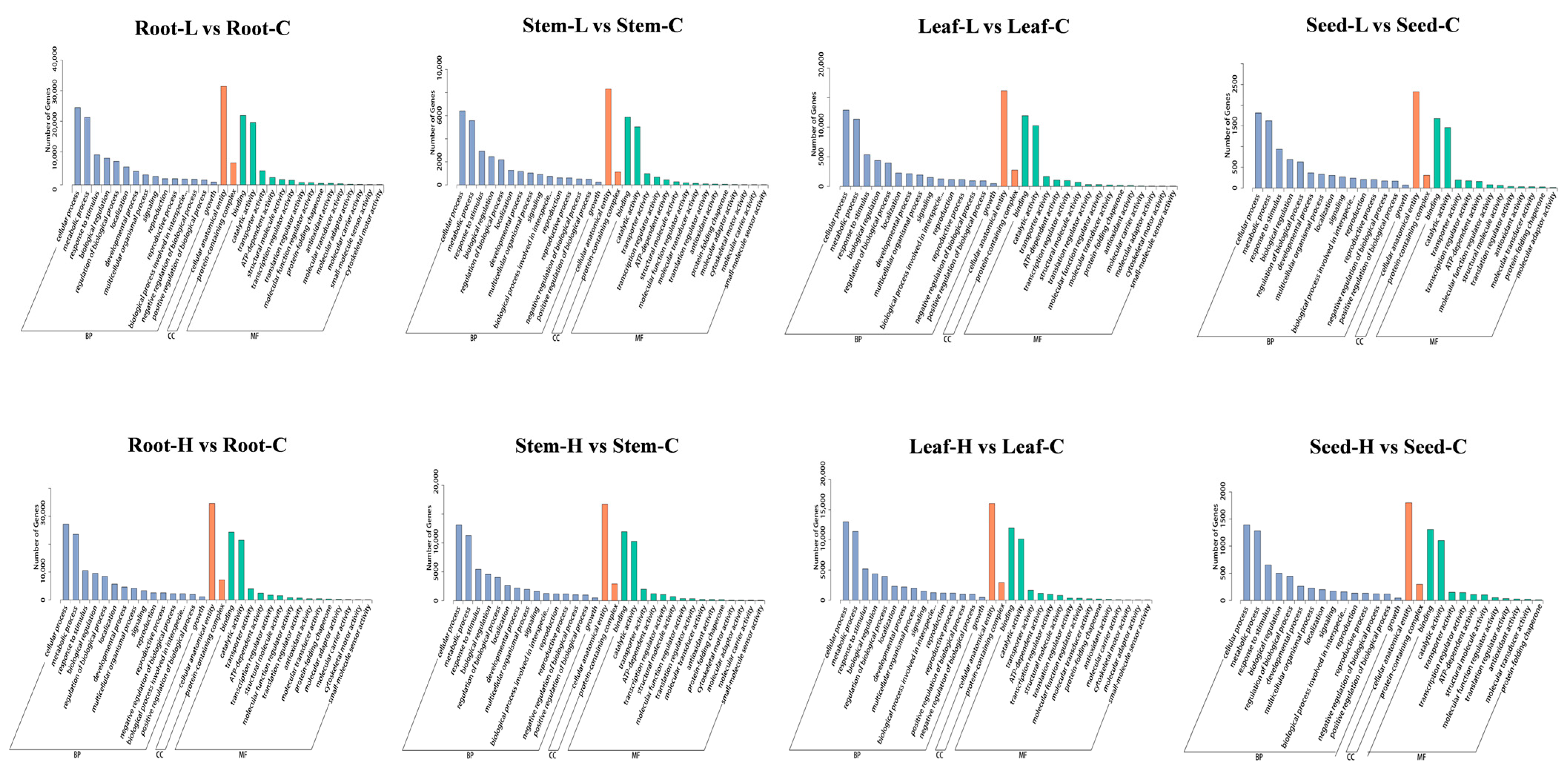
3.6. Differential Gene Expression GO Analysis
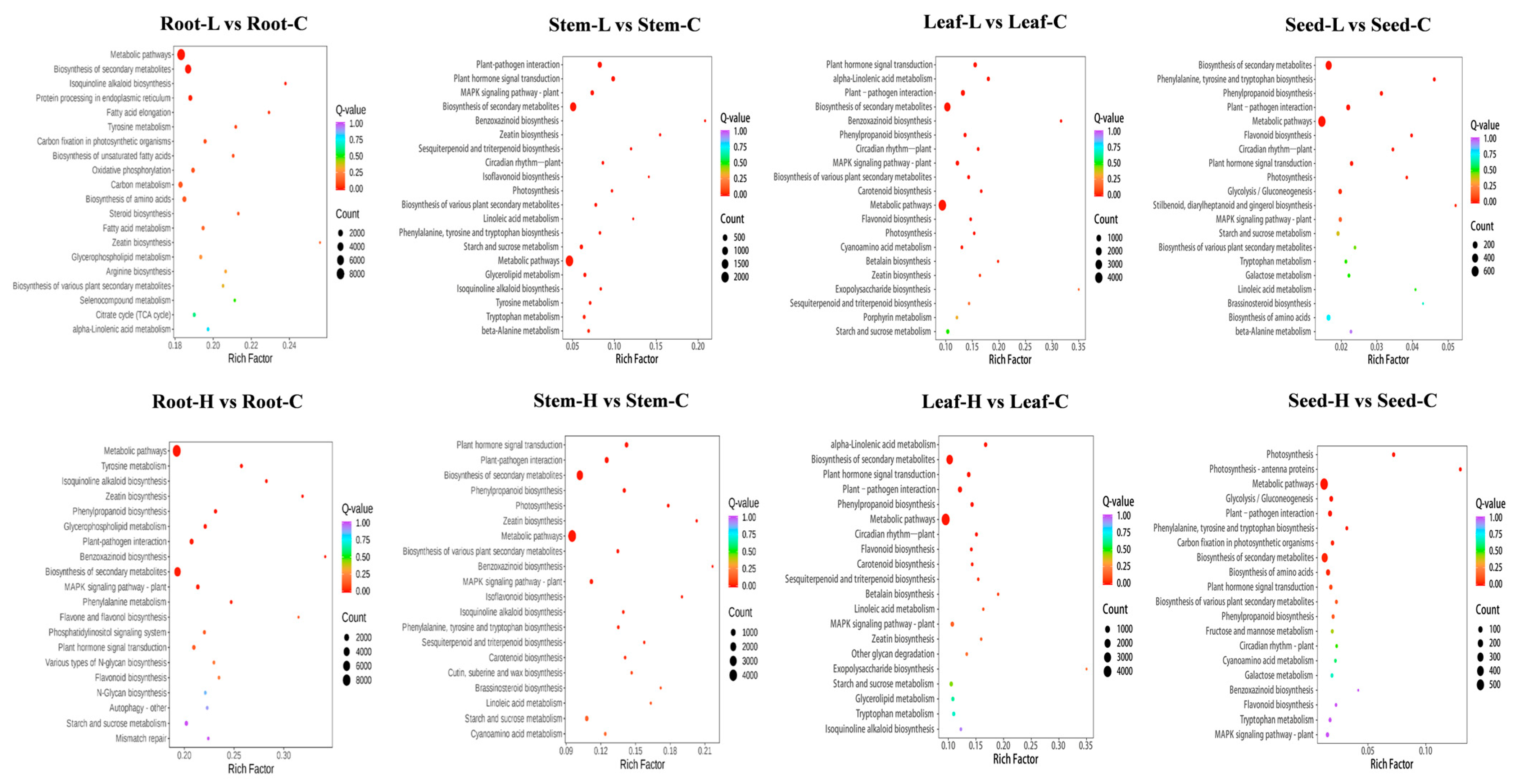
3.7. Differential Gene Expression KEGG Analysis
3.8. DEGs Related to the Biological Accumulation of Coixol Under SA Treatment
3.9. qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Corke, H.; Huang, Y.; Li, J.S. Coix: Overview. In Encyclopedia of Food Grains, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 2016; pp. 184–189. [Google Scholar]
- Guo, C.; Wang, Y.; Yang, A.; He, J.; Xiao, C.; Lv, S.; Han, F.; Yuan, Y.; Yuan, Y.; Dong, X.; et al. The coix genome provides insights into panicoideae evolution and papery hull domestication. Mol. Plant 2020, 13, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, L.; Ball, T.; Yu, L.; Li, Y.; Xing, F. Revealing a 5000-y-old beer recipe in China. Proc. Natl. Acad. Sci. USA 2016, 113, 6444–6448. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.H.; Yang, C.; Meng, Q.; Liu, F.; Shen, G.; Zhou, M.; Ao, M. Genetic diversity and structure of Coix lacryma-jobi L. from its world secondary diversity center, Southwest China. Int. J. Genom. 2019, 2019, 9815697. [Google Scholar]
- Yu, J.; Wang, X.; Yao, X.; Wu, X. Safety Evaluation of heavy metal contamination and pesticide residues in coix seeds in Guizhou province, China. Foods 2022, 11, 2286. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Jiang, L.P. Starch grain analysis reveals ancient diet at Kuahuqiao site, Zhejiang Province. Chin. Sci. Bull. 2010, 55, 1150–1156. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, X.; Fu, Y.; Wang, H.; Yu, C.; Chu, J.; Jiang, B.; Zhu, J. Genome-wide identification and expression analysis of VQ gene family under abiotic stress in Coix lacryma-jobi L. BMC Plant Biol. 2023, 23, 327. [Google Scholar] [CrossRef]
- Zhang, P.; Meng, X.; Tang, X.; Ren, L.; Liang, J. The effect of a coix seed oil injection on cancer pain relief. Support. Care Cancer 2018, 27, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Coix: Chemical composition and health effects. Trends Food Sci. Technol. 2017, 61, 160–175. [Google Scholar] [CrossRef]
- Tian, Y.; Zheng, S.; He, L.; Li, C.; Qiao, S.; Tao, H.; Wang, X.; Zeng, X. The effects of Trametes versicolor fermented Rosa roxburghii tratt and coix seed quild on the nutrition, sensory characteristics and physical and chemical parameters of yogurt. Food Chem. X 2023, 20, 100969. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Peng, L.; Yin, F.; Fang, J.; Cai, L.; Zhang, C.; Xiang, Z.; Zhao, Y.; Zhang, S.; Sheng, H.; et al. Research on Coix seed as a food and medicinal resource, it’s chemical components and their pharmacological activities: A review. J. Ethnopharmacol. 2024, 319, 117309. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Xu, M.; Zhang, H.; He, D.; Kong, Y.; Chen, L.; Song, H. Transcriptome and proteome analyses of the molecular mechanisms associated with coix seed nutritional quality in the process of breeding. Food Chem. 2019, 272, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, X.; Rong, Y.Z.; Wu, J.H.; Yang, Y.J.; Wang, Z.W. Rapid determination of fat, protein and amino acid content in coix seed using near-infrared spectroscopy technique. Food Anal. Methods 2014, 8, 334–342. [Google Scholar] [CrossRef]
- Chang, C.C.; Huang, L.H.; Chiang, W.; Hsia, S.M. Hexane fraction of adlay (Coix lachryma-jobi L.) testa ethanolic extract inhibits human uterine sarcoma cancer cells growth and chemosensitizes human uterine sarcoma cells to doxorubicin. Phytomedicine 2018, 47, 69–80. [Google Scholar] [CrossRef]
- Xia, T.; Liu, C.S.; Hu, Y.N.; Luo, Z.Y.; Chen, F.L.; Yuan, L.X.; Tan, X.M. Coix seed polysaccharides alleviate type 2 diabetes mellitus via gut microbiota-derived short-chain fatty acids activation of IGF1/PI3K/AKT signaling. Food Res. Int. 2021, 150, 110717. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Zhang, Y.; Zhang, S.; Zhao, H.; Wang, Y.; Kan, D.; Zhang, Y.; Guo, L.; Lv, J.; Hao, Q.; et al. Coix lacryma-jobi seed oil reduces fat accumulation in nonalcoholic fatty liver disease by inhibiting the activation of the p-AMPK/SePP1/apoER2 pathway. J. Oleo Sci. 2021, 70, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Choi, C.W.; Hong, S.H.; Park, S.K.; Oh, J.S.; Lee, D.; Hong, S.S. Coixlachryside B: A new benzoxazinoid glycoside from the roots of Coix lachryma-jobi var. ma-yuen (Gramineae). J. Asian Nat. Prod. Res. 2019, 21, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Kim, B.; Choi, B.S.; Lee, H.O.; Kim, N.H.; Lee, S.J.; Kim, H.S.; Shin, M.J.; Kim, H.W.; Nam, K.; et al. Genome assembly and annotation of soft-shelled adlay (Coix lacryma-jobi Variety ma-yuen), a cereal and medicinal crop in the poaceae family. Front. Plant Sci. 2020, 11, 630. [Google Scholar] [CrossRef]
- Lee, H.J.; Ryu, J.; Park, S.H.; Seo, E.K.; Han, A.R.; Lee, S.K.; Kim, Y.S.; Hong, J.H.; Seok, J.H.; Lee, C.J. Suppressive effects of coixol, glyceryl trilinoleate and natural products derived from Coix Lachryma-Jobi var. ma-yuen on gene expression, production and secretion of airway MUC5AC mucin. Arch. Pharmacal Res. 2015, 38, 620–627. [Google Scholar] [CrossRef]
- Cui, E.; Qian, S.; Li, J.; Jiang, X.; Wang, H.; Du, S.; Du, L. Discovery of coixol derivatives as potent anti-inflammatory agents. J. Nat. Prod. 2023, 86, 1950–1959. [Google Scholar] [CrossRef]
- Lin, Y.; Tsai, C.E. A study of adlay on lowering serum and liver lipids in hamsters. J. Food Lipids 2008, 15, 176–189. [Google Scholar] [CrossRef]
- Xi, X.J.; Zhu, Y.G.; Tong, Y.P.; Yang, X.L.; Tang, N.N.; Ma, S.M.; Li, S.; Cheng, Z. Assessment of the genetic diversity of different coix (Coix lacryma-jobi L.) accessions and the active composition and anticancer effect of its seed oil. PLoS ONE 2016, 11, e0153269. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chen, L.; Ali, B.; Yang, N.; Chen, Y.; Wu, F.; Jin, Z.; Xu, X. Impact of germination on nutritional and physicochemical properties of adlay seed (Coix lachryma-jobi L.). Food Chem. 2017, 229, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Deepshikha, P.; Navneet, K.; Jeena, G.; Paranjeet, K.; Gopal, L.K. Design, synthesis, and biological evaluation of coixol-based derivatives as potential antidiabetic agents. J. Mol. Struct. 2023, 1277, 134861. [Google Scholar]
- Lu, C.; Wu, S.; Ke, L.; Liu, F.; Shang, W.; Deng, X.; Huang, Y.; Zhang, Q.; Cui, X.; Mentis, A.A.; et al. Kanglaite (Coix seed extract) as adjunctive therapy in cancer: Evidence mapping overview based on systematic reviews with meta-analyses. Front. Pharmacol. 2022, 13, 901875. [Google Scholar] [CrossRef] [PubMed]
- Bharti, A.; Garg, N. SA and AM symbiosis modulate antioxidant defense mechanisms and asada pathway in chickpea genotypes under salt stress. Ecotoxicol. Environ. Saf. 2019, 178, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Mao, J.; Wu, T.; Xiong, T.; Huang, Q.; Wu, H.; Hu, G. Transcriptomic analysis of SA promoting seed germination of melon under salt stress. Horticulturae 2023, 9, 375. [Google Scholar] [CrossRef]
- Han, J.; Zhang, X.; Zhang, D.; Liu, X.; Wang, C.; Liu, X. Expression analysis of OsbZIP transcription factors in resistance response by the rice blast resistance gene Pi36-mediated. Afr. J. Biotechnol. 2013, 12, 5294–5302. [Google Scholar]
- Mostofa, M.G.; Rahman, M.M.; Siddiqui, M.N.; Fujita, M.; Tran, L.P. Salicylic acid antagonizes selenium phytotoxicity in rice: Selenium homeostasis, oxidative stress metabolism and methylglyoxal detoxification. J. Hazard. Mater. 2020, 394, 1225–1238. [Google Scholar] [CrossRef]
- Chavoushi, M.; Najafi, F.; Salimi, A.; Angaji, S.A. Effect of salicylic acid and sodium nitroprusside on growth parameters, photosynthetic pigments and secondary metabolites of safflower under drought stress. Sci. Hortic. 2020, 259, 108823. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A. Effects of exogenously applied salicylic acid and putrescine alone and in combination with rhizobacteria on the phytoremediation of heavy metals and chickpea growth in sandy soil. Int. J. Phytoremediation 2018, 20, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Qin, S.; Zhang, T.; Wang, Z.; Li, H.; Li, Y.; Nie, Y. Comparative phosphoproteomic analysis of blast resistant and susceptible rice cultivars in response to SA. BMC Plant Biol. 2019, 19, 454. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Shu, P.; Zhang, Z.; Wu, Y.; Chen, Y.; Li, K.; Deng, H.; Zhang, J.; Zhang, X.; Wang, J.; Liu, Z.; et al. A comprehensive metabolic map reveals major quality regulations in red-flesh kiwifruit (Actinidia chinensis). New Phytol. 2023, 238, 2064–2079. [Google Scholar] [CrossRef]
- Zhu, L.; Huang, T.; Liu, J.; Xu, X.; Zhang, Z. Transcriptome analysis reveals the potential mechanism of methyl jasmonate alleviated ripening disorder in mango fruit at low temperature. Food Chem. 2024, 463, 141093. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, G.; Chen, N.; Zhang, J.; He, C. Metabolomic and transcriptomic analyses provide insights into the flavonoid biosynthesis in sea buckthorn (Hippophae rhamnoides L.). LWT 2023, 187, 115276. [Google Scholar] [CrossRef]
- Kiss, T.; Karácsony, Z.; Gomba-Tóth, A.; Szabadi, K.L.; Spitzmüller, Z.; Hegyi-Kaló, J.; Cels, T.; Otto, M.; Golen, R.; Hegyi, Á.I. A modified CTAB method for the extraction of high-quality RNA from mono-and dicotyledonous plants rich in secondary metabolites. Plant Methods 2024, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yang, J.; Li, X.; Zhang, Y. Salicylic acid: Biosynthesis and signaling. Annu. Rev. Plant Biol. 2021, 72, 761–791. [Google Scholar] [CrossRef]
- Janda, T.; Szalai, G.; Pál, M. SA signalling in plants. Int. J. Mol. Sci. 2020, 21, 2655. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Ugurlar, F.; Ashraf, M.; Ahmad, P. Salicylic acid interacts with other plant growth regulators and signal molecules in response to stressful environments in plants. Plant Physiol. Biochem. 2023, 196, 431–443. [Google Scholar] [CrossRef]
- Bagautdinova, Z.Z.; Omelyanchuk, N.; Tyapkin, A.V.; Kovrizhnykh, V.V.; Lavrekha, V.V.; Zemlyanskaya, E.V. SA in root growth and development. Int. J. Mol. Sci. 2022, 23, 2228. [Google Scholar] [CrossRef]
- Frey, M.; Schullehner, K.; Dick, R.; Fiesselmann, A.; Gierl, A. Benzoxazinoid biosynthesis, a model for evolution of secondary metabolic pathways in plants. Phytochemistry 2009, 70, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, H.M. Hydroxamic acids derived from 2-hydroxy-2H-1,4-benzoxazin-3(4H)-one: Key defense chemicals of cereals. J. Agric. Food Chem. 2009, 57, 1677–1696. [Google Scholar] [CrossRef]
- Makowska, B.; Bakera, B.; Rakoczy-Trojanowska, M. The genetic background of benzoxazinoid biosynthesis in cereals. Acta Physiol. Plant. 2015, 37, 176. [Google Scholar] [CrossRef]
- Kudjordjie, E.N.; Sapkota, R.; Steffensen, S.K.; Fomsgaard, I.S.; Nicolaisen, M. Maize synthesized benzoxazinoids affect the host associated microbiome. Microbiome 2019, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Richter, A.; Jander, G. Beyond defense: Multiple functions of benzoxazinoids in maize metabolism. Plant Cell Physiol. 2018, 59, 1528–1537. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, Q.; Liu, T.; Liu, Z. Coixol suppresses NF-κB, MAPK pathways and NLRP3 inflammasome activation in lipopolysaccharide-induced RAW 264.7 cells. Molecules 2020, 25, 894. [Google Scholar] [CrossRef]
- Meihls, L.N.; Handrick, V.; Glauser, G.; Barbier, H.; Kaur, H.; Haribal, M.M.; Lipka, A.E.; Gershenzon, J.; Buckler, E.S.; Erb, M.; et al. Natural variation in maize aphid resistance is associated with 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one glucoside methyltransferase activity. Plant Cell 2013, 25, 2341–2355. [Google Scholar] [CrossRef] [PubMed]


| Type | Number (n) | Mean Length (bp) | N50 (bp) | N90 (bp) | Total Bases (n) |
|---|---|---|---|---|---|
| Transcript | 740,306 | 984 | 1778 | 372 | 728,126,344 |
| Unigene | 378,420 | 1406 | 2078 | 619 | 532,088,427 |
| Groups | Metabolic Pathway | koID | Enzyme | Number of DEGs |
|---|---|---|---|---|
| Root-L vs. Root-C | Benzoxazinoid biosynthesis | ko00402 | Indole-3-glycerol-phosphate lyase | 5 |
| Indole-2-monooxygenase | 5 | |||
| Indolin-2-one monooxygenase | 4 | |||
| 2,4-dihydroxy-1,4-benzoxazin-3-one-glucoside dioxygenase | 7 | |||
| 2,4,7-trihydroxy-1,4-benzoxazin-3-one-glucoside 7-O-methyltransferase | 1 | |||
| UDP-glucosyltransferase | 8 | |||
| Root-H vs. Root-C | Benzoxazinoid biosynthesis | ko00402 | Indole-3-glycerol-phosphate lyase | 6 |
| Indole-2-monooxygenase | 8 | |||
| Indolin-2-one monooxygenase | 8 | |||
| 2,4-dihydroxy-1,4-benzoxazin-3-one-glucoside dioxygenase | 6 | |||
| 2,4,7-trihydroxy-1,4-benzoxazin-3-one-glucoside 7-O-methyltransferase | 2 | |||
| UDP-glucosyltransferase | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Ye, H.; Luo, X.; Li, Z.; Zheng, C.; Sun, D. Transcriptomic Analysis Reveals the Biosynthesis Mechanism of Coixol Under Salicylic Acid Treatment. Horticulturae 2025, 11, 234. https://doi.org/10.3390/horticulturae11030234
Wang Y, Ye H, Luo X, Li Z, Zheng C, Sun D. Transcriptomic Analysis Reveals the Biosynthesis Mechanism of Coixol Under Salicylic Acid Treatment. Horticulturae. 2025; 11(3):234. https://doi.org/10.3390/horticulturae11030234
Chicago/Turabian StyleWang, Yao, Hanli Ye, Xuqin Luo, Ziwei Li, Chuanqi Zheng, and Dali Sun. 2025. "Transcriptomic Analysis Reveals the Biosynthesis Mechanism of Coixol Under Salicylic Acid Treatment" Horticulturae 11, no. 3: 234. https://doi.org/10.3390/horticulturae11030234
APA StyleWang, Y., Ye, H., Luo, X., Li, Z., Zheng, C., & Sun, D. (2025). Transcriptomic Analysis Reveals the Biosynthesis Mechanism of Coixol Under Salicylic Acid Treatment. Horticulturae, 11(3), 234. https://doi.org/10.3390/horticulturae11030234






