Root Systems of Five Clonal Avocado Genotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Establishment
2.3. Root System Monitoring
2.4. Image Analysis and Measurement Program
2.5. Replications, Experimental Design and Statistical Analysis
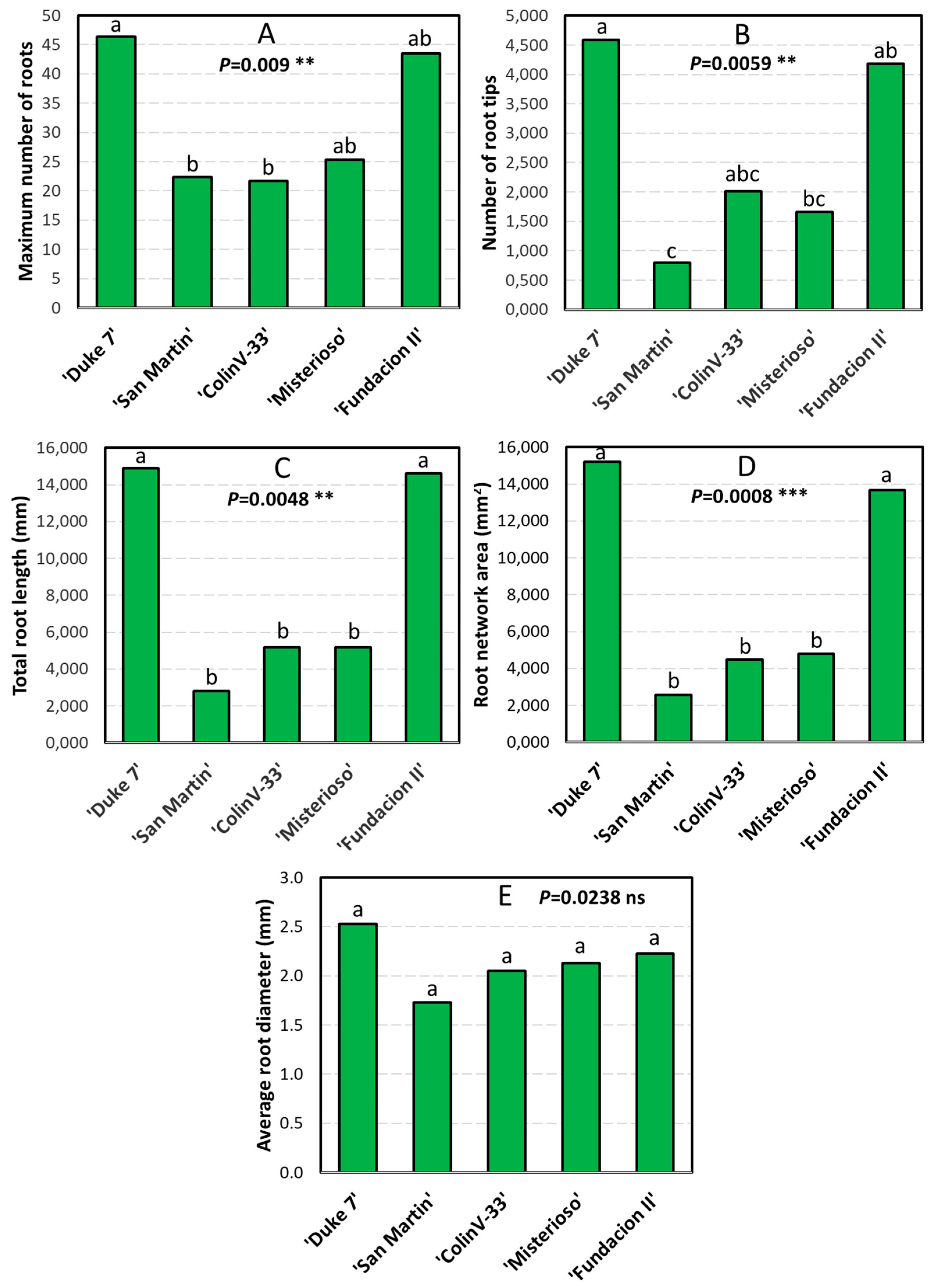
3. Results
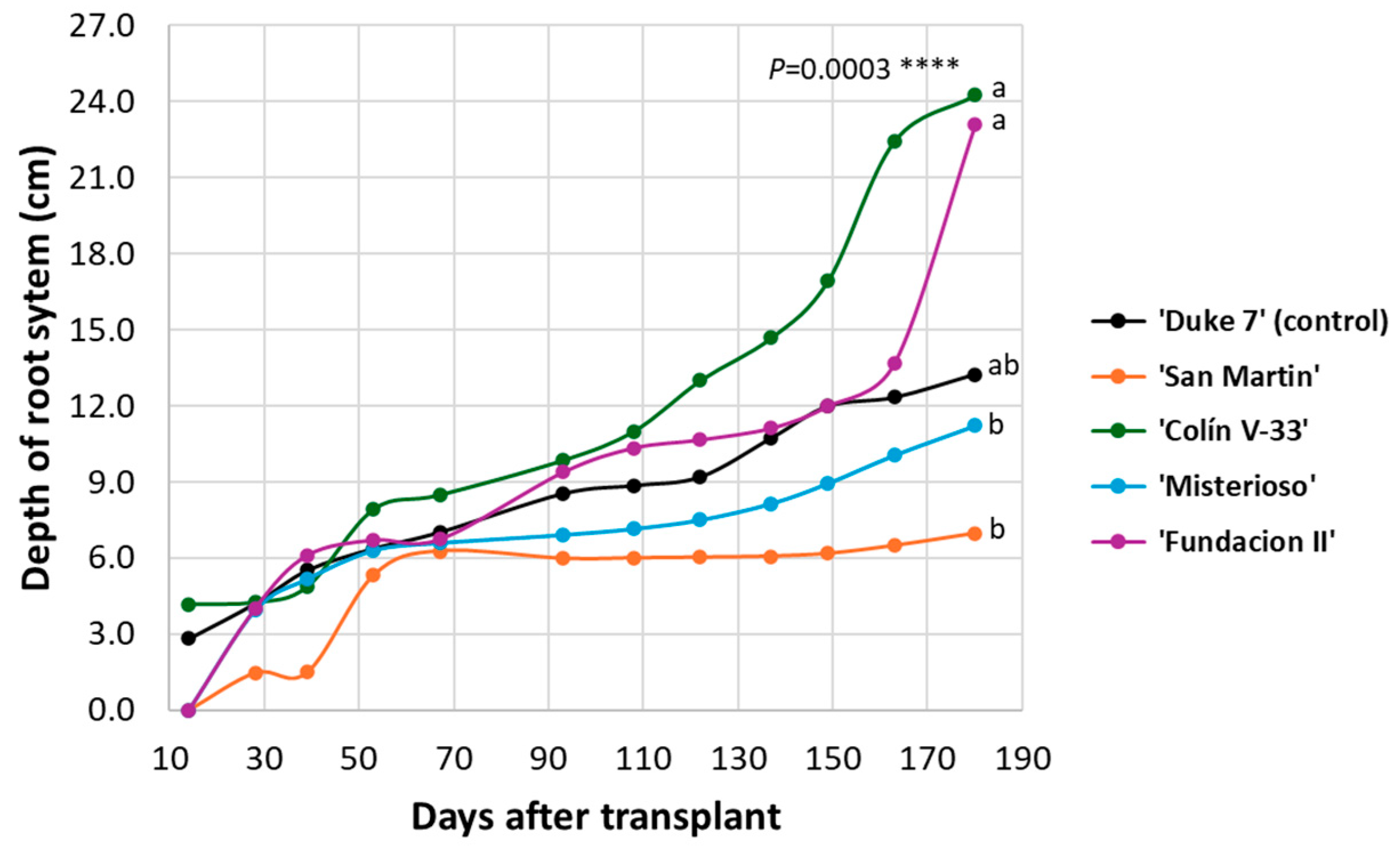
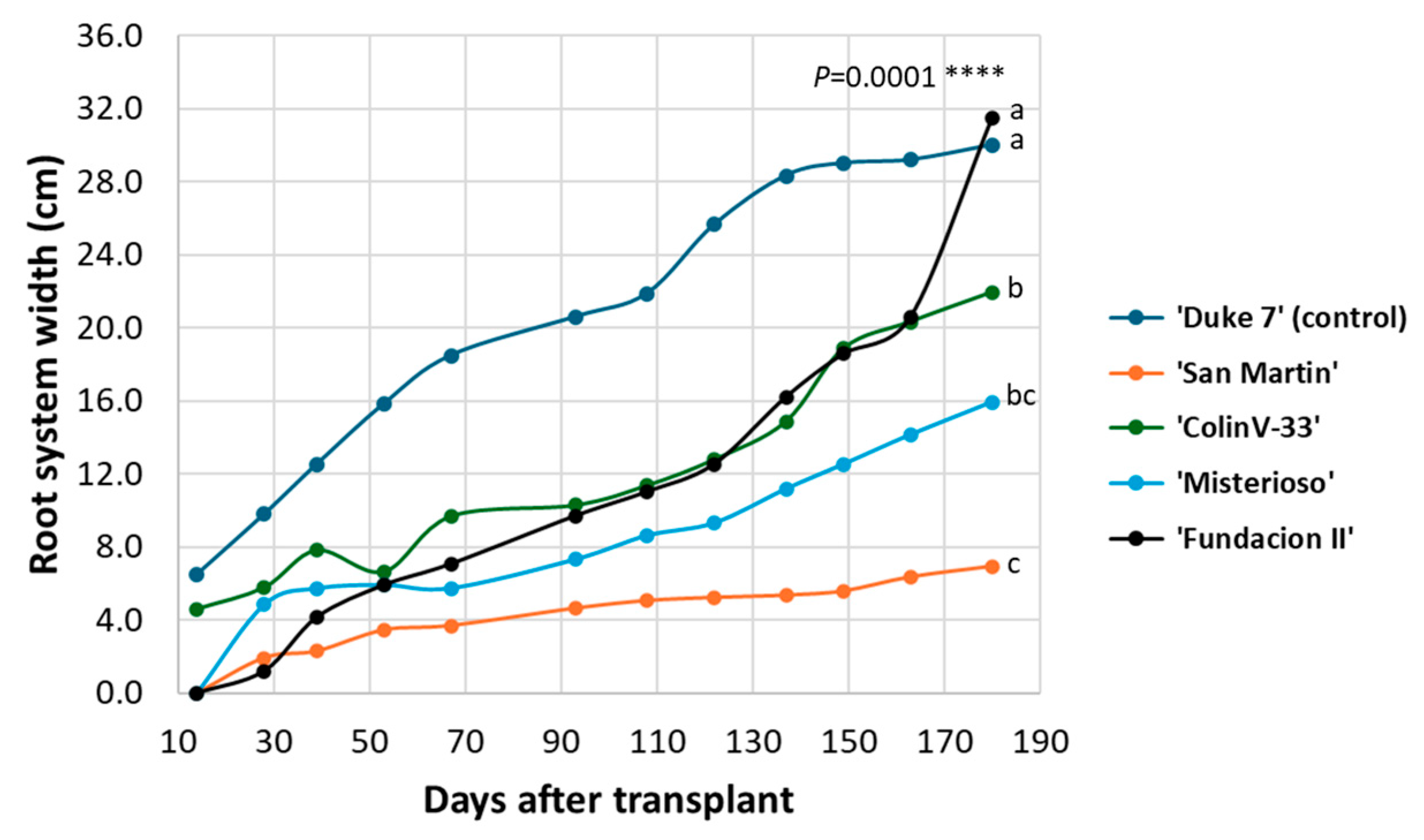
3.1. Follow-up of Complete Root System in Rhizotron
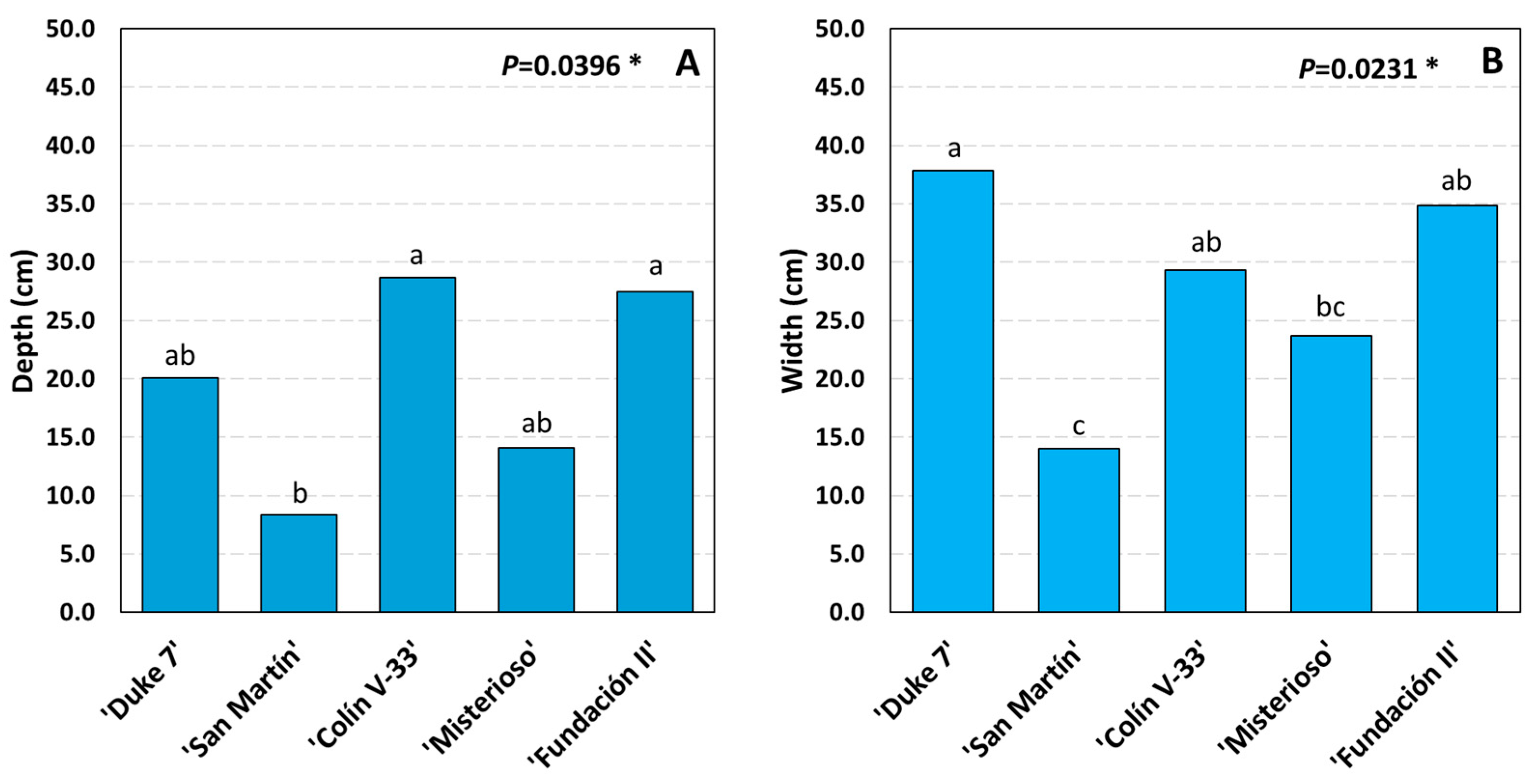
3.2. Complete Root System Outside the Rhizotron
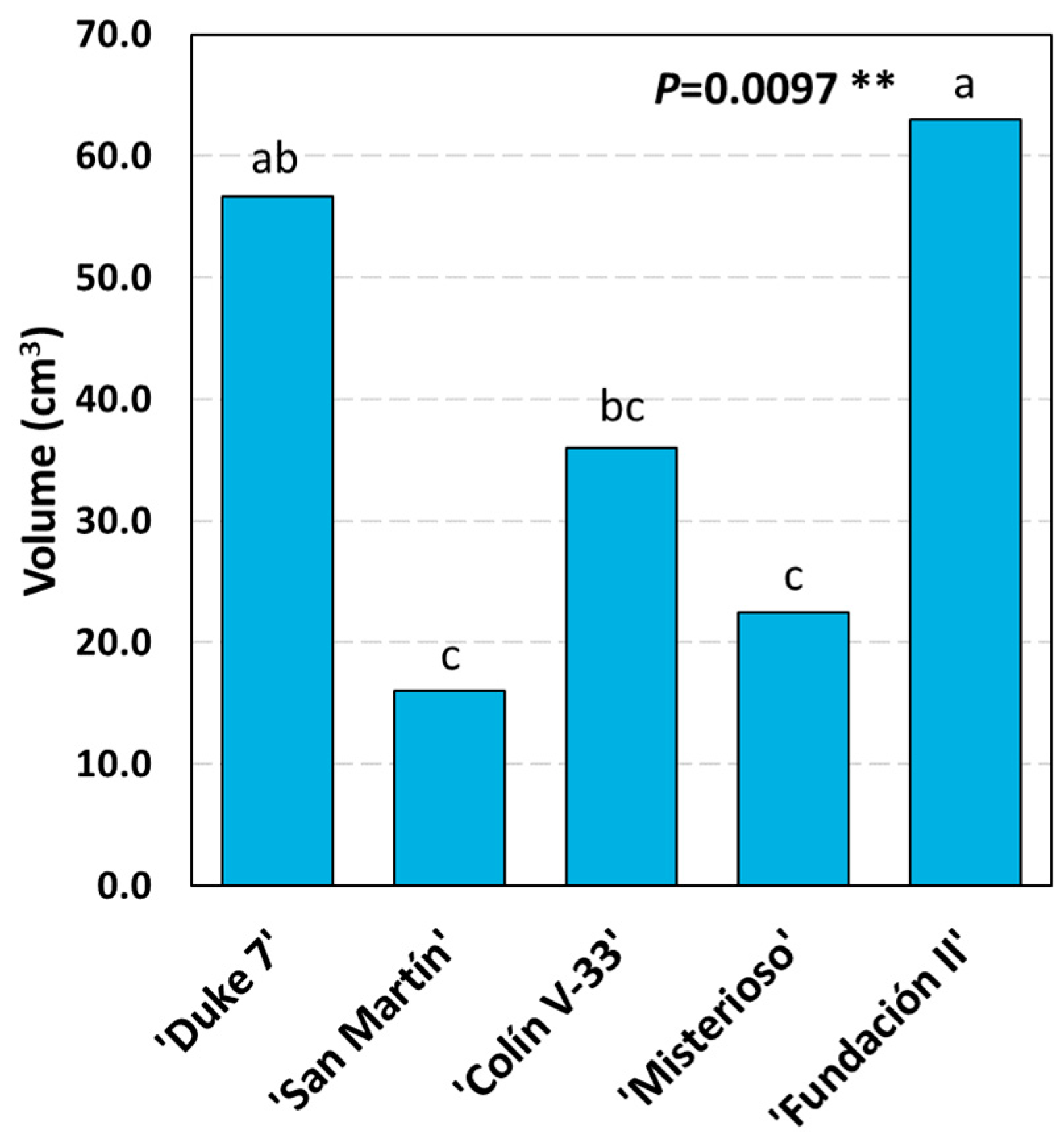
3.3. Main Variables of Root Growth Through Image Analysis
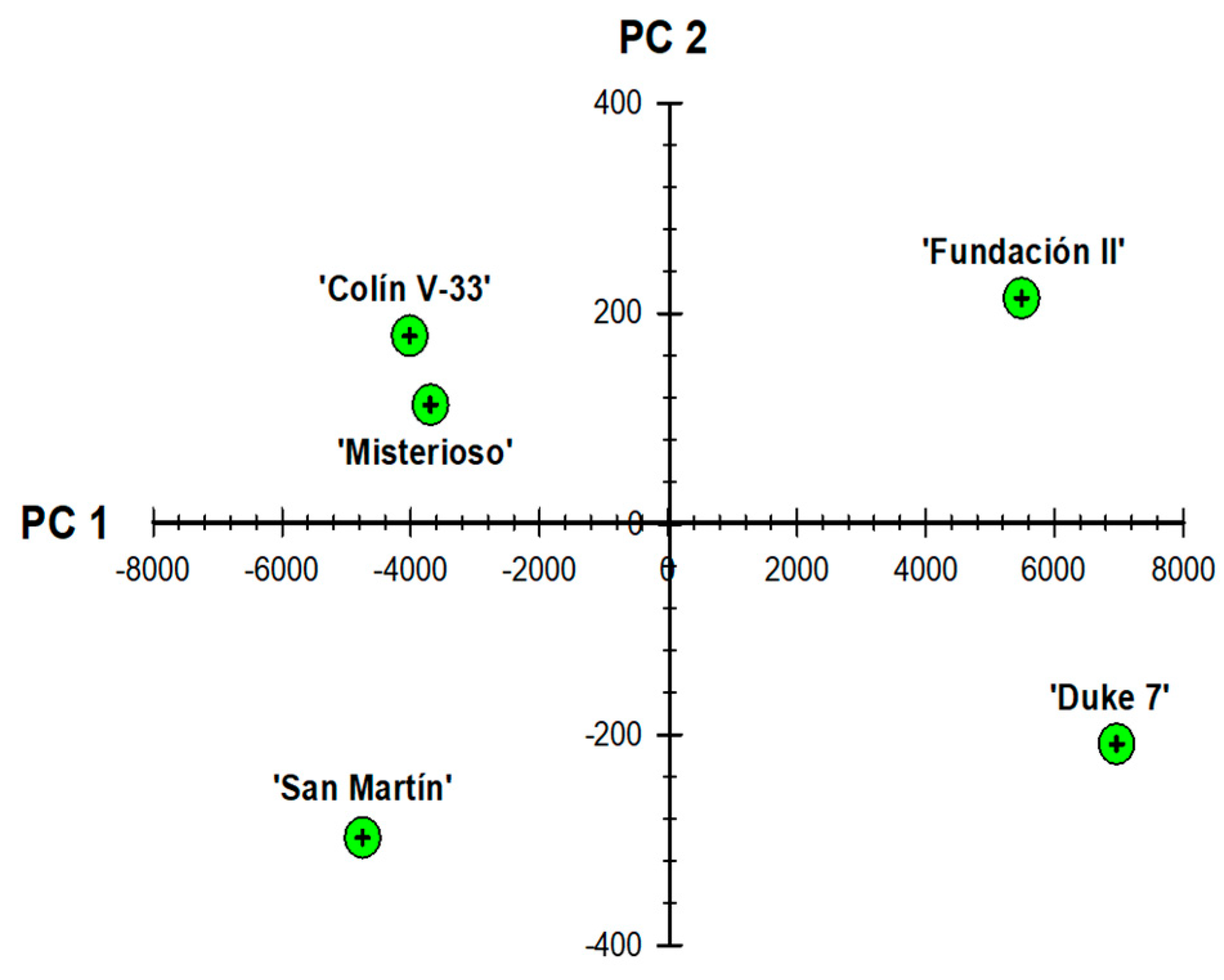
3.4. Depuration and Principal Component Analysis (PCA) of Root Growth Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darrouy Palacios, N.; Castro Valdebenito, M.; Cautín Morales, R.; Kort Silva, L.; Bozzolo Artaza, R. Efecto de la posición de la yema y de la poda en plantas de aguacate destinadas a la clonación. Rev. Fitotec. Mex. 2010, 33, 249–256. [Google Scholar] [CrossRef]
- González-Calderón, V.M.; Barrientos-Priego, A.F.; Núñez-Colín, C.A.; Ramírez-Ramírez, S.P.; Arpaia, M.L. Anatomía de la lámina de hoja en ocho cultivares de aguacate. Rev. Mexicana Cienc. Agríc. 2011, 2, 733–744. [Google Scholar] [CrossRef][Green Version]
- Meza-Castillo, E.; Barrientos-Priego, A.F.; Rodríguez-Pérez, J.E.; Reyes-Alemán, J.C.; Borys, M.W.; Espíndola-Barquera, M.C. Caracterización morfológica e histológica de ápices de raíz de portainjertos de aguacate y su resistencia a factores adversos en el suelo. Bioagro 2018, 30, 107–116. [Google Scholar]
- Ibarra-López, A.; Ojeda-Zacarías, M.D.; García-Zambrano, E.; Gutiérrez-Diez, A. Inducción in vitro de brotes de dos cultivares de aguacate raza Mexicana Persea americana var. drymifolia Schltdl. & Cham. Rev. Mexicana Cienc. Agríc. 2016, 7, 337–347. [Google Scholar]
- Freire Alberti, M.; Amaral Brogio, B.D.; Rodrigues da Silva, S.; Cantuarias-Avilés, T.; Fassio, C. Avances en la propagación de aguacate. Rev. Bras. Frutic. 2018, 40, e-782. [Google Scholar]
- Fassio, C.; Cautin, R.; Pérez-Donoso, A.; Bonomelli, C.; Castro, M. Propagation techniques and grafting modify the morphological traits of roots and biomass allocation in avocado trees. Horttechnology 2016, 26, 63–69. [Google Scholar] [CrossRef]
- Lesmes-Vesga, R.A.; Chaparro, J.X.; Sarkhosh, A.; Ritenour, M.A.; Cano, L.M.; Rossi, L. Effect of propagation systems and indole-3-butyric acid potassium salt (K-IBA) concentrations on the propagation of peach rootstocks by stem cuttings. Plants 2021, 10, 1151. [Google Scholar] [CrossRef]
- Salazar-García, S.; Medina-Torres, R.; Ibarra-Estrada, M.E.; González-Valdivia, J. Influencia de portainjertos clonales sobre la concentración foliar de nutrimentos en aguacate ‘Hass’ cultivado sin riego. Rev. Chapingo Ser. Hort. 2016, 22, 161–175. [Google Scholar]
- Brokaw, W.H. Avocado clonal propagation. Proc. Int. Plant Prop. Soc. 1987, 37, 97–103. [Google Scholar]
- Barrientos-Priego, A.F.; Muñoz-Pérez, R.; Reyes-Alemán, J.C.; Borys, M.W.; Martínez-Damián, M.T. Taxonomía, cultivares y portainjertos. In El Aguacate y su Manejo Integrado, 2nd ed.; Téliz, D., Mora, A.C., Eds.; Mundi Prensa: Distrito Federal, México, 2007; pp. 31–62. [Google Scholar]
- Silber, A.; Israeli, Y.; Levi, M.; Keinan, A.; Shapira, O.; Chudi, G.; Golan, A.; Noy, M.; Levkovitch, I.; Assouline, S. Response of ‘Hass’ avocado trees to irrigation management and root constraint. Agric. Water Manag. 2012, 104, 95–103. [Google Scholar] [CrossRef]
- Beyer, C.P.; Cuneo, I.F.; Alvaro, J.E.; Pedreschi, R. Evaluation of aerial and root plant growth behavior, water and nutrient use efficiency and carbohydrate dynamics for Hass avocado grown in a soilless and protected growing system. Sci. Hortic. 2021, 277, 109830. [Google Scholar] [CrossRef]
- Foster, T.M.; Celton, J.M.; Chagné, D.; Tustin, D.S.; Gardiner, S.E. Two quantitative trait loci, Dw1 and Dw2, are primarily responsible for rootstock-induced dwarfing in apple. Hortc. Res. 2015, 2, 15001. [Google Scholar] [CrossRef] [PubMed]
- Fassio, C.; Cautin, R.; Perez-Donoso, A.G.; Castro, M. Comparative branching order and root anatomy of clonal and seed grown avocado trees (Persea americana Mill.). Int. J. Agric. Nat. Resour. 2020, 41, 134–144. [Google Scholar] [CrossRef]
- Alzugaray, P.; Haase, D.; Rose, R. Efecto del volumen radicular y la tasa de fertilización sobre el comportamiento en terreno de plantas de pino Oregón (Pseudotsuga menziesii (Mirb.) Franco producidas con el método 1+1. Bosques 2004, 25, 17–33. [Google Scholar] [CrossRef]
- Castro, M.; Fassio, C.; Darrouy, N. Portainjertos de aguacate en Chile. Hortic. Intern. 2008, 62, 42–46. [Google Scholar]
- Cabeza, R.A.; Claassen, N. Sistemas radicales de cultivos: Extensión, distribución y crecimiento. Agro Sur 2017, 45, 31–45. [Google Scholar] [CrossRef]
- Holzgreve, H.; Eick, M.; Stöhr, C. Protease activity in the rhizosphere of tomato plants is independent from nitrogen status. In Root Biology-Growth, Physiology, and Functions; Ohyama, T., Ed.; IntechOpen: London, UK, 2019; pp. 3–16. [Google Scholar]
- Lesmes-Vesga, R.A.; Cano, L.M.; Ritenour, M.A.; Sarkhosh, A.; Chaparro, J.X.; Rossi, L. Rhizoboxes as rapid tools for the study of root systems of Prunus seedlings. Plants 2022, 11, 2081. [Google Scholar] [CrossRef]
- Frolich, E.; Platt, R. Use of the etiolation technique in rooting avocado cuttings. Calif. Avocado Soc. Yearb. 1972, 55, 97–109. [Google Scholar]
- Bràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Intern. 2004, 11, 36–42. [Google Scholar]
- Seethepalli, A.; Dhakal, K.; Griffiths, M.; Guo, H.; Freschet, G.T.; York, L.M. RhizoVision Explorer: Open-source software for root image analysis and measurement standardization. AoB Plants 2021, 13, plab056. [Google Scholar] [CrossRef]
- SAS. SAS® Studio 3.8: Administrator’s Guide; SAS Institute Inc.: Cary, NC, USA, 2023; 58p. [Google Scholar]
- López-Santiago, J.; Nieto-Ángel, R.; Barrientos-Priego, A.F.; Rodríguez-Pérez, E.; Colinas-León, M.T.; Borys, M.W.; González-Andrés, F. Selección de variables morfológicas para la caracterización del tejocote (Crataegus spp.). Rev. Chapingo Ser. Hort. 2008, 14, 97–111. [Google Scholar]
- Núñez-Colín, C.A.; Escobedo-López, D. Uso correcto del análisis clúster en la caracterización de germoplasma vegetal. Agron. Mesoam. 2011, 22, 415–427. [Google Scholar] [CrossRef][Green Version]
- Hammer, Ø.; Harper, D.A. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 4. [Google Scholar]
- Lesmes-Vesga, R.A.; Cano, L.M.; Ritenour, M.A.; Sarkhosh, A.; Chaparro, J.X.; Rossi, L. Variation in the root system architecture of peach×(peach×almond) backcrosses. Plants 2023, 12, 1874. [Google Scholar] [CrossRef] [PubMed]
- Cichi, M.; Gheorghiu, N. Aspects regarding variety/rootstock relationship in some species of fruit species. Ann. Univ. Craiova-Agri. Montanol. Cadastre Ser. 2022, 52, 73–78. [Google Scholar] [CrossRef]
- Carr, M.K.V. The water relations and irrigation requirements of avocado (Persea americana Mill.): A review. Exp. Agric. 2013, 49, 256–278. [Google Scholar] [CrossRef]
- Lynch, J.P. Harnessing root architecture to address global challenges. Plant J. 2021, 109, 415–431. [Google Scholar] [CrossRef]
- Atkinson, D.; Wilson, S.A. The root-soil interface and its significance for fruit tree roots of different ages. In The Soil–Root Interface; Harley, J.L., Russell, R.S., Eds.; Academic Press: London, UK, 1979; pp. 259–271. [Google Scholar]
- Meneses, T.N.; Coelho Filho, M.A.; Santos Filho, H.P.; Santos, L.L.D.A.; Gesteira, A.D.S.; Soares Filho, W.D.S. Rootstocks and planting types on root architecture and vegetative vigor of ‘Pera’ sweet orange trees. Rev. Bras. Engn. Agríc. Ambient. 2020, 24, 685–693. [Google Scholar] [CrossRef]
- He, W.; Luo, C.; Wang, Y.; Wen, X.; Wang, Y.; Li, T.; Chen, G.; Zhao, K.; Li, X.; Fan, C. Response strategies of root system architecture to soil environment: A case study of single-species Cupressus funebris plantations. Front. Plant Sci. 2022, 13, 822223. [Google Scholar] [CrossRef]
- Yang, C.; Fredua-Agyeman, R.; Hwang, S.F.; Gorim, L.Y.; Strelkov, S.E. Genome-wide association studies of root system architecture traits in a broad collection of Brassica genotypes. Front. Plant Sci. 2024, 15, 1389082. [Google Scholar] [CrossRef]
- Petersson, S.V.; Johansson, A.I.; Kowalczyk, M.; Makoveychuk, A.; Wang, J.Y.; Moritz, T.; Grebe, M.; Benfey, P.N.; Sandberg, G.; Ljung, K. An auxin gradient and maximum in the Arabidopsis root apex shown by high-resolution cell-specific analysis of IAA distribution and synthesis. Plant Cell 2009, 21, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Barker, R.; Fernandez Garcia, M.N.; Powers, S.J.; Vaughan, S.; Bennett, M.J.; Phillips, A.L.; Thomas, S.G.; Hedden, P. Mapping sites of gibberellin biosynthesis in the Arabidopsis root tip. New Phytol. 2021, 229, 1521–1534. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, S.; Meyering, B.; Bowman, K.D.; Albrecht, U. Horticultural attributes and root architectures of field-grown ‘Valencia’ trees grafted on different rootstocks propagated by seed, cuttings, and tissue culture. HortScience 2021, 56, 163–172. [Google Scholar] [CrossRef]
- Blois, L.; de Miguel, M.; Bert, P.F.; Ollat, N.; Rubio, B.; Voss-Fels, K.P.; Schmid, J.; Marguerit, E. Dissecting the genetic architecture of root related traits in a grafted wild Vitis berlandieri population for grapevine rootstock breeding. Theor. Appl. Genet. 2023, 136, 223. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.; Wunsch, A.; Sabety, J.; van Zoeren, J.; Basedow, M.; Miranda Sazo, M.; Fuchs, M.; Khan, A. The comparative root system architecture of declining and non-declining trees in two apple orchards in New York. Plants 2023, 12, 2644. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Frégeau-Reid, J. Breeding line selection based on multiple traits. Crop Sci. 2008, 48, 417–423. [Google Scholar] [CrossRef]
- Dutta, C.; Sarma, R.N. Role of root traits and root phenotyping in drought tolerance. Int. J. Environ. Clim. 2022, 12, 2300–2309. [Google Scholar] [CrossRef]
- Katuuramu, D.N.; Wechter, W.P.; Washington, M.L.; Horry, M.; Cutulle, M.A.; Jarret, R.L.; Levi, A. Phenotypic diversity for root traits and identification of superior germplasm for root breeding in watermelon. HortScience 2020, 55, 1272–1279. [Google Scholar] [CrossRef]



| Genotype | Origin and Race | Characteristic |
|---|---|---|
| ‘Duke 7’ | California, USA. ‘Duke’ seedling, Mexican race. | Normal growth with medium-length internodes, tall size tree. |
| ‘San Martín’ | Tezuitlán, Puebla. Mexico. Mexican race seedling. | Dwarf growth with rather short internodes, extremely small tree. |
| ‘Colín V-33’ | Ixtapan de la Sal, Estado de México, Mexico. ‘Fuerte’ seedling, hybrid Mexican-Guatemalan race. | Dwarf growth, medium-length internodes, lateral extensive branch growth, loss of apical dominance, small tree. |
| ‘Misterioso’ | Coatepec Harinas, Estado de México, Mexico. ‘Frazer’ seedling, hybrid Mexican-Guatemalan race. | Dwarf growth with rather short internodes, extremely small tree. |
| ‘Fundación II’ | Coatepec Harinas, Estado de México, Mexico. ‘Hass’ seedling, hybrid Mexican-Guatemalan race. | Dwarf growth, medium-length internodes, lateral extensive branch growth, loss of apical dominance, small tree. |
| Abbreviation | Variable | |
|---|---|---|
| 1 | MinNR | Median Number of Roots |
| 2 | MaxNR | Maximum Number of Roots |
| 3 | NRT | Number of Root Tips |
| 4 | TRL | Total Root Length (mm) |
| 5 | Depth | Depth (mm) |
| 6 | MW | Maximum Width (mm) |
| 7 | WDR | Width-to-Depth Ratio |
| 8 | NA | Network Area (mm2) |
| 9 | CA | Convex Area (mm2) |
| 10 | S | Solidity |
| 11 | LRA | Lower Root Area (mm2) |
| 12 | AD | Average Diameter (mm) |
| 13 | MedD | Median Diameter (mm) |
| 14 | MaxD | Maximum Diameter (mm) |
| 15 | P | Perimeter (mm) |
| 16 | Vol | Volume (mm3) |
| 17 | SA | Surface Area (mm2) |
| 18 | H | Holes |
| 19 | AH | Average Hole Size (mm2) |
| 20 | ARO | Average Root Orientation |
| 21 | SAF | Shallow Angle Frequency |
| 22 | MAF | Medium Angle Frequency |
| 23 | SteepAF | Steep Angle Frequency |
| 24–26 | RLDR 1–3 layer | Root Length Diameter (mm) |
| 27–29 | PADR 1–3 layer | Projected Area Diameter (mm2) |
| 30–32 | SADR 1–3 layer | Surface Area Diameter (mm2) |
| 33–35 | VDR 1–3 layer | Volume Diameter (mm3) |
| Principal Component | Eigen Value | Variance (%) |
|---|---|---|
| 1 | 3.27 × 107 | 99.821 |
| 2 | 55,768.3 | 0.17004 |
| 3 | 1877.25 | 0.0057237 |
| 4 | 930.56 | 0.0028373 |
| Variable | PC 1 | PC 2 |
|---|---|---|
| Maximum number of roots | 0.0021239 | −0.0002322 |
| Root depth (mm) | 0.0046799 | 0.20023 |
| Maximum broadness of root system (mm) | 0.013135 | 0.039346 |
| Area of root network (mm2) | 0.96847 | −0.24493 |
| Solidity of root system | 1.58 × 10−6 | −0.0003678 |
| Average diameter of roots (mm) | −3.64 × 10−7 | −0.0019959 |
| Projected diameter of roots in the second layer (mm) | 0.24873 | 0.94782 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Florez, N.E.; Barrientos-Priego, A.F.; Campos-Rojas, E.; Colinas y León, M.T.B.; Sánchez García, P. Root Systems of Five Clonal Avocado Genotypes. Horticulturae 2025, 11, 232. https://doi.org/10.3390/horticulturae11030232
Gonzalez-Florez NE, Barrientos-Priego AF, Campos-Rojas E, Colinas y León MTB, Sánchez García P. Root Systems of Five Clonal Avocado Genotypes. Horticulturae. 2025; 11(3):232. https://doi.org/10.3390/horticulturae11030232
Chicago/Turabian StyleGonzalez-Florez, Nancy Elena, Alejandro Facundo Barrientos-Priego, Eduardo Campos-Rojas, María Teresa Beryl Colinas y León, and Prometeo Sánchez García. 2025. "Root Systems of Five Clonal Avocado Genotypes" Horticulturae 11, no. 3: 232. https://doi.org/10.3390/horticulturae11030232
APA StyleGonzalez-Florez, N. E., Barrientos-Priego, A. F., Campos-Rojas, E., Colinas y León, M. T. B., & Sánchez García, P. (2025). Root Systems of Five Clonal Avocado Genotypes. Horticulturae, 11(3), 232. https://doi.org/10.3390/horticulturae11030232






