Effect of Exogenous Melatonin Application on Maintaining Physicochemical Properties, Phytochemicals, and Enzymatic Activities of Mango Fruits During Cold Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials, Chemicals, and Reagents
2.2. Melatonin Treatment and Storage
2.3. Quality Analysis
2.3.1. Determination of Weight Loss
2.3.2. Determination of Decay Rate
2.3.3. Determination of Color Characteristics
2.3.4. Determination of Respiration Rate
2.3.5. Determination of Ethylene Production
2.3.6. Determination of Firmness
2.3.7. Determination of Malondialdehyde (MDA) Content
2.3.8. Determination of Starch Content
2.3.9. Determination of Total Soluble Solids
2.3.10. Determination of Titratable Acidity
2.3.11. Determination of Phytochemicals and Antioxidant Activities
Determination of Ascorbic Acid Content (AsA)
Determination of Phenolic Contents and Antioxidant Activities
2.3.12. Determination of Browning-Related Enzymes
Determination of Polyphenol Oxidase Activity
Determination of Peroxidase Activity
2.3.13. Enzyme Activities
Determination of Cell Wall-Degrading Enzymes
- Determination of polygalacturonase activity
- 2.
- Determination of pectin methylesterase activity
- 3.
- Determination of lipoxygenase activity
2.3.14. Determination of Antioxidant Enzyme Activities
Determination of Superoxide Dismutase Activity
Determination of Ascorbate Peroxidase Activity
2.4. Statistical Analysis
3. Results and Discussion
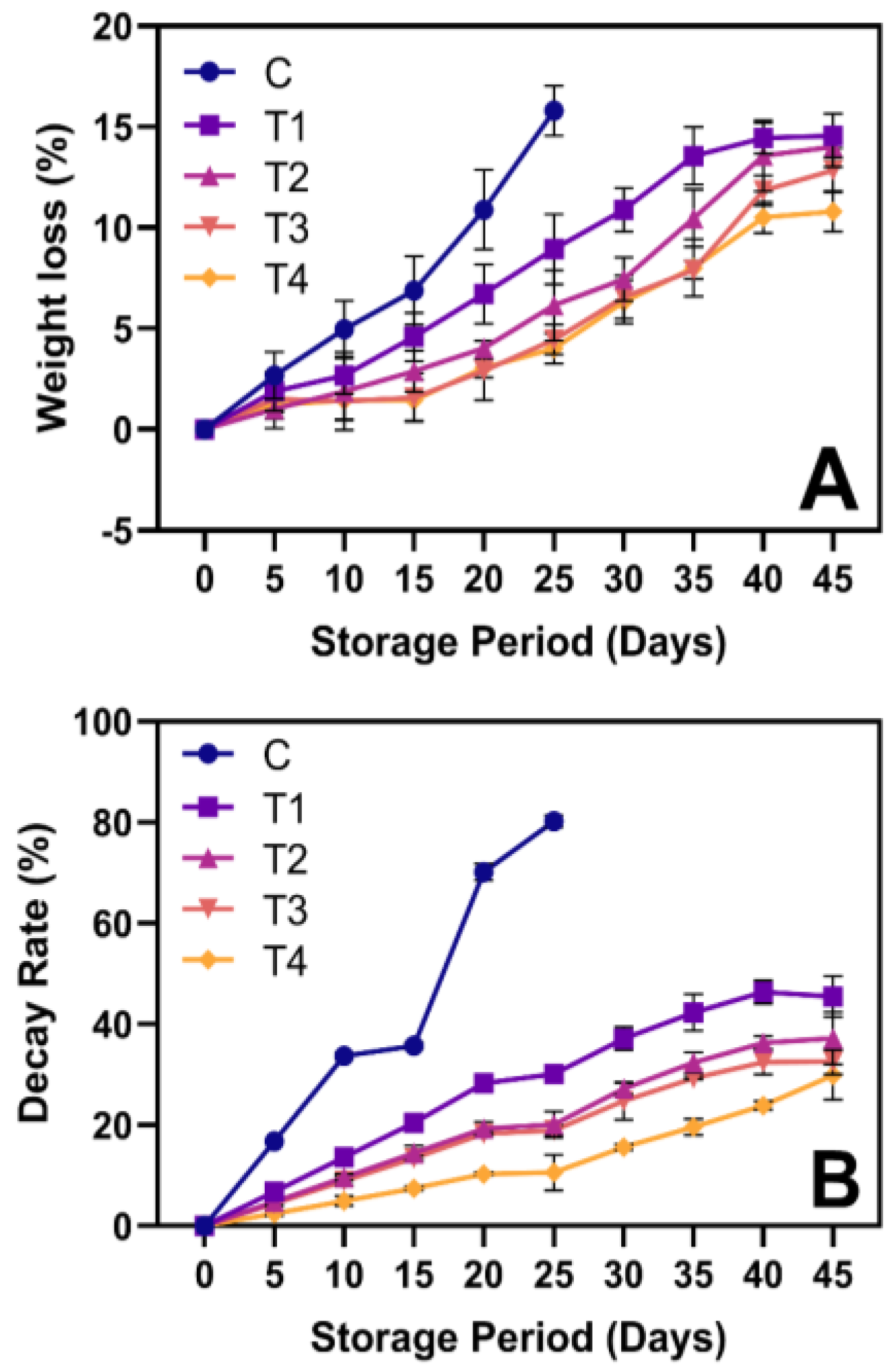
3.1. WL and DR
3.2. Color Characteristics
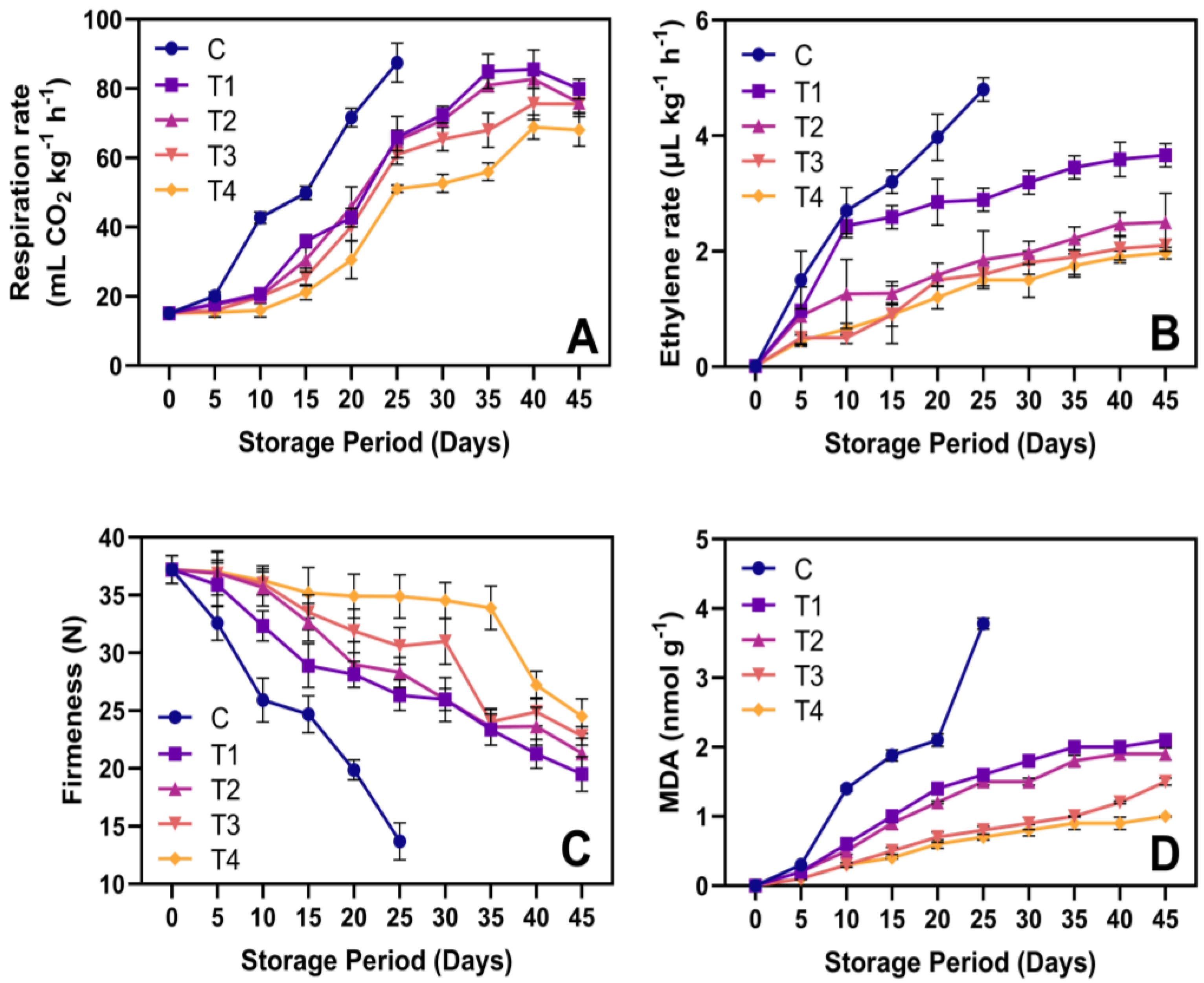
3.3. Respiration Rate, Ethylene Production, Firmness, and MDA Content
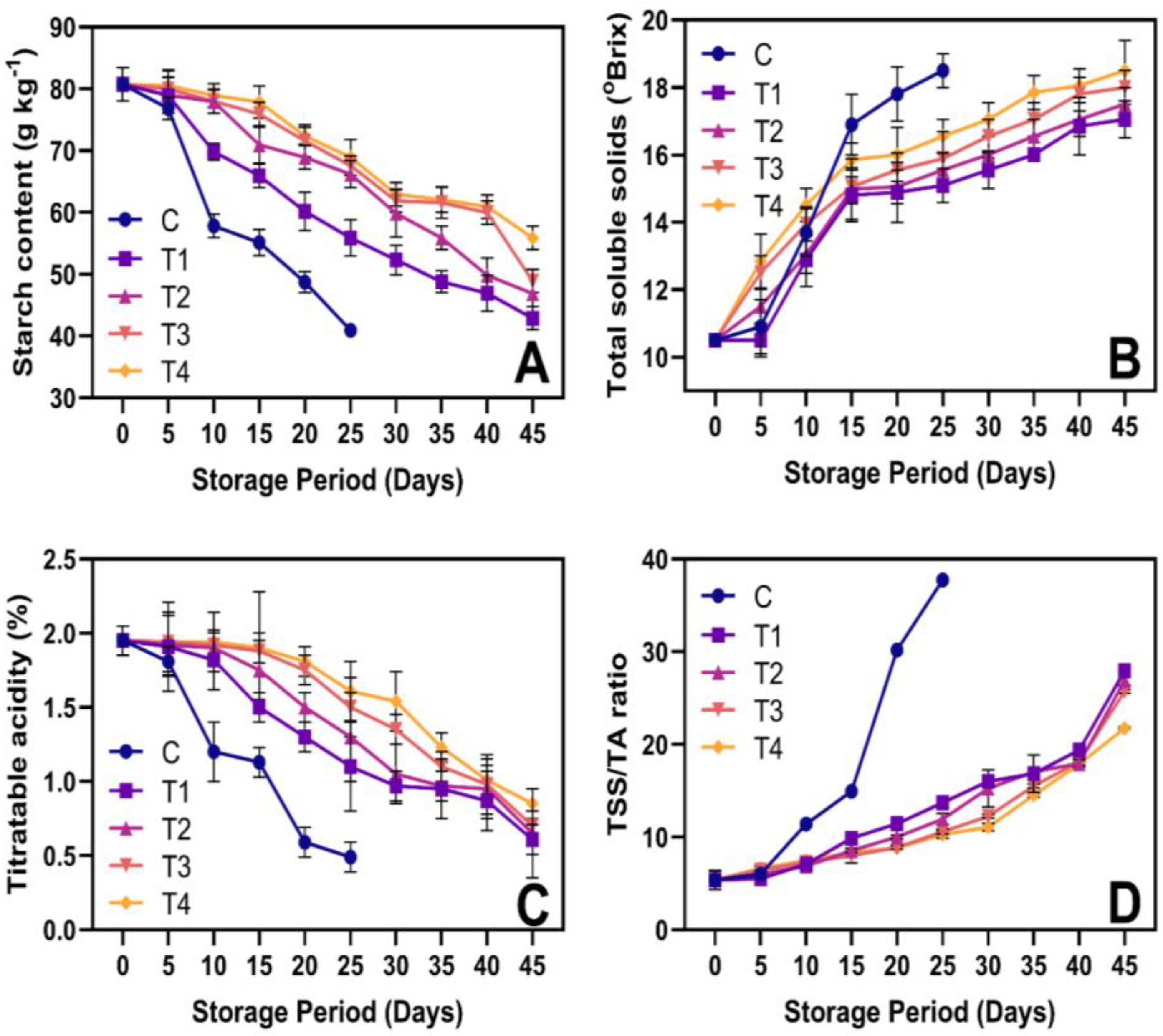
3.4. SC, TSS, TA, and TSS/TA Ratio
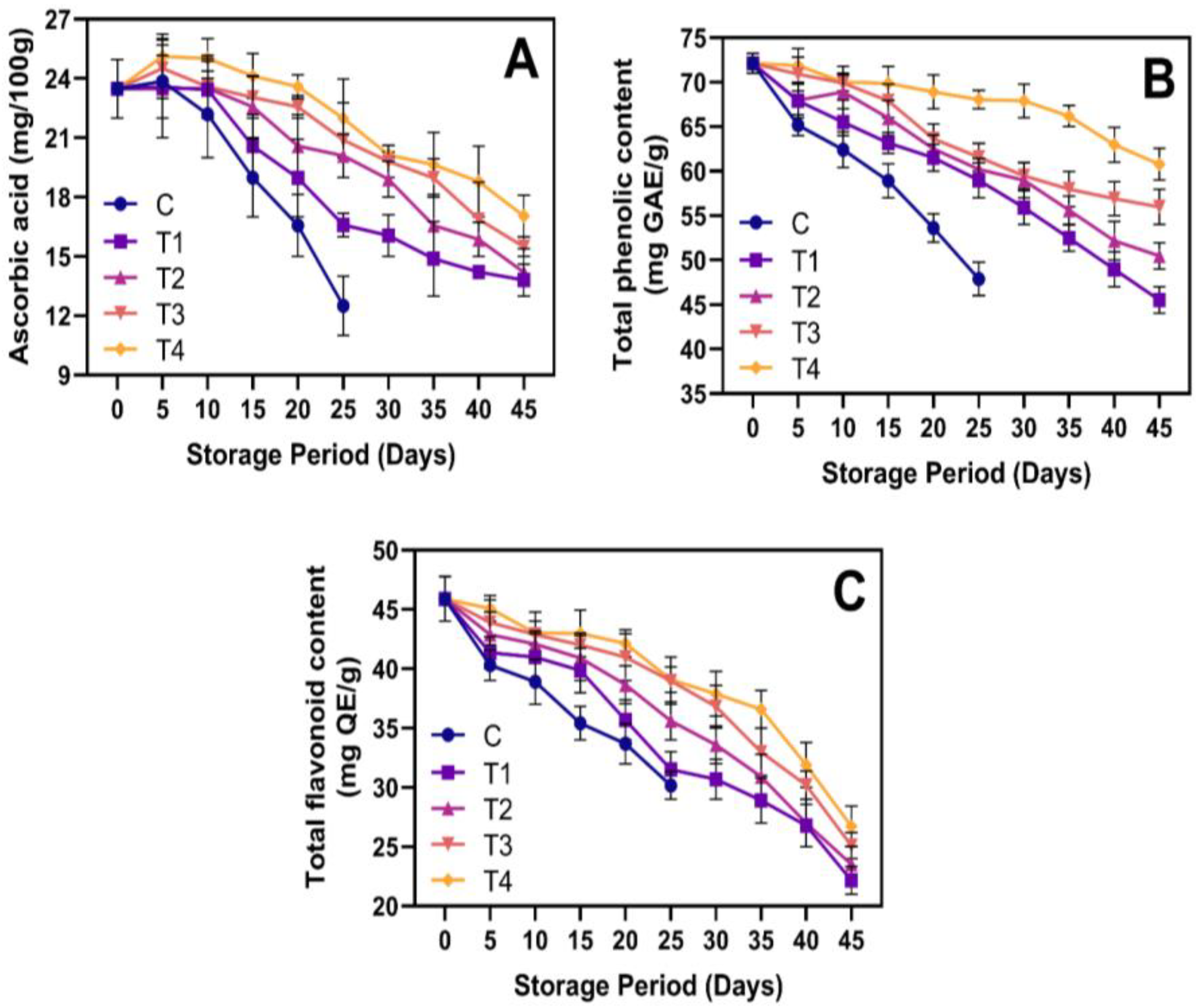
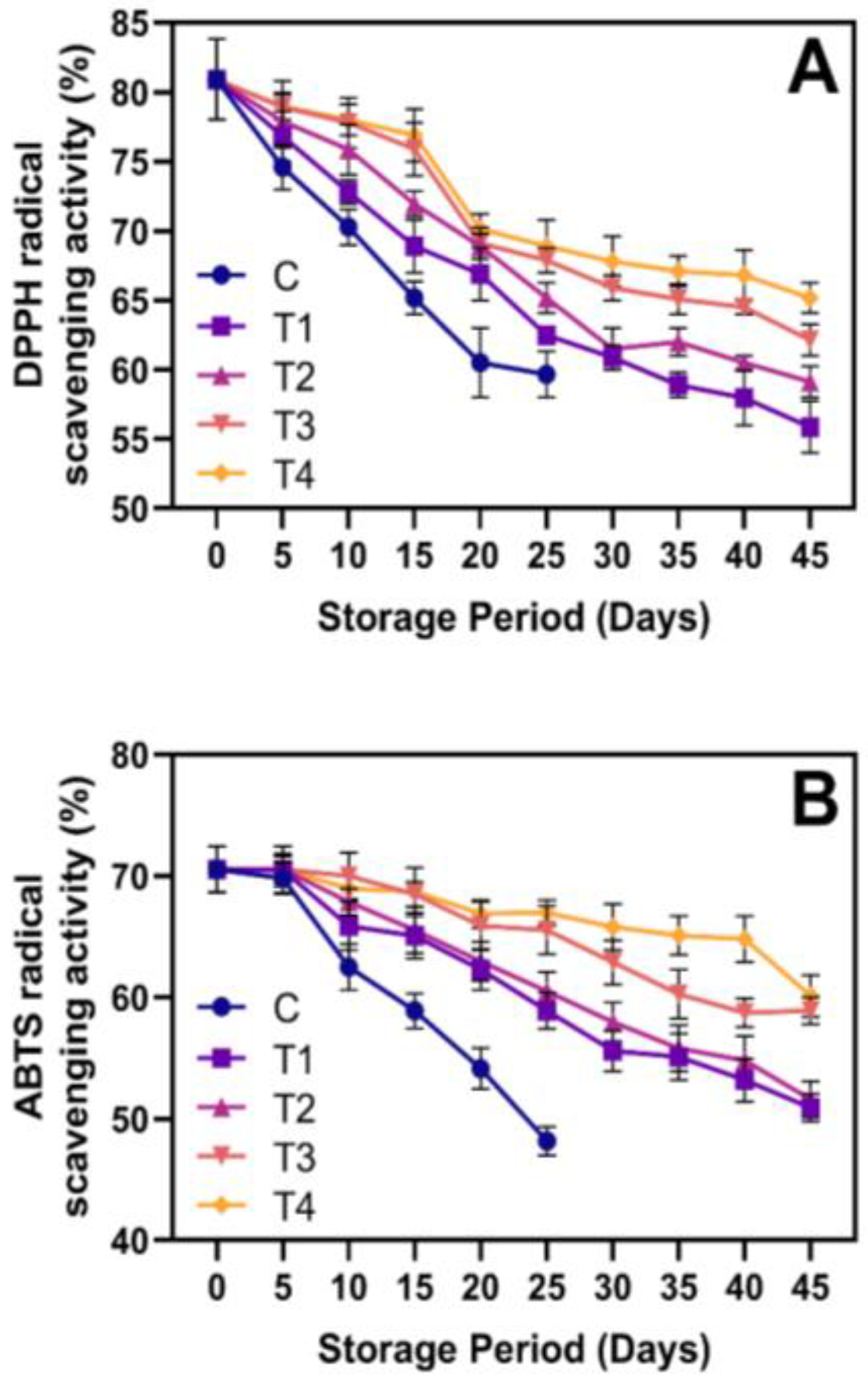
3.5. Phytochemicals and Antioxidant Activities
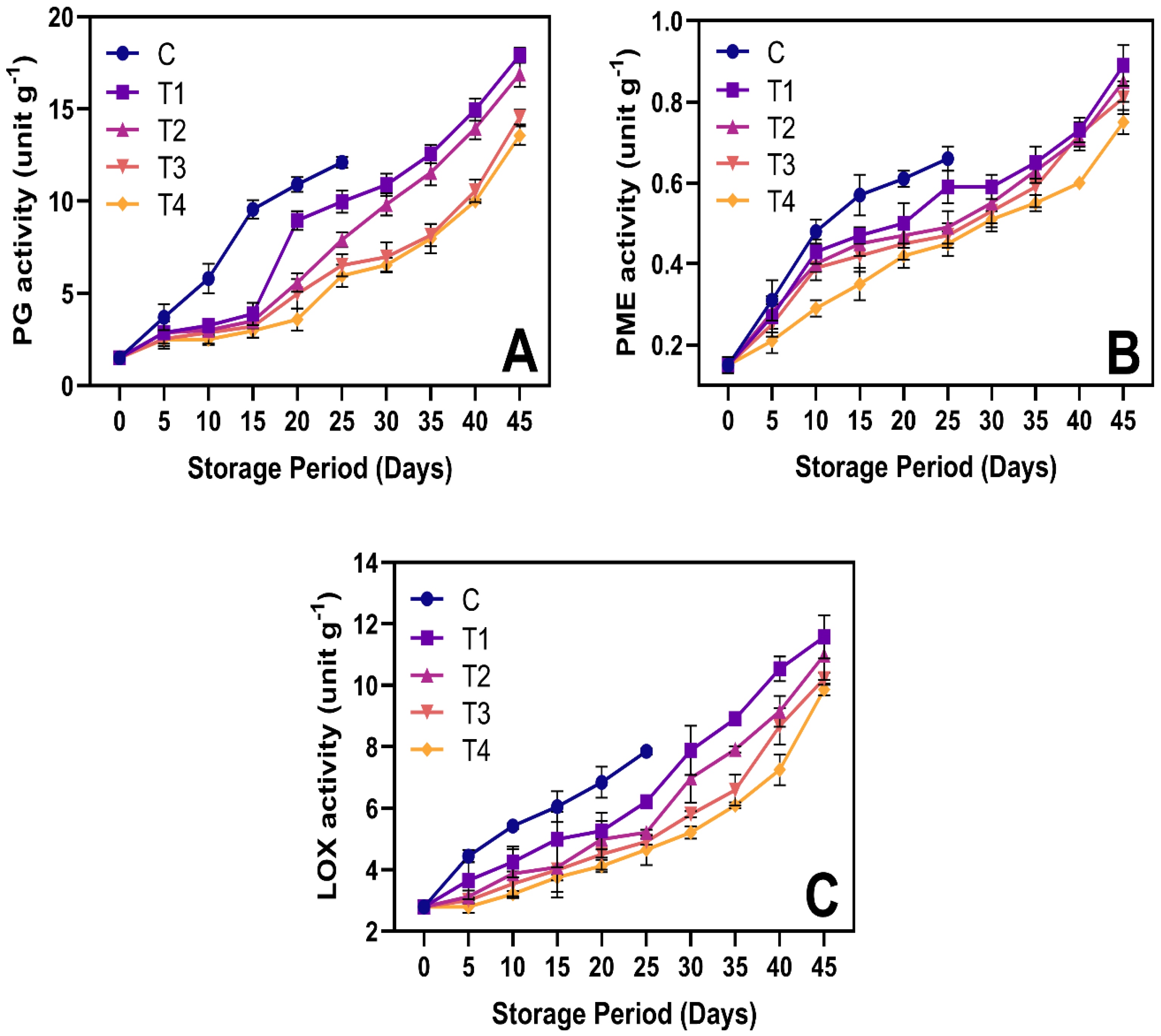
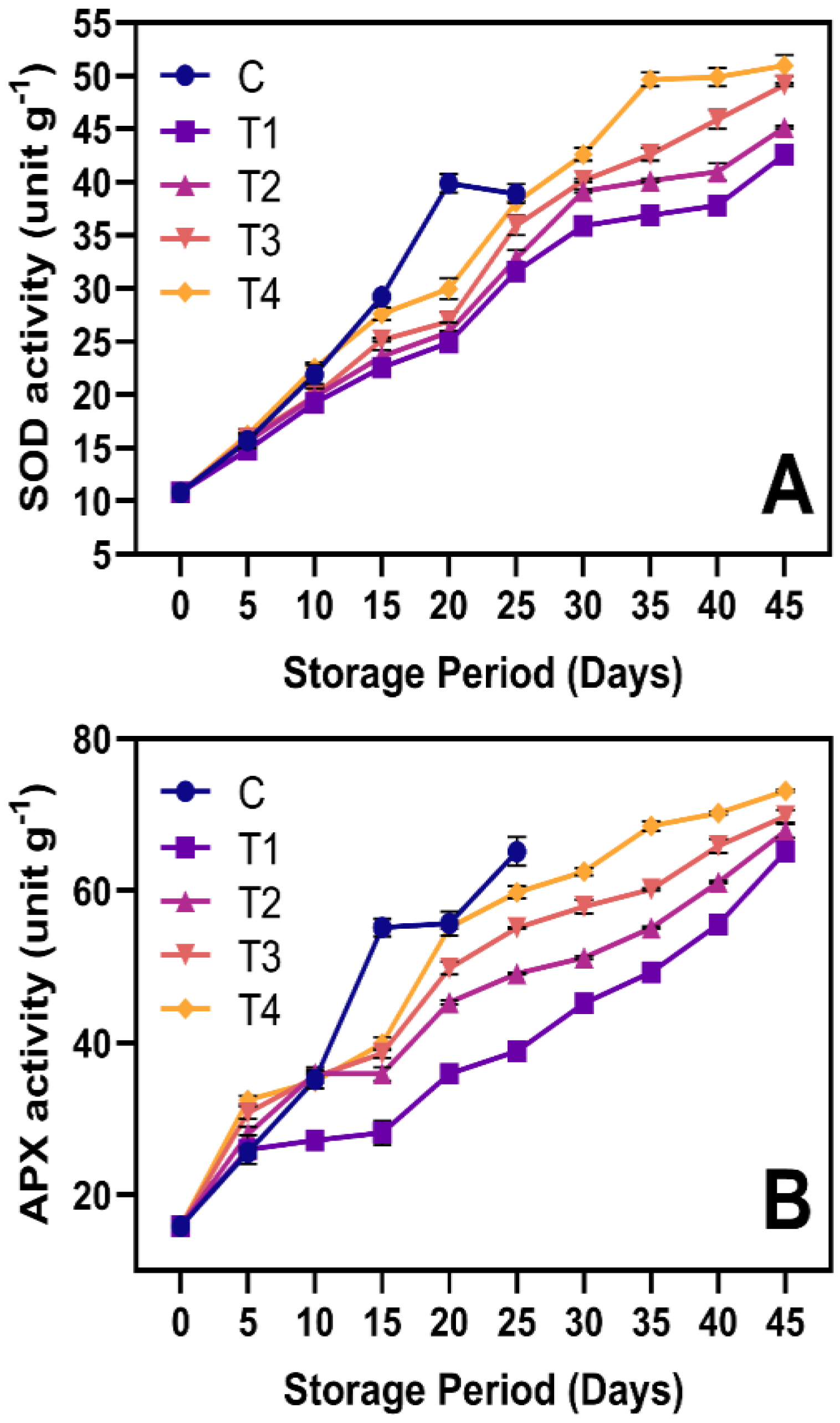
3.6. Enzyme Activities
3.6.1. Browning-Related Enzyme Activities
3.6.2. Cell Wall-Degrading Enzyme Activities
3.6.3. Antioxidant Activities
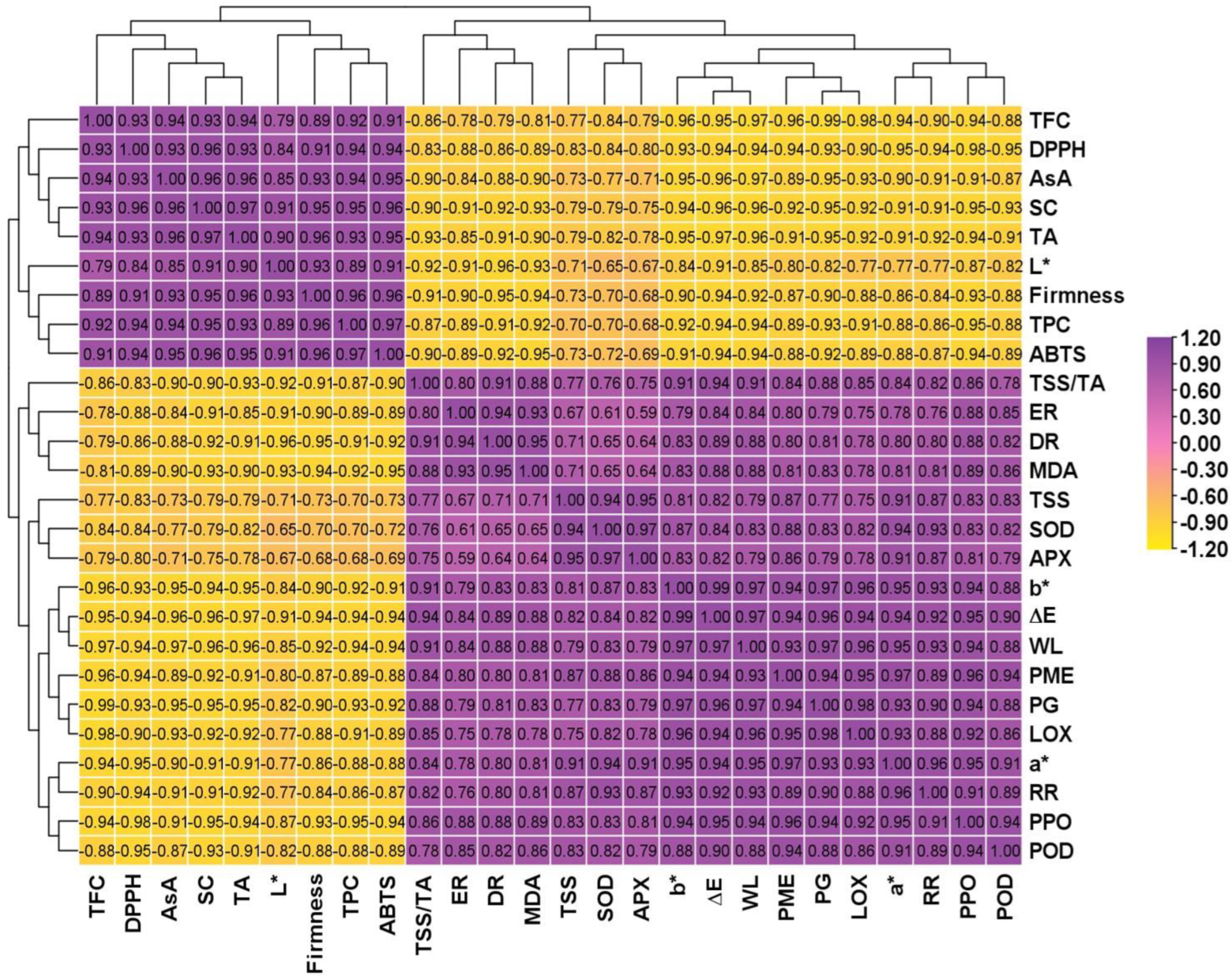
3.7. Correlation Coefficient and Hierarchical Clustering
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Priyadarsani, S.; Kar, A. Influence of temperature on natural ripening of mango. Indian J. Hortic. 2022, 79, 495–501. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Pareek, S.; Mani, S.; Domínguez-Avila, J.A.; González-Aguilar, G.A. A melatonin treatment delays postharvest senescence, maintains quality, reduces chilling injury, and regulates antioxidant metabolism in mango fruit. J. Food Qual. 2022, 1, 2379556. [Google Scholar] [CrossRef]
- Yeo, M.A.; Coulibaly, G.N.; Yeo, S.O.; Kouakou, K.L.; Gogbeu, D.G.L.; Kouakou, T.H.; Coulibaly, L. Changes of physico-chemical parameters in relation to storage time and temperature of mango fruit cv. Kent harvested in northern Côte d’Ivoire for export purposes. World J. Adv. Res. Rev. 2022, 16, 58–73. [Google Scholar] [CrossRef]
- Rastegar, S.; Khankahdani, H.H.; Rahimzadeh, M. Effects of melatonin treatment on the biochemical changes and antioxidant enzyme activity of mango fruit during storage. Sci. Hortic. 2020, 259, 108835. [Google Scholar] [CrossRef]
- Hailu, Z. Effects of controlled atmosphere storage and temperature on quality attributes of mango. J. Chem. Eng. Process Technol. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Dautt-Castro, M.; López-Virgen, A.G.; Ochoa-Leyva, A.; Contreras-Vergara, C.A.; Sortillón-Sortillón, A.P.; Martínez-Téllez, M.A.; González-Aguilar, G.A.; Casas-Flores, J.S.; Sañudo-Barajas, A.; Kuhn, D.N.; et al. Genome-wide identification of mango (Mangifera indica L.) polygalacturonases: Expression analysis of family members and total enzyme activity during fruit ripening. Front. Plant Sci. 2019, 10, 969. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, J.; Lu, X.; Huang, M.; Yu, W.; Li, C. The role of melatonin in delaying senescence and maintaining quality in postharvest horticultural products. Plant Biol. 2024, 27, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Khedr, E.H.; Khedr, N.; Abdel-Haleem, M. Harnessing the metabolic modulatory and antioxidant power of 1-(3-Phenyl-Propyl) cyclopropane and melatonin in maintaining mango fruit quality and prolongation storage life. BMC Plant Biol. 2023, 23, 464. [Google Scholar] [CrossRef]
- Njie, A.; Dong, X.; Liu, Q.; Lu, C.; Pan, X. Melatonin treatment inhibits mango fruit (Cv. ‘Guiqi’) softening by maintaining cell wall and reactive oxygen metabolisms during cold storage. Postharvest Biol. Technol. 2023, 205, 112500. [Google Scholar] [CrossRef]
- Kakaei, S.; Saba, M.K.; Mansouri, S.; Darvishi, H. Melatonin postharvest spray influences white mulberry browning, storage life, and biochemical changes. Postharvest Biol. Technol. 2024, 213, 112947. [Google Scholar] [CrossRef]
- Gao, H.; Lu, Z.; Yang, Y.; Wang, D.; Yang, T.; Cao, M.; Cao, W. Melatonin treatment reduces chilling injury in peach fruit through its regulation of membrane fatty acid contents and phenolic metabolism. Food Chem. 2018, 245, 659–666. [Google Scholar] [CrossRef]
- Dong, J.; Kebbeh, M.; Yan, R.; Huan, C.; Jiang, T.; Zheng, X. Melatonin treatment delays ripening in mangoes associated with maintaining the membrane integrity of fruit exocarp during postharvest. Plant Physiol. Biochem. 2021, 169, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Adeseko, C.J.; Sanni, D.M.; Lawal, O.T. Biochemical studies of enzyme-induced browning of African bush mango (Irvingia gabonensis) fruit pulp. Prep. Biochem. Biotechnol. 2022, 52, 835–844. [Google Scholar] [CrossRef]
- Wan, J.; Wu, Y.; Tong, Z.; Su, W.; Lin, H.; Fan, Z. Melatonin treatment alleviates chilling injury of Loquat fruit via modulating ROS metabolism. Foods 2024, 13, 3050. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Zhou, Q.; Yu, Y.; Chen, W.; Yang, Z.; Cao, S.; Shi, L. Melatonin treatment induces DNA methylation to alleviate chilling induced-browning in cold stored peach fruit. Postharvest Biol. Technol. 2024, 208, 112686. [Google Scholar] [CrossRef]
- Cao, S.; Qu, G.; Ma, C.; Ba, L.; Ji, N.; Meng, L.; Lei, J.; Wang, R. Effects of melatonin treatment on the physiological quality and cell wall metabolites in kiwifruit. Food Sci. Technol. 2021, 42, e85421. [Google Scholar] [CrossRef]
- Mandal, D.; Ennio, N.; Lalhruaitluangi, N.; Fanai, A.V. Response of melatonin on postharvest qualities and shelf life of pineapple cv. Kew at ambient storage. J. Appl. Nat. Sci. 2024, 16, 794–804. [Google Scholar] [CrossRef]
- Anchana, K.; Kavitha, C.; Shanmugasundaram, K.A.; Djanaguiraman, M.; Johnson, I. Role of exogenous melatonin in enhancing shelf life of traditional banana varieties. Int. J. Environ. Clim. Change 2023, 13, 992–998. [Google Scholar] [CrossRef]
- Caleb, O.; Mahajan, P.; Opara, U.; Witthuhn, C. Modeling the respiration rates of Pomegranate fruit and arils. Postharvest Biol. Technol. 2012, 64, 49–54. [Google Scholar] [CrossRef]
- Mesa, K.; Serra, S.; Masia, A.; Gagliardi, F.; Bucci, D.; Musacchi, S. Seasonal trends of starch and soluble carbohydrates in fruits and leaves of ‘Abbé Fétel’ pear trees and their relationship to fruit quality parameters. Sci. Hortic. 2016, 211, 60–69. [Google Scholar] [CrossRef]
- Keawpeng, I.; Paulraj, B.; Venkatachalam, K. Antioxidant and antimicrobial properties of mung bean phyto-film combined with longkong pericarp extract and sonication. Membranes 2022, 12, 379. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.S.S.; Shehata, A.S.F.; Banora, M.Y. Impact of some agrochemical products on early fruit drop of certain Egyptian mango cultivars induced by fungal infection. Egyptian J. Phytopathol. 2024, 52, 67–82. [Google Scholar] [CrossRef]
- Zhang, Y.; Huber, D.J.; Hu, M.; Jiang, G.; Gao, Z.; Xu, X.; Jiang, Y.; Zhang, Z. Delay of postharvest browning in litchi fruit by melatonin via the enhancing of antioxidative processes and oxidation repair. J. Agric. Food Chem. 2018, 66, 7475–7484. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, M.; Zhang, W.; Gao, Y.; Ma, X.; Cheng, S.; Chen, G. Exogenous melatonin activates the antioxidant system and maintains postharvest organoleptic quality in Hami melon (Cucumis. melo var. inodorus Jacq.). Front. Plant Sci. 2023, 14, 1274939. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Mei, J.; Xie, J. Effects of different carbon dioxide-modified atmosphere packaging and low-temperature storage at 13 ºC on the quality and metabolism in mango (Mangifera indica L.). Agriculture 2021, 11, 636. [Google Scholar] [CrossRef]
- Charoenphun, N.; Pham, N.H.; Rattanawut, J.; Venkatachalam, K. Exogenous application of melatonin on the preservation of physicochemical and enzymatic qualities of Pepper Fruit from chilling injury. Horticulturae 2024, 10, 550. [Google Scholar] [CrossRef]
- Qu, G.; Ba, L.; Wang, R.; LI, J.; Ma, C.; Ji, N.; Cao, S. Effects of melatonin on blueberry fruit quality and cell wall metabolism during low temperature storage. Food Sci. Technol. 2022, 42, e40822. [Google Scholar] [CrossRef]
- Lu, D.; Ren, Y.; Yan, T.; Jia, X.; Xu, H.; Yang, B.; Zhang, X.; He, J. Melatonin improves the postharvest anthracnose resistance of mango fruit by regulating antioxidant activity, the phenylpropane pathway and cell wall metabolism. Eur. J. Plant Pathol. 2024, 171, 17–36. [Google Scholar] [CrossRef]
- Xiao, Y.; Xie, J.; Wu, C.; He, J.; Wang, B. Effects of melatonin treatment on browning alleviation of fresh-cut foods. J. Food Biochem. 2021, 45, e13798. [Google Scholar] [CrossRef]
- Brizzolara, S.; Manganaris, G.A.; Fotopoulos, V.; Watkins, C.B.; Tonutti, P. Primary metabolism in fresh fruits during storage. Front. Plant Sci. 2020, 11, 80. [Google Scholar] [CrossRef]
- Sun, B.; Kuang, X.; Lin, H.; Lin, M.; Chen, Y.; Zeng, L.; Lin, Y.; Chen, Y.; Wang, H.; Fan, Z. The role of respiratory metabolism in chilling injury development of Chinese olive fruit during cold storage. Postharvest Biol. Technol. 2023, 205, 112489. [Google Scholar] [CrossRef]
- Burg, S.P.; Burg, E.A. Ethylene action and the ripening of fruits. Science 1965, 148, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Lal, M.K.; Tiwari, R.K.; Lal, P.; Kumar, A.; Kumar, R. Regulatory role of melatonin in post-harvest management of vegetables and fruits. In Melatonin in Plants: A Regulator for Plant Growth and Development; Kumar, R., Altaf, M.A., Lal, M.K., Tiwari, R.K., Eds.; Springer: Singapore, 2023; pp. 219–244. [Google Scholar] [CrossRef]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. 2004, 57, 453–455. [Google Scholar]
- Silva, A.P.F.B.; Nascimento, J.; Lajolo, F.; Cordenunsi, B. Starch mobilization and sucrose accumulation in the pulp of Keitt mangoes during postharvest ripening. J. Food Biochem. 2008, 32, 384–395. [Google Scholar] [CrossRef]
- Sivankalyani, V.; Sela, N.; Feygenberg, O.; Zemach, H.; Maurer, D.; Alkan, N. Transcriptome dynamics in mango fruit peel reveals mechanisms of chilling stress. Front. Plant Sci. 2016, 7, 1579. [Google Scholar] [CrossRef] [PubMed]
- Hor, S.; Léchaudel, M.; Mith, H.; Bugaud, C. Fruit density: A reliable indicator of sensory quality for mango. Sci. Hortic. 2020, 272, 109548. [Google Scholar] [CrossRef]
- Gill, P.P.S.; Jawandha, S.K.; Kaur, N.; Singh, N. Physico-chemical changes during progressive ripening of mango (Mangifera indica L.) cv. Dashehari under different temperature regimes. J. Food Sci. Technol. 2017, 54, 1964–1970. [Google Scholar] [CrossRef]
- Njie, A.; Zhang, W.E.; Dong, X.; Lu, C.; Pan, X.; Liu, Q. Effect of melatonin on fruit quality via decay inhibition and enhancement of antioxidative enzyme activities and genes expression of two mango cultivars during cold storage. Foods 2022, 11, 3209. [Google Scholar] [CrossRef]
- Malundo, T.; Shewfelt, R.; Ware, G.; Baldwin, E. Sugars and acids influence flavor properties of mango (Mangifera indica). J. Am. Soc. Hortic. Sci. 2001, 126, 115–121. [Google Scholar] [CrossRef]
- Wongkhot, A.; Rattanapanone, N.; Chanasut, U. BrimA, total acidity and total soluble solids correlate to total carotenoid content as indicators of the ripening process of six Thai mango fruit cultivars. CMU J. Nat. Sci. 2012, 11, 97–103. [Google Scholar]
- Medina-Santamarina, J.; Serrano, M.; Lorente-Mento, J.M.; García-Pastor, M.; Zapata, P.; Valero, D.; Guillén, F. Melatonin treatment of pomegranate trees increases crop yield and quality parameters at harvest and during storage. Agronomy 2021, 11, 861. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, J.; Xiang, M.; Zeng, J.; Chen, J.; Chen, M. Exogenous melatonin treatment affects ascorbic acid metabolism in postharvest ‘Jinyan’ kiwifruit. Front. Nutr. 2022, 9, 1081476. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Kul, R.; Esringü, A.; Dadasoglu, E.; Sahin, Ü.; Turan, M.; Örs, S.; Ekinci, M.; Agar, G.; Yildirim, E. Melatonin: Role in increasing plant tolerance in abiotic stress conditions. Abiotic Biot. Stress Plants 2019, 1, 19. [Google Scholar] [CrossRef]
- Junmatong, C.; Chomkitichai, W.; Boonyakiat, D.; Uthaibutra, J.; Saengnil, K. Reduction of Free Radical Content and Chilling Injury in ‘Nam Dok Mai no. 4’ Mango Fruit with Methyl Jasmonate during Low Temperature Storage. Acta Hortic. 2015, 1088, 107–112. [Google Scholar] [CrossRef]
- Foluso, A.O.; Makinde, A.; Adeyemi, I.; Timothy, V. Bioactive components, antioxidative properties and inhibition of Fe2+-induced lipid peroxidation of mango peel as affected by the storage of mango fruit. Int. J. Food Stud. 2016, 5, 131–145. [Google Scholar] [CrossRef]
- Koirala, P.; Chunhavacharatorn, P.; Suttisansanee, U.; Benjakul, S.; Katewongsa, K.; Al-Asmari, F.; Nirmal, N. Antioxidant and antimicrobial activities of mango peel and radish peel-a comparative investigation. Front. Sustain. Food Syst. 2024, 8, 1354393. [Google Scholar] [CrossRef]
- Umamahesh, K.; Sivudu, S.N.; Reddy, O.V.S. Evaluation of antioxidant activity, total phenolics and total flavonoids in peels of five cultivars of mango (Mangifera indica) fruit. J. Med. Plants Stud. 2016, 4, 200–203. [Google Scholar]
- Merhan, O. Biochemistry and antioxidant effects of melatonin. In Melatonin-Recent Updates; Gelen, V., Şengül, E., Kükürt, A., Eds.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Faizan, M.; Sultan, H.; Alam, P.; Karabulut, F.; Cheng, S.H.; Rajput, V.D.; Minkina, T.; Hayat, S.; Khan, M.N.; Nie, L. Melatonin and its cross-talk with other signaling molecules under abiotic stress. Plant Stress 2024, 11, 100410. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Y.; Liu, G.; Wang, C.; Jiang, J.; Yang, C. Cloning of ten peroxidase (POD) genes from Tamarix hispida and characterization of their responses to abiotic stress. Plant Mol. Biol. Rep. 2010, 28, 77–89. [Google Scholar] [CrossRef]
- Kebbeh, M.; Dong, J.-x.; Huan, C.; Liu, Y.; Zheng, X.-l. Melatonin treatment alleviates chilling injury in mango fruit ‘Keitt’by modulating proline metabolism under chilling stress. J. Integr. Agric. 2023, 22, 935–944. [Google Scholar] [CrossRef]
- García-Gago, J.A.; Posé, S.; Muñoz-Blanco, J.; Quesada, M.; Mercado, J. The polygalacturonase FaPG1 gene plays a key role in strawberry fruit softening. Plant Signal. Behav. 2009, 4, 766–768. [Google Scholar] [CrossRef] [PubMed]
- Jolie, R.P.; Duvetter, T.; Van Loey, A.V.; Hendrickx, M. Pectin methylesterase and its proteinaceous inhibitor: A review. Carbohydr. Res. 2010, 345, 2583–2595. [Google Scholar] [CrossRef] [PubMed]
- Bal, E. Impact of chitosan-melatonin composite coating on postharvest quality of sweet cherry. Appl. Fruit Sci. 2023, 66, 763–770. [Google Scholar] [CrossRef]
- Lester, G. Lipoxygenase activity of hypodermal- and middle-mesocarp tissues from netted muskmelon fruit during maturation and storage. J. Am. Soc. Hortic. Sci. 1990, 115, 612–615. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Wang, P.; Zhang, S. Role of melatonin in reducing chilling injury and maintaining quality in long green pepper fruit. Horticulturae 2023, 10, 550. [Google Scholar]
- Masia, A. Superoxide dismutase and catalase activities in apple fruit during ripening and post-harvest and with special reference to ethylene. Physiol. Plant. 1998, 104, 668–672. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charoenphun, N.; Lekjing, S.; Venkatachalam, K. Effect of Exogenous Melatonin Application on Maintaining Physicochemical Properties, Phytochemicals, and Enzymatic Activities of Mango Fruits During Cold Storage. Horticulturae 2025, 11, 222. https://doi.org/10.3390/horticulturae11020222
Charoenphun N, Lekjing S, Venkatachalam K. Effect of Exogenous Melatonin Application on Maintaining Physicochemical Properties, Phytochemicals, and Enzymatic Activities of Mango Fruits During Cold Storage. Horticulturae. 2025; 11(2):222. https://doi.org/10.3390/horticulturae11020222
Chicago/Turabian StyleCharoenphun, Narin, Somwang Lekjing, and Karthikeyan Venkatachalam. 2025. "Effect of Exogenous Melatonin Application on Maintaining Physicochemical Properties, Phytochemicals, and Enzymatic Activities of Mango Fruits During Cold Storage" Horticulturae 11, no. 2: 222. https://doi.org/10.3390/horticulturae11020222
APA StyleCharoenphun, N., Lekjing, S., & Venkatachalam, K. (2025). Effect of Exogenous Melatonin Application on Maintaining Physicochemical Properties, Phytochemicals, and Enzymatic Activities of Mango Fruits During Cold Storage. Horticulturae, 11(2), 222. https://doi.org/10.3390/horticulturae11020222






